Submitted:
12 November 2024
Posted:
15 November 2024
You are already at the latest version
Abstract
Keywords:
1. Introduction
1.1. Description and Epidemiology
1.2. Pathophysiology
1.3. Types of Glaucoma
1.4. Prevalence and Risk Factors
1.5. Therapeutic Options (Medical and Surgical)
2. Stem Cells Basics (Types, Sources, Advantages and Limitations)
3. Stem Cells in Glaucoma Treatment
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| AD- | Adipose Tissue |
| AD-MSCs | Adipose Tissue Mesenchymal Stem Cells |
| ADRC | Adipose-Derived Regenerative Cells |
| BDNF | Brain-Derived Neurotrophic Factor |
| BM- | Bone Marrow |
| BM-MSCs | Bone Marrow Mesenchymal Stem Cells |
| CD | Clusters of Differentiation |
| CM | Conditioned Media |
| CNS | Central Nervous System |
| CNTF | Ciliary Neurotrophic Factor |
| cUC-MSCs | Canine Umbilical Cord Mesenchymal Stem Cells |
| EGF | Epidermal Growth Factor |
| ESCs | Embryonic Stem Cells |
| FDA | Food and Drug Administration |
| FGF | Fibroblast Growth Factor |
| HLA | Human Leukocyte Antigen |
| HSCs | Hematopoietic Stem Cells |
| ICTRP | International Clinical studies Registry Platform |
| IGF-1 | Insulin-like Growth Factor 1 |
| IL-6/-8 | Interleukin-6/8 |
| IOP | Intraocular Pressure |
| iPSCs | Induced Pluripotent Stem Cells |
| ISCT | International Society for Cellular Therapy |
| MHC-I/-II | Major histocompatibility complex I/II |
| MSCs | Mesenchymal Stem Cells |
| MYOC | Myocilin |
| ONH | Optic Nerve Head |
| OPTN | Optineurin |
| PACG | Primary Angle-Closure Glaucoma |
| POAG | Primary Open-Angled Glaucoma |
| PRF | Platelet-rich Fibrin |
| RGCs | Retinal Ganglion CellsTBK1 |
| TANK-binding kinase 1 | |
| TGF-β1 | Transforming Growth Factor-beta 1 |
| TNF-α | Tumor Necrosis Factor Alpha |
| UC | Umbilical Cord |
| UC-MSCs | Umbilical Cord Mesenchymal Stem Cells |
| UCB | Umbilical cord blood |
| VEGF-A | Vascular Endothelial Growth Factor A |
| VMDB | Veterinary Medical Data Base |
References
- Quigley, H. A. Glaucoma: Macrocosm to Microcosm. The Friedenwald Lecture. Invest Ophthalmol Vis Sci 2005, 46 (8), 2663–2670. [CrossRef]
- Tham, Y. C.; Li, X.; Wong, T. Y.; Quigley, H. A.; Aung, T.; Cheng, C. Y. Global Prevalence of Glaucoma and Projections of Glaucoma Burden through 2040: A Systematic Review and Meta-Analysis. Ophthalmology 2014, 121 (11), 2081–2090. [CrossRef]
- Quigley, H.; Broman, A. T. The Number of People with Glaucoma Worldwide in 2010 and 2020. British Journal of Ophthalmology 2006, 90 (3), 262–267. [CrossRef]
- Gelatt, K. N.; MacKay, E. O. Secondary Glaucomas in the Dog in North America. Vet Ophthalmol 2004, 7 (4), 245–259. [CrossRef]
- Gelatt, K. N.; MacKay, E. O. Prevalence of the Breed-Related Glaucomas in Pure-Bred Dogs in North America. Vet Ophthalmol 2004, 7 (2), 97–111. [CrossRef]
- Gelatt, K. N.; Whitley, R. D. Surgery of the Orbit. Veterinary Ophthalmic Surgery 2011, 51–88. [CrossRef]
- Frezzotti, R. The Glaucoma Mystery from Ancient Times to the 21st Century. The Glaucoma Mystery: Ancient Concepts. Acta Ophthalmol Scand Suppl 2000, 78 (232), 14–18. [CrossRef]
- Reinstein, S. L.; Rankin, A. J.; Allbaugh, R. Canine Glaucoma: Pathophysiology and Diagnosis. Compend Contin Educ Vet 2009, 31 (10).
- Wrześniewska, K.; Madany, J.; Winiarczyk, D. Comparison of Intraocular Pressure Measurement with Schiotz Tonometer and Tono-Pen Vet Tonometer in Healthy Dogs. J Vet Res 2018, 62 (2), 243–247. [CrossRef]
- Pizzirani, S. Definition, Classification, and Pathophysiology of Canine Glaucoma. Vet Clin North Am Small Anim Pract 2015, 45 (6), 1127–1157. [CrossRef]
- Dielemans, I.; Vingerling, J. R.; Wolfs, R. C. W.; Hofman, A.; Grobbee, D. E.; de Jong, P. T. V. M. The Prevalence of Primary Open-Angle Glaucoma in a Population-Based Study in The Netherlands. The Rotterdam Study. Ophthalmology 1994, 101 (11), 1851–1855. [CrossRef]
- Trick, G. L. Visual Dysfunction in Normotensive Glaucoma. Doc Ophthalmol 1993, 85 (2), 125–133. [CrossRef]
- Komaromy, A. M.; Sherwood, M. B.; Dawson, W. W.; Sapp, H. L.; Nelson, G.; Kubilis, P. S.; Brooks, D. E. Diurnal Intraocular Pressure Curves in Healthy Rhesus Macaques (Macaca Mulatta) and Rhesus Macaques With Normotensive and Hypertensive Primary Open-Angle Glaucoma. J Glaucoma 1998, 2 (7), 131.
- Johnson, M.; McLaren, J. W.; Overby, D. R. Unconventional Aqueous Humor Outflow: A Review. Experimental Eye Research. Academic Press May 1, 2017, pp 94–111. [CrossRef]
- Chang, E. E.; Goldberg, J. L. Glaucoma 2.0: Neuroprotection, Neuroregeneration, Neuroenhancement. Ophthalmology 2012, 119 (5), 979. [CrossRef]
- Maggio, F. Glaucomas. Top Companion Anim Med 2015, 30 (3), 86–96. [CrossRef]
- Morgan, R. Glaucoma in Dogs and Cats. https://veterinarypartner.vin.com/doc/?id=6097123&pid=19239.
- Slater, M. R.; Erb, H. N. Effects of Risk Factors and Prophylactic Treatment on Primary Glaucoma in the Dog. J Am Vet Med Assoc 1986, 188 (9), 1028–1030.
- Mendicino, M.; Bailey, A. M.; Wonnacott, K.; Puri, R. K.; Bauer, S. R. Cell Stem Cell Forum MSC-Based Product Characterization for Clinical Trials: An FDA Perspective. 2014. [CrossRef]
- Gelatt, K. N.; MacKay, E. O. Prevalence of the Breed-Related Glaucomas in Pure-Bred Dogs in North America. Vet Ophthalmol 2004, 7 (2), 97–111. [CrossRef]
- Gelatt, K. N.; Mackay, E. O. Prevalence of the Breed-Related Glaucomas in Pure-Bred Dogs in North America; 2004; Vol. 7.
- Soundarya, T. C.; Kshama, M. A.; Aishwarya, N. Studies on Occurrence of Ocular Diseases in Dogs with Emphasis on Occurrence of Glaucoma. Int J Curr Microbiol Appl Sci 2020, 9 (2), 2787–2795. [CrossRef]
- Sigle, K.; Nasisse, M. Long-Term Complications after Phacoemulsification for Cataract Removal in Dogs: 172 Cases (1995–2002); 2006.
- Biros, D.; Gelatt, K.; Brooks, D.; Kubilis, P.; Andrew, S.; Strubbe, D.; Whigham, H. Development of Glaucoma after Cataract Surgery in Dogs: 220 Cases (1987–1998); 2000.
- Lannek, EB.; Miller, P. Development of Glaucoma after Phacoemulsification for Removal of Cataracts in Dogs: 22 Cases (1987–1997); 2001.
- Scott, E. M.; Esson, D. W.; Fritz, K. J.; Dubielzig, R. R. Major Breed Distribution of Canine Patients Enucleated or Eviscerated Due to Glaucoma Following Routine Cataract Surgery as Well as Common Histopathologic Findings within Enucleated Globes. Vet Ophthalmol 2013, 16 (SUPPL.1), 64–72. [CrossRef]
- Foote, B. C.; Pederson, S. L.; Welihozkiy, A.; Stine, J. M.; Carastro, S. M.; Andrew, S. E.; Michau, T. M. Retinal Detachment and Glaucoma in the Boston Terrier and Shih Tzu Following Phacoemulsification (135 Patients): 2000–2014. Vet Ophthalmol 2018, 21 (3), 240–248. [CrossRef]
- Newbold, G. M.; Kelch, W. J.; Chen, T.; Ward, D. A.; Hendrix, D. V. H. Phacoemulsification Outcomes in Boston Terriers as Compared to Non-Boston Terriers: A Retrospective Study (2002–2015). Vet Ophthalmol 2018, 21 (4), 353–361. [CrossRef]
- Moeller, E.; Blocker, T.; Esson, D.; Madsen, R. Postoperative Glaucoma in the Labrador Retriever: Incidence, Risk Factors, and Visual Outcome Following Routine Phacoemulsification. Vet Ophthalmol 2011, 14 (6), 385–394. [CrossRef]
- Beamer, G.; Reilly, C. M.; Pizzirani, S. Microscopic Lesions in Canine Eyes with Primary Glaucoma. Vet Clin North Am Small Anim Pract 2015, 45 (6), 1213–1233. [CrossRef]
- Miller, P. E.; Bentley, E. Clinical Signs and Diagnosis of the Canine Primary Glaucomas. Vet Clin North Am Small Anim Pract 2015, 45 (6), 1183–1212. [CrossRef]
- Wiggs, J. L.; Pasquale, L. R. Genetics of Glaucoma. Human Molecular Genetics. Oxford University Press August 1, 2017, pp R21–R27. [CrossRef]
- Cohen, C. S.; Allingham, R. R. The Dawn of Genetic Testing for Glaucoma; 2004. www.theorator.
- Challa, P. Glaucoma Genetics: Advancing New Understandings of Glaucoma Pathogenesis.
- Almasieh, M.; Zhou, Y.; Casanova, C.; Polo, A. Di. Structural and Functional Neuroprotection in Glaucoma: Role of Galantamine-Mediated Activation of Muscarinic Acetylcholine Receptors. Cell Death Dis 2010, 27. [CrossRef]
- Mandell, D. C. Ophthalmic Emergencies. Clin Tech Small Anim Pract 2000, 15 (2), 94–100. [CrossRef]
- Alario, A. F.; Strong, T. D.; Pizzirani, S. Medical Treatment of Primary Canine Glaucoma. Vet Clin North Am Small Anim Pract 2015, 45 (6), 1235–1259. [CrossRef]
- Willis, A. M.; Diehl, K. A.; Robbin, T. E. Advances in Topical Glaucoma Therapy. Vet Ophthalmol 2002, 5 (1), 9–17. [CrossRef]
- U.S. Food and Drug Administration. Latanoprostene Bunod (VyzultaTM) and Netarsudil (RhopressaTM): Drug Approval Package. https://www.fda.gov/drugs/development-approval-process-drugs/drug-approvals-and-databases (accessed 2024-09-25).
- Krauss, A. H. P.; Impagnatiello, F.; Toris, C. B.; Gale, D. C.; Prasanna, G.; Borghi, V.; Chiroli, V.; Chong, W. K. M.; Carreiro, S. T.; Ongini, E. Ocular Hypotensive Activity of BOL-303259-X, a Nitric Oxide Donating Prostaglandin F2α Agonist, in Preclinical Models. Exp Eye Res 2011, 93 (3), 250–255. [CrossRef]
- Borghi, V.; Bastia, E.; Guzzetta, M.; Chiroli, V.; Toris, C. B.; Batugo, M. R.; Carreiro, S. T.; Chong, W. K. M.; Gale, D. C.; Kucera, D. J.; Jia, L.; Prasanna, G.; Ongini, E.; Krauss, A. H. P.; Impagnatiello, F. A Novel Nitric Oxide Releasing Prostaglandin Analog, NCX 125, Reduces Intraocular Pressure in Rabbit, Dog, and Primate Models of Glaucoma. J Ocul Pharmacol Ther 2010, 26 (2), 125–131. [CrossRef]
- Impagnatiello, F.; Borghi, V.; Gale, D. C.; Batugo, M.; Guzzetta, M.; Brambilla, S.; Carreiro, S. T.; Chong, W. K. M.; Prasanna, G.; Chiroli, V.; Ongini, E.; Krauss, A. H. P. A Dual Acting Compound with Latanoprost Amide and Nitric Oxide Releasing Properties, Shows Ocular Hypotensive Effects in Rabbits and Dogs. Exp Eye Res 2011, 93 (3), 243–249. [CrossRef]
- Cavet, M. E.; Decory, H. H. The Role of Nitric Oxide in the Intraocular Pressure Lowering Efficacy of Latanoprostene Bunod: Review of Nonclinical Studies. J Ocul Pharmacol Ther 2018, 34 (1–2), 52–60. [CrossRef]
- Lin, C. W.; Sherman, B.; Moore, L. A.; Laethem, C. L.; Lu, D. W.; Pattabiraman, P. P.; Rao, P. V.; Delong, M. A.; Kopczynski, C. C. Discovery and Preclinical Development of Netarsudil, a Novel Ocular Hypotensive Agent for the Treatment of Glaucoma. J Ocul Pharmacol Ther 2018, 34 (1–2), 40–51. [CrossRef]
- Rao, P. V.; Pattabiraman, P. P.; Kopczynski, C. Role of the Rho GTPase/Rho Kinase Signaling Pathway in Pathogenesis and Treatment of Glaucoma: Bench to Bedside Research. Exp Eye Res 2017, 158, 23–32. [CrossRef]
- Wang, S. K.; Chang, R. T. An Emerging Treatment Option for Glaucoma: Rho Kinase Inhibitors. Clin Ophthalmol 2014, 8, 883–890. [CrossRef]
- Rao, P. V.; Deng, P.-F.; Kumar, J.; Epstein, D. L. Modulation of Aqueous Humor Outflow Facility by the Rho Kinase-Specific Inhibitor Y-27632.
- Kiel, J. W.; Kopczynski, C. C. Effect of AR-13324 on Episcleral Venous Pressure in Dutch Belted Rabbits. J Ocul Pharmacol Ther 2015, 31 (3), 146–151. [CrossRef]
- Miyagi, H.; Kim, S.; Li, J.; Murphy, C. J.; Thomasy, S. M. Topical Rho-Associated Kinase Inhibitor, Y27632, Accelerates Corneal Endothelial Regeneration in a Canine Cryoinjury Model. Cornea 2019, 38 (3), 352–359. [CrossRef]
- U.S. Food and Drug Administration. RocklatanTM: Drug Approval Package. https://www.fda.gov/drugs/development-approval-process-drugs/drug-approvals-and-databases (accessed 2024-09-25).
- U.S. Food and Drug Administration. DurystaTM: Drug Approval Package. https://www.fda.gov/drugs/development-approval-process-drugs/drug-approvals-and-databases (accessed 2024-10-01).
- U.S. Food and Drug Administration. OmlontiTM (Omidenepag Isopropyl): Drug Approval Package. https://www.fda.gov/drugs/development-approval-process-drugs/drug-approvals-and-databases (accessed 2024-10-01).
- U.S. Food and Drug Administration. iDose® TR: Drug Approval Package. https://www.fda.gov/drugs/development-approval-process-drugs/drug-approvals-and-databases (accessed 2024-10-03).
- Schehlein, E. M.; Novack, G.; Robin, A. L. New Pharmacotherapy for the Treatment of Glaucoma. Expert Opinion on Pharmacotherapy. Taylor and Francis Ltd. December 12, 2017, pp 1939–1946. [CrossRef]
- Lin, T. Y.; Lai, Y. F.; Chen, Y. H.; Lu, D. W. The Latest Evidence of Erythropoietin in the Treatment of Glaucoma. International Journal of Molecular Sciences. MDPI December 1, 2022. [CrossRef]
- Silva, B.; Gonçalves, L. M.; Braz, B. S.; Delgado, E. Chitosan and Hyaluronic Acid Nanoparticles as Vehicles of Epoetin Beta for Subconjunctival Ocular Delivery. Mar Drugs 2022, 20 (2). [CrossRef]
- Silva, B.; Gonçalves, L. M.; Braz, B. S.; Delgado, E. Topical Administration of a Nanoformulation of Chitosan-Hyaluronic Acid-Epoetin Beta in a Rat Model of Glaucoma. Pharmaceuticals (Basel) 2023, 16 (2). [CrossRef]
- Silva, B.; Marto, J.; Braz, B. S.; Delgado, E.; Almeida, A. J.; Gonçalves, L. New Nanoparticles for Topical Ocular Delivery of Erythropoietin. Int J Pharm 2020, 576. [CrossRef]
- Silva, B.; Gonçalves, L. M.; São Braz, B.; Delgado, E. Topical Ocular Delivery of Nanoparticles with Epoetin Beta in Wistar Hannover Rats. Sci Rep 2023, 13 (1). [CrossRef]
- Resende, A. P.; Rosolen, S. G.; Nunes, T.; São Braz, B.; Delgado, E. Functional and Structural Effects of Erythropoietin Subconjunctival Administration in Glaucomatous Animals. Biomed Hub 2018, 3 (2), 1–11. [CrossRef]
- Beidoe, G.; Mousa, S. A. Current Primary Open-Angle Glaucoma Treatments and Future Directions. Clinical Ophthalmology. October 19, 2012, pp 1699–1707. [CrossRef]
- Mckinnon, S.; Goldberg, L. D.; Peeples, P.; Walt, J.; Bramley, T. Current Management of Glaucoma and the Need for Complete Therapy. American Journal of Managed Care 2008.
- Okeke, C. O.; Quigley, H. A.; Jampel, H. D.; Ying, G. shuang; Plyler, R. J.; Jiang, Y.; Friedman, D. S. Adherence with Topical Glaucoma Medication Monitored Electronically the Travatan Dosing Aid Study. Ophthalmology 2009, 116 (2), 191–199. [CrossRef]
- Friedman, D. S.; Quigley, H. A.; Gelb, L.; Tan, J.; Margolis, J.; Shah, S. N.; Kim, E. E.; Zimmerman, T.; Hahn, S. R. Using Pharmacy Claims Data to Study Adherence to Glaucoma Medications: Methodology and Findings of the Glaucoma Adherence and Persistency Study (GAPS). Invest Ophthalmol Vis Sci 2007, 48 (11), 5052–5057. [CrossRef]
- Miller, P. E.; Schmidt, G. M.; Vainisi, S. J.; Swanson, J. F.; Herrmann, M. K. The Efficacy of Topical Prophylactic Antiglaucoma Therapy in Primary Closed Angle Glaucoma in Dogs: A Multicenter Clinical Trial. J Am Anim Hosp Assoc 2000, 36 (5), 431–438. [CrossRef]
- Aref, A. A. Sustained Drug Delivery for Glaucoma: Current Data and Future Trends. Curr Opin Ophthalmol 2017, 28 (2), 169–174. [CrossRef]
- Brandt, J. D.; DuBiner, H. B.; Benza, R.; Sall, K. N.; Walker, G. A.; Semba, C. P. Long-Term Safety and Efficacy of a Sustained-Release Bimatoprost Ocular Ring. Ophthalmology 2017, 124 (10), 1565–1566. [CrossRef]
- Lee, S. S.; Burke, J.; Shen, J.; Almazan, A.; Orilla, W.; Hughes, P.; Zhang, J.; Li, H.; Struble, C.; Miller, P. E.; Robinson, M. R. Bimatoprost Sustained-Release Intracameral Implant Reduces Episcleral Venous Pressure in Dogs. Vet Ophthalmol 2018, 21 (4), 376–381. [CrossRef]
- Komaromy, A. M.; Koehl, K.; Harman, C. D.; Stewart, G.; Wolinski, N.; Norris, T. N.; Valade, D.; Chekhtman, I.; Lambert, J. N.; Donohue, A. C.; Tait, R. Long-Term Intraocular Pressure (IOP) Control by Means of a Novel Biodegradable Intracameral (IC) Latanoprost Free Acid (LFA) Implant. Invest Ophthalmol Vis Sci 2017, 58 (8), 4591–4591.
- Robeson, R.; Verhoeven, R. S.; Garcia, A.; Das, S.; Hamby, K.; Hernandez, M.; Gum, G. G.; Yerxa, B. R.; Navratil, T. A 12-Month Study of the ENV515 (Travoprost) Intracameral Implant on Intraocular Pressure in Beagle Dogs. Invest Ophthalmol Vis Sci 2017, 58 (8), 1072–1072.
- Seal, J. R.; Robinson, M. R.; Burke, J.; Bejanian, M.; Coote, M.; Attar, M. Intracameral Sustained-Release Bimatoprost Implant Delivers Bimatoprost to Target Tissues with Reduced Drug Exposure to Off-Target Tissues. J Ocul Pharmacol Ther 2019, 35 (1), 50–57. [CrossRef]
- Barachetti, L.; Rampazzo, A.; Mortellaro, C. M.; Scevola, S.; Gilger, B. C. Use of Episcleral Cyclosporine Implants in Dogs with Keratoconjunctivitis Sicca: Pilot Study. Vet Ophthalmol 2015, 18 (3), 234–241. [CrossRef]
- Graham, K. L.; Hall, E. J. S.; Caraguel, C.; White, A.; Billson, F. A.; Billson, F. M. Comparison of Diode Laser Trans-Scleral Cyclophotocoagulation versus Implantation of a 350-Mm2 Baerveldt Glaucoma Drainage Device for the Treatment of Glaucoma in Dogs (a Retrospective Study: 2010-2016). Vet Ophthalmol 2018, 21 (5), 487–497. [CrossRef]
- Bras, D.; Maggio, F. Surgical Treatment of Canine Glaucoma: Cyclodestructive Techniques. Vet Clin North Am Small Anim Pract 2015, 45 (6), 1283–1305. [CrossRef]
- Pumphrey, S. Canine Secondary Glaucomas. Vet Clin North Am Small Anim Pract 2015, 45 (6), 1335–1364. [CrossRef]
- Komáromy, A. M.; Bras, D.; Esson, D. W.; Fellman, R. L.; Plummer, C. E.; Sapienza, J. S.; Storey, E. S.; Teixeira, L. B.; Toris, C. B.; Webb, T. R.; András Komáromy, C. M. The Future of Canine Glaucoma Therapy. 2019. [CrossRef]
- Komáromy, A. M.; Bras, D.; Esson, D. W.; Fellman, R. L.; Grozdanic, S. D.; Kagemann, L.; Miller, P. E.; Moroi, S. E.; Plummer, C. E.; Sapienza, J. S.; Storey, E. S.; Teixeira, L. B.; Toris, C. B.; Webb, T. R. The Future of Canine Glaucoma Therapy. Vet Ophthalmol 2019, 22 (5), 726–740. [CrossRef]
- Evans, M. J.; Kaufman, M. H. Establishment in Culture of Pluripotential Cells from Mouse Embryos. Nature 1981 292:5819 1981, 292 (5819), 154–156. [CrossRef]
- Takahashi, K.; Yamanaka, S. Induction of Pluripotent Stem Cells from Mouse Embryonic and Adult Fibroblast Cultures by Defined Factors. Cell 2006, 126 (4), 663–676. [CrossRef]
- Wagers, A. J.; Weissman, I. L. Plasticity of Adult Stem Cells. Cell 2004, 116 (5), 639–648. [CrossRef]
- Barry, F. P.; Murphy, J. M. Mesenchymal Stem Cells: Clinical Applications and Biological Characterization. Int J Biochem Cell Biol 2004, 36 (4), 568–584. [CrossRef]
- Bianco, P.; Robey, P. G.; Simmons, P. J. Cell Stem Cell Commentary Mesenchymal Stem Cells: Revisiting History, Concepts, and Assays. Cell Stem Cell 2008, 2 (4), 313–319. [CrossRef]
- Dominici, M.; Le Blanc, K.; Mueller, I.; Slaper-Cortenbach, I.; Marini, F. C.; Krause, D. S.; Deans, R. J.; Keating, A.; Prockop, D. J.; Horwitz, E. M. Minimal Criteria for Defining Multipotent Mesenchymal Stromal Cells. The International Society for Cellular Therapy Position Statement. Cytotherapy 2006, 8 (4), 315–317. [CrossRef]
- Mendicino, M.; Bailey, A. M.; Wonnacott, K.; Puri, R. K.; Bauer, S. R. Cell Stem Cell Forum MSC-Based Product Characterization for Clinical Trials: An FDA Perspective. 2014. [CrossRef]
- Pittenger, M. F.; Mackay, A. M.; Beck, S. C.; Jaiswal, R. K.; Douglas, R.; Mosca, J. D.; Moorman, M. A.; Simonetti, D. W.; Craig, S.; Marshak, D. R. Multilineage Potential of Adult Human Mesenchymal Stem Cells. Science (1979) 1999, 284 (5411), 143–147. [CrossRef]
- Tögel, F.; Weiss, K.; Yang, Y.; Hu, Z.; Zhang, P.; Westenfelder, C. Vasculotropic, Paracrine Actions of Infused Mesenchymal Stem Cells Are Important to the Recovery from Acute Kidney Injury. Am J Physiol Renal Physiol 2007, 292, 1626–1635. [CrossRef]
- Suga, H.; Eto, H.; Shigeura, T.; Inoue, K.; Aoi, N.; Kato, H.; …; Yoshimura, K. IFATS Collection: Fibroblast Growth Factor-2-Induced Hepatocyte Growth Factor Secretion by Adipose-Derived Stromal Cells Inhibits Postinjury Fibrogenesis Through a c-Jun N-Terminal Kinase-Dependent Mechanism. Stem Cells 2009, 27 (1), 238–249. [CrossRef]
- Blobe, G. C.; Schiemann, W. P.; Lodish, H. F. Role of Transforming Growth Factor β in Human Disease. N Engl J Med 2009, 342 (18), 1350–1358. [CrossRef]
- Kalinski, P. Regulation of Immune Responses by Prostaglandin E2. Journal of Immunology 2012, 188 (1), 21–28. [CrossRef]
- Pereira, T.; Armada-Da Silva, P. A. S.; Amorim, I.; Rêma, A.; Caseiro, A. R.; Gärtner, A.; Rodrigues, M.; Lopes, M. A.; Bártolo, P. J.; Santos, J. D.; Luís, A. L.; Maurício, A. C.; Frias, R. Effects of Human Mesenchymal Stem Cells Isolated from Wharton’s Jelly of the Umbilical Cord and Conditioned Media on Skeletal Muscle Regeneration Using a Myectomy Model. Stem Cells Int 2014, 2014, 16. [CrossRef]
- Carvalho, M. M.; Teixeira, F. G.; Reis, R. L.; Sousa, N.; Salgado, A. J. Mesenchymal Stem Cells in the Umbilical Cord: Phenotypic Characterization, Secretome and Applications in Central Nervous System Regenerative Medicine. Curr Stem Cell Res Ther 2011, 6 (3), 221–228. [CrossRef]
- Vizoso, F. J.; Eiro, N.; Cid, S.; Schneider, J.; Perez-Fernandez, R. Molecular Sciences Mesenchymal Stem Cell Secretome: Toward Cell-Free Therapeutic Strategies in Regenerative Medicine. J. Mol. Sci 2017, 18 (8), 1852. [CrossRef]
- Volarevic, V.; Simovic Markovic, B.; Gazdic, M.; Volarevic, A.; Jovicic, N.; Arsenijevic, N.; Armstrong, L.; Djonov, V.; Lako, M.; Stojkovic, M. Ethical and Safety Issues of Stem Cell-Based Therapy. Int. J. Med. Sci 2018, 15 (1), 36–45. [CrossRef]
- Carrade, D. D.; Owens, S. D.; Galuppo, L. D.; Vidal, M. A.; Ferraro, G. L.; Librach, F.; Buerchler, S.; Friedman, M. S.; Walker, N. J.; Borjesson, D. L. Clinicopathologic Findings Following Intra-Articular Injection of Autologous and Allogeneic Placentally Derived Equine Mesenchymal Stem Cells in Horses. Cytotherapy 2011, 13 (4), 419–430. [CrossRef]
- Mensing, N.; Gasse, H.; Hambruch, N.; Haeger, J. D.; Pfarrer, C.; Staszyk, C. Isolation and Characterization of Multipotent Mesenchymal Stromal Cells from the Gingiva and the Periodontal Ligament of the Horse. BMC Vet Res 2011, 7, 1–13. [CrossRef]
- Webb, T. L.; Quimby, J. m.; Dow, S. W. In Vitro Comparison of Feline Bone Marrow-Derived and Adipose Tissue-Derived Mesenchymal Stem Cells. J Feline Med Surg 2012, 14 (2), 165–168. [CrossRef]
- Prado, A. A. F.; Favaron, P. O.; da Silva, L. C. L. C.; Baccarin, R. Y. A.; Miglino, M. A.; Maria, D. A. Characterization of Mesenchymal Stem Cells Derived from the Equine Synovial Fluid and Membrane. BMC Vet Res 2015, 11 (1), 1–13. [CrossRef]
- Sato, K.; Yamawaki-Ogata, A.; Kanemoto, I.; Usui, A.; Narita, Y. Isolation and Characterisation of Peripheral Blood-Derived Feline Mesenchymal Stem Cells. The Veterinary Journal 2016, 216, 183–188. [CrossRef]
- Friedenstein, A. J.; Piatetzky-Shapiro, I. I.; Petrakova, K. V. Osteogenesis in Transplants of Bone Marrow Cells. J Embryol Exp Morphol 1966, 16 (3), 381–390.
- Herthel DJ. Suspensory Desmitis Therapies. Proc 12th ACVS Vet Sympo 2002, 165–167.
- da Silva Meirelles, L.; Chagastelles, P. C.; Nardi, N. B. Mesenchymal Stem Cells Reside in Virtually All Post-Natal Organs and Tissues. J Cell Sci 2006, 119 (11), 2204–2213. [CrossRef]
- Wu, L. W.; Wang, Y. L.; Christensen, J. M.; Khalifian, S.; Schneeberger, S.; Raimondi, G.; Cooney, D. S.; Lee, W. P. A.; Brandacher, G. Donor Age Negatively Affects the Immunoregulatory Properties of Both Adipose and Bone Marrow Derived Mesenchymal Stem Cells. Transpl Immunol 2014, 30 (4), 122–127. [CrossRef]
- Richardson, S. M.; Kalamegam, G.; Pushparaj, P. N.; Matta, C.; Memic, A.; Khademhosseini, A.; Mobasheri, R.; Poletti, F. L.; Hoyland, J. A.; Mobasheri, A. Mesenchymal Stem Cells in Regenerative Medicine: Focus on Articular Cartilage and Intervertebral Disc Regeneration. Methods 2016, 99, 69–80. [CrossRef]
- Kim, J. H.; Jo, C. H.; Kim, H. R.; Hwang, Y. Il. Comparison of Immunological Characteristics of Mesenchymal Stem Cells from the Periodontal Ligament, Umbilical Cord, and Adipose Tissue. Stem Cells Int 2018, 2018. [CrossRef]
- Fong, C. Y.; Subramanian, A.; Gauthaman, K.; Venugopal, J.; Biswas, A.; Ramakrishna, S.; Bongso, A. Human Umbilical Cord Wharton’s Jelly Stem Cells Undergo Enhanced Chondrogenic Differentiation When Grown on Nanofibrous Scaffolds and in a Sequential Two-Stage Culture Medium Environment. Stem Cell Rev Rep 2012, 8 (1), 195–209. [CrossRef]
- Gauthaman, K.; Fong, C. Y.; Suganya, C. A.; Subramanian, A.; Biswas, A.; Choolani, M.; Bongso, A. Extra-Embryonic Human Wharton’s Jelly Stem Cells Do Not Induce Tumorigenesis, Unlike Human Embryonic Stem Cells. Reprod Biomed Online 2012, 24 (2), 235–246. [CrossRef]
- Sultana, T.; Lee, S.; Yoon, H. Y.; Lee, J. I. Current Status of Canine Umbilical Cord Blood-Derived Mesenchymal Stem Cells in Veterinary Medicine. Stem Cells Int 2018, 2018. [CrossRef]
- Arutyunyan, I.; Fatkhudinov, T.; Sukhikh, G. Umbilical Cord Tissue Cryopreservation: A Short Review. Stem Cell Res Ther 2018, 9 (1), 1–7. [CrossRef]
- Fortier, L. A.; Travis, A. J. Stem Cells in Veterinary Medicine. Stem Cell Research & Therapy 2011 2:1 2011, 2 (1), 1–6. [CrossRef]
- Voga, M.; Adamic, N.; Vengust, M.; Majdic, G. Stem Cells in Veterinary Medicine—Current State and Treatment Options. Front Vet Sci 2020, 7 (May), 1–20. [CrossRef]
- de Girolamo, L.; Lucarelli, E.; Alessandri, G.; Antonietta Avanzini, M.; Ester Bernardo, M.; Biagi, E.; Teresa Brini, A.; D’Amico, G.; Fagioli, F.; Ferrero, I.; Locatelli, F.; Maccario, R.; Marazzi, M.; Parolini, O.; Pessina, A.; ; Italian Mesenchymal Stem Cell Group (GISM), M. Mesenchymal Stem/Stromal Cells: A New “Cells as Drugs” Paradigm. Efficacy and Critical Aspects in Cell Therapy. Curr Pharm Des 2013, 19 (13), 2459–2473. [CrossRef]
- Álvarez-Viejo, M.; Menéndez-Menéndez, Y.; Otero-Hernández, J. CD271 as a Marker to Identify Mesenchymal Stem Cells from Diverse Sources before Culture. World J Stem Cells 2015, 7 (2), 470–476. [CrossRef]
- Fitter, S.; Gronthos, S.; Ooi, S. S.; Zannettino, A. C. W. The Mesenchymal Precursor Cell Marker Antibody STRO-1 Binds to Cell Surface Heat Shock Cognate 70. Stem Cells 2017, 35 (4), 940–951. [CrossRef]
- Bianco, P. “Mesenchymal” Stem Cells. Annu Rev Cell Dev Biol 2014, 30 (Volume 30, 2014), 677–704. [CrossRef]
- Cen, L. P.; Ng, T. K.; Liang, J. J.; Zhuang, X.; Yao, X.; Yam, G. H. F.; Chen, H.; Cheung, H. S.; Zhang, M.; Pang, C. P. Human Periodontal Ligament-Derived Stem Cells Promote Retinal Ganglion Cell Survival and Axon Regeneration After Optic Nerve Injury. Stem Cells 2018, 36 (6), 844–855. [CrossRef]
- Trounson, A.; McDonald, C. Stem Cell Therapies in Clinical Trials: Progress and Challenges. Cell Stem Cell 2015, 17 (1), 11–22. [CrossRef]
- Musiał-Wysocka, A.; Kot, M.; Majka, M. The Pros and Cons of Mesenchymal Stem Cell-Based Therapies. Cell Transplant 2019, 28 (7), 801–812. [CrossRef]
- Johnson, T. V.; Bull, N. D.; Martin, K. R. Stem Cell Therapy for Glaucoma: Possibilities and Practicalities. Expert Rev Ophthalmol 2011, 6 (2), 165–174. [CrossRef]
- Phelps, J.; Sanati-Nezhad, A.; Ungrin, M.; Duncan, N. A.; Sen, A. Bioprocessing of Mesenchymal Stem Cells and Their Derivatives: Toward Cell-Free Therapeutics. Stem Cells Int 2018, 2018 (1), 9415367. [CrossRef]
- Takahashi, K.; Tanabe, K.; Ohnuki, M.; Narita, M.; Ichisaka, T.; Tomoda, K.; Yamanaka, S. Induction of Pluripotent Stem Cells from Adult Human Fibroblasts by Defined Factors. Cell 2007, 131 (5), 861–872. [CrossRef]
- De Almeida, P. E.; Ransohoff, J. D.; Nahid, A.; Wu, J. C. Immunogenicity of Pluripotent Stem Cells and Their Derivatives. Circ Res 2013, 112 (3), 549–561. [CrossRef]
- Ding, D. C.; Shyu, W. C.; Lin, S. Z. Mesenchymal Stem Cells. Cell Transplant 2011, 20 (1), 5–14. [CrossRef]
- Johnson, T. V.; Martin, K. R. Cell Transplantation Approaches to Retinal Ganglion Cell Neuroprotection in Glaucoma. Curr Opin Pharmacol 2013, 13 (1), 78–82. [CrossRef]
- Jin, S.; Trope, G. E.; Buys, Y. M.; Badley, E. M.; Thavorn, K.; Yan, P.; Nithianandan, H.; Jin, Y. P. Reduced Social Participation among Seniors with Self-Reported Visual Impairment and Glaucoma. PLoS One 2019, 14 (7), e0218540. [CrossRef]
- Jiang, D.; Xiong, G.; Feng, H.; Zhang, Z.; Chen, P.; Yan, B.; Chen, L.; Gandhervin, K.; Ma, C.; Li, C.; Han, S.; Zhang, Y.; Liao, C.; Lee, T. L.; Tse, H. F.; Fu, Q. L.; Chiu, K.; Lian, Q. Donation of Mitochondria by IPSC-Derived Mesenchymal Stem Cells Protects Retinal Ganglion Cells against Mitochondrial Complex I Defect-Induced Degeneration. Theranostics 2019, 9 (8), 2395–2410. [CrossRef]
- Emre, E.; Yüksel, N.; Duruksu, G.; Pirhan, D.; Subaşi, C.; Erman, G.; Karaöz, E. Neuroprotective Effects of Intravitreally Transplanted Adipose Tissue and Bone Marrow–Derived Mesenchymal Stem Cells in an Experimental Ocular Hypertension Model. Cytotherapy 2015, 17 (5), 543–559. [CrossRef]
- Inoue, Y.; Iriyama, A.; Ueno, S.; Takahashi, H.; Kondo, M.; Tamaki, Y.; Araie, M.; Yanagi, Y. Subretinal Transplantation of Bone Marrow Mesenchymal Stem Cells Delays Retinal Degeneration in the RCS Rat Model of Retinal Degeneration. Exp Eye Res 2007, 85 (2), 234–241. [CrossRef]
- Jiang, Y.; Zhang, Y.; Zhang, L.; Wang, M.; Zhang, X.; Li, X. Therapeutic Effect of Bone Marrow Mesenchymal Stem Cells on Laser-Induced Retinal Injury in Mice. International Journal of Molecular Sciences 2014, Vol. 15, Pages 9372-9385 2014, 15 (6), 9372–9385. [CrossRef]
- Zhang, Y.; Wang, W. Effects of Bone Marrow Mesenchymal Stem Cell Transplantation on Light-Damaged Retina. Invest Ophthalmol Vis Sci 2010, 51 (7), 3742–3748. [CrossRef]
- Harper, M. M.; Grozdanic, S. D.; Blits, B.; Kuehn, M. H.; Zamzow, D.; Buss, J. E.; Kardon, R. H.; Sakaguchi, D. S. Transplantation of BDNF-Secreting Mesenchymal Stem Cells Provides Neuroprotection in Chronically Hypertensive Rat Eyes. Invest Ophthalmol Vis Sci 2011, 52 (7), 4506–4515. [CrossRef]
- Hu, Y.; Tan, H. B.; Wang, X. M.; Rong, H.; Cui, H. P.; Cui, H. Bone Marrow Mesenchymal Stem Cells Protect against Retinal Ganglion Cell Loss in Aged Rats with Glaucoma. Clin Interv Aging 2013, 8, 1467–1470. [CrossRef]
- Manuguerra-Gagné, R.; Boulos, P. R.; Ammar, A.; Leblond, F. A.; Krosl, G.; Pichette, V.; Lesk, M. R.; Roy, D. C. Transplantation of Mesenchymal Stem Cells Promotes Tissue Regeneration in a Glaucoma Model Through Laser-Induced Paracrine Factor Secretion and Progenitor Cell Recruitment. Stem Cells 2013, 31 (6), 1136–1148. [CrossRef]
- Hernandez, M. R.; Miao, H.; Lukas, T. Astrocytes in Glaucomatous Optic Neuropathy. Prog Brain Res 2008, 173, 353–373. [CrossRef]
- Karl, M. O. The Potential of Stem Cell Research for the Treatment of Neuronal Damage in Glaucoma. Cell Tissue Res 2013, 353 (2), 311–325. [CrossRef]
- Evangelho, K.; Mogilevskaya, M.; Losada-Barragan, M.; Vargas-Sanchez, J. K. Pathophysiology of Primary Open-Angle Glaucoma from a Neuroinflammatory and Neurotoxicity Perspective: A Review of the Literature. Int Ophthalmol 2019, 39 (1), 259–271. [CrossRef]
- Fang, C. E. H.; Guo, L.; Hill, D.; Yap, T. E.; Cordeiro, M. F. Neuroprotective Strategies in Glaucoma-Translation to Clinical Trials. OBM Neurobiology. LIDSEN Publishing Inc 2020. [CrossRef]
- Storgaard, L.; Tran, T. L.; Freiberg, J. C.; Hauser, A. S.; Kolko, M. Glaucoma Clinical Research: Trends in Treatment Strategies and Drug Development. Front Med (Lausanne) 2021, 8. [CrossRef]
- Kuriyan, A. E.; Albini, T. A.; Townsend, J. H.; Rodriguez, M.; Pandya, H. K.; Leonard, R. E.; Parrott, M. B.; Rosenfeld, P. J.; Flynn, H. W.; Goldberg, J. L. Vision Loss after Intravitreal Injection of Autologous “Stem Cells” for AMD. N Engl J Med 2017, 376 (11), 1047–1053. [CrossRef]
- Özmert, E.; Arslan, U. Management of Retinitis Pigmentosa by Wharton’s Jelly Derived Mesenchymal Stem Cells: Preliminary Clinical Results. Stem Cell Res Ther 2020, 11 (1). [CrossRef]
- Zhao, T.; Liang, Q.; Meng, X.; Duan, P.; Wang, F.; Li, S.; Liu, Y.; Yin, Z. Q. Intravenous Infusion of Umbilical Cord Mesenchymal Stem Cells Maintains and Partially Improves Visual Function in Patients with Advanced Retinitis Pigmentosa. Stem Cells Dev 2020, 29 (16), 1029–1037. [CrossRef]
- Sung, Y.; Lee, S. M.; Park, M.; Choi, H. J.; Kang, S.; Choi, B. I.; Lew, H. Treatment of Traumatic Optic Neuropathy Using Human Placenta-Derived Mesenchymal Stem Cells in Asian Patients. Regenerative Med 2020, 15 (10), 2163–2179. [CrossRef]
- Weiss, J. N.; Levy, S.; Malkin, A. Stem Cell Ophthalmology Treatment Study (SCOTS) for Retinal and Optic Nerve Diseases: A Preliminary Report. Neural Regen Res 2015, 10 (6), 982–988. [CrossRef]
- Weiss, J. N.; Levy, S.; Benes, S. C. Stem Cell Ophthalmology Treatment Study (SCOTS) for Retinal and Optic Nerve Diseases: A Case Report of Improvement in Relapsing Auto-Immune Optic Neuropathy. Neural Regen Res 2015, 10 (9), 1507–1515. [CrossRef]
- Weiss, J. N.; Benes, S. C.; Levy, S. Stem Cell Ophthalmology Treatment Study (SCOTS): Improvement in Serpiginous Choroidopathy Following Autologous Bone Marrow Derived Stem Cell Treatment. Neural Regen Res 2016, 11 (9), 1512–1516. [CrossRef]
- Weiss, J. N.; Levy, S.; Benes, S. C. Stem Cell Ophthalmology Treatment Study (SCOTS): Bone Marrow-Derived Stem Cells in the Treatment of Leber’s Hereditary Optic Neuropathy. Neural Regen Res 2016, 11 (10), 1685–1694. [CrossRef]
- Weiss, J. N.; Levy, S.; Benes, S. C. Stem Cell Ophthalmology Treatment Study: Bone Marrow Derived Stem Cells in the Treatment of Non-Arteritic Ischemic Optic Neuropathy (NAION). Stem Cell Investig 2017, 4 (11). [CrossRef]
- Weiss, J. N.; Levy, S. Stem Cell Ophthalmology Treatment Study: Bone Marrow Derived Stem Cells in the Treatment of Retinitis Pigmentosa. Stem Cell Investig 2018, 5 (June). [CrossRef]
- Weiss, J. N.; Levy, S. Dynamic Light Scattering Spectroscopy of the Retina-a Non-Invasive Quantitative Technique to Objectively Document Visual Improvement Following Ocular Stem Cell Treatment. Stem Cell Investig 2019, 6 (April). [CrossRef]
- Weiss, J. N.; Levy, S. Stem Cell Ophthalmology Treatment Study (SCOTS): Bone Marrow Derived Stem Cells in the Treatment of Usher Syndrome. Stem Cell Investig 2019, 6 (September). [CrossRef]
- Weiss, J. N.; Levy, S. Stem Cell Ophthalmology Treatment Study (SCOTS): Bone Marrow Derived Stem Cells in the Treatment of Dominant Optic Atrophy. Stem Cell Investig 2019, 6. [CrossRef]
- Weiss, J. N.; Levy, S. Stem Cell Ophthalmology Treatment Study (SCOTS): Bone Marrow-Derived Stem Cells in the Treatment of Age-Related Macular Degeneration. Medicines (Basel) 2020, 7 (4), 16. [CrossRef]
- Weiss, J. N.; Levy, S. Stem Cell Ophthalmology Treatment Study (SCOTS): Bone Marrow-Derived Stem Cells in the Treatment of Stargardt Disease. Medicines (Basel) 2021, 8 (2), 10. [CrossRef]
- Vilela, C. A. P.; Messias, A.; Calado, R. T.; Siqueira, R. C.; Silva, M. J. L.; Covas, D. T.; Paula, J. S. Retinal Function after Intravitreal Injection of Autologous Bone Marrow-Derived Mesenchymal Stromal Cells in Advanced Glaucoma. Doc Ophthalmol 2021, 143 (1), 33–38. [CrossRef]
- Weiss, J. N.; Levy, S.; Malkin, A. Stem Cell Ophthalmology Treatment Study (SCOTS) for Retinal and Optic Nerve Diseases: A Preliminary Report. Neural Regen Res 2015, 10 (6), 982–988. [CrossRef]
- Weiss, J. N.; Levy, S.; Benes, S. C. Stem Cell Ophthalmology Treatment Study (SCOTS) for Retinal and Optic Nerve Diseases: A Case Report of Improvement in Relapsing Auto-Immune Optic Neuropathy. Neural Regen Res 2015, 10 (9), 1507–1515. [CrossRef]
- Weiss, J. N.; Benes, S. C.; Levy, S. Stem Cell Ophthalmology Treatment Study (SCOTS): Improvement in Serpiginous Choroidopathy Following Autologous Bone Marrow Derived Stem Cell Treatment. Neural Regen Res 2016, 11 (9), 1512–1516. [CrossRef]
- Kasetty, M. A.; Hedges, T. R.; Witkin, A. J. BILATERAL EPIRETINAL MEMBRANE FORMATION AFTER INTRAVITREAL INJECTIONS OF AUTOLOGOUS MESENCHYMAL STEM CELLS. Retin Cases Brief Rep 2022, 16 (5), 561–564. [CrossRef]
- Weiss, J. N.; Levy, S.; Benes, S. C. Stem Cell Ophthalmology Treatment Study (SCOTS): Bone Marrow-Derived Stem Cells in the Treatment of Leber’s Hereditary Optic Neuropathy. Neural Regen Res 2016, 11 (10), 1685–1694. [CrossRef]
- Yang, N.; Zeng, S.; Yang, J.; Lu, G.; Du, L. Application of Platelet-Rich Fibrin Transplantation for Large Macular Hole. Curr Eye Res 2022, 47 (5), 770–776. [CrossRef]
- Yang, N.; Xing, Y.; Zhao, Q.; Zeng, S.; Yang, J.; Du, L. Application of Platelet-Rich Fibrin Grafts Following Pterygium Excision. Int J Clin Pract 2021, 75 (10). [CrossRef]
- Roubeix, C.; Godefroy, D.; Mias, C.; Sapienza, A.; Riancho, L.; Degardin, J.; Fradot, V.; Ivkovic, I.; Picaud, S.; Sennlaub, F.; Denoyer, A.; Rostene, W.; Sahel, J. A.; Parsadaniantz, S. M.; Brignole-Baudouin, F.; Baudouin, C. Intraocular Pressure Reduction and Neuroprotection Conferred by Bone Marrow-Derived Mesenchymal Stem Cells in an Animal Model of Glaucoma. Stem Cell Res Ther 2015, 6 (1). [CrossRef]
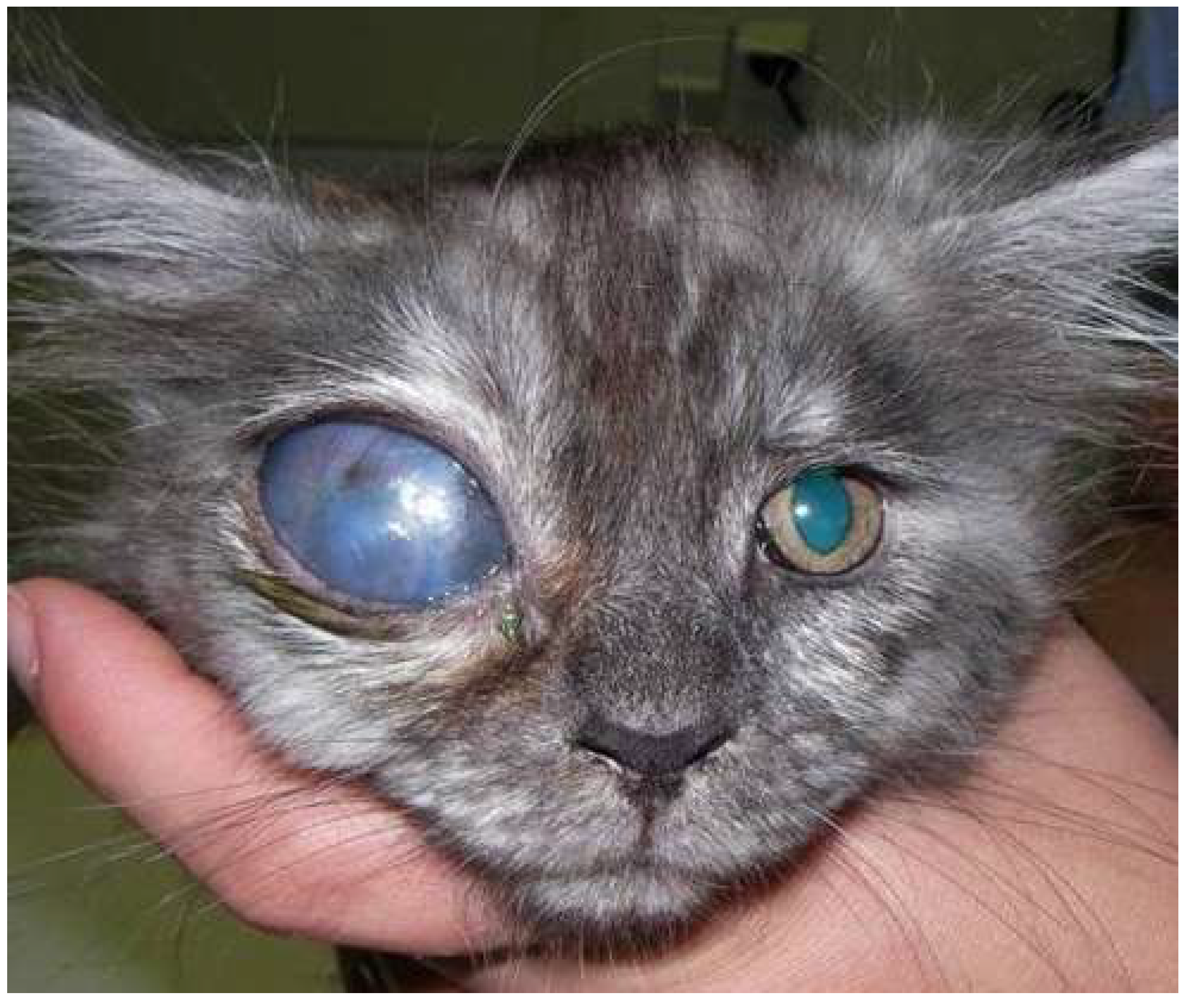
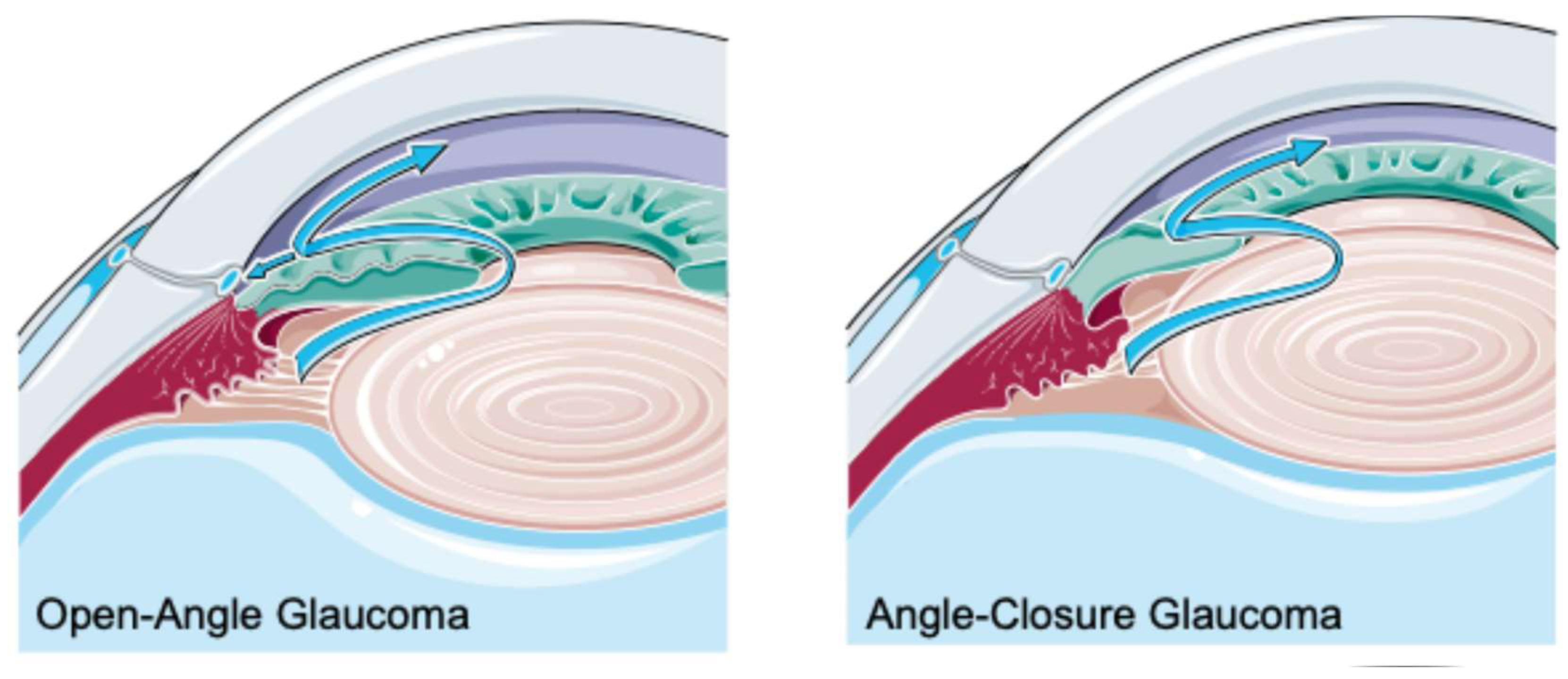
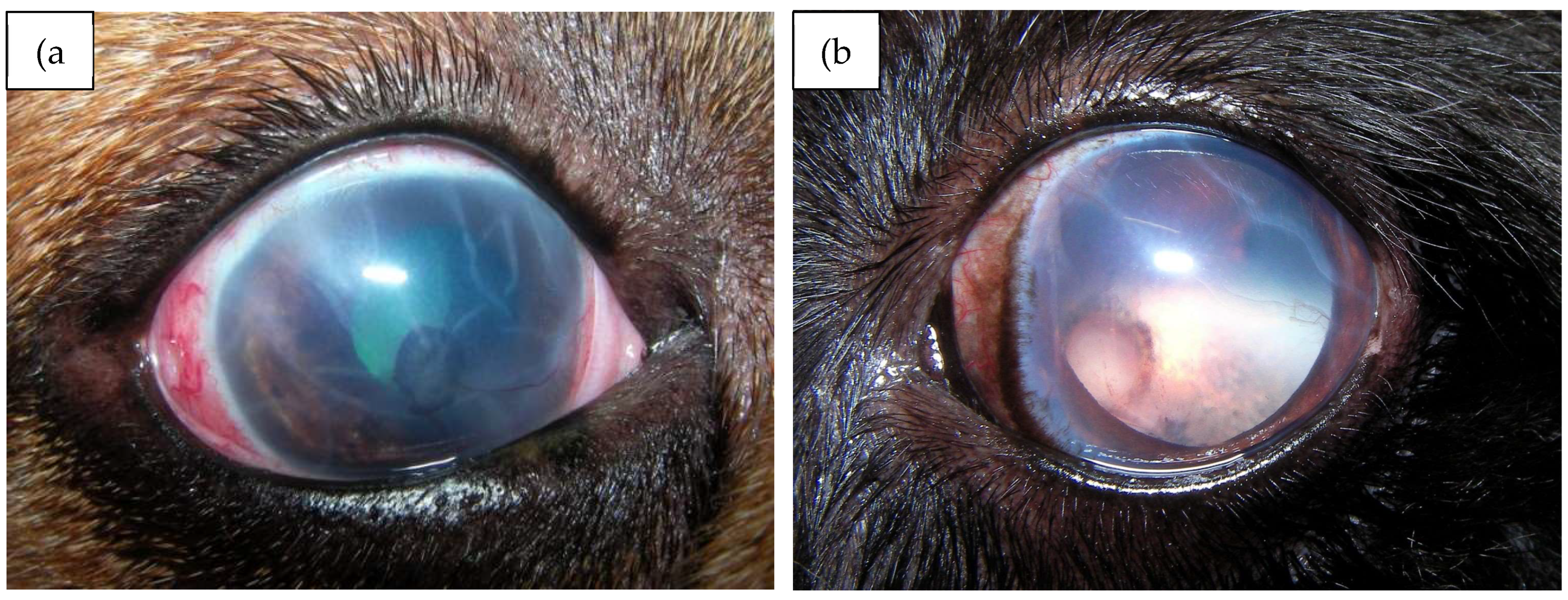
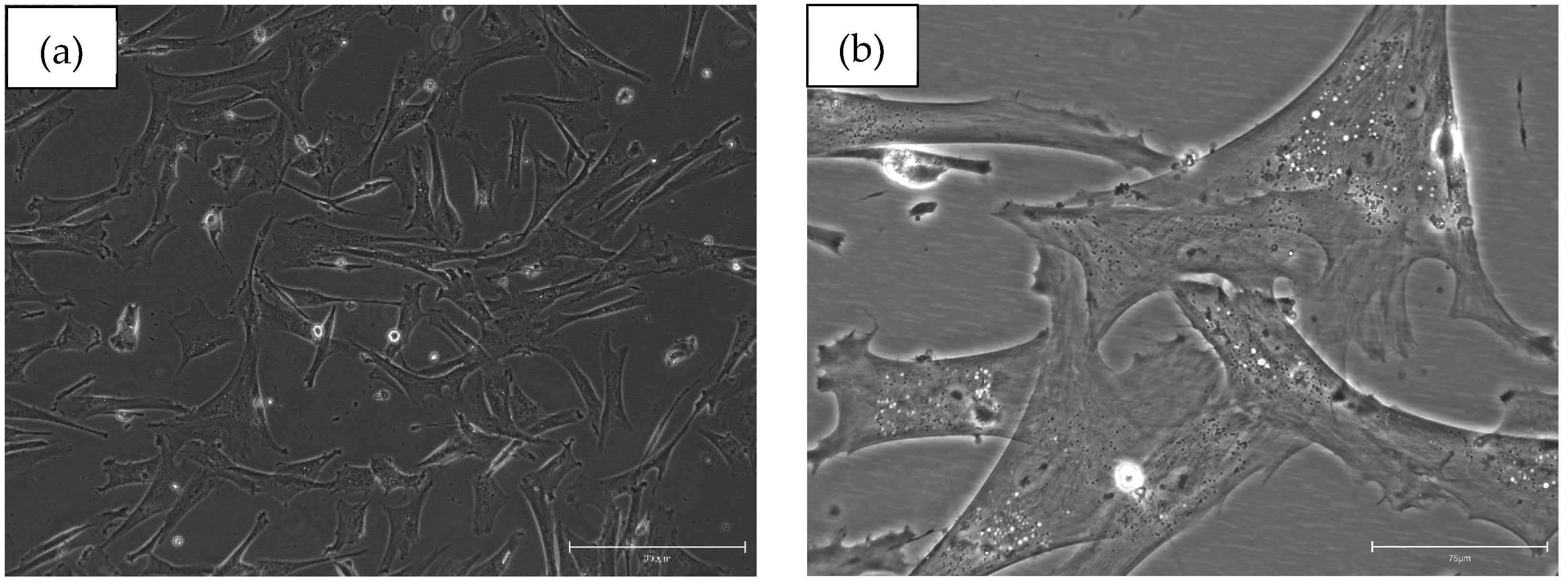
| Study public title | NCT number, location and date | Eye conditions | Treatment | Status | Sample size/target | Results |
|---|---|---|---|---|---|---|
| Safety of Cultured Allogeneic Adult Umbilical Cord Derived Mesenchymal Stem Cells for Eye Diseases | NCT05147701 Antigua, Barbuda, Argentina (2022-2026) |
Macular Degeneration Optic Atrophy Retinitis Pigmentosa Eye Diseases Traumatic Optic Neuropathy Diabetic Retinopathy Glaucoma |
AlloRx (cultured allogeneic adult umbilical cord derived MSCs, intravenous and sub-tenon delivery, total dose of 100 million cells) |
Recruiting | 20 | [139,140,141] |
| Stem Cell Ophthalmology Treatment Study II (SCOTS2) | NCT03011541 United States, United Arab Emirates (2016-2026) |
Age-Related Macular Degeneration Glaucoma Retinopathy Hereditary Optic Neuropathy Macular Degeneration Blindness (…) |
Arm 1 (Autologous bone marrow derived stem cells provided retrobulbar, subtenon and intravenous for one or both eyes) |
Recruiting | 500 | [142,143,144,145,146,147,148,149,150,151,152] |
| Intravitreal Mesenchymal Stem Cell Transplantation in Advanced Glaucoma. | NCT02330978 Brazil (2014-2019) |
Retinal degeneration POAG |
Intravitreal transplantation of autologous bone-marrow mesenchymal stem cells (BM-MSCs) | Completed | 2 | [153] |
| Stem Cell Ophthalmology Treatment Study (SCOTS) | NCT01920867 United States, United Arab Emirates (2013-2019) |
Macular Degeneration Hereditary Retinal Dystrophy Optic Nerve Disease Glaucoma Retinal Disease |
Intraocular, retrobulbar, intravenous, subtenon, intravitreal transplantation of autologous BM-MSCs | Unknown | 300 | [154,155,156,157,158] |
| Platelet-rich Fibrin (PRF) Membrane in Ophthalmic Diseases | NCT06200727 China (2023-2025) |
Platelet-rich Fibrin Macular Holes Pterygium Glaucoma |
Autologous PRF membrane grafting/ amniotic membrane to cover the exposed sclera after trabeculectomy for glaucoma. |
Active, not recruiting |
170 | [159,160] |
| Effectiveness and Safety of Adipose-Derived Regenerative Cells for Treatment of Glaucomatous Neurodegeneration | NCT02144103 Moscow, Russian federations (2014-2019) |
Retinal degeneration POAG |
ADRC injection: liposuction to isolate adipose-derived regenerative cells (ADRC). The concentrated ADRCs will then be injected into the subtenon space of the patient’s eye. |
Unknown | 16 | No results reported yet |
| Efficacy of NSAID vs. Steroid-NSAID Combo Post-Selective Laser Trabeculoplasty: Phase 4, Single-Center RCT (CES-NSLT) | NCT06498440 Canada (2024-2025) |
Open Angle Glaucoma Ocular Hypertension Postoperative Inflammation |
Ketorolac 0.5% eye drops; Ketorolac 0.5% and Fluorometholone 0.1% eye drops |
Not yet recruiting |
126 | No results reported yet |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





