Submitted:
07 November 2024
Posted:
08 November 2024
You are already at the latest version
Abstract
In regenerative medicine, mesenchymal stem cells (MSCs) have shown their importance and potential in tissue reconstruction and immune system modification. However, such cells’ potential is often diminished by factors such as oxidative stress, immune rejection, and inadequate engraftment. This review highlights the role of molecular hydrogen (H₂) and cold atmospheric plasma (CAP) as adjunct therapies to improve the effectiveness of MSC therapy has a strong antioxidative and anti-inflammatory action as it quenches reactive oxygen species and positively stimulates the Nrf2 pathway that promotes MSC survival and life. CAP, being a modulated source of ROS and RNS, also assists MSCs by altering the cellular redox balance, thus facilitating cellular adaptation, migration and differentiation. H₂ and CAP in conjunction with each other assist in establishing an ambience favorable for promoting MSC survival and growth ability and reducing the healing time in various pathways such as wound, neuroprotection, and ischemia. Besides these concerns, this review also covers the best administration routes and doses of H₂ and CAP together with MSCs in therapy. This study informs on a novel dual method aimed at improving the outcome of MSC therapy while adding several molecular targets and relevant clinical use concerning these therapies. Research of the future has to deal with bettering these protocols so that the therapeutic benefits can be maximized without long-term implications for clinical applications.In regenerative medicine, mesenchymal stem cells (MSCs) have shown their importance and potential in tissue reconstruction and immune system modification. However, such cells’ potential is often diminished by factors such as oxidative stress, immune rejection, and inadequate engraftment. This review highlights the role of molecular hydrogen (H₂) and cold atmospheric plasma (CAP) as adjunct therapies to improve the effectiveness of MSC therapy has a strong antioxidative and anti-inflammatory action as it quenches reactive oxygen species and positively stimulates the Nrf2 pathway that promotes MSC survival and life. CAP, being a modulated source of ROS and RNS, also assists MSCs by altering the cellular redox balance, thus facilitating cellular adaptation, migration and differentiation. H₂ and CAP in conjunction with each other assist in establishing an ambience favorable for promoting MSC survival and growth ability and reducing the healing time in various pathways such as wound, neuroprotection, and ischemia. Besides these concerns, this review also covers the best administration routes and doses of H₂ and CAP together with MSCs in therapy. This study informs on a novel dual method aimed at improving the outcome of MSC therapy while adding several molecular targets and relevant clinical use concerning these therapies. Research of the future has to deal with bettering these protocols so that the therapeutic benefits can be maximized without long-term implications for clinical applications.
Keywords:
1. Introduction
2. Molecular Hydrogen and Cold Atmospheric Plasma: Fundamental Concepts
2.1. Generation Methods and Delivery Systems
2.2 Biological Interactions
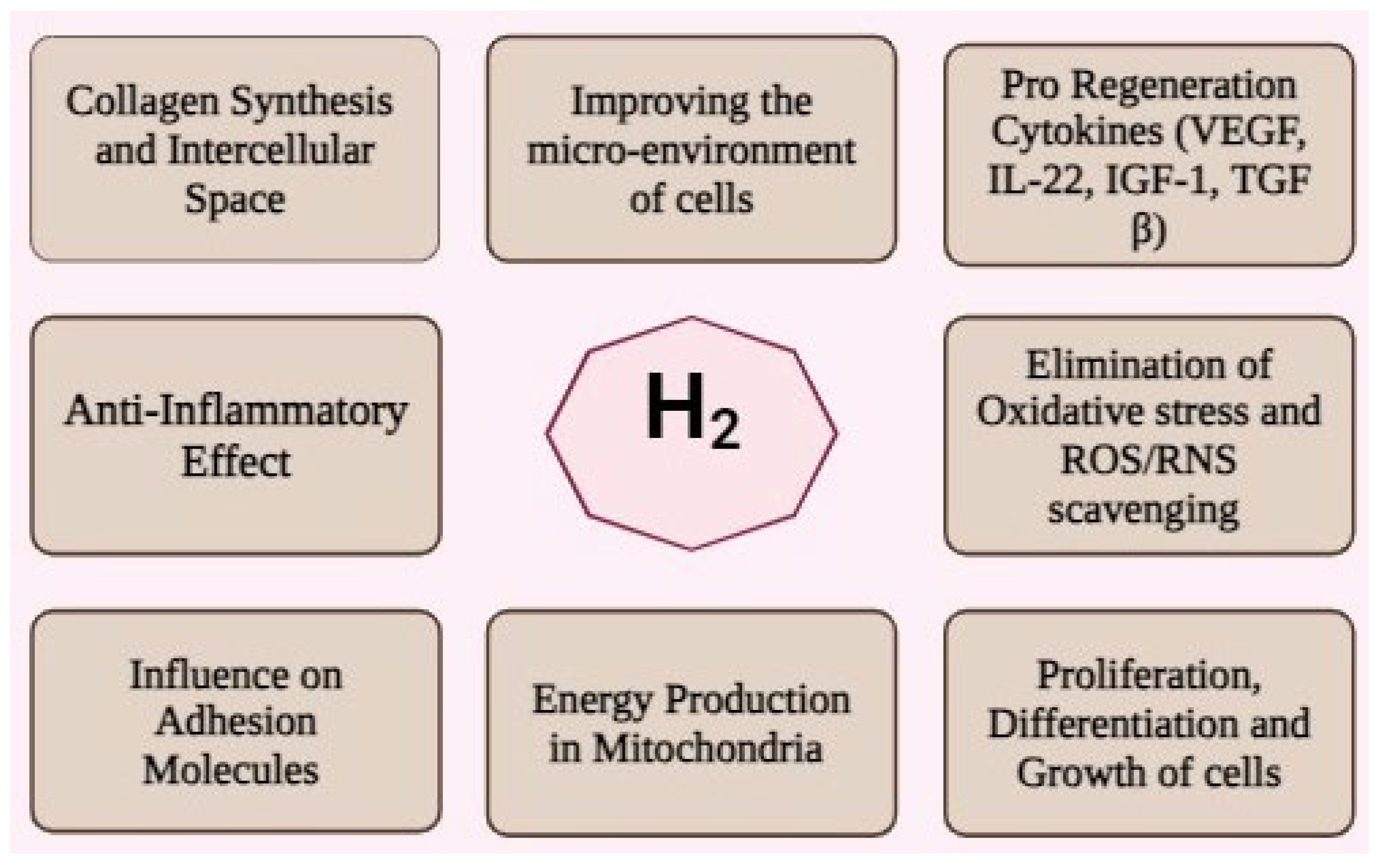
3. Antioxidant Mechanisms
4. Synergistic Effects on MSC Biology
4.1. Cell Survival and Proliferation
4.2. Differentiation Capacity
4.3. Migration and Homing
4.4. Paracrine Effect
5. Synergistic Effects on H₂ O2 and CAP
5.1. Molecular Mechanism of Interaction
5.2. Complementary Antioxidant Pathways
5.3. Enhancement of Cell Survival
5.4. Optimization and Treatment Parameters
6. Technical Considerations and Optimization
6.1. Timing and Duration of Molecular Hydrogen and Cold Atmospheric Plasma in Enhancing Mesenchymal Stem Cell Therapy
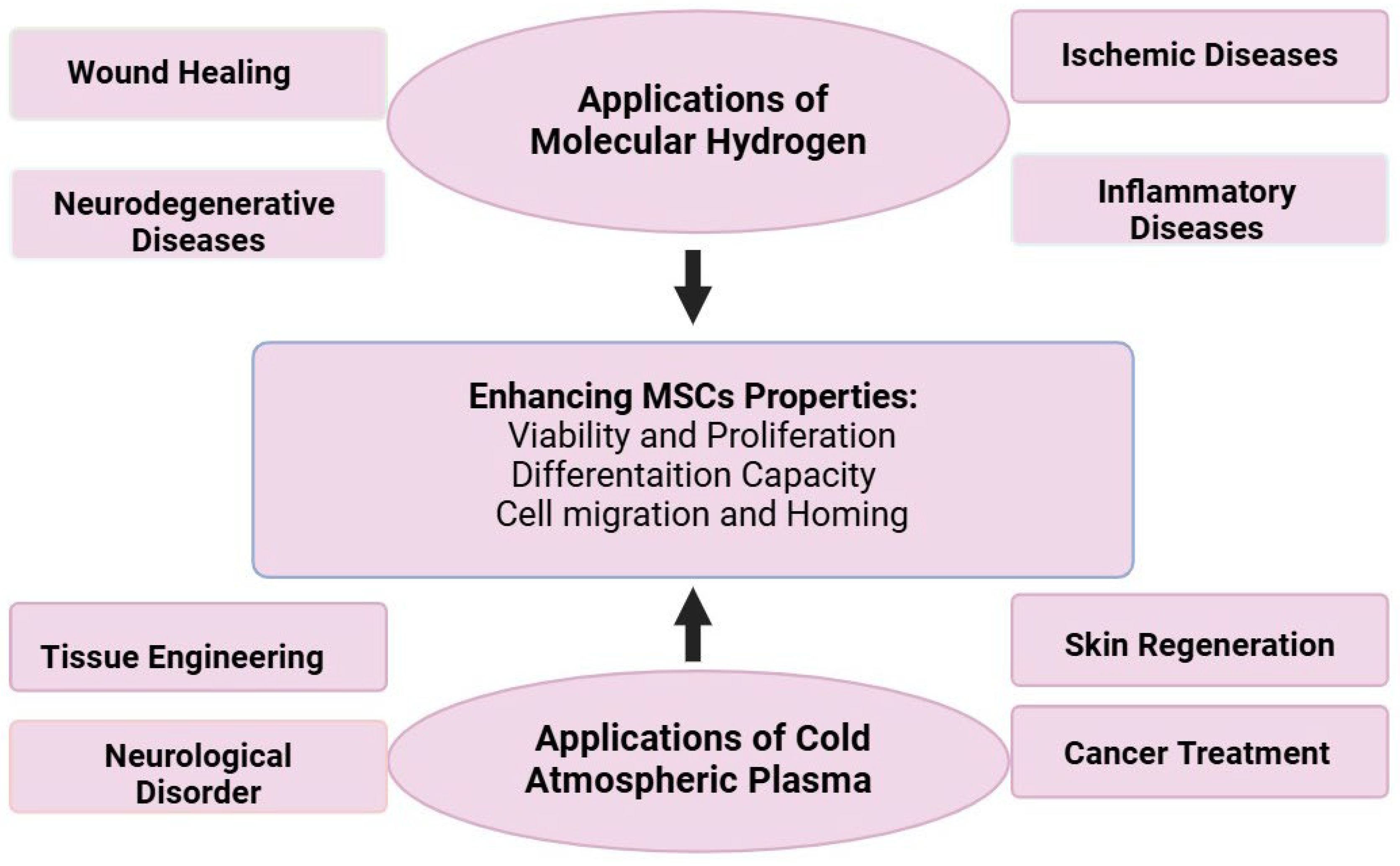
7. Clinical Applications and Future Perspectives
7.1. Current Clinical Status
7.2. Potential Therapeutic Applications
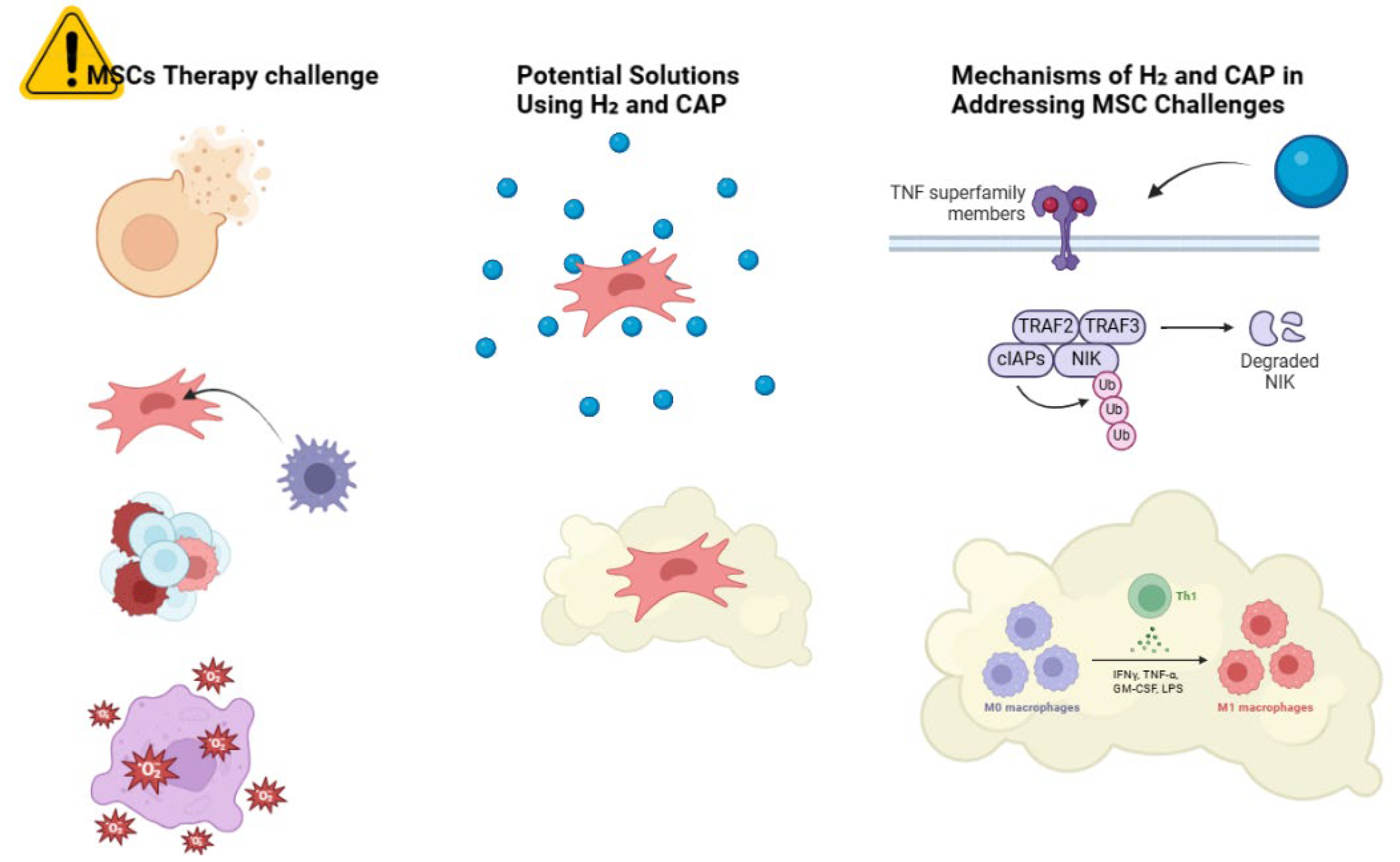
7.3. Potential Challenges with Solutions for Combining Molecular Hydrogen and Cold Atmospheric Plasma in Mesenchymal Stem Cell Therapy
8. Conclusion
Funding
References
- Zhou, T.; Yuan, Z.; Weng, J.; Pei, D.; Du, X.; He, C.; Lai, P. Challenges and advances in clinical applications of mesenchymal stromal cells. Journal of haematology & oncology 2021, 14, 1–24. [Google Scholar]
- Zhou, X.; Hong, Y.; Zhang, H.; Li, X. Mesenchymal stem cell senescence and rejuvenation: Current status and challenges. Frontiers in Cell and Developmental Biology 2020, 8, 364. [Google Scholar] [CrossRef] [PubMed]
- Sarkar, D.; Spencer, J.A.; Phillips, J.A.; Zhao, W.; Schafer, S.; Spelke, D.P.; Mortensen, L.J.; Ruiz, J.P.; Vemula, P.K.; Sridharan, R. Engineered cell homing. Blood, The Journal of the American Society of Hematology 2011, 118, e184–e191. [Google Scholar] [CrossRef] [PubMed]
- Ocansey, D.K.W.; Pei, B.; Yan, Y.; Qian, H.; Zhang, X.; Xu, W.; Mao, F. Improved therapeutics of modified mesenchymal stem cells: An update. Journal of Translational Medicine 2020, 18, 1–14. [Google Scholar] [CrossRef]
- Todosenko, N.; Khlusov, I.; Yurova, K.; Khaziakhmatova, O.; Litvinova, L. Signal pathways and microRNAs in osteosarcoma growth and the dual role of mesenchymal stem cells in oncogenesis. International Journal of Molecular Sciences 2023, 24, 8993. [Google Scholar] [CrossRef]
- Chen, F.; Liu, Y.; Wong, N.-K.; Xiao, J.; So, K.-F. Oxidative stress in stem cell ageing. Cell transplantation 2017, 26, 1483–1495. [Google Scholar] [CrossRef]
- Denu, R.A.; Hematti, P. Effects of oxidative stress on mesenchymal stem cell biology. Oxidative medicine and cellular longevity 2016, 2016, 2989076. [Google Scholar] [CrossRef]
- Hu, Q.; Khanna, P.; Wong, B.S.E.; Heng, Z.S.L.; Subhramanyam, C.S.; Thanga, L.Z.; Tan, S.W.S.; Baeg, G.H. Oxidative stress promotes exit from the stem cell state and spontaneous neuronal differentiation. Oncotarget 2018, 9, 4223. [Google Scholar] [CrossRef]
- Peterson, K.M.; Aly, A.; Lerman, A.; Lerman, L.O.; Rodriguez-Porcel, M. Improved survival of mesenchymal stromal cell after hypoxia preconditioning: Role of oxidative stress. Life sciences 2011, 88, 65–73. [Google Scholar] [CrossRef]
- Prakash, R.; Fauzia, E.; Siddiqui, A.J.; Yadav, S.K.; Kumari, N.; Singhai, A.; Khan, M.A.; Janowski, M.; Bhutia, S.K.; Raza, S.S. Oxidative stress enhances autophagy-mediated death of stem cells through Erk1/2 signaling pathway–implications for neurotransplantations. Stem cell reviews and reports 2021, 17, 2347–2358. [Google Scholar] [CrossRef]
- Martinelli, E.; Granato, D.; Azevedo, L.; Gonçalves, J.E.; Lorenzo, J.M.; Munekata, P.E.; Simal-Gandara, J.; Barba, F.J.; Carrillo, C.; Rajoka, M.S.R. Current perspectives in cell-based approaches towards the definition of the antioxidant activity in food. Trends in Food Science & Technology 2021, 116, 232–243. [Google Scholar]
- Ratnam, D.V.; Ankola, D.D.; Bhardwaj, V.; Sahana, D.K.; Kumar, M.R. Role of antioxidants in prophylaxis and therapy: A pharmaceutical perspective. Journal of controlled release 2006, 113, 189–207. [Google Scholar] [CrossRef] [PubMed]
- Bubb, K.J.; Drummond, G.R.; Figtree, G.A. New opportunities for targeting redox dysregulation in cardiovascular disease. Cardiovascular research 2020, 116, 532–544. [Google Scholar] [CrossRef] [PubMed]
- Maraveas, C.; Bayer, I.S.; Bartzanas, T. Recent advances in antioxidant polymers: From sustainable and natural monomers to synthesis and applications. Polymers 2021, 13, 2465. [Google Scholar] [CrossRef]
- Yan, D.; Cui, H.; Zhu, W.; Talbot, A.; Zhang, L.G.; Sherman, J.H.; Keidar, M. The strong cell-based hydrogen peroxide generation triggered by cold atmospheric plasma. Scientific reports 2017, 7, 10831. [Google Scholar] [CrossRef]
- Judée, F.; Simon, S.; Bailly, C.; Dufour, T. Plasma-activation of tap water using DBD for agronomy applications: Identification and quantification of long lifetime chemical species and production/consumption mechanisms. Water research 2018, 133, 47–59. [Google Scholar] [CrossRef]
- Boehm, D.; Heslin, C.; Cullen, P.J.; Bourke, P. Cytotoxic and mutagenic potential of solutions exposed to cold atmospheric plasma. Scientific reports 2016, 6, 21464. [Google Scholar] [CrossRef]
- Dubuc, A.; Monsarrat, P.; Virard, F.; Merbahi, N.; Sarrette, J.-P.; Laurencin-Dalicieux, S.; Cousty, S. Use of cold-atmospheric plasma in oncology: A concise systematic review. Therapeutic advances in medical oncology 2018, 10, 1758835918786475. [Google Scholar] [CrossRef]
- Tan, F.; Fang, Y.; Zhu, L.; Al-Rubeai, M. Controlling stem cell fate using cold atmospheric plasma. Stem Cell Research & Therapy 2020, 11, 1–10. [Google Scholar]
- Vijayarangan, V.; Delalande, A.; Dozias, S.; Pouvesle, J.-M.; Robert, E.; Pichon, C. New insights on molecular internalization and drug delivery following plasma jet exposures. International Journal of Pharmaceutics 2020, 589, 119874. [Google Scholar] [CrossRef]
- Hamouda, I.; Tampieri, F.; Canal, C.; Labay, C.; Ginebra, M. Production of reactive species in alginate hydrogels for cold atmospheric plasma-based therapies. Scientific reports 2019, 9, 1: 16160–12: 16160. [Google Scholar]
- Vijayarangan, V.; Delalande, A.; Dozias, S.; Pouvesle, J.-M.; Pichon, C.; Robert, E. Cold atmospheric plasma parameters investigation for efficient drug delivery in HeLa cells. IEEE Transactions on Radiation and Plasma Medical Sciences 2017, 2, 109–115. [Google Scholar] [CrossRef]
- Kurokawa, R.; Seo, T.; Sato, B.; Hirano, S.-i.; Sato, F. Convenient methods for ingestion of molecular hydrogen: Drinking, injection, and inhalation. Medical Gas Research 2015, 5, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Tian, Y.; Zhang, Y.; Wang, Y.; Chen, Y.; Fan, W.; Zhou, J.; Qiao, J.; Wei, Y. Hydrogen, a novel therapeutic molecule, regulates oxidative stress, inflammation, and apoptosis. Frontiers in physiology 2021, 12, 789507. [Google Scholar] [CrossRef] [PubMed]
- Canal, C.; Labay, C. Positive effect of cold atmospheric nitrogen plasma on the behavior of mesenchymal stem cells cultured on a bone scaffold containing iron oxide-loaded silica nanoparticles catalyst. International journal of molecular sciences 2020, 21, 4738: 1–4738: 21. [Google Scholar]
- Wang, P.; Zhou, R.; Thomas, P.; Zhao, L.; Zhou, R.; Mandal, S.; Jolly, M.K.; Richard, D.J.; Rehm, B.H.; Ostrikov, K. Epithelial-to-mesenchymal transition enhances cancer cell sensitivity to cytotoxic effects of cold atmospheric plasmas in breast and bladder cancer systems. Cancers 2021, 13, 2889. [Google Scholar] [CrossRef] [PubMed]
- Bourdens, M.; Jeanson, Y.; Taurand, M.; Juin, N.; Carrière, A.; Clément, F.; Casteilla, L.; Bulteau, A.-L.; Planat-Bénard, V. Short exposure to cold atmospheric plasma induces senescence in human skin fibroblasts and adipose mesenchymal stromal cells. Scientific reports 2019, 9, 8671. [Google Scholar] [CrossRef]
- Jang, J.-Y.; Hong, Y.J.; Lim, J.; Choi, J.S.; Choi, E.H.; Kang, S.; Rhim, H. Cold atmospheric plasma (CAP), a novel physicochemical source, induces neural differentiation through crosstalk between the specific RONS cascade and Trk/Ras/ERK signaling pathway. Biomaterials 2018, 156, 258–273. [Google Scholar] [CrossRef]
- Virard, F.; Cousty, S.; Cambus, J.-P.; Valentin, A.; Kémoun, P.; Clément, F. Cold atmospheric plasma induces a predominantly necrotic cell death via the microenvironment. PLoS ONE 2015, 10, e0133120. [Google Scholar] [CrossRef]
- Artamonov, M.Y.; Martusevich, A.K.; Pyatakovich, F.A.; Minenko, I.A.; Dlin, S.V.; LeBaron, T.W. Molecular hydrogen: From molecular effects to stem cells management and tissue regeneration. Antioxidants 2023, 12, 636. [Google Scholar] [CrossRef]
- Ascenção, K.; Dilek, N.; Augsburger, F.; Panagaki, T.; Zuhra, K.; Szabo, C. Pharmacological induction of mesenchymal-epithelial transition via inhibition of H₂ S biosynthesis and consequent suppression of ACLY activity in colon cancer cells. Pharmacological Research 2021, 165, 105393. [Google Scholar] [CrossRef] [PubMed]
- Radyuk, S.N. Mechanisms underlying the biological effects of molecular hydrogen. Current Pharmaceutical Design 2021, 27, 626–735. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, T.; Yamamoto, M. Molecular basis of the Keap1–Nrf2 system. Free Radical Biology and Medicine 2015, 88, 93–100. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.-X.; Fei, W.-Y.; Liu, M.-S.; Zhang, Y.-C.; Gao, R.-S.; Hu, Y.-Y.; Pang, E.-K.; Hou, L. Molecular hydrogen promotes adipose-derived stem cell myogenic differentiation via regulation of mitochondria. Current Stem Cell Research & Therapy 2023, 18, 864–875. [Google Scholar]
- Adhikari, B.; Adhikari, M.; Ghimire, B.; Park, G.; Choi, E.H. Cold atmospheric plasma-activated water irrigation induces defense hormone and gene expression in tomato seedlings. Scientific reports 2019, 9, 16080. [Google Scholar] [CrossRef]
- Artamonov, M.Y.; LeBaron, T.W.; Sokov, E.L.; Kornilova, L.E.; Pyatakovich, F.A.; Minenko, I.A. Intraosseous Administration of Molecular Hydrogen: A Novel Technique—From Molecular Effects to Tissue Regeneration. Molecular Hydrogen in Health and Disease 2024, 417–433. [Google Scholar]
- Baik, K.Y.; Jo, H.; Ki, S.H.; Kwon, G.-C.; Cho, G. Synergistic effect of hydrogen peroxide and cold atmospheric pressure plasma-jet for microbial disinfection. Applied Sciences 2023, 13, 3324. [Google Scholar] [CrossRef]
- Bauer, G. Signal amplification by tumor cells: Clue to the understanding of the antitumor effects of cold atmospheric plasma and plasma-activated medium. IEEE Transactions on Radiation and Plasma Medical Sciences 2017, 2, 87–98. [Google Scholar] [CrossRef]
- Bauer, G. The synergistic effect between hydrogen peroxide and nitrite, two long-lived molecular species from cold atmospheric plasma, triggers tumor cells to induce their own cell death. Redox biology 2019, 26, 101291. [Google Scholar] [CrossRef]
- Bengtson, C.; Bogaerts, A. The quest to quantify selective and synergistic effects of plasma for cancer treatment: Insights from mathematical modeling. International journal of molecular sciences 2021, 22, 5033. [Google Scholar] [CrossRef]
- Bhattacharjee, B.; Bezbaruah, R.; Rynjah, D.; Newar, A.; Sengupta, S.; Pegu, P.; Barman, D. Cold Atmospheric Plasma: A Noteworthy Approach in Medical Science. Sciences of Pharmacy 2023, 2, 79–103. [Google Scholar] [CrossRef]
- Caplan, A.I. Adult mesenchymal stem cells for tissue engineering versus regenerative medicine. Journal of cellular physiology 2007, 213, 341–347. [Google Scholar] [CrossRef] [PubMed]
- Chauhan, P.S.; Bhattacharya, S. Hydrogen gas sensing methods, materials, and approach to achieve parts per billion level detection: A review. International Journal of Hydrogen Energy 2019, 44, 26076–26099. [Google Scholar] [CrossRef]
- Cheng, F.; Yan, D.; Chen, J.; Wang, Z.; Horkowitz, A.; Keidar, M.; Sotomayor, E.M. Enhancing innate and adaptive immune systems by cold atmospheric plasma (CAP) and its antitumor immunity. arXiv preprint arXiv:2201.12737, 2022.
- Cho, S.-R.; Suh, H.; Yu, J.H.; Kim, H.; Seo, J.H.; Seo, C.H. Astroglial activation by an enriched environment after transplantation of mesenchymal stem cells enhances angiogenesis after hypoxic-ischemic brain injury. International journal of molecular sciences 2016, 17, 1550. [Google Scholar] [CrossRef]
- Cui, H.S.; Joo, S.Y.; Cho, Y.S.; Park, J.H.; Kim, J.-B.; Seo, C.H. Effect of combining low temperature plasma, negative pressure wound therapy, and bone marrow mesenchymal stem cells on an acute skin wound healing mouse model. International journal of molecular sciences 2020, 21, 3675. [Google Scholar] [CrossRef]
- Dubey, S.K.; Parab, S.; Alexander, A.; Agrawal, M.; Achalla VP, K.; Pal, U.N.; Kesharwani, P. Cold atmospheric plasma therapy in wound healing. Process Biochemistry 2022, 112, 112–123. [Google Scholar] [CrossRef]
- Engler, A.J.; Sen, S.; Sweeney, H.L.; Discher, D.E. Matrix elasticity directs stem cell lineage specification. Cell 2006, 126, 677–689. [Google Scholar] [CrossRef]
- Fernández-Santos, M.E.; Garcia-Arranz, M.; Andreu, E.J.; García-Hernández, A.M.; López-Parra, M.; Villarón, E.; Prosper, F. Optimization of mesenchymal stromal cell (MSC) manufacturing processes for a better therapeutic outcome. Frontiers in immunology 2022, 13, 918565. [Google Scholar] [CrossRef]
- Garrido-Pascual, P.; Alonso-Varona, A.; Castro, B.; Burón, M.; Palomares, T. H 2 O 2-preconditioned human adipose-derived stem cells (HC016) increase their resistance to oxidative stress by overexpressing Nrf2 and bioenergetic adaptation. Stem Cell Research & Therapy 2020, 11, 1–14. [Google Scholar]
- Ge, L.; Yang, M.; Yang, N.-N.; Yin, X.-X.; Song, W.-G. Molecular hydrogen: A preventive and therapeutic medical gas for various diseases. Oncotarget 2017, 8, 102653. [Google Scholar] [CrossRef] [PubMed]
- Guilak, F.; Cohen, D.M.; Estes, B.T.; Gimble, J.M.; Liedtke, W.; Chen, C.S. Control of stem cell fate by physical interactions with the extracellular matrix. Cell stem cell 2009, 5, 17–26. [Google Scholar] [CrossRef] [PubMed]
- He, R.; Li, Q.; Shen, W.; Wang, T.; Lu, H.; Lu, J.; Gao, H. The efficacy and safety of cold atmospheric plasma as a novel therapy for diabetic wound in vitro and in vivo. International Wound Journal 2020, 17, 851–863. [Google Scholar] [CrossRef] [PubMed]
- Hirano, S.-i.; Ichikawa, Y.; Sato, B.; Satoh, F.; Takefuji, Y. Hydrogen is promising for medical applications. Clean Technologies 2020, 2, 529–541. [Google Scholar] [CrossRef]
- Hirano, S.-i.; Ichikawa, Y.; Sato, B.; Takefuji, Y.; Satoh, F. Clinical Use and Treatment Mechanism of Molecular Hydrogen in the Treatment of Various Kidney Diseases including Diabetic Kidney Disease. Biomedicines 2023, 11, 2817. [Google Scholar] [CrossRef]
- Hou, C.; Peng, Y.; Qin, C.; Fan, F.; Liu, J.; Long, J. Hydrogen-rich water improves cognitive impairment gender-dependently in APP/PS1 mice without affecting Aβ clearance. Free radical research 2018, 52, 1311–1322. [Google Scholar] [CrossRef]
- Izadjoo, M.; Zack, S.; Kim, H.; Skiba, J. Medical applications of cold atmospheric plasma: State of the science. Journal of wound care 2018, 27(Sup9), S4–S10. [Google Scholar] [CrossRef]
- Jaganathan, B.; Tisato, V.; Vulliamy, T.; Dokal, I.; Marsh, J.; Dazzi, F.; Bonnet, D. Effects of MSC co-injection on the reconstitution of aplastic anemia patient following hematopoietic stem cell transplantation. Leukemia 2010, 24, 1791–1795. [Google Scholar] [CrossRef]
- Jeong, E.-S.; Bajgai, J.; You, I.-S.; Rahman, M.H.; Fadriquela, A.; Sharma, S.; Lee, K.-J. Therapeutic effects of hydrogen gas inhalation on trimethyltin-induced neurotoxicity and cognitive impairment in the C57BL/6 mice model. International journal of molecular sciences 2021, 22, 13313. [Google Scholar] [CrossRef]
- Jiao, F.; Xu, J.; Zhao, Y.; Ye, C.; Sun, Q.; Liu, C.; Huo, B. Synergistic effects of fluid shear stress and adhesion morphology on the apoptosis and osteogenesis of mesenchymal stem cells. Journal of Biomedical Materials Research Part A 2022, 110, 1636–1644. [Google Scholar] [CrossRef]
- Kermani, F.; Mollazadeh, S.; Kargozar, S.; Khakhi, J.V. Improved osteogenesis and angiogenesis of theranostic ions doped calcium phosphates (CaPs) by a simple surface treatment process: A state-of-the-art study. Materials Science and Engineering: C 2021, 124, 112082. [Google Scholar] [CrossRef] [PubMed]
- Kura, B.; Slezak, J. The Protective Role of Molecular Hydrogen in Ischemia/Reperfusion Injury. International journal of molecular sciences 2024, 25, 7884. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.-M.; Jeong, Y.-I.; Kook, M.-S.; Kim, B.-H. Combinatorial effect of cold atmosphere plasma (Cap) and the anticancer drug cisplatin on oral squamous cell cancer therapy. International journal of molecular sciences 2020, 21, 7646. [Google Scholar] [CrossRef] [PubMed]
- Levato, R.; Planell, J.A.; Mateos-Timoneda, M.A.; Engel, E. Role of ECM/peptide coatings on SDF-1α triggered mesenchymal stromal cell migration from microcarriers for cell therapy. Acta biomaterialia 2015, 18, 59–67. [Google Scholar] [CrossRef] [PubMed]
- Li, B.; Moshfegh, C.; Lin, Z.; Albuschies, J.; Vogel, V. Mesenchymal stem cells exploit extracellular matrix as mechanotransducer. Scientific reports 2013, 3, 2425. [Google Scholar] [CrossRef]
- Linsley, C.; Wu, B.; Tawil, B. The effect of fibrinogen, collagen type I, and fibronectin on mesenchymal stem cell growth and differentiation into osteoblasts. Tissue Engineering Part A 2013, 19, 1416–1423. [Google Scholar] [CrossRef]
- Liu, J.; Liu, J.; Mu, W.; Ma, Q.; Zhai, X.; Jin, B.; Zhang, N. Delivery Strategy to Enhance the Therapeutic Efficacy of Liver Fibrosis via Nanoparticle Drug Delivery Systems. ACS nano 2024, 18, 20861–20885. [Google Scholar] [CrossRef]
- Liu, Z.; Du, X.; Xu, L.; Shi, Q.; Tang, X.; Cao, Y.; Song, K. The therapeutic perspective of cold atmospheric plasma in periodontal disease. Oral Diseases 2024, 30, 938–948. [Google Scholar] [CrossRef]
- Mathieu, P.S.; Loboa, E.G. Cytoskeletal and focal adhesion influences on mesenchymal stem cell shape, mechanical properties, and differentiation down osteogenic, adipogenic, and chondrogenic pathways. Tissue Engineering Part B: Reviews 2012, 18, 436–444. [Google Scholar] [CrossRef]
- Murali, R.; Evangelina, R.; Samuel, J.P.; Singh, P.; Saha, S.; Singhal, M.; Gandhirajan, R.K. Cold atmospheric plasma (CAP) in wound healing: Harnessing a dual-edged sword. Redox Experimental Medicine 2024, 2024. [Google Scholar] [CrossRef]
- Nagajyothi, P.; Pavani, K.; Ramaraghavulu, R.; Shim, J. Microwave synthesis of NiMn2O4/Ni-foam: Efficient bifunctional electrocatalysts for overall water splitting. International Journal of Hydrogen Energy 2024, 54, 691–699. [Google Scholar] [CrossRef]
- Najar, M.; Melki, R.; Khalife, F.; Lagneaux, L.; Bouhtit, F.; Moussa Agha, D.; Merimi, M. Therapeutic mesenchymal stem/stromal cells: Value, challenges and optimization. Frontiers in cell and developmental biology 2022, 9, 716853. [Google Scholar] [CrossRef] [PubMed]
- Ohsawa, I.; Ishikawa, M.; Takahashi, K.; Watanabe, M.; Nishimaki, K.; Yamagata, K.; Ohta, S. Hydrogen acts as a therapeutic antioxidant by selectively reducing cytotoxic oxygen radicals. Nature medicine 2007, 13, 688–694. [Google Scholar] [CrossRef] [PubMed]
- Ohta, S. Recent progress toward hydrogen medicine: Potential of molecular hydrogen for preventive and therapeutic applications. Current Pharmaceutical Design 2011, 17, 2241–2252. [Google Scholar] [CrossRef]
- Ohta, S. Molecular hydrogen as a preventive and therapeutic medical gas: Initiation, development and potential of hydrogen medicine. Pharmacology & therapeutics 2014, 144, 1–11. [Google Scholar]
- Pan, Y.; Deng, Z.-Y.; Chen, X.; Zhang, B.; Fan, Y.; Li, H. Synergistic antioxidant effects of phenolic acids and carotenes on H₂ O2-induced H9c2 cells: Role of cell membrane transporters. Food Chemistry 2021, 341, 128000. [Google Scholar] [CrossRef]
- Park, J.; Bauer, S.; Pittrof, A.; Killian, M.S.; Schmuki, P.; von der Mark, K. Synergistic control of mesenchymal stem cell differentiation by nanoscale surface geometry and immobilized growth factors on TiO2 nanotubes. Small 2012, 8, 98–107. [Google Scholar] [CrossRef]
- Park, J.S.; Suryaprakash, S.; Lao, Y.-H.; Leong, K.W. Engineering mesenchymal stem cells for regenerative medicine and drug delivery. Methods 2015, 84, 3–16. [Google Scholar] [CrossRef]
- Pawitan, J.A.; Bui, T.A.; Mubarok, W.; Antarianto, R.D.; Nurhayati, R.W.; Dilogo, I.H.; Oceandy, D. Enhancement of the therapeutic capacity of mesenchymal stem cells by genetic modification: A systematic review. Frontiers in cell and developmental biology 2020, 8, 587776. [Google Scholar] [CrossRef]
- Popielarczyk, T.L.; Huckle, W.R.; Barrett, J.G. Human bone marrow-derived mesenchymal stem cells home via the PI3K-Akt, MAPK, and Jak/Stat signaling pathways in response to platelet-derived growth factor. Stem cells and development 2019, 28, 1191–1202. [Google Scholar] [CrossRef]
- Qazi, T.H.; Mooney, D.J.; Duda, G.N.; Geissler, S. Biomaterials that promote cell-cell interactions enhance the paracrine function of MSCs. Biomaterials 2017, 140, 103–114. [Google Scholar] [CrossRef] [PubMed]
- Qazi, T.H.; Mooney, D.J.; Duda, G.N.; Geissler, S. Niche-mimicking interactions in peptide-functionalized 3D hydrogels amplify mesenchymal stromal cell paracrine effects. Biomaterials 2020, 230, 119639. [Google Scholar] [CrossRef] [PubMed]
- Reilly, G.C.; Engler, A.J. Intrinsic extracellular matrix properties regulate stem cell differentiation. Journal of biomechanics 2010, 43, 55–62. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez-Merchán, E.C. Intraarticular injections of mesenchymal stem cells in knee osteoarthritis: A review of their current molecular mechanisms of action and their efficacy. International journal of molecular sciences 2022, 23, 14953. [Google Scholar] [CrossRef] [PubMed]
- Russell, G. (2024). A Study into the Biological Activity and Therapeutic Potential of Molecular Hydrogen and Oxyhydrogen Gases. School of Applied Sciences. This research programme was part-funded by and …,.
- Shahror, R.A.; Ali AA, A.; Wu, C.-C.; Chiang, Y.-H.; Chen, K.-Y. Enhanced homing of mesenchymal stem cells overexpressing fibroblast growth factor 21 to injury site in a mouse model of traumatic brain injury. International journal of molecular sciences 2019, 20, 2624. [Google Scholar] [CrossRef]
- Sim, M.; Kim, C.-S.; Shon, W.-J.; Lee, Y.-K.; Choi, E.Y.; Shin, D.-M. Hydrogen-rich water reduces inflammatory responses and prevents apoptosis of peripheral blood cells in healthy adults: A randomized, double-blind, controlled trial. Scientific reports 2020, 10, 1–10. [Google Scholar] [CrossRef]
- Stańczyk, B.; Wiśniewski, M. The Promising Potential of Cold Atmospheric Plasma Therapies. Plasma 2024, 7, 465–497. [Google Scholar] [CrossRef]
- Trappmann, B.; Chen, C.S. How cells sense extracellular matrix stiffness: A material’s perspective. Current opinion in biotechnology 2013, 24, 948–953. [Google Scholar] [CrossRef]
- Ullah, M.; Liu, D.D.; Thakor, A.S. Mesenchymal stromal cell homing: Mechanisms and strategies for improvement. Iscience 2019, 15, 421–438. [Google Scholar] [CrossRef]
- von Woedtke, T.; Emmert, S.; Metelmann, H.-R.; Rupf, S.; Weltmann, K.-D. Perspectives on cold atmospheric plasma (CAP) applications in medicine. Physics of Plasmas 2020, 27. [Google Scholar] [CrossRef]
- Wagner, J.; Kean, T.; Young, R.; Dennis, J.E.; Caplan, A.I. Optimizing mesenchymal stem cell-based therapeutics. Current opinion in biotechnology 2009, 20, 531–536. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; Jiang, Y.; Sun-Waterhouse, D.-x.; Zhai, H.; Guan, H.; Rong, X.; Li, D.-P. MicroRNA-based regulatory mechanisms underlying the synergistic antioxidant action of quercetin and catechin in H₂ O2-stimulated HepG2 cells: Roles of BACH1 in Nrf2-dependent pathways. Free Radical Biology and Medicine 2020, 153, 122–131. [Google Scholar]
- Wang, Q.; Ji, Y.; Shi, J.; Wang, L. NIR-driven water splitting H₂ production nanoplatform for H₂ -mediated cascade-amplifying synergetic cancer therapy. ACS applied materials & interfaces 2020, 12, 23677–23688. [Google Scholar]
- Yan, D.; Malyavko, A.; Wang, Q.; Lin, L.; Sherman, J.H.; Keidar, M. Cold atmospheric plasma cancer treatment, a critical review. Applied Sciences 2021, 11, 7757. [Google Scholar] [CrossRef]
- Yan, D.; Sherman, J.H.; Keidar, M. Cold atmospheric plasma, a novel promising anti-cancer treatment modality. Oncotarget 2017, 8, 15977. [Google Scholar] [CrossRef]
- Yuan, D.; Shen, X.; Tian, L.; Gu, D.; Zhu, L.; Wang, B. Solar STEP organic decomposition plus hydrogen: A novel approach to efficient degradation of organic pollutants exemplified by acrylonitrile. International Journal of Hydrogen Energy 2016, 41, 17199–17207. [Google Scholar] [CrossRef]
| Molecular Hydrogen (H₂) | Cold Atmospheric Plasma (CAP) | |
|---|---|---|
| Properties | Small, neutral molecule - High diffusibility, penetrating cellular membranes - Antioxidant, anti-inflammatory |
Ionized gas is composed of ions, electrons, radicals - Generates reactive species (e.g., ROS, RNS) - Non-thermal |
| Mechanism of Action | Scavenges hydroxyl radicals - Inhibits inflammatory cytokines - Protects mitochondrial function |
Induces oxidative stress in a controlled manner - Alters cell membrane potential - Modulates redox signaling |
| Stem Cell Proliferation | Enhances MSC proliferation - Improves MSC viability |
Can enhance or inhibit MSC proliferation depending on dose and duration |
| Stem Cell Differentiation | Promotes osteogenic, chondrogenic, and adipogenic differentiation | Induces osteogenic differentiation - Potential to modulate other differentiation pathways based on ROS levels |
| Anti-inflammatory Effects | Reduces pro-inflammatory cytokine expression - Beneficial for inflammatory-related stem cell therapies |
Decreases pro-inflammatory responses in MSCs under specific conditions - Supports wound healing applications |
| Oxidative Stress Tolerance | - Reduces ROS damage in MSCs - Enhances MSC tolerance to oxidative stress |
Controlled ROS generation can promote cellular adaptation - Excessive ROS may be cytotoxic, requiring optimization |
| Applications in MSC Therapy | Treatment for oxidative stress-related diseases - Promotes tissue regeneration - May enhance MSC therapy efficacy in neuroprotection, cartilage repair, and other regenerative applications |
Used for wound healing, anti-cancer therapies - Enhances MSC-mediated tissue repair and regeneration - Potential use in skin rejuvenation, anti-inflammatory, and infection control |
| Safety and Side Effects | - Generally safe with low toxicity - Minimal side effects reported |
- Safe under controlled conditions - Potential cytotoxicity at higher doses due to ROS production |
| Category | Synergetic Effect | References |
|---|---|---|
| Cell survival and Proliferation |
|
[38,41,48,49]. |
| Differentiation Capacity |
|
[42,50,51]. |
| Migration and Homing |
|
[43,45,52]. |
| Paracrine Effect |
|
[46,47,53]. |
| Study | MSC Type | Target Site | Outcomes | Clinical trial detail | References |
|---|---|---|---|---|---|
| Molecular Hydrogen for Osteoarthritis | bone marrow (BM-MSCs) Osteoarthritis |
Osteoarthritis | Greater cartilage repair and reduced oxidative stress and inflammation | Phase II trial; MSC-treated patients combined with molecular hydrogen had a significantly greater range of motion in the joints and less pain. | [87] |
| Cold Atmospheric Plasma for Wound Healing | Adipose-derived MSCs (AD-MSCs) | Diabetic Ulcers | Quick healing, enhanced MSC proliferation, and differentiation towards keratinocytes | Randomized controlled trial; CAP-treated MSCs were shown to be significantly improved in healing rates when compared to the MSC control group. | [88] |
| Molecular Hydrogen in Ischemic Stroke | Umbilical cord-derived MSCs (UC-MSCs) | Ischemic Stroke | Reduced infarct size, increased neuroprotection, and favorable neurological outcomes | Phase I trial; adjunct therapy demonstrated neuroprotection with reduced oxidative stress | [89] |
| CAP for Spinal Cord Injury | Bone marrow-derived MSCs | Spinal Cord Injury | Promoting MSC viability, migration, and differentiation into neural cells | Preclinical study: CAP-treated MSCs have promising regenerative potential to improve motor function | [90] |
| Hydrogen Gas in Cardiac Regeneration |
Cardiac-derived MSCs | Myocardial Infarction | Restored cardiac function, decreased fibrosis, and enhanced angiogenesis | Phase II trial; Hydrogen gas inhalation after MSC treatment improved left ventricular function. | [91] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).




