1. Introduction
Market authorization of cosmetic ingredients differ among jurisdictions, but in general include the assessment of the potential risk of the cosmetic ingredients to the user under the expected use patterns. The cosmetic sector has been proactive regarding self-regulation. The International Fragrance Association (IFRA) (
www.ifrafragrance.org accessed on 14 May 2024) with the technical support of the Research Institute for Fragrance Materials (RIFM) (
www.rifm.org accessed on 14 May 2024) establish safety standards for fragrance ingredients, prohibiting or restricting the use of certain ingredients, which are compulsory for all IFRA members. RIFM has developed a safety assessment program with seven human health endpoints completed with environmental concerns, that currently includes over 250 standards. In addition, the EU and other jurisdictions have developed specific legislations regulating the scientific assessment of cosmetic ingredients and the marketing of the final product. A leading aspect of the EU legislation is the ban on the use of animal studies for assessing the safety of cosmetic ingredients. This has triggered specific efforts by the EU Scientific Committee on Consumer Safety (SCCS), Member State institutions and research organizations to develop risk assessment methodologies not requiring animal testing. The assessment of dermal absorption and topical effects, including skin and eye irritation and sensitization, is facilitated through recent OECD (Organization for Economic Cooperation and Development) test guidelines based on alternative methods. If absorption is confirmed, the assessment of systemic toxicity using alternative methods to animal testing is still under development. New Approach Methodologies (NAM) including high-throughput in vitro bioactivity methods measuring are proposed as the basis for hypothesis driven Next Generation Risk Assessments (NGRA). In addition to avoid or at least minimize animal testing, NGRA approaches can also be used to address one of the key limitations of the current animal-based risk assessment paradigm, to extend the assessment from healthy populations to those with specific pathologies that could increase their vulnerability. The assessment of endocrine activity is an emerging issue, with increasing relevance in the area of cosmetics [
1]. The susceptibility to endocrine disruptors (EDs) is particularly high during specific time windows of human development, including pregnancy and fetal stage, childhood, puberty or menopause. In addition, diseases such as cancer or atopic dermatitis triggers concerns on the enhanced vulnerability to ED in cosmetics due to a combination of increased use/absorption and enhanced sensitivity to endocrine mediated toxicity mechanisms [
2].
The current chemical risk assessment paradigm focuses on setting acceptability toxicity thresholds for the “healthy” general population. Despite the scientific and legal efforts for minimizing and replacing the use of animal testing for assessing the safety of cosmetic ingredients, many cosmetic hazard assessments for systemic toxicity are still based on Points of Departure (PoD) from animal studies. The applicability a PoD based on of apical endpoints observed in healthy animals under controlled conditions should be considered with caution for assessing the risk to vulnerable human populations with specific diseases. In fact, as the patients are under medical supervision, identifying the risk drivers for each vulnerable group and passing the information to the patient and the responsible medical services could be much more relevant than setting generic PoDs and acceptability threshold levels. Consequently, we propose the use of hypothesis driven NGRA approaches, combining the available traditional assessments for the general population with other available information. The assessment includes endocrine mediated effects as well as other relevant pathways. In particular, key elements from the toxicokinetic and toxicodynamic studies are combined with the available NAMs, for identifying potential vulnerability drivers for the different groups of patients, in order to improve their medical supervision.
A key element for this innovative approach is that the risk characterization does not put the focus on selecting acceptable thresholds, but on identifying and communicating disease-related vulnerabilities that should trigger specific recommendations regarding the use of cosmetics containing ingredients that could lead to adverse effects in these vulnerable groups.
The UV filter octocrylene has been selected for this proof of concept due to the unresolved assessment on endocrine potential and its confirmed presence in marketed cosmetics intended for oncology patients and other vulnerable groups [
2]. The use of sunscreens is a preventive measure against skin cancer [
3]. Oncology patients require chronic and effective photoprotection due to the high incidence of skin cancer developing following cancer therapies. The EU risk assessment for the UV filter octocrylene was conducted by the SCCS using available animal oral toxicity studies for selecting the PoD for the hazard assessment [
4]. The SCCS approach for the risk characterization is to estimate the Margin of Exposure (MoE) between the selected PoD and the estimated exposure levels, considering an acceptable risk level for the general population when the MoE is around 100 or higher.
2. Materials and Methods
The problem formulation focused on the risk for two vulnerable groups: oncological patients and those with atopic dermatitis. The hypothesis-driven conceptual model used information extracted from the EU assessment for the general “healthy” population of cosmetic users, conducted by the SCCS, as background for selecting potential concerns and triggering the risk hypothesis.
In vitro bioactivity results for octocrylene were extracted from the USEPA CompTox Dashboard, using the Excel download option for further handling of the selected data. The assays with octocrylene activity were selected and assessed to identify mechanistic effects other than general cytotoxicity, endocrine mediated effects and non-monotonic dose responses. These assessments were the basis for the identification of specific concerns regarding the vulnerability of subpopulation groups with specific diseases, focusing on oncological patients and those with atopic dermatitis.
For the hazard and exposure assessments, the data extracted from the references reported in the SCCS (2021) risk assessment [
4] were complemented through a literature search in PubMed using “octocrylene” as search term.
Exposure and hazard drivers were extracted independently from the SCCS assessment and from the ToxCast in vitro bioactivity; and then integrated as specific vulnerability concerns for the selection of the most relevant risk hypothesis. When possible, the risk hypothesis was analyzed and implemented into evidence-based recommendations. The risk characterization method considered both deterministic and probabilistic approaches and was selected according to the available information for assessing each hypothesis. The evaluation was complemented with an uncertainty assessment and the identification of data gaps and testing needs.
In addition, the methodology developed by the EU project HBM4EU was used for proposing human health reference values for the general “healthy” population, and a discussion regarding possible adaptations for covering vulnerable groups. The methodology for deriving the HBM-GV for octocrylene follows option developed in the framework of the HBM4EU project [
5] based on a defined external toxicity reference value, selecting the PoD in line with the final recommendations and the current EU approach [
6,
7]. Considering the toxicokinetic information, the selected biomarker was the CDAA metabolite.
The calculation considers the toxicity reference value (TVR), daily urinary excretion rate of the metabolite, molar corrections, and body weight-adjusted daily urine flow, formula (1)[
5]:
3. Results and Discussion
3.1. Drivers and Identification of Risk Hypothesis
Two parallel assessments, one extracted from the SCCS (2021) opinion [
4] and another from ToxCast in vitro bioactivity (
https://comptox.epa.gov/dashboard/chemical/invitrodb/DTXSID9025299 accessed on January 2024) were conducted for the identification of risk drivers and indications of potential vulnerability for oncological and atopic dermatitis patients.
The main background hypothesis extracted from the SCCS assessment [
4] are summarized in
Table 1.
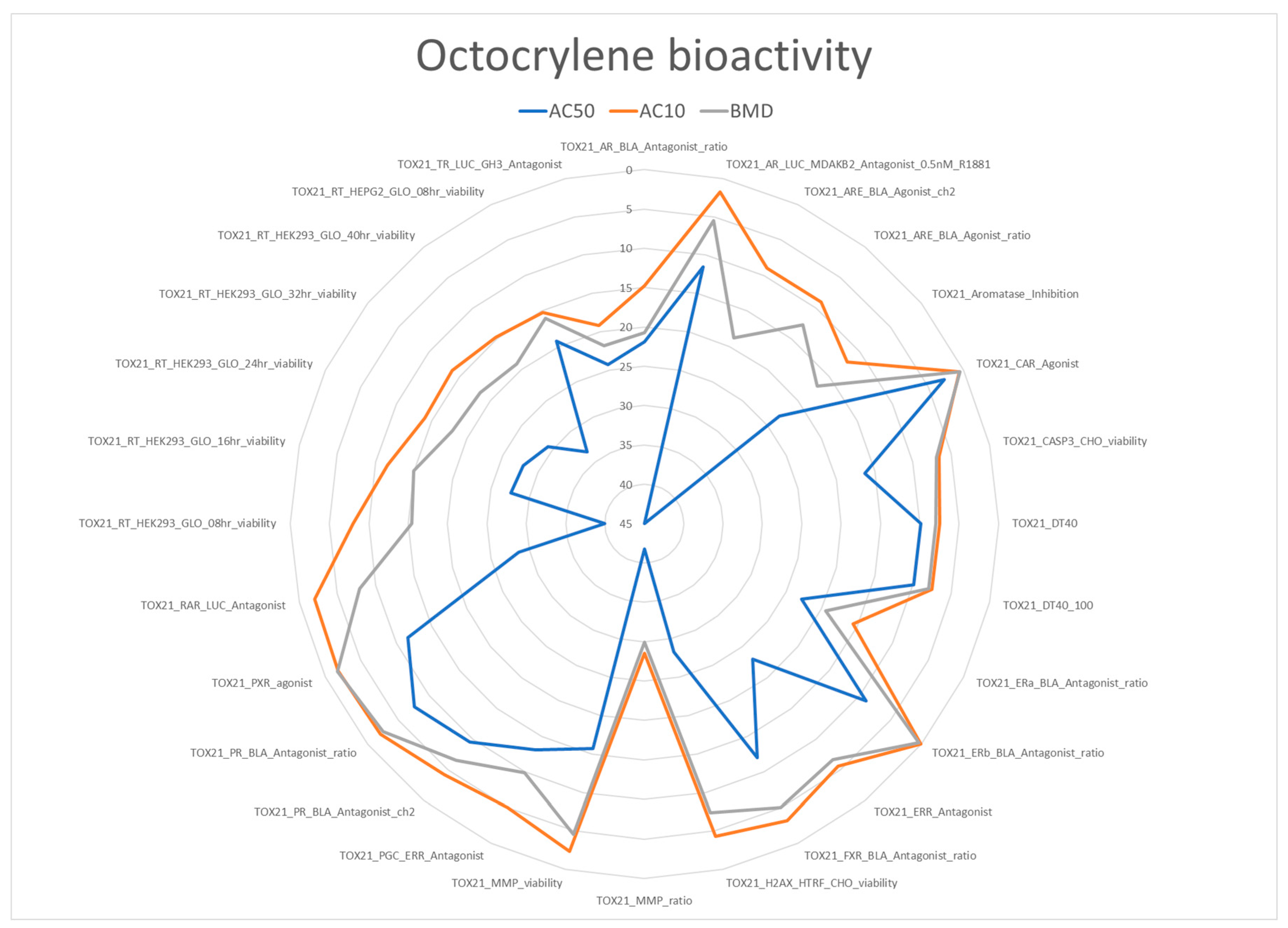
The ToxCast database extraction included 218 bioactivity studies for octocrylene; bioactivity was observed in 28 studies, including 17 linked to specific genetic targets.
Figure 1 summarizes the in vitro bioactivity of octocrylene. Most AC50s were around 5-20 µM, the most potent bioactivity was for reported for TOX21_CAR_Agonist, 2.72 µM, followed by TOX21_PR_BLA_Antagonist_ratio, 7.62 µM. In addition to general (cito)toxicity, bioactivity drivers were observed for the four endocrine modalities (EAST, Estrogen, Androgen, Steroidogenesis and Thyroid), although inconsistent and not triggering the ToxCast ER (estrogen receptor) and AR (androgen receptor) models. Activation of hepatic CYP3A4 linked to drug metabolism and disruption of metabolism and energetic homeostasis were also reported. The bioactivity observations were used for identifying additional risk and vulnerability drivers, which are summarized in
Table 2. Regarding bioactivity gaps, it should be noted that the available studies did not include those specific for assessing immunotoxicity and skin effects. Both are clear limitations regarding the identification of disease-related vulnerability drivers for the selected illnesses. In addition, the available data only covers the parent compound, no information on the bioactivity of the metabolite CDAA (2-cyano-3,3-diphenylacrylic acid) was found.
The interpretation of the in vitro ToxCast data requires the consideration of the “cytotoxicity burst” as many stress responses are activated in a nonspecific way at concentrations close to those resulting in cell death [
8]. A cytotoxicity burst of 9.01 µM has been suggested for octocrylene [
9]; three bioactivities are still below this value, suggesting that at least these three assays should be consider as specific activity linked to octocrylene mechanisms of action. One bioassay, CAR Agonist, is linked to activation of Cyt-P450 with increased drug metabolism and also implication on glucose and lipid metabolism, the other two are associated with nuclear receptors regulating the activity of transcription factors for the progesterone and estrogen receptors. Therefore, all risk and vulnerability drivers identified in
Table 2 are confirmed by specific bioactivities observed at concentrations below the cytotoxicity burst.
The literature search identified 325 PubMed (accessed on 03/10/2024) documents published between 2010 and 2024, 63 mentioned octocrylene in the title. Following a screening by title and abstracts, five relevant publications were included in the analysis of the risk drivers. Several publications provide further confirmation regarding human exposure of susceptible groups and systemic distribution, detecting octocrylene in human mother milk at 27.50 ± 22.15 ng/g lipids [
10], and their metabolites in urine [
11,
12]. The most relevant publication explores the use of ToxCast for assessing endocrine disruption potential [
9].
3.2. Analysis of Risk Hypothesis, Vulnerabilities and Data Gaps
In line with the problem formulation, the hypothesis driven assessment for the general population was followed by the identification and assessment of potential specific concerns for two vulnerable groups, oncological patients and those with atopic dermatitis.
SCCS exposure assessment follows the standard methodology for assessing external exposure, expressed as mg/kg bw per day, for different use patterns and routes. Dermal absorption was estimated as 0.97 µg/cm
2 from an OECD TG 428 in vitro skin absorption (Unpublished study report by Fabian and Landsiedel, 2020, summarized in SCCS (2021) [
4]. Systemic absorption was confirmed in studies with healthy human volunteers, averaged maximum plasmatic levels ranged from 0.6 to 11.7 µg/L (0.002 to 0.032 µM/L) covering different products and use patterns [
13,
14,
15]. The additional literature search confirmed systemic distribution with a continuous increase in octocrylene exposure levels in the German general population since 1996 based on measured urinary CDAA levels [
16]; and the presence of octocrylene in maternal milk, with measured values of 27.50 ± 22.15 g/g lipids [
10].
The integration of use patterns and physicochemical properties revealed dermal accumulation and transfer to blood as the main exposure drivers for local and systemic assessments respectively. The adaptation of the exposure drivers considered the potential for both, specific use patterns and higher dermal absorption. The dermal distress of patients with atopic dermatitis is expected to increase both the absorption and the application frequency. As octocrylene is lipophilic, both conditions may be associated not only with increased systemicity but also with increased accumulation potential. No information for proposing an absorption value for these patients could be retrieved except the generic indication for drugs of an almost twofold increase compared to healthy individuals from a non-updated systematic review [
17]. For oncological patients, adaptation is less obvious and will depend on specific recommendations from physicians. Avoidance of sun exposure is a typical recommendation; if this is mainly achieved by physical barriers (hats and long-sleeve clothing), exposure will be reduced, while if the option is cosmetic protection, an increased frequency should be expected.
The adaptation of the toxicodynamic drivers considered the mechanisms associated with the assays with observed bioactivity and their potential links with enhanced vulnerability for the selected diseases. The SCCS evaluation and ToxCast bioactivity identified two common concerns, unconclusive potential for endocrine activity and clear evidence induction of CYP450 and other liver enzymes involved in hepatic metabolism. In addition, the ToxCast extraction suggested concerns regarding drivers for metabolism and energetic homeostasis, and lack of information on immunotoxicity and skin disruption drivers. As previously reported [
9], the bioactivity AC
50s are two orders of magnitude below the plasmatic levels measured in human volunteers, confirming low concerns for healthy individuals in line with the SCCS opinion. However, these generic assessments do not cover the disease-related vulnerabilities described in
Table 2.
Endocrine activity is a vulnerability driver for oncological patients as several oncological processes are mediated by or associated to endocrine processes, the bioactivity is observed in some assays related to the four EAST modalities, and require further assessment. Current approaches for identifying endocrine potential using ToxCast data focuses on the combination of different assays measuring different elements of the EAST pathway to explore the overall agonistic or antagonistic activities; the estrogen [
18] and androgen [
19] models are well developed and included in regulatory guidance. However, in the case of disease-related vulnerabilities, a different approach is needed, as the vulnerability can be associated to a specific endocrine-related activity in a particular organ, tissue or cell type within a specific time window. Therefore, the assessment should focus on the relationship of the observed bioactivities with the pathogenesis of the disease, and the possible consequences in terms of treatment and prognosis. The health status may also influence the dose-response slope, as a consequence, preventive measures should be considered even in case of bioactivity observed at levels above those expected or measured in healthy individuals. The combination of the potential for increased skin absorption and the systemic bioactivity linked to increased drug metabolism and endocrine related effects should be communicated to the professionals responsible for monitoring the health status of the vulnerable groups.
Regarding the identified data gaps, the three key areas mentioned in
Table 2 require further investigation. In fact, in vitro studies with CDAA, and completing the high-throughput battery with the specific assays investigating immunotoxicity and effects on skin, should be suggested as a first step in an Integrated Approach to Testing and Assessment (IATA) focused on vulnerable groups.
3.3. Estimation of Human Biomonitoring Guideline Values (HBM_GV) for Octocrylene
The human biomonitoring guideline value for general population (HBM_GV) is a health-related guideline limit that refers to the internal body burden. It is derived for the general population and refers to the concentration of a substance or its metabolites in human biological material at which there is no risk of expected health effects during lifetime exposure [
5]. Health-based HBM-GVs can be used directly to interpret HBM data and thus provide a better health risk assessment than a risk assessment based solely on external intake estimates. Health-based HBM-GVs have been developed for several substances within the HBM4EU Joint European Program [
5,
6] and various international bodies, bit nor yet for octocrylene.
Our estimation is based on the toxicity value (TVR) derivation used as PoD the oral NOAEL for octocrylene (0.765 mg/kg bw/day proposed by the SCCS [
4], divided by the standard uncertainty factor of 100 (10 intraspecific variability x 10 interspecific variability), and the proportional urinary excretion factor (Fue) of 0.45 (45%) [
20]. Default daily urinary flow rates adjusted for body weight; 30 ml/kg bw/d and 20 ml/kg bw/d for children and adults, respectively, were used [
5], see
Table 3.
The value for adults is three orders of magnitude higher than the average measured values in the German adult population based on human biomonitoring data in urine from the German population in winter [
16], and 60 times higher than the average maximum value. However, higher HBM values are expected in situations of increased skin permeability due to altered skin barrier function, and maximal exposure, such as during the summer, or throughout the year, as in the case of sun sensitive patients, such as oncological patients.
The comparative results indicate little concern for the general population considering a standard risk assessment for healthy population that does not specifically addressed risk related to endocrine disruption potential, confirming the need for a specific approach using an NGRA assessment to evaluate endocrine disruption concerns in vulnerable groups.
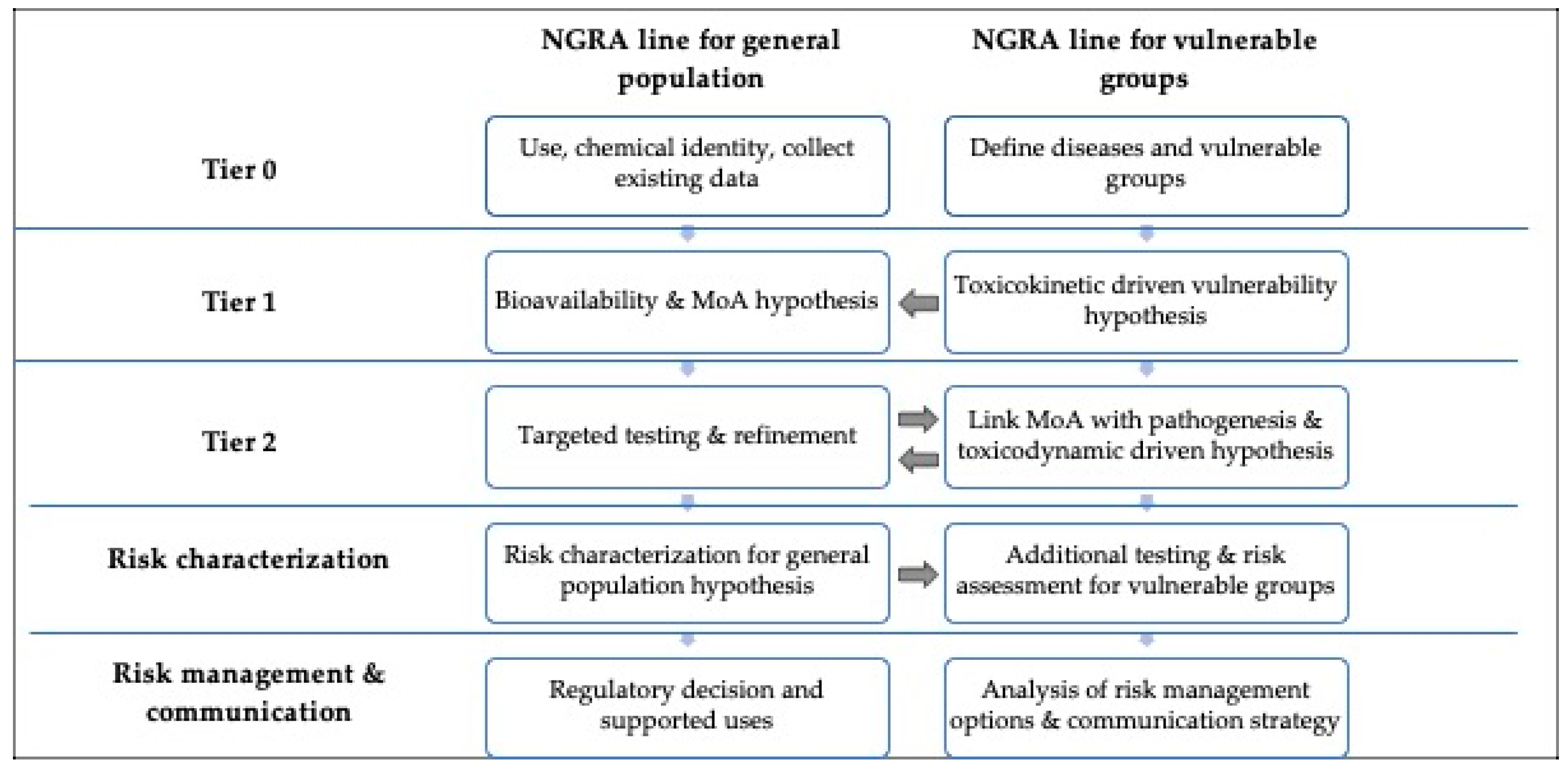
4. NGRA and Risk Management Options for Vulnerable Groups
The current regulatory risk assessment practice for cosmetic ingredients focuses on healthy users; when used in products recommended for vulnerable groups, a complementary assessment should be conducted to refine the risk. This proof of concept for the UV filter octocrylene confirms the benefits of using hypothesis driving NGRA for assessing the available information and identifying priorities for additional studies within an IATA strategy specifically formulated for addressing disease-related vulnerabilities. The SCCS Notes of Guidance updated in 2023 [
21] integrated the definition of NGRA as a human-relevant, exposure-led, hypothesis-driven risk assessment designed to prevent harm, and the tiered framework proposed by Berggren et al. (2017) [
22] adapted to cosmetics by Dent et al. (2018) [
23]. The framework can be adapted to cover disease-related vulnerabilities through the incorporation of an additional column, as presented in
Figure 2.
One additional element for assessments focusing on vulnerable groups is the consideration of the best risk management and risk communication options. The identification of concerns should be followed by specific indications, recommending vulnerable groups not use the product or to follow specific measures to mitigate the risk. In addition, in line with the precautionary principle, the recommendations should be extended to unconclusive assessments. Depending on the concern, its severity and the uncertainties, another option for patients under health status control is to include a dedicated monitoring scheme of the potential consequences for the identified concerns. This approach could be the best option when the vulnerable group is small and well characterized, as well as when there are clear benefits related to the use of cosmetic products and there are no alternatives with confirmed low concerns. This requires an awareness campaign and specific efforts to pass the relevant information to the health professionals that are controlling the patients’ status.
5. Conclusions
The use of the UV filter octocrylene as cosmetic ingredients was assessed by the SCCS and no main concerns for healthy users were identified, although the assessment of potential for endocrine disruption was inconclusive. Cosmetic products containing this ingredient include, as recommended users, population groups with specific pathologies, such as atopic dermatitis and oncological patients [
2], triggering the need for a dedicated assessment for these vulnerable groups. Hypothesis-driven NGRA has been applied to the existing information as the first steps of the IATA strategy. Following the assessment of vulnerability drivers, concerns have identified related to increased absorption in patients with skin alterations, inconclusive assessment regarding the potential for disrupting endocrine pathways, and confirmed capacity for increasing drug metabolism, which may affect the treatment of oncological patients. The main data gaps for confirming vulnerability drivers are linked immunotoxicity and dermal alterations related to local accumulation following repeated use. An assessment fully based on in vitro methods would also requires a parallel toxicological evaluation of the metabolite CDAA, which reaches much higher systemic concentrations than the parent under normal use patterns.
Following the analysis of this proof of concept, a proposal is presented for extending the NGRA framework to cover disease-related vulnerabilities. To avoid confusion, we have previously proposed to distinguish between susceptible and vulnerable groups, the first covers groups within the general healthy population with specific concerns linked to physiological conditions due to the developmental status (fetus, children, puberty, pregnancy, elderly); the second, is linked to persons with specific diseases that may increase the exposure and/or effects of the ingredient exposure, and which currently are not covered in the standard regulatory risk assessments.
Author Contributions
Conceptualization, methodology JVT; formal analysis, investigation, and data curation, EFM; writing—original draft preparation, EFM and JVT; writing—review and editing, JVT. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Data Availability Statement
All data supporting the reported results are publicly available and can be found in the references and websites indicated in through the document.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Fernández-Martín, M.-E.; Tarazona, J.V. Cosmetics, Endocrine Disrupting Ingredients. In Reference Module in Biomedical Sciences; Elsevier, 2023; p. B9780128243152011854 ISBN 978-0-12-801238-3.
- Fernández-Martín, M.-E.; Tarazona, J.V. Market Analysis of the Presence of Endocrine Disrupting Chemicals in Cosmetic Products Intended for Oncological Patients and Other Vulnerable Groups. Eur J Dermatol 2024, 34, 40–50. [CrossRef]
- Sander, M.; Sander, M.; Burbidge, T.; Beecker, J. The Efficacy and Safety of Sunscreen Use for the Prevention of Skin Cancer. CMAJ 2020, 192, E1802–E1808. [CrossRef]
- EU SCCS SCCS/1627/21 Final Opinion. Scientific Committee on Consumer Safety SCCS Opinion on Octocrylene. 2021.
- Apel, P.; Rousselle, C.; Lange, R.; Sissoko, F.; Kolossa-Gehring, M.; Ougier, E. Human Biomonitoring Initiative (HBM4EU) - Strategy to Derive Human Biomonitoring Guidance Values (HBM-GVs) for Health Risk Assessment. International Journal of Hygiene and Environmental Health 2020, 230, 113622. [CrossRef]
- Apel, P.; Lamkarkach, F.; Lange, R.; Sissoko, F.; David, M.; Rousselle, C.; Schoeters, G.; Kolossa-Gehring, M. Human Biomonitoring Guidance Values (HBM-GVs) for Priority Substances under the HBM4EU Initiative – New Values Derivation for Deltamethrin and Cyfluthrin and Overall Results. International Journal of Hygiene and Environmental Health 2023, 248, 114097. [CrossRef]
- Santonen, T.; Mahiout, S.; Alvito, P.; Apel, P.; Bessems, J.; Bil, W.; Borges, T.; Bose-O’Reilly, S.; Buekers, J.; Cañas Portilla, A.I.; et al. How to Use Human Biomonitoring in Chemical Risk Assessment: Methodological Aspects, Recommendations, and Lessons Learned from HBM4EU. International Journal of Hygiene and Environmental Health 2023, 249, 114139. [CrossRef]
- Escher, B.I.; Henneberger, L.; König, M.; Schlichting, R.; Fischer, F.C. Cytotoxicity Burst? Differentiating Specific from Nonspecific Effects in Tox21 in Vitro Reporter Gene Assays. Environmental Health Perspectives 2020, 128, 077007. [CrossRef]
- Onyango, D.O.; Selman, B.G.; Rose, J.L.; Ellison, C.A.; Nash, J.F. Comparison between Endocrine Activity Assessed Using ToxCast/Tox21 Database and Human Plasma Concentration of Sunscreen Active Ingredients/UV Filters. Toxicological Sciences 2023, 196, 25–37. [CrossRef]
- Schlumpf, M.; Kypke, K.; Wittassek, M.; Angerer, J.; Mascher, H.; Mascher, D.; Vökt, C.; Birchler, M.; Lichtensteiger, W. Exposure Patterns of UV Filters, Fragrances, Parabens, Phthalates, Organochlor Pesticides, PBDEs, and PCBs in Human Milk: Correlation of UV Filters with Use of Cosmetics. Chemosphere 2010, 81, 1171–1183. [CrossRef]
- Bury, D.; Belov, V.N.; Qi, Y.; Hayen, H.; Volmer, D.A.; Brüning, T.; Koch, H.M. Determination of Urinary Metabolites of the Emerging UV Filter Octocrylene by Online-SPE-LC-MS/MS. Anal. Chem. 2018, 90, 944–951. [CrossRef]
- Gu, J.; Yuan, T.; Ni, N.; Ma, Y.; Shen, Z.; Yu, X.; Shi, R.; Tian, Y.; Zhou, W.; Zhang, J. Urinary Concentration of Personal Care Products and Polycystic Ovary Syndrome: A Case-Control Study. Environmental Research 2019, 168, 48–53. [CrossRef]
- Hiller, J.; Klotz, K.; Meyer, S.; Uter, W.; Hof, K.; Greiner, A.; Göen, T.; Drexler, H. Systemic Availability of Lipophilic Organic UV Filters through Dermal Sunscreen Exposure. Environ Int 2019, 132, 105068. [CrossRef]
- Matta, M.K.; Florian, J.; Zusterzeel, R.; Pilli, N.R.; Patel, V.; Volpe, D.A.; Yang, Y.; Oh, L.; Bashaw, E.; Zineh, I.; et al. Effect of Sunscreen Application on Plasma Concentration of Sunscreen Active Ingredients: A Randomized Clinical Trial. JAMA 2020, 323, 256. [CrossRef]
- Matta, M.K.; Zusterzeel, R.; Pilli, N.R.; Patel, V.; Volpe, D.A.; Florian, J.; Oh, L.; Bashaw, E.; Zineh, I.; Sanabria, C.; et al. Effect of Sunscreen Application Under Maximal Use Conditions on Plasma Concentration of Sunscreen Active Ingredients: A Randomized Clinical Trial. JAMA 2019, 321, 2082–2091. [CrossRef]
- Bury, D.; Weber, T.; Ebert, K.E.; Zülz, S.; Brüning, T.; Koch, H.M.; Kolossa-Gehring, M. Increasing Exposure to the UV Filters Octocrylene and 2-Ethylhexyl Salicylate in Germany from 1996 to 2020: Human Biomonitoring in 24-h Urine Samples of the German Environmental Specimen Bank (ESB). Environment International 2023, 182, 108334. [CrossRef]
- Halling-Overgaard, A. -S.; Kezic, S.; Jakasa, I.; Engebretsen, K.A.; Maibach, H.; Thyssen, J.P. Skin Absorption through Atopic Dermatitis Skin: A Systematic Review. British Journal of Dermatology 2017, 177, 84–106. [CrossRef]
- Browne, P.; Judson, R.S.; Casey, W.M.; Kleinstreuer, N.C.; Thomas, R.S. Screening Chemicals for Estrogen Receptor Bioactivity Using a Computational Model. Environ Sci Technol 2015, 49, 8804–8814. [CrossRef]
- Mansouri, K.; Kleinstreuer, N.; Abdelaziz, A.M.; Alberga, D.; Alves, V.M.; Andersson, P.L.; Andrade, C.H.; Bai, F.; Balabin, I.; Ballabio, D.; et al. CoMPARA: Collaborative Modeling Project for Androgen Receptor Activity. Environmental Health Perspectives 2020, 128, 027002. [CrossRef]
- Bury, D.; Modick-Biermann, H.; Leibold, E.; Brüning, T.; Koch, H.M. Urinary Metabolites of the UV Filter Octocrylene in Humans as Biomarkers of Exposure. Arch Toxicol 2019, 93, 1227–1238. [CrossRef]
- EU SCCS SCCS/1647/22 Corrigendum. SCCS (Scientific Committee on Consumer Safety), SCCS Notes of Guidance for the Testing of Cosmetic Ingredients and Their Safety Evaluation 12th Revision, 15 May 2023, Corrigendum 26 October 2023. 2023.
- Berggren, E.; White, A.; Ouedraogo, G.; Paini, A.; Richarz, A.-N.; Bois, F.Y.; Exner, T.; Leite, S.; Grunsven, L.A. van; Worth, A.; et al. Ab Initio Chemical Safety Assessment: A Workflow Based on Exposure Considerations and Non-Animal Methods. Computational Toxicology 2017, 4, 31–44. [CrossRef]
- Dent, M.; Amaral, R.T.; Da Silva, P.A.; Ansell, J.; Boisleve, F.; Hatao, M.; Hirose, A.; Kasai, Y.; Kern, P.; Kreiling, R.; et al. Principles Underpinning the Use of New Methodologies in the Risk Assessment of Cosmetic Ingredients. Computational Toxicology 2018, 7, 20–26. [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).