1. Introduction
Since short-term use of oral glucocorticoids (GC) (defined as courses for <=14 days) was considered safe, its prevalence has rarely been measured. However, there is mounting evidence of its detrimental impact. Seminal research reported in 2017 that within 30 days of initiating GC bursts, the risk for fracture, thromboembolism, or sepsis was doubled, tripled, or increased by a factor of five, respectively [
1]. Subsequently, these findings have been confirmed in systematic reviews [
2]. To conclude, descriptive research on over one million children who were prescribed GC bursts has proved truly alarming in concluding that this treatment increases the risk of pneumonia or sepsis by about 100% within one month of starting the therapy at issue [
3].
Hence, our first objective was to explore whether there is an unnecessary prescription of GC bursts to outpatient children in our area, and our secondary objective was to explore variations of GC bursts dispensing linked to physicians’ settings.
2. Materials and Methods
Design and setting. This is a retrospective cohort study to evaluate GC outpatient prescriptions for children. It is a secondary analysis of a pilot research study on paediatric heart rhythm disturbances between Spring and Fall 2022 [
4] undertaken at La Vileta General Practice, Mallorca. La Vileta includes ambulatory clinics and emergency care for 27,000 people, of whom 4,500 are children under 15 Participants. We recruited all children aged seven and twelve who attended three paediatric clinics of our medical centre during the follow-up that is scheduled for every healthy child. Only children under prescriptions for long-term GC treatment were excluded from this study.
Data Collection
We extracted details on oral glucocorticoids (dexamethasone, methylprednisolone, prednisolone, and hydrocortisone) and inhaled salbutamol treatment of these children from birth to their well-child visits at the age of 7 or 12 from the local admission dataset. Ambulatory by-appointment and out-of-hours (OOH) prescriptions of inhaled salbutamol in this sample were taken as a proxy of these two types of consultations in our study, given that 98% of ten-year-old European children have received inhaled salbutamol at some point [
6]. Majorcan proportion of out-of-hospital by-appointment and OOH medical care was taken from the local public health care register [
5] to confirm the previous estimation based on salbutamol prescriptions.
Definitions
Wheeze is a sign of bronchiolitis, bronchitis, and asthma that indicates airflow limitation. There is no agreed-upon definition of wheeze. This paper accepts wheeze as a high-pitched intrathoracic sound heard and recorded by a health care professional [
7]. In addition, we have classified wheezing episodes as mild when they meet the requirement of rapid symptom relief, according to a proposal by the updated 2024 GINA Report [
8] for a commonly used definition with obvious clinical utility in primary care.
Statistical Analysis. We used convenience sampling of subjects who participated in the original research, which enabled this secondary analysis of previous data. We analysed the data using R Version 4.3.3. The chi-square test was used for dichotomous variables, and the t-test was used for continuous variables.
Ethics
The Balearic Institutional Review Board approved the study’s protocol for Health and Medical Research (ECIAP-2023), and the parents of all children were required to give formal written consent.
3. Results
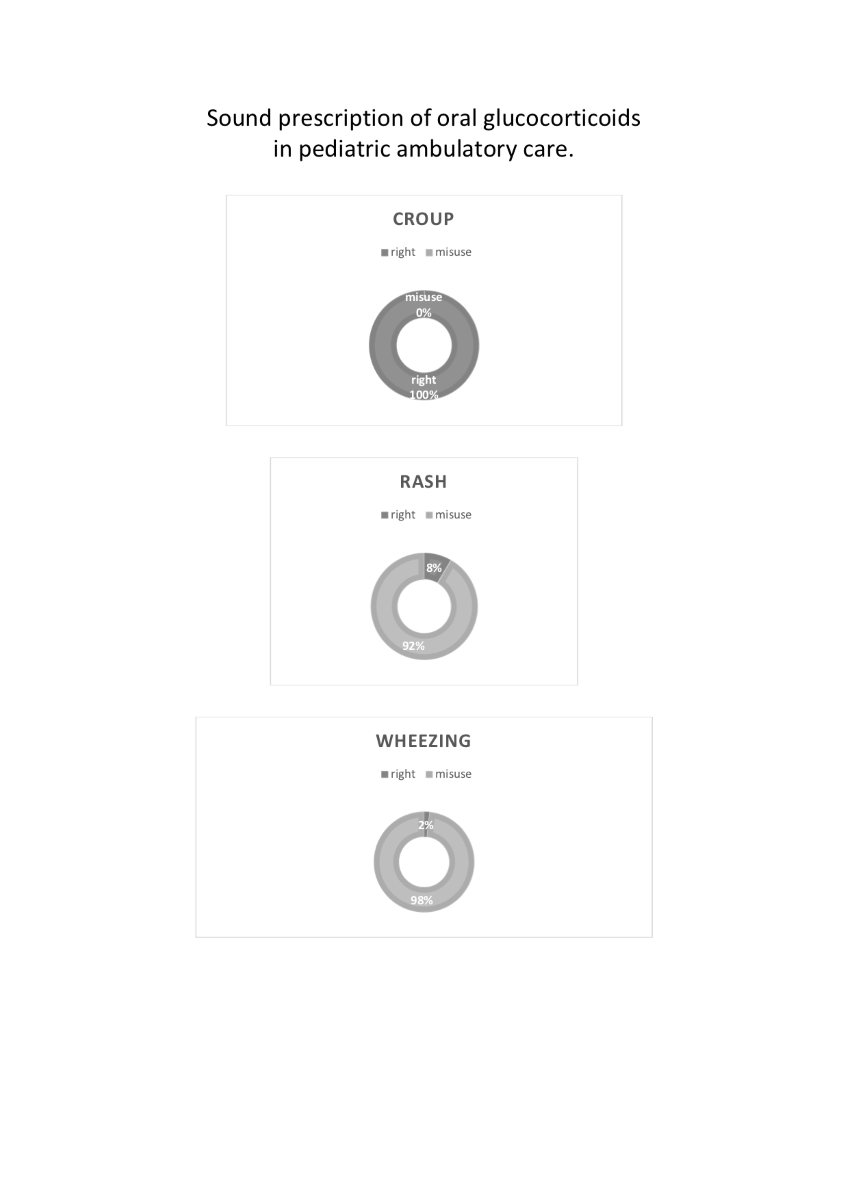
We recruited 95 children, who received, on average, five and a half days of GC in roughly eleven years of life. In total, the children of our sample have received 102 GC prescriptions, 12 for rash (12 children), 45 for croup (16 children), 59 for wheezing (20 children), and 7 for upper airway infection. Sometimes there was overlapping of diagnoses, a few prescriptions were due to the child having more than one of these diagnoses at the same time. Forty-five children (47.3%) received at least one GC burst. We found no significant differences in baseline or somatometric characteristics between ever and never-GC-prescribed children (
Table 1). Among GC users, the median number of days per short course was 3, and the range of short courses was 1-10 (
Table 2).
The most common indication for GC bursts was wheezing, followed by croup, and rash. Five out of twenty children on short courses of GC had moderate or severe wheezing; only one of them was of school age. Most children prescribed GC for skin conditions had an insect bite or dermatitis (42% and 33%, respectively), and the remaining 25% were equally divided between urticaria, cellulitis, and miliaria (
Table 3).
A reasonable estimate of paediatric treatments prescribed by OOH services in our area has been derived from figures of the population at large (children and adults): primary care consultations by appointment account for 85.4%, and OOH consultations account for 14.6% of the ambulatory health care burden [
6]. The 17.4% proportion of paediatric salbutamol prescriptions by OOH services in our sample confirms this estimate [OR=0.962; 95%CI:0.615-1.503; P=0.862]. Conversely, our 22.4% of OOH GC prescriptions deviates from the overall proportion of OOH consultations, and shows a trend toward a higher prescription of GC during OOH visits compared to scheduled primary care visits [OR=0.638; 95%CI:0.401-1.016; P=0.056] (
Table 4).
4. Discussion
Among our prescription of GC over time and space have been described. There is an annual rise in GC use, with a 30% increase in the UK over two decades and a 14% increase in France over one decade [
9]. GC prescription rates vary from region to region from 0.5%-21% [
10]. Until aged eleven, 11% of Swedish children [
11] and 26% of Dutch children have been prescribed GC [
6], which is less than our findings. Besides that, our prevalence is lower than 13% or 24% 1-year prevalence of GC use among Italian or French children, respectively [
12,
13].
Wheezing
Twenty children in our sample were prescribed GC bursts to treat wheezing, and most of them received repeated GC prescriptions. These findings align with previous reports of 15%-50% of children receiving more than one GC prescription [
6]. The use of GC, given for decades to treat wheezy toddlers, was based on an extrapolation from studies conducted in asthmatic older children and adolescents who responded to this class of drugs. It is worth emphasizing that the response to GC differs depending on the different phenotypes of wheezing in childhood; in preschool wheezers, airway inflammation is mostly due to a neutrophilic response to viral triggers, while school-age children more frequently present with eosinophilic, highly steroid-responsive airway infiltration A meta-analysis on the role of GC in toddlers with wheezing exacerbations [
14] found no difference between GC and placebo regarding unscheduled visits, and that outpatient toddlers treated with GC had a higher hospitalization rate. Accordingly, current guidelines remind us of the strong evidence against GC bursts in preschool children with acute wheezing [
15]. For schoolchildren, guidelines state that although clear evidence is lacking to support GC use for those with mild exacerbations, closely monitored GC should be a part of the initial treatment in case of moderate to severe asthma [
16]. Just one of the GC prescriptions in our sample was indicated for a school-aged child with a moderate wheezing crisis.
Croup
Sixteen (17%) of 95 children in our cohort received at least one GC burst to treat croup. The 2023 Cochrane systematic review on this topic concluded that it reduces croup symptoms, length of hospital stays, and the rate of return consultations [
17]
Rash
Eleven children in this study were treated with GC for skin conditions: 42% for insect bites, a third for dermatitis, and a quarter for cellulitis, urticaria, or miliaria (
Table 3). Current guidelines recommend against GC in atopic dermatitis [
18]. Whether GC are beneficial in acute urticaria is controversial [
19]. There is strong evidence on the efficacy of concurrent systemic steroid use in children with orbital cellulitis. We have not found references to support GC prescription against miliaria or insect bite inflammation.
To summarize, GC bursts did follow current guidelines in one wheezy child, in sixteen children with croup, and in one child with cellulitis, which totals 18 appropriate indications out of 45 participants (40%) that were prescribed GC. These findings deepen previous reports on the inappropriate use of GC in primary care, which may account for 63% of total prescriptions, especially by young physicians or patients under 15 [
20].
Health Care Setting
The escalation of inappropriate GC prescriptions has been linked to multimorbidity, economic and geographical factors, or physicians’ preferences. Parents who make appointments for acute conditions are usually seen within 24 hours in our health area. This is quite adequate when comparing OOH services with scheduled primary care. We have found one-third more GC prescriptions in OOH than in scheduled primary care consultations. While our results, at the P=0.056 level, do not reach statistical significance, they show a trend towards greater ease in prescribing GC by emergency physicians compared to paediatricians in primary care. The data presented in this study are limited to Southeast Spain and, therefore, cannot be considered representative of GC’s short-term usage. Our conclusions may be limited by the sample size, and the fact that we investigated GC misuse indirectly through medical records prescriptions. The retrospective nature of this study does not permit causal inferences. Finally, accurate estimation of the dose and treatment duration is beyond the scope of our work.
5. Conclusions
It is unclear whether the ubiquitous use of GC, from neonatal to pubertal stages, can be attributed to the incidence of these medications’ approved indications or to irrational outpatient drug use. There is a trend towards higher rates of GC prescriptions the further away from Northern Europe and the closer to Southern Europe. Most ever-GC-prescribed wheezy children in our study received repeated doses of GC, a red flag for poor bronchospasm control. This study’s most extensive indications for prescribing GC do not conform to currently available recommendations and evidence. Emergency physicians are more likely to prescribe GC bursts than primary care physicians.
Author Contributions
Conceptualization, S.V. and M.M.; Methodology, E.S. and A.F.; Validation, M.M.; Formal Analysis, A-E.T.; Investigation, SV. and I.P..; Resources, A-E.T.; Data Curation, A-E.T.; Writing—Original Draft Preparation, A.F. and I.P.; Writing—Review & Editing, S.V.; Visualization, ALL AUTHORS; Supervision, M.M.
Funding
This research received no external funding.
Institutional Review Board Statement
The study was conducted in accordance with the Declaration of Helsinki, and approved by the Balearic Islands Review Board (ECIAP-2023).
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data availability statement
The data underlying this article will be shared on reasonable request to the corresponding author.
Acknowledgments
The authors would like to extend their thanks to all the emergency physicians, patients and research assistants who collaborated on this project.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Waljee, A. K.; Rogers, M. A. M.; Lin, P.; Singal, A. G.; Stein, J. D.; Marks, R. M.; Ayanian, J. Z.; Nallamothu, B. K. Short Term Use of Oral Corticosteroids and Related Harms Among Adults in the United States: Population Based Cohort Study. BMJ 2017, 357, j1415. [CrossRef]
- Bleecker, E. R.; Menzies-Gow, A. N.; Price, D. B.; Bourdin, A.; Sweet, S.; Martin, A. L.; Alacqua, M.; Tran, T. N. Systematic Literature Review of Systemic Corticosteroid Use for Asthma Management. Am. J. Respir. Crit. Care Med. 2020, 201 (3), 276–293. [CrossRef]
- Yao, T.-C.; Wang, J.-Y.; Chang, S.-M.; Chang, Y.-C.; Tsai, Y.-F.; Wu, A. C.; Huang, J.-L.; Tsai, H.-J. Association of Oral Corticosteroid Bursts with Severe Adverse Events in Children. JAMA Pediatr. 2021, 175 (7), 723–729. [CrossRef]
- Costa, J.-A.; Rodriguez-Trabal, C.; Pareja, I.; Tur, A.; Mambié, M.; Fernandez-Hidalgo, M.; Verd, S. P-Wave Axis of Schoolchildren Who Were Once Breastfed. Children (Basel) 2023, 10 (7), 1255. [CrossRef]
- Arabkhazaeli, A.; Vijverberg, S. J. H.; van der Ent, C. K.; Raaijmakers, J. A. M.; Maitland-van der Zee, A. H. High Incidence of Oral Corticosteroids Prescriptions in Children with Asthma in Early Childhood. J. Asthma 2016, 53 (10), 1012–1017. [CrossRef]
- Servei de Salut. El Número de Consultas en los Centros de Atención Primaria del Servicio de Salud. https://www.ibsalut.es/es/actualidad-ibsalut/noticias-para-la-ciudadania/1699-el-numero-de-consultas-en-los-centros-de-atencion-primaria-del-servicio-de-salud-aumentan-un-1-75-en-el-ultimo-ano (accessed October 26, 2024).
- Katz, M. A.; Marangu, D.; Attia, E. F.; Bauwens, J.; Bont, L. J.; Bulatovic, A.; Crane, J.; Doroshenko, A.; Ebruke, B. E.; Edwards, K. M.; Fortuna, L.; Jagelaviciene, A.; Joshi, J.; Kemp, J.; Kovacs, S.; Lambach, P.; Lewis, K. D. C.; Ortiz, J. R.; Simões, E. A. F.; Turner, P.; Tagbo, B. N.; Vaishnavi, V.; Bonhoeffer, J.; Brighton Collaboration Wheeze Working Group. Acute Wheeze in the Pediatric Population: Case Definition & Guidelines for Data Collection, Analysis, and Presentation of Immunization Safety Data. Vaccine 2019, 37 (2), 392–399. [CrossRef]
- Global Strategy for Asthma Management and Prevention, 2024. GINA Main Report. https://ginasthma.org/2024-report/Accessed (accessed October 26, 2024).
- Li, X.; Zeng, Z.; Fan, X.; Wang, W.; Luo, X.; Yang, J.; Chang, Y., et sl. Trends and Patterns of Systemic Glucocorticoid Prescription in Primary Care Institutions in Southwest China, from 2018 to 2021. Risk Manag. Healthc. Policy 2023, 16, 2849–2868. [CrossRef]
- Bjornsdottir, H. H.; Einarsson, Ó. B.; Gröndal, G.; Gudbjornsson, B. Nationwide Prevalence of Glucocorticoid Prescriptions Over 17 Years and Osteoporosis Prevention Among Long-Term Users. SAGE Open Med. 2024, 12, 20503121241235056. [CrossRef]
- Einarsdottir, M. J.; Ekman, P.; Trimpou, P.; Olsson, D. S.; Johannsson, G.; Ragnarsson, O. High Prescription Rate of Oral Glucocorticoids in Children and Adults: A Retrospective Cohort Study from Western Sweden. Clin. Endocrinol. (Oxf.) 2020, 92 (1), 21–28. [CrossRef]
- Clavenna, A.; Berti, A.; Gualandi, L.; Rossi, E.; De Rosa, M.; Bonati, M. Drug Utilisation Profile in the Italian Paediatric Population. Eur. J. Pediatr. 2009, 168 (2), 173–180. [CrossRef]
- Bénard-Laribière, A.; Pariente, A.; Pambrun, E.; Bégaud, B.; Fardet, L.; Noize, P., et sl. Prevalence and Prescription Patterns of Oral Glucocorticoids in Adults: A Retrospective Cross-Sectional and Cohort Analysis in France. BMJ Open 2017, 7 (7), e015905. [CrossRef]
- Castro-Rodriguez, J. A.; Beckhaus, A. A.; Forno, E. Efficacy of Oral Corticosteroids in the Treatment of Acute Wheezing Episodes in Asthmatic Preschoolers: Systematic Review with Meta-analysis. Pediatr. Pulmonol. 2016, 51 (8), 868–876. [CrossRef]
- Fainardi, V.; Caffarelli, C.; Deolmi, M.; Skenderaj, K.; Meoli, A.; Morini, R.; Bergamini, B. M.; Bertelli, L.; Biserna, L.; Bottau, P.; Corinaldesi, E.; De Paulis, N.; Dondi, A.; Guidi, B.; Lombardi, F.; Magistrali, M. S.; Marastoni, E.; Pastorelli, S.; Piccorossi, A.; Poloni, M.; Tagliati, S.; Vaienti, F.; Gregori, G.; Sacchetti, R.; Mari, S.; Musetti, M.; Antodaro, F.; Bergomi, A.; Reggiani, L.; Caramelli, F.; De Fanti, A.; Marchetti, F.; Ricci, G.; Esposito, S.; Emilia-Romagna Asthma (ERA) Study Group. Management of Preschool Wheezing: Guideline from the Emilia-Romagna Asthma (ERA) Study Group. J. Clin. Med. 2022, 11 (16), 4763. [CrossRef]
- Canadian Pediatric Society. Position Statement. Managing an Acute Asthma Exacerbation in Children, 2021. https://cps.ca/documents/position/managing-an-acute-asthma-exacerbation (accessed October 26, 2024).
- Aregbesola, A.; Tam, C. M.; Kothari, A.; Le, M.-L.; Ragheb, M.; Klassen, T. P. Glucocorticoids for Croup in Children. Cochrane Database Syst. Rev. 2023, 1 (1) (1), Art. No.: CD001955. [CrossRef]
- AAAAI/ACAAI JTF Atopic Dermatitis Guideline Panel; Chu, D. K.; Schneider, L.; Asiniwasis, R. N.; Boguniewicz, M.; De Benedetto, A.; Ellison, K.; Frazier, W. T.; Greenhawt, M.; Huynh, J.; Kim, E.; LeBovidge, J.; Lind, M. L.; Lio, P.; Martin, S. A.; O’Brien, M.; Ong, P. Y.; Silverberg, J. I.; Spergel, J. M.; Wang, J.; Wheeler, K. E.; Guyatt, G. H.; Patient Groups: Global Parents for Eczema Research; Capozza, K.; National Eczema Association; Begolka, W. S.; Evidence in Allergy Group; Chu, A. W. L.; Zhao, I. X.; Chen, L.; Oykhman, P.; Bakaa, L.; AAAAI/ACAAI Joint Task Force on Practice Parameters; Golden, D.; Shaker, M.; Bernstein, J. A.; Greenhawt, M.; Horner, C. C.; Lieberman, J.; Stukus, D.; Rank, M. A.; Wang, J.; Ellis, A.; Abrams, E.; Ledford, D.; Chu, D. K. Atopic Dermatitis (Eczema) Guidelines: 2023 American Academy of Allergy, Asthma and Immunology/American College of Allergy, Asthma and Immunology Joint Task Force on Practice Parameters GRADE- and Institute of Medicine-Based Recommendations. Ann. Allergy Asthma Immunol. 2024, 132 (3), 274–312. [CrossRef]
- Zuberbier, T.; Abdul Latiff, A. H.; Abuzakouk, M.; Aquilina, S.; Asero, R.; Baker, D.; Ballmer-Weber, B.; Bangert, C.; Ben-Shoshan, M.; Bernstein, J. A.; Bindslev-Jensen, C.; Brockow, K.; Brzoza, Z.; Chong Neto, H. J.; Church, M. K.; Criado, P. R.; Danilycheva, I. V.; Dressler, C.; Ensina, L. F.; Fonacier, L.; Gaskins, M.; Gáspár, K.; Gelincik, A.; Giménez-Arnau, A.; Godse, K.; Gonçalo, M.; Grattan, C.; Grosber, M.; Hamelmann, E.; Hébert, J.; Hide, M.; Kaplan, A.; Kapp, A.; Kessel, A.; Kocatürk, E.; Kulthanan, K.; Larenas-Linnemann, D.; Lauerma, A.; Leslie, T. A.; Magerl, M.; Makris, M.; Meshkova, R. Y.; Metz, M.; Micallef, D.; Mortz, C. G.; Nast, A.; Oude-Elberink, H.; Pawankar, R.; Pigatto, P. D.; Ratti Sisa, H.; Rojo Gutiérrez, M. I.; Saini, S. S.; Schmid-Grendelmeier, P.; Sekerel, B. E.; Siebenhaar, F.; Siiskonen, H.; Soria, A.; Staubach-Renz, P.; Stingeni, L.; Sussman, G.; Szegedi, A.; Thomsen, S. F.; Vadasz, Z.; Vestergaard, C.; Wedi, B.; Zhao, Z.; Maurer, M. The International EAACI/GA²LEN/EuroGuiDerm/APAAACI Guideline for the Definition, Classification, Diagnosis, and Management of Urticaria. Allergy 2022, 77 (3), 734–766. [CrossRef]
- Ashcroft, D. M.; Lewis, P. J.; Tully, M. P.; Farragher, T. M.; Taylor, D.; Wass, V.; Williams, S. D.; Dornan, T. Prevalence, Nature, Severity and Risk Factors for Prescribing Errors in Hospital Inpatients: Prospective Study in 20 UK Hospitals. Drug Saf. 2015, 38 (9), 833–843. [CrossRef]
Table 1.
Demographic and somatometric data by glucocorticoid prescription. Data are mean (SD) unless otherwise specified.
Table 1.
Demographic and somatometric data by glucocorticoid prescription. Data are mean (SD) unless otherwise specified.
| VARIABLE |
No prescribed oral glucocorticoids |
Prescribed oral
glucocorticoids |
P value |
| Number |
50 |
45 |
|
| Age (years) |
11.18 (3.00) |
11.00 (3.03) |
NS |
| Girls//boys |
22/28 |
25/20 |
NS |
| Weight percentile |
51.80 (28.09) |
58.25 (26.67) |
NS |
| Height percentile |
63.76 (27.07) |
65.10 (24.54) |
NS |
| BMI percentile |
46.24 (27.73) |
49.47 (27.01) |
NS |
Table 2.
Oral glucocorticoids prescription (days and short courses) to 95 children of our sample. Data are mean (SD) unless otherwise specified.
Table 2.
Oral glucocorticoids prescription (days and short courses) to 95 children of our sample. Data are mean (SD) unless otherwise specified.
| Illness |
Days of oral glucocorticoids |
Short courses of oral glucocorticoids |
| Wheezing |
2.14 (5.68) |
0.66 (1.86) |
| Crup |
2.28 (9.76) |
0.52 (1.50) |
| Rash |
0.82 (2.41) |
0.15 (0.39) |
| Total |
5.55 (1.50) |
1.51 (2.63) |
Table 3.
Oral glucocorticoid use for acute cutaneous disease. Data are mean (SD) unless otherwise specified.
Table 3.
Oral glucocorticoid use for acute cutaneous disease. Data are mean (SD) unless otherwise specified.
| gender |
Age (years) |
season |
diagnosis |
prescribing physician’s specialty |
| girl |
2 |
spring |
Atopic dermatitis |
Primary care Paediatrician |
| girl |
10 |
summer |
Atopic dermatitis |
Primary care Paediatrician |
| boy |
1 |
winter |
Atopic dermatitis |
Emergency physician |
| girl |
11 |
winter |
Atopic dermatitis |
Unknown |
| girl |
7 |
spring |
Urticaria |
Emergency physician |
| boy |
10 |
winter |
Miliaria (sweat rash) |
Primary care Paediatrician |
| boy |
7 |
summer |
Insect bite |
Primary care Paediatrician |
| boy |
3 |
winter |
Insect bite |
Primary care Paediatrician |
| girl |
2 |
winter |
Insect bite |
Primary care Paediatrician |
| boy |
3 |
spring |
Insect bite |
Unknown |
| boy |
6 |
summer |
Scarlet fever |
Emergency physician |
| boy |
6 |
winter |
Cellulitis (dermis infection) |
Primary care Paediatrician |
Table 4.
Comparison between nominal figures of medical care and actual salbutamol or oral glucocorticoids orders by outpatients’ setting.
Table 4.
Comparison between nominal figures of medical care and actual salbutamol or oral glucocorticoids orders by outpatients’ setting.
| |
Primary care |
Out-of-hours |
| Salbutamol paediatric prescriptions |
119 (84%) |
23 (16%) |
| Annual medical consultations versus salbutamol paediatric prescriptions |
P=0.862 |
| Ambulatory annual medical consultations1
|
5,922,161 (85.4%) |
1,101,542 (14.6%) |
| Annual medical consultations versus oral glucocorticoids paediatric prescriptions |
P=0.056 |
| Oral glucocorticoids paediatric prescriptions |
79 (77%) |
23 (23%) |
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).