1. Introduction
The World Health Organization (WHO) defines abortion as the involuntary termination of pregnancy up to 22 weeks’ gestation, which, in quantified terms, would correspond to a fetal weight of approximately 500 grams [
1]. Spontaneous abortion affects a large number of women, with most occurring in the first 12 weeks of pregnancy [
2].
It is estimated that 25% of miscarriages could be prevented if risk factors could be reduced. However, approximately 50% of miscarriages have unknown causes [
3].
Studies have shown that viruses such as cytomegalovirus (CMV), herpes simplex virus (HSV-1/2), human papillomavirus B19 (B19V), enterovirus, adenovirus, and varicella zoster virus are the most common causes of miscarriage [
4,
5,
6,
7]. However, other causes may be associated with miscarriage.
The reported consequences of maternal infection caused by some arboviruses include placental transmission, miscarriage, congenital malformations, stillbirths, intrauterine growth restriction, and preterm delivery [
8].
In recent years, several evidences have led to the belief that arboviruses are present in the maternal and child context and should be monitored, seeking preventive strategies and better clinical management for pregnant women and their concept, or neonate, who may become ill [
9,
10,
11].
Arboviruses are characterized by a group of viral diseases, transmitted by vectors (arthropod-borne viruses) to susceptible vertebrate hosts. Arboviruses are viruses transmitted to humans by the bite of hematophagous arthropod vectors, as part of the viral replicative cycle occurs in these arthropods [
12].
Currently, the most prevalent viruses in Brazil are the
Orthoflavivirus denguei (DENV),
Chikungunya virus (CHIKV) and the
Orthoflavivirus zikaense (ZIKV) [
13]. DENV and ZIKV belong to the
Flaviviridae family, while CHIKV belongs to the
Togaviridae family [
14].
For fetal infection by ZIKV to occur and cause fetal malformations, the highest probability occurs during the first trimester of pregnancy, in which the trophoblast is more permissive to ZIKV, presenting immaturity of the villi, reducing its defenses in controlling infections, causing cause the virus to attack fetal neural tissue, causing neurodevelopmental abnormalities. Spontaneous abortions, fetal losses and neonatal deaths have also been observed. These occurrences are related to the gestational period in which there was exposure to the virus [
15].
Infection with ZIKV has been identified as the cause of spontaneous abortion, taking into account the vertical transmission from the positive mother during pregnancy [
16].
Another arbovirus associated with maternal-infant complications is CHIKV, as there is evidence of a high risk of abortion in the first trimester and maternal-fetal transmission in the last trimester [
13,
17].
Among the obstetric complications related to CHIKV were spontaneous abortion in the first trimester, pre-eclampsia in the second and third historical trimesters and hospitalization in the Intensive Care Unit (ICU), due to viral sepsis caused by CHIKV. Among the obstetric pathologies, premature rupture of membranes, intrauterine growth restriction, pre-eclampsia, preterm delivery and postpartum hemorrhage have been reported. Among pregnant women infected with CHIKV (15%) required ICU and (89%) were in the third trimester of pregnancy [
18].
It is noted that there is still no effective treatment that can be used safely in pregnant women who are affected by such arboviruses. Several researches are underway, trying to get better results to reduce the damage caused by the triple viral infection in an attempt to reduce its effects as well as to alleviate the complications for the pregnant woman and her fetus [
19].
Conducting epidemiologic and clinical research to improve knowledge of prevention and care for women who are affected by spontaneous abortion is the basis of this study. Now, it is known that many women are assisted in hospitals for having had an abortion, however, most of the time, it is not known why this abortion happened.
However, it is necessary to better investigate the etiology of these spontaneous abortions, especially if there is a correlation with ZIKV and CHIKV infection, as they may be capable of causing abortion.
Therefore, this study aims to investigate the association of spontaneous abortions with the occurrence of infections by the Zika virus and Chikungunya virus.
2. Methods
2.1. Study Design and Sampling
This is a descriptive, longitudinal, retrospective, qualitative-quantitative study. According to Gil (2017), descriptive research is used when it aims to describe the characteristics of certain populations or phenomena [
20]. The longitudinal study is intended to study a process over time to examine changes, that is, reflect a sequence of facts. These are studies that investigate the prevalence of new cases of a particular disease in a population, and can be conducted at different time intervals [
21]. In the case of a retrospective study, the researcher can collect past information on the exposure factor(s) (hence the term retrospective) and follow the subjects over a period of time (the cohort) [
22].
The sample universe consisted of pregnant women who had a miscarriage in the years 2015, 2016 and 2017, comprising an “N” sample of 30 women who received care at the FSCMPA. The study material comprises tissue block samples in paraffin containing material from the miscarriage, corresponding to placental and/or fetal remains.
The study period was chosen because the arboviruses under investigation were circulating at the same time.
Women who had a spontaneous abortion, with a gestational age up to 22 weeks, or the product of the abortion weighing less than 500g, without a previously known cause, who underwent curettage or MVA procedures and who were examined for histopathologic evaluation, were included in the research. corresponding to the samples in paraffin blocks, and this one was sent to the Ruth Brazão Laboratory.
Pregnant women who underwent curettage or MVA procedures and examination collection for histopathologic evaluation were excluded from the study, and this was sent to another laboratory other than the Ruth Brazão Laboratory and pregnant women who did not have suspicious or suggestive symptoms of ZIKV infection. and CHIKV, such as fever, headache, retro-orbital pain, muscle and/or joint pain, among others.
2.2. Data Collection
The data collected consisted of the following five steps:
a) Step 1- The data collection was carried out through the analysis of medical records, during the aforementioned period, by filling in a pre-prepared instrument (APPENDIX A), which was used to organize the data and included the following study variables: Sociodemographic characteristics (origin, age group, race and marital status); Reproductive characteristics (pregnancy history, parity history, abortion history, gestational age at death); Clinical conditions (symptoms); Type of abortion (threatened abortion, complete abortion, incomplete abortion, missed abortion, and habitual or recurrent abortion); method of abortion (curettage, MVA). The women who were included in the study, according to the inclusion criteria, had their medical records filled in, as well as the order number of the histopathological examination, so that they could be identified in the external laboratory (Ruth Brazão Laboratory), associated with the FSCMPA, to which the collection of histopathological material was sent;
b) Step 2- In the aforementioned laboratory, the histopathologic material, already embedded in paraffin, was located, according to the numbering of the histopathologic examination work order contained in the medical record of each selected patient, according to the inclusion criteria;
c) Step 3 - In the pathology laboratory of the IEC, 5 μm sections were made from the paraffin blocks of each selected patient, which were fixed on 8 different slides for the immunohistopathological analyses. The histologic sections obtained were stained and examined by optical microscopy in the aforementioned laboratory. The remainder of the blocked material was returned to the laboratory, in order to preserve the sample;
d) Step 4 - The 8 slides prepared from each patient were examined by the techniques of hematoxylin-eosin and immunohistochemistry for ZIKV and CHIKV, one of which was kept in reserve, in case the experiment needed to be repeated and;
e) Step 5: After the completion of the steps, the resulting waste, corresponding to the sharps was properly disposed in a sharps collector (discard) according to the biosafety criteria contained in RDC No. 222, dated March 28, 2018, which regulates the good practices of waste management in health care, among other provisions.
2.3. Histopathology
After fixation of the tissue samples obtained from curettage or MVA, they were processed for histopathologic analysis according to the routine Hematoxylin-Eosin (HE) staining protocol of the pathology laboratory. First, the samples were dehydrated in graded baths of 70% to 100% alcohol for one hour and twenty minutes each step, followed by two immersions in a xylene bath at room temperature and successive baths in liquid paraffin, maintained at a temperature of 60°C. The material was then embedded to form paraffin blocks. In this study, the paraffin blocks were already prepared and located to perform the microtomy of the samples in 5 μm sections, performed in a LEICA histotechnician (TP 1020). The histologic sections were then stained by the HE technique and examined under an optical microscope.
2.4. Immunohistochemistry (IHC)
For the detection of viral antigens of ZIKV and CHIKV in histological sections, the immunohistochemistry technique was performed [
23]. 5 μm sections of the blocks were harvested on slides previously prepared with 3-amino-propyltriethoxy-silane adhesive solution (Sigma). Then, this material was deparaffinized in two xylene baths at room temperature for 5 minutes each and then rehydrated in decreasing sequence with absolute alcohol (100%, 95% and 70%) for five minutes each and distilled water.
Subsequently, antigen retrieval was performed, placing the slides on a plastic support and adding EDTA inside the metallic cuvettes until the tissue was completely covered. Then, the metallic cuvettes were placed in 500 ml of distilled water in an electric pressure cooker for 15 minutes at a temperature of 110ºC. After removing the cuvettes from the pressure cooker, a jet of distilled water was poured over them. The tissue was incubated with nonspecific reaction blocking solution (Background Punisher) for 10 minutes at room temperature. In the next step, the samples were incubated with the polyclonal antibody produced “in house” in adult mice by the intraperitoneal route against the aforementioned viruses and incubated for 30 minutes at room temperature, with a titration of 1:200.
Slides were washed 2 times with TBS (TRIS Saline Buffer) solution. Subsequently, the tissue was incubated with secondary antibody from the commercial kit (M4CH4 Universal AP-Prob) for 10 minutes at room temperature. The slides were washed with TBS solution and incubated with enzymatic polymer for 15 min and then washed 2 more times with TBS solution. The reaction was developed with chromogen chromogen solution (Fast Red) for 20 minutes at room temperature, then washed with distilled water. For counterstaining, hematoxylin was added for 2 minutes, then washed with distilled water for 1 minute. Slides were mounted using Permount resin (Fisher Scientific).
Photodocumentation was performed by means of a photographic record with a camera attached to the Axio autoimage Z2 microscope (Zeiss, Oberkochen, Germany), at 400X magnification in the spontaneous abortion samples.
2.5. Statistical Analysis
The data obtained during the study were stored in Microsoft Office Excel (2010) Spreadsheets. The method used to analyze the quantitative data, obtained from the medical records is based on descriptive statistics through absolute and relative frequencies, seeking to verify if the data converge to a particular differential or if there is a tendency in their distribution. Comparisons between the distributions of the variables were performed using the Chi-square test for tendency/adherence, wich is a hypothesis test designed to verify whether or not there is a tendency in the distribution of nominal and ordinal variables [
24]. adopting a significance level of p-value < 0.05. Statistical analyses were carried out using the Bioestat 5.4 software.
2.6. Ethical Statement
The present study was developed in the Pathology Department of the Evandro Chagas Institute (IEC) in collaboration with the Santa Casa de Misericórdia do Pará Foundation (FSCMP) and Ruth Brazão Laboratory. It began after approval by the IEC’s Committee for Ethics in Human Research (CEP) and the CEP of the FSCMPA. The study was submitted for review and submission according to Resolution 466 of 2012 of the National Health Council of the Ministry of Health, approved by the Certificate of Ethical Assessment (CAAE) under the numbers 18613819.3.0000.0019 and 18613819.3.3001.5171.
4. Discussion
The etiology of miscarriage remains poorly understood wich may be due to inconclusive, incomplete and unreported nature of data on spontaneous abortion. It is imperative to raise awareness among healthcare professionals so that they can correctly and completely investigate situations of spontaneous abortion so that it is possible to identify the causes and risk factors in order to prevent the future fetal damages like miscarriage. This study will facilitate the comprehension one of possible risk factors that may be associated miscarriages during ZIKV and CHIKV infections of pregnant women [
25].
Regarding the sociodemographic, clinical and obstetric data portrayed in our study, spontaneous abortion usually occurs in the first gestational trimester [
26]. Being that understanding its causes can be an important aspect as a preventive way. This corroborates the data of our study, in wich the abortion took place in the first trimester of pregnancy, corresponding to 12 weeks of gestation. In estimated that 25% of spontaneous abortions could be avoided if the risk factors could be reduced [
1]. However, approximately 50% of miscarriages have unknown causes. There may be causes related to genetic factors and non-genetic causes.
It highlights the non-genetic causes of spontaneous abortion, such as socio-demographic factors associated with advanced maternal age (> 35 years), in those over 35 years [
1]. High maternal age, over 35 years, has been considered a risk factor for miscarriage and fetal malformations due to the senility of the oocyte, which is more prone to chromosomal alterations in the event of fertilization [
27]. A 5-year increase in maternal age increases the risk of miscarriage by 1.5 times [
3,
28,
29].
However, some studies found a contrary association. One explanation for this divergence lies in the fact that studies that associated maternal age over 35 years with abortion were carried out in developed countries, where women are likely to become pregnant at an older age; and studies that address maternal age below 35 years as a risk factor were carried out in developing countries, where women become pregnant earlier, increasing the likelihood of miscarriage [
30,
31,
32]. This corroborates the data of our study, in which women aged between 20-29 years predominated.
Our study showed that women who had a spontaneous abortion at the maternity referral hospital were from the state capital (Belém). The high-risk maternity referral hospital of the present study receives a large number of pregnant women through spontaneous demand, referred from the metropolitan region of Belém and municipalities in the interior of the Pará State. A study by Durex et al. (2016) shows the number of hospital admissions for spontaneous abortion in the regions of Brazil from 2010 to 2014, in which the North region had 5.79 admissions per 1000 women of childbearing age (10 - 49 years) [
33]. The scarcity of studies involving our region showing data on spontaneous abortion is notorious.
In the study conducted, most women declared themselves to be brown. According to the study carried out by Soares and Cançado (2018) at Santa Casa de Misericórdia de Passos, in Minas Gerais (MG), the majority of women who had spontaneous abortions, according to ethnicity, 47.5% were white, unlike the finding in our research [
34]. However, the highest prevalence of abortion was observed in mulatto women (66%), followed by whites (28%) and blacks (6%) [
35]. corroborating the finding of our study. Thus, these studies show that ethnicity may differ according to the study population evaluated.
Another fact highlighted in that study was the absence of a steady partner in 44% of the women who suffered a miscarriage [
35]. This data corroborates our research, in which the majority of women who had a miscarriage were single This can be explained by the lack of affective support and economic instability that women who do not have a stable relationship are susceptible to [
36].
Most infections caused by arboviruses are asymptomatic, but 20% to 25% of infected people have nonspecific clinical manifestations, giving rise to the need for a differential laboratory diagnosis between chikungunya and dengue (SHARP et al., 2019). ZIKV or CHIKV infection should be suspected if two or more of the following symptoms occur: absence or low-grade fever ≤38°C (1-2 days of low-grade fever) in ZIKV and high fever >38°C (2-3 days) in CHIKV, skin rashes, muscle pain, joint pain, conjunctivitis, headache, among others. In our study, most women had headache followed by fever, ranging between 37-38°C at the time of hospitalization for abortion in the reference maternity hospital. Thus, it is suggested that they may have been affected by arboviruses, despite the non-specific symptoms [
37].
In our study, most women had a miscarriage for the 1st time and already had a previous pregnancy, which corroborates the study by Soares and Cançado (2018) [
36]. in which in the previous history of the patients, 82.5% had the first pregnancy, pregnancy loss and 60% had a previous pregnancy. Data evidenced in the literature in which there is a higher rate of abortions the greater the number of children [
36,
38].
In our study, the majority of women had uterine curettage as an abortion procedure. A study on therapeutic approaches in the uterine abortion process identified that uterine curettage was the second most observed treatment, accounting for 175 (37.55%) indications for this therapy. MVA was responsible for 85 (18.24%) indications for uterine evacuation, although 370 (79.40%) patients had early miscarriage [
39].
It can be concluded that the obstetric profile of women who experience pregnancy loss does not differ from that found nationally, curettage was the most used final therapy, plus some unfavorable outcomes, such as longer hospital stay and excessive exposure to medication [
39].
It is possible to show that MVA is a good alternative in the management of first trimester abortion due to its lower costs, fewer complications, high efficiency in the resolution of the pathology and great satisfaction on the part of users. According to international organizations, the use of uterine curettage should be eliminated from obstetric practice, replacing it with manual or electric aspiration, or the use of medication [
43].
However, deciding on the best conduct in the treatment of miscarriage is still a major challenge for prescribers today, reflecting the lack of information based on scientific evidence that allows for a safe and effective conduct [
44].
Our study showed that most women had incomplete abortion. Conduct in abortion, the main conduct was incomplete abortion, accounting for 44.85% (n=209) hospitalizations for uterine emptying, followed by fetal death, with 34.98% (n=163). Regarding the treatment of choice, misoprostol was the most used 59.01% followed by the curettage procedure 37.55%, both used associated or not [
45].
Regarding the histopathological and immunohistochemical aspects evidenced, the vertical transmission of arboviruses that may have caused spontaneous abortions, described in our research, has already been described in other arboviruses. Vertical transmission of arboviruses, caused by flaviviruses including West Nile, Dengue and Zika, where they found viral replication in the placenta of humans and mice [
46,
47].
The pathogenesis of the process of transplacental transmission of ZIKV, although well accepted in the literature, is still poorly understood [
48]. As well as the detection of the CHIKV genome in placentas of pregnant women with virus infections has already been reported in the literature previously [
49,
50,
51,
52]. The association between ZIKV infection and miscarriage has also been demonstrated in a study with non-human primates (Imbeloni, et al., 2021; Alcantara, et al., 2021)
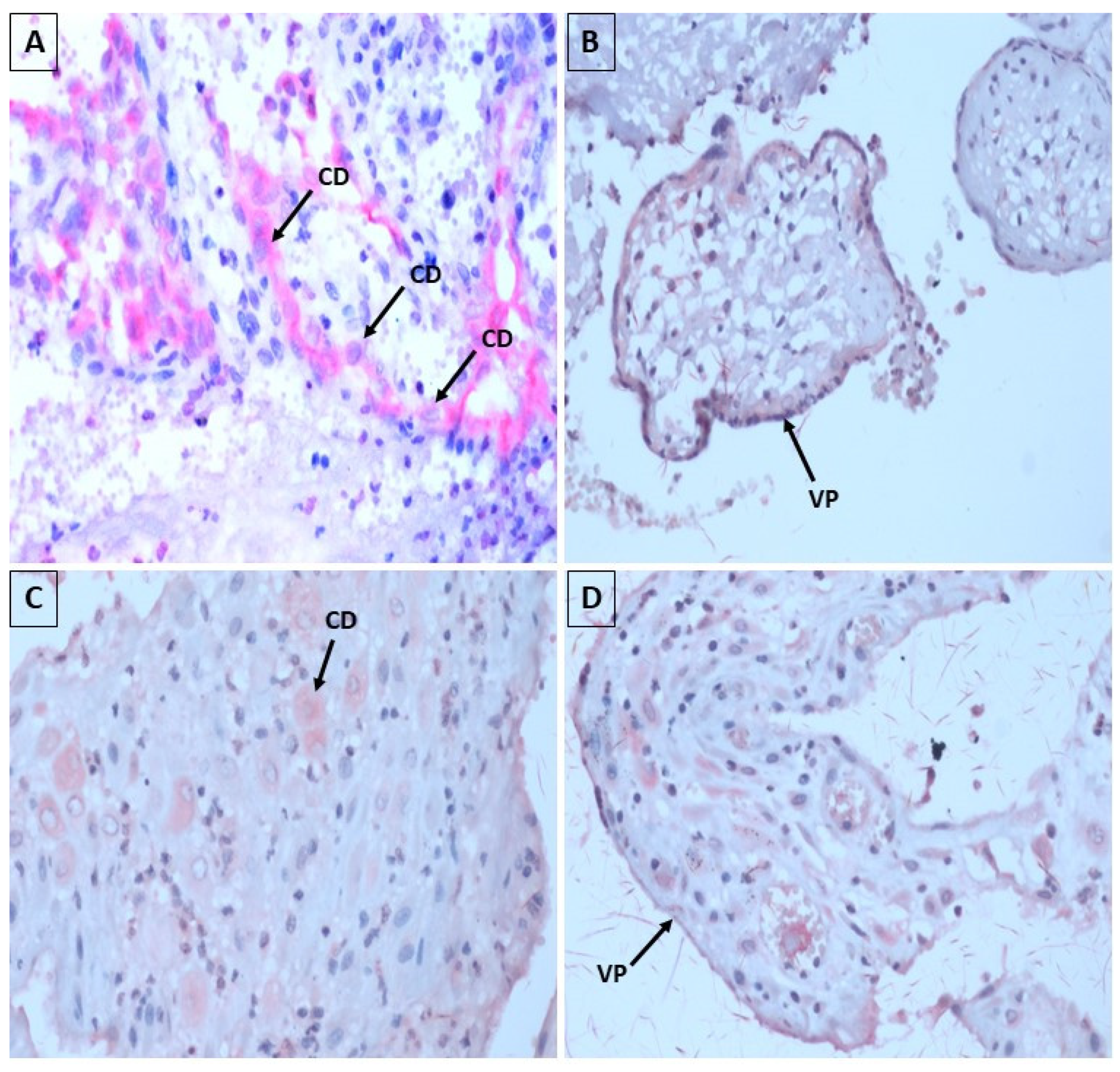
In our research, with the use of immunohistochemistry, in 40% of the samples the antibodies against ZIKV and antibodies against CHIKV revealed focal positivity, with predominance for CHIKV, with reddish granular material in the cytoplasm of the decidua cells and placental villi, elucidating that the virus may be present in these placental regions, which was also evidenced in the study by Annemiek et al. (2022) in which in situ hybridization using ZIKV-specific probes, compared to control probes, revealed that placental amniotic epithelium was positive for ZIKV RNA, while fetal chorion and trophoblasts and maternal decidua were not. showed evidence of ZIKV infection [
53].
It was possible to observe placental villi coated with STB and CTB, containing swollen conjunctival mesh, in the spontaneous abortion samples. described that ZIKV seems to be able to induce vascular damage followed by apoptosis in the placental tissue, making the placenta more permeable, facilitating the entry of the virus into syncytiotrophoblastic cells. Once in the placental tissue, ZIKV can replicate in other cell types, such as macrophages and fetal endothelial cells, acting as true deposits of the virus, allowing its dissemination in fetal blood [
53].
Using immunohistochemical techniques, the CHIKV antigen was detected in the epithelial cells of the endometrial glands and in the decidual cells in the case of spontaneous abortion. In addition, CHIKV antigen was also detected in some chorionic villus STB. Likewise, our findings corroborate those of the aforementioned author [
55].
STBs are also an important cell type that undertakes the endocrine function of the human placenta to drive the physiological and metabolic adaptations to pregnancy. CTBs have a high proliferation capacity and form a monolayer of polarized stem cells, which eventually differentiate via cell-cell fusion into STBs that cover the entire surface [
56].
Different subtypes of trophloblasts have different susceptibilities to pathogenic infections. Although the mechanisms are still poorly understood, STBs have been shown to be resistant to infections by several pathogens. As well as the arboviruses reported in our study. In a recent pandemic, it was proposed that SRS-CoV-2 also prefers to target STB, probably due to the high abundance of viral receptors on its apical surface [
56].
It is worth mentioning that syncytiotrophoblastic cells produce human chorionic gonadotropin (hCG), a very important hormone to stimulate the corpus luteum in the continuous production of progesterone, which in turn is necessary to maintain a pregnancy. Once infected, production can be altered, leading to miscarriage. Perhaps, the involvement of the STBs found impaired the production of hCG, which possibly culminated in the fatal outcome of the spontaneous abortion cases reported in our study [
57].
A study conducted by Martines et al., (2016) described two cases of miscarriage by ZIKV. One of the reported cases was a previously healthy 37-year-old woman who developed fever and rash at 8 weeks’ gestation and subsequently miscarried at 11 weeks’ gestation. It is important to mention that no drug or chemical exposures have been reported. Prenatal serologic testing was negative for cytomegalovirus, rubella virus, toxoplasma gondii, herpes simplex virus and HIV. Placental blocks were available for evaluation. Placental tissue showed dense and heterogeneous chorionic villi with calcification, sclerosis, edema, increased perivillous fibrin deposition and irregular lymphohistiocytic intervillous inflammation. Immunohistochemistry of placental tissue was positive for Zika virus, with antigens observed on Hofbauer cells in the chorionic villi [
58].
In another study carried out on Réunion Island, vertical transmission of CHIKV was first observed in June 2005 during the CHIKV epidemic that affected more than one-third of the local population from March 2005 to July 2006. However, evidence of transplacental infection has been reported by recovery of the CHIKV viral genome from amniotic fluid, placenta and fetal brain of stillborn fetuses on Réunion Island when fetal loss occurred less than 16 weeks of gestational age. The maternal diagnosis of chikungunya was confirmed by RT-PCR detection in maternal blood two weeks before the diagnosis of fetal loss; these detections of viral genome in placenta and amniotic fluid confirmed transplacental transmission (vertical transmission) of CHIKV as well as its persistence after fetal death, demonstranting once again the viral tropism of arboviruses for placental tissues [
49].
The other case was a previously healthy 31-year-old woman who presented with fever and rash at 8 weeks gestation. No drug or chemical exposures were reported. She had miscarried at 13 weeks’ gestation. Ultrasound at 6 weeks’ gestation showed no abnormalities, and prenatal serologic tests for HIV, herpes simplex virus, hepatitis B and C viruses, cytomegalovirus (IgM), rubella virus (IgM), and Toxoplasma gondii were negative. Paraffin blocks from the curettage specimen were available for examination [
58].
The specimens showed predominantly endometrial tissue with Arias-Stella reaction. Tiny pieces of placental tissue showed no significant findings. Immunohistochemistry was negative for ZIKV. ZIKV RT-PCR was positive and sequence analysis showed 100% identity with ZIKV strains isolated in Brazil in 2015 [
53]. The Arias-Stella reaction observed in the aforementioned study was also found in fragments of endometrium in our study, often showing secretion by decapitation of the apical portion of the epithelium.
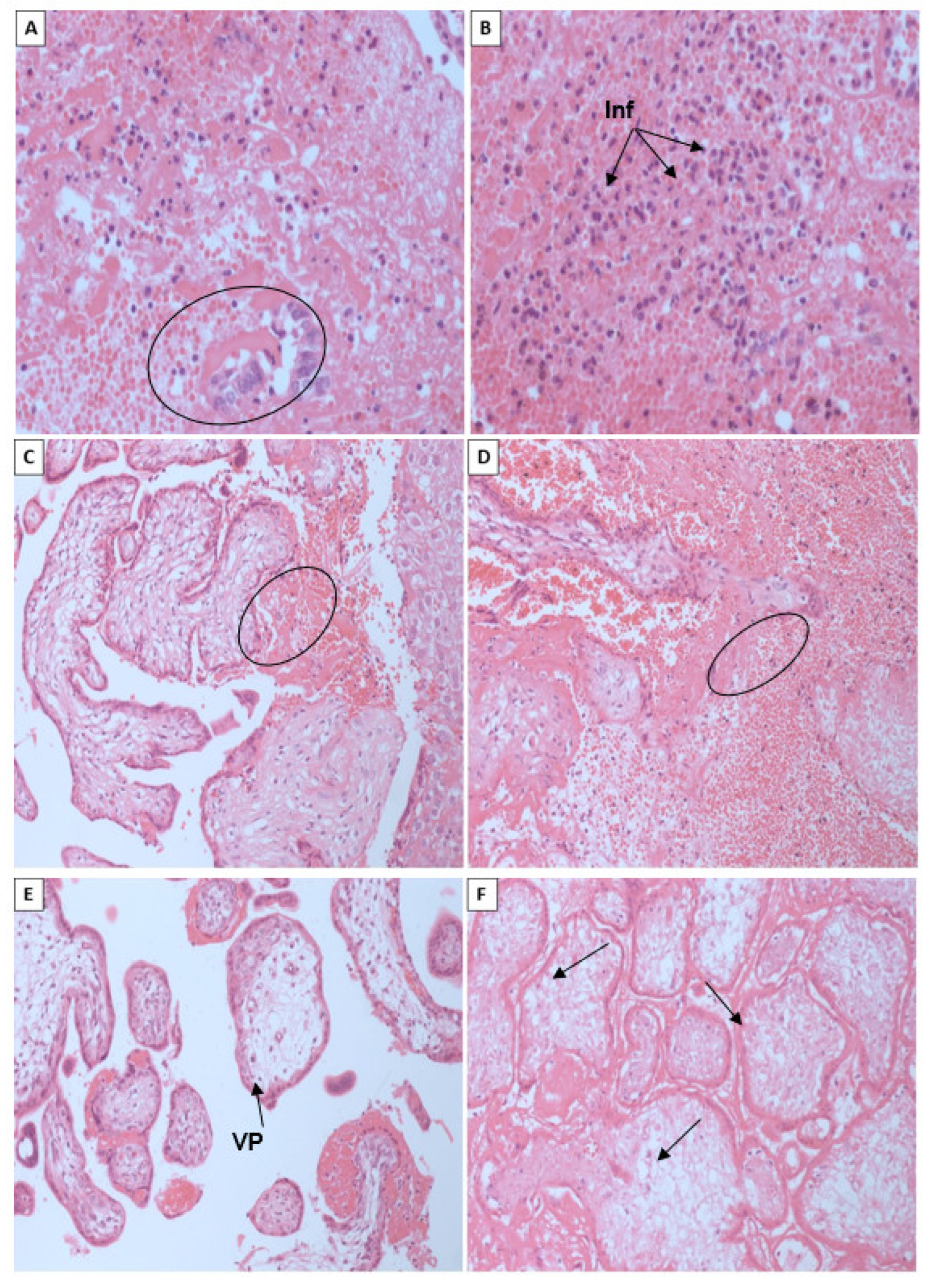
We also found intense areas of inflammatory infiltrate in fragments of the decidua, forming areas rich in neutrophils, necrotic tissue and hemorrhages. In the chorioamnionic membranes, the cells showed inflammation with the presence of neutrophils and lymphocytes. A study in a woman with suspected ZIKV infection who had a spontaneous abortion, in which amniocentesis was performed, followed by dilation and curettage, histopathologic examination and immunohistochemistry using CD45 and CD3 antibodies to detect inflammation showed no evidence of increased infiltration of inflammatory cells [
53]. This contradicts the findings of our study.
However, in another study in a pregnant woman diagnosed with incomplete abortion and with a positive serologic test for CHIKV, the histopathologic findings showed that there was an extensive area of inflammatory infiltrate in the decidua, composed mainly of lymphocytes and neutrophils, characterized by their morphology in infected tissue. The chorionic villi showed changes such as inflammatory infiltrate and areas of calcification, edema and deposition of fibrinoid material [
55].
In the same study reported, another pregnant woman with a positive serologic test for CHIKV had a spontaneous abortion, in which curettage was performed. The chorionic villi also showed areas of fibrin deposition and edema, except for calcifications and inflammatory infiltrate. In addition, the intervillous space showed infiltrate of lymphocytes [
55]. Therefore, the presence of inflammatory infiltrate may be a factor associated with abortion, but it is not a single factor and requires the presence of other factors.
In a report on spontaneous abortion, irregularities were observed in the chorionic villi. These irregularities included edema wich is recognizable by open spaces in the cytoplasm of intervillous cells and in the interstitium of villi. This edema could be a possible cause of prenatal hypoxia [
59]. It is noteworthy that the swelling observed in regions of the placental villus is also described in our study. Placental tissue from spontaneous abortion caused by ZIKV infection may exhibit features such as edema, deposition of fibrinoid material and calcification. Swollen conjunctive meshes may also be observed in cases of spontaneous abortion [
60].
In our study, extensive associated necrosis was observed, and areas represented by blood clots were frequently associated with an extensive inflammatory component. The results of deaths of stillbirths and neonates caused by ZIKV infection showed an intrinsic and complex relationship between the immune response and the occurrence of cell damage. The main types of cell death found were necrosis and apoptosis [
61].
The are few studies showing that CHIKV can cause long-term morbidity and death in fetuses and neonates infected during pregnancy [
62]. (inseri no comentário alguns artigos na literatura).
We observed that three pregnant women with both the ZIKV and CHIKV coinfection culminated in spontaneous abortion. Coinfection of ZIKV and other arboviruses has been previously documented with: DENV and CHIKV in Colombia, DENV, ZIKV, CHIKV in some areas of Mexico [Eligio-García, et al., 2020]. and with DENV in French Polynesia and New Caledonia [
63]. The magnitude of arbovirus co-infection during pregnancy is complex and is not yet clear, especially due to the high frequency of asymptomatic infections and cross reactions between flaviviruses, such as ZIKV and DENV
. And this impact of co-infection caused by arboviruses is little known, even though a fatal co-infection caused by DENV and CHIKV has already been reported in Colombia [
64].
A study showed that a woman in her second trimester of pregnancy was diagnosed by RT-PCR with a co-infection caused by CHIKV and ZIKV after an abnormal ultrasound, with evidence of the absence of fetal heartbeat. After fetal autopsy, it was possible to observe low birth weight, along with renal and placental calcifications. Such findings are in agreement with our cases of co-infections reported in our study with ZIKV and CHIKV. It is worth mentioning that complementary studies such as the detection of the viral genome by RT-PCR of the study samples need to be performed so that we can affirm these findings more strongly [
52].
Thus, the infection possibly caused by arboviruses in the placenta, may cause placental dysfunction, leading to adverse effects on the fetus, making further research on this placental collection necessary. Additionally, the scarcity of updated regional studies on the subject as well as maternal clinical data are essentially necessary for the acquisition of new data on arboviruses that have already been associated or known in pregnancies, as well as the etiology of abortions in the metropolitan area of Belém and other regions of Pará state.