1. Introduction
The impact of different maize cropping systems on pests and diseases in maize may vary, potentially influencing yield and food safety in different ways. Maize-legume intercropping, a common practice among smallholder farmers in East Africa, holds the potential to significantly boost land and labour utilization [
1]. The positive outcomes of intercropping maize with leguminous crops such as common bean (
Phaseolus vulgaris), mung bean (
Vigna radiata), fava bean (
Vicia faba), and soya bean (
Glycine max) are well-documented, including enhanced soil fertility, reduced disease occurrence, and increased overall productivity [2, 3, 4]. This promising approach offers a ray of hope for the future of crop management and food safety.
Maize-legume intercropping also significantly improves the diversity of the beneficial entomofauna in smallholder agricultural production systems [
5]. Several theories have been proposed to explain the enhanced arthropod diversity and abundance in intercrop systems. Intercropping has been associated with an increase in the population of beneficial insects and a decrease in the population of certain insect pests, such as the budworm (
Heliothis spp.), the corn borer (
Ostrinia nubilalis), the leafhopper (
Cicadulina mbila), and the maize stalk borer (
Busseola fusca) [6, 7, 8]. This enlightening aspect of intercrop systems underscores their potential in sustainable crop management.
The push-pull technique is an innovative agricultural method widely used in Africa to enhance food security and sustainable farming practices. This approach involves integrating the use of specific plant varieties that repel pests (push) and those that attract beneficial insects (pull). By strategically planting these crops together, farmers can reduce pest damage, improve soil health, and increase overall yields [9, 10]. Farmers in western Kenya have embraced the push-pull technology, which uses nappier grass, Pennisetum purpureum Schumach, as the ‘pull’ and Desmodium spp. as the ‘push.' This technology has significantly reduced aflatoxin contamination in maize [11, 12]. Maize intercropping with Desmodium (a repellent crop) and fields surrounded with Napier grass (an attractive trap crop) has been shown to reduce maize crop damage by Lepidopteran pests [9, 10].
Trichoderma harzianum is a beneficial fungus widely recognized for its role in combating aflatoxins. This biocontrol agent works by outcompeting harmful fungi for resources, thereby inhibiting their growth and reducing aflatoxin production. Additionally,
T. harzianum enhances soil health and promotes plant growth, making it a valuable tool in integrated pest management strategies [
13].
Trichoderma harzianum has been shown to parasitize on
Aspergillus flavus as well as to colonize the fungal entry points [
14]. Additionally,
T. harzianum biodegrades aflatoxin B1 in maize grains [
15]. In Kenya, the use of
T. harzianum is often combined with maize-legume intercropping.
Aspergillus flavus infection in maize and the potential contamination with aflatoxin, which poses a significant health risk to humans and animals, are challenges of significant concern. The susceptibility of maize to
A. flavus infection is influenced by various factors, including insect infestation, grain damage, and environmental conditions [
16]. Insect damage coupled with favorable climatic conditions like high temperatures and drought stress usually results in enhanced aflatoxin contamination [
17]. The European corn borer,
Ostrinia nubilalis (Hubner); the corn earworm,
Helicoverpa zea (Boddie)
; and the Fall armyworm,
Spodoptera frugiperda (J.E. Smith)
, have been identified as significant contributors to
A. flavus infection and subsequent aflatoxin contamination of preharvest maize [
16]. Climate change has led to extended drought stresses and high temperatures, conditions that favour
Aspergillus infection and subsequent aflatoxin contamination in maize [
18]. In addition, climate change increases the geographic range and population densities of insect pests [
19].
While previous studies have explored the benefits of maize-legume intercrops in terms of productivity per unit area, soil fertility improvement, soil conservation, and related economic benefits [5, 20, 21] our study takes a unique approach. We delve into the effect of maize-legume intercrops on the occurrence of insect pests, population densities, and their role in enhancing Aspergillus and aflatoxin contamination.
The objective of this study was to test the hypothesis that intercropping maize with legumes, T. harzianum application, and the push-pull method will reduce damage to maize by herbivores, and reduce the dispersal of mycotoxigenic fungi and associated aflatoxin contamination, while maintaining or increasing productivity. The study had two main objectives: firstly, to evaluate the insects that are potential vectors for Aspergillus in pre-harvest maize, and secondly, to determine the mechanisms of mycotoxigenic fungi dispersal. The results of the study could inform the development of more sustainable pest management strategies and contribute to the reduction of aflatoxin contamination in maize.
2. Materials and Methods
2.1. Description of the Study Sites and Trial Establishment
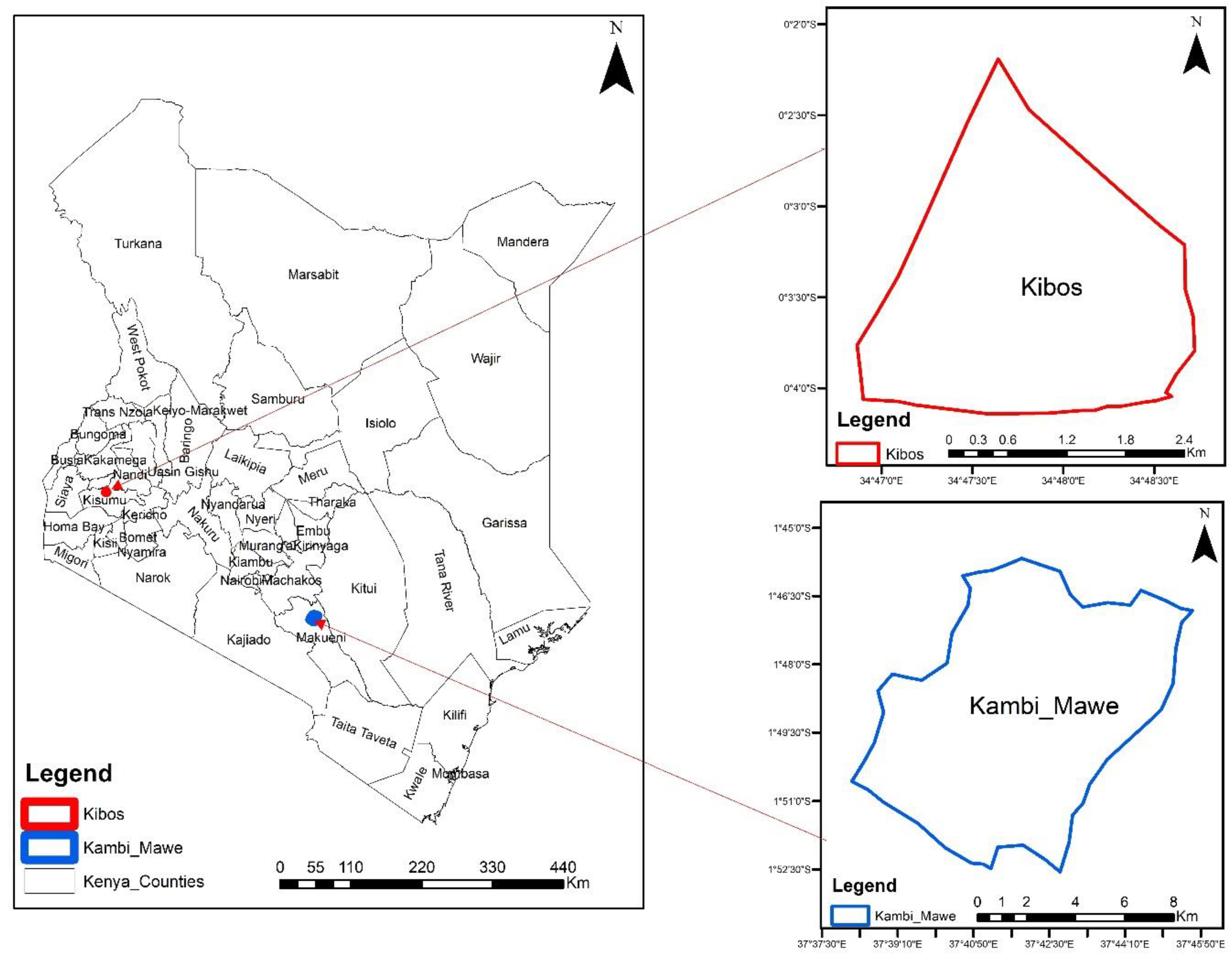
The study was conducted at the Kenya Agricultural Research Organization (KALRO) farms in Kibos, Kisumu County (0
o 2’11’’S, 34
o49’17’’E) and Kambi ya Mawe, Makueni County (01
o 37’S, 37
o 40’E) as shown in
Figure 1. The treatments were: (1) maize monocrop, (2) maize-bean intercrop, (3) maize-bean intercrop sprayed with
Trichoderma harzianum-T22, and (4) Push-pull technique (maize intercropped with
Desmodium intortum and with three rows of napier grass (
Pennisetum purpureum). The
Desmodium and napier grass were pre-germinated for planting to ensure survival in Makueni, which gets lower amounts of rain. The intercrop crops were planted at the ratio of one row of legume to two rows of maize on the dates and spacing as in
Table 1 below. The land was prepared using a tractor-mounted disc harrow, and the trial was established in a randomized complete block design (RCBD) with three replicates. The plots were 30m long and 30m wide. Two seeds were placed per hole and thinned to one plant per spot after germination. The varieties planted, spacing, and rainfall data are shown in
Table 1. In Kisumu, planting was done on 6/4/2021 and 11/10/2021 for the long and short rain cropping seasons respectively, while in Makueni it was done on 3/4/21 and 23/11/2021. Di-ammonium Phosphate fertilizer (18:46:0, NPK) was used at planting at the rate of 125kg/ha and topdressed 30 days after planting with Calcium Ammonium Nitrate fertilizer with 21% nitrogen at 200kg/ha. Weeding was done by hand and no pesticides were used in the trial so as not to affect the arthropods.
2.2.Collection, Identification, and Enumeration of Arthropods
Insects were captured fortnightly during the generative stage of maize (BBCH 69-89) [
22] between 0800h and 1200h to ensure comparable results. Only insects on the silks or the ears were captured. The insects were singly placed in 1.5ml centrifuge tubes and labelled with the plot numbers and dates for further analysis. Insects were captured using the hand-picking method or using an aspirator.
The arthropods were identified to the lowest possible taxonomic level using morphological characters under a stereo-dissecting microscope (Wild M38, Leica, Heerbrugg, Switzerland) with the help of keys and catalogues [
23] and confirmation done by Mr. Morris Mutua an entomologist at the National Museums of Kenya. A list of the arthropods sampled from the plots was made with each taxon means per treatment (Riungu et al., in prep.).
Since the FAW was noted as the main insect pest across the two regions and records of effective management using the push-pull method have been reported, data on the incidence and severity of the attack was determined for this insect. Incidence on foliage or ears was determined as the percentage of plants or ears showing an attack by the larvae respectively, while the severity of attack was estimated using a scale (1 = low to 5 = high) [
24],
2.3. Aspergillus Isolation from Insects Captured on Maize Ears
The insects were processed as described by Yamoah et al. [
25] and Awad et al. [
26] with modifications. Twenty individuals from each species caught per farm were washed off separately to dislodge fungi from the insect’s exoskeleton. Each beetle was placed in a sterile universal bottle containing 3 ml of 100 mmol Potassium phosphate buffer (pH 7.0) + 0.01% Tween 80 and shaken for three minutes on a vortex machine (Vortex-Genie 2, Scientific Industries, USA). The washed insect samples were serially diluted 100 fold by successively pipetting 1 ml of the sample into a sterile tube and topping it up with 9 ml of sterile distilled water. A 1ml aliquot of each dilution series (0, 10
-1, and 10
-2) was placed on Petri dishes containing potato dextrose agar (PDA) with chloramphenicol (39 g of PDA, Oxoid, UK, and 250 mg of chloramphenicol). Incubation of the plates was at room temperature (25 ± 2˚C) and a 12-hour photoperiod for five days, after which the colony-forming units (CFUs) were counted and the population expressed as CFUs per insect.
2.4. Detection of Viable Aspergillus and Fusarium Spores on Beetles in Preharvest Maize
A slightly different isolation technique [
27] with modifications was used to determine the mode of spread (zoochory or endozoochory) of
Aspergillus and
Fusarium spores. Twenty
Sitophilus zeamais,
Carpophilus spp, and
Forficula spp. individuals each captured in Makueni County from maize at BBCH 75, 85 and 87 developmental stages were put into pre-sterilized Petri dishes. The insects were incubated for 36 hours at 25±2°C, 16h photoperiod, and 72 ± 10% RH. The insects were shaded with a paperboard and water supplied with a slightly moist sterile cotton bud. After 36 hours, the insects were put into a refrigerator at 4°C for 1 hour to slow down their metabolic activity. Fecal pellets dropped in the Petri dish during the 36h incubation were picked aseptically using a sterile scalpel and placed on PDA with chloramphenicol. The head, elytra, and guts were aseptically detached from the insects using sterile forceps in a laminar flow with the aid of a stereo-dissecting microscope. The head and elytra samples were cultivated directly onto media as described above, whereas the gut was surface sterilized in 70% ethanol for 10s, rinsed in sterile distilled water, and punctured before plating as described above.
The fungi were incubated for 5 days at 25°C. Following this incubation period, colonies that were morphologically identified as either
Aspergillus [
28] or
Fusarium [
29] were enumerated and subcultured on PDA for confirmation purposes.
2.5. Maize and Legume Harvesting, Sample Handling, and Analysis
Maize was harvested manually at physiological maturity (BBCH 89). Five ears from each pre-tagged plant were harvested from each batch and dehusked in order to evaluate the extent of ear rot. This was done through a visual assessment of the grain colour and development, with scores ranging from 1 (indicating no damage or discolouraton to 5 (indicating severe damage or discolouration) [
30]. The second batch was subjected to a manual shelling process, followed by a sun-drying procedure (using a Twist Grain Pro device, manufactured in Draminski, Poland) until the moisture content was reduced to below 13%. Subsequently, the kernels underwent a fine milling process using a coffee and spice grinder (AR1100, Moulinex, United Kingdom). To prevent cross-contamination, the blender was cleaned and rinsed between samples with 70% ethanol. Grain yield was quantified by multiplying the average grain yield of the ten pre-tagged plants in each plot by the number of plants in one hectare (44,000 plants ha
-1). The weight was determined using an analytical balance (Nimbus 1602E, Adam Equipment, United Kingdom). The percent spoilt grain was determined by counting the spoilt grain from a random sample of 100 kernels in a bag in four replicates.
The 100-seed weight was determined by averaging the weight of the four replicates of 100 seeds used to determine the maize spoilage. The weights were measured using an analytical balance (Nimbus 1602E, Adam Equipment, United Kingdom). The bean and Desmodium yields were determined by averaging the bean grain and Desmodium forage harvested from four replicates of randomly chosen 1m2 areas and extrapolated by multiplying by 10,000 m2 (the size of a hectare of land).
2.6. Total Aflatoxin Content Determination
The total aflatoxin content of 10 grams of flour was determined using the total aflatoxin assay (Helica, Biosystems Inc.). The assay is based on a solid-phase competitive inhibition enzyme immunoassay with an aflatoxin-specific antibody optimized to cross-react with all four subtypes of aflatoxin (B1, B2, G1, and G2) in grain [
31].
Aflatoxin extraction was conducted using 70% methanol (300 ml de-ionized water was added to 700 ml methanol) as the extraction solvent. Five grams of milled maize flour was added to 25 ml of the extraction solvent, 1.5 (weight by volume) (w/v) ratio. The mixture was agitated in an orbital shaker for a period of two minutes, after which it was left to stand for a further two minutes to allow any particulate matter to settle. Five 10 ml of the supernatant was filtered using Whatman #1 filter paper into a clean beaker [
31].
For the assay, aliquots of 100 µl of the sample or standard solution, in duplicates, were added to a mixing well with 200 µl of the aflatoxin-HRP conjugate and mixed by priming the pipettor thrice. From the mix, 100 µl of the solution was pipetted into corresponding wells in an antibody-coated microtiter well and incubated at room temperature for 15 minutes. The contents of the wells were then discarded, and the microwells were washed off five times by filling each of the wells with phosphate-buffered saline-tween (PBS-Tween) buffer. The microtiter plates were dried by inverting them on absorbent paper towels.100µl of substrate reagent was added to each well. The plates were incubated in a dark chamber for 5 minutes to avoid direct light, and the reaction stopped by adding 120µl of the stop solution to each well. Each microwell's optical density (O.D.) readings (Eliza Reader, ELx 808, Biotek, USA) at 450 nm filter were noted. A standard curve was constructed using the mean relative absorbance of the standard references against their concentrations in ng/ml on a logarithmic curve. Mean sample relative absorbance values were extrapolated to the corresponding concentrations.
The formula for relative absorbance:
2.7. Data Analysis
Data collected were subjected to SAS version 9 for analysis of variance (ANOVA) at P ≤ 0.05. The mean±SE number of arthropods per plant in the specific cropping system and maize development stages (BBCH) was calculated. Data on maize yield, grain spoilage, kernel weight, bean yields, aflatoxin levels, and fungal colonization were subjected to ANOVA. Because the grain yield, % spoilt grain, CFU/g, and aflatoxin levels (ppb) were not normally distributed, they were log-transformed (log10). Post hoc tests were performed using Tukey’s Honestly Significant Difference (Tukey HSD) procedure at P ≤ 0.05 level of significance for each trait determined whenever the main effects were significant.
3. Results
3.1. Fall Armyworm Incidence and Damage on Maize Foliage and Cobs
Fall armyworms (FAW) were identified as the most damaging insects in maize. The fall armyworm larvae attacked the maize foliage and later moved into the cobs as the crop matured (
Figure 2), (
Table 2). The percent of foliage exhibiting damage was found to be significantly influenced by location (F=29.4, df=1, P< 0.001), season (F=15.2, df=1, P< 0.001), and treatments (F=29.4, df=3, P< 0.001). The severity differed between the two locations (F=132.2, df=1, P< 0.001), and treatments (F=42.7, df=3 P< 0.001). The highest incidence of damage was in Makueni in the long rain cropping season (85.8±7.6 %) and the least in Kisumu in the long rain cropping season (45.7±3.9 %).
Damage to maize foliage and the incidence on the foliage and cobs were highest in the maize monocrop treatment and significantly differed from those in the maize-legume intercropping systems. The FAW incidence on foliage was highest at 75 % in maize monocrops and lowest (41 %) in the push-pull cropping system. A similar trend was observed concerning the severity of damage in the foliage and the percent incidence on cobs. In the long and short rain seasons, the highest FAW incidence and severity were recorded in Makueni. Incidences of 100% were recorded on the cobs and the foliage, particularly during the long rain season. In Kisumu, the damage by the FAW was lower than that in Makueni, and the cobs were not heavily attacked.
3.2. Recovery of Microorganisms from Insects Captured from Maize
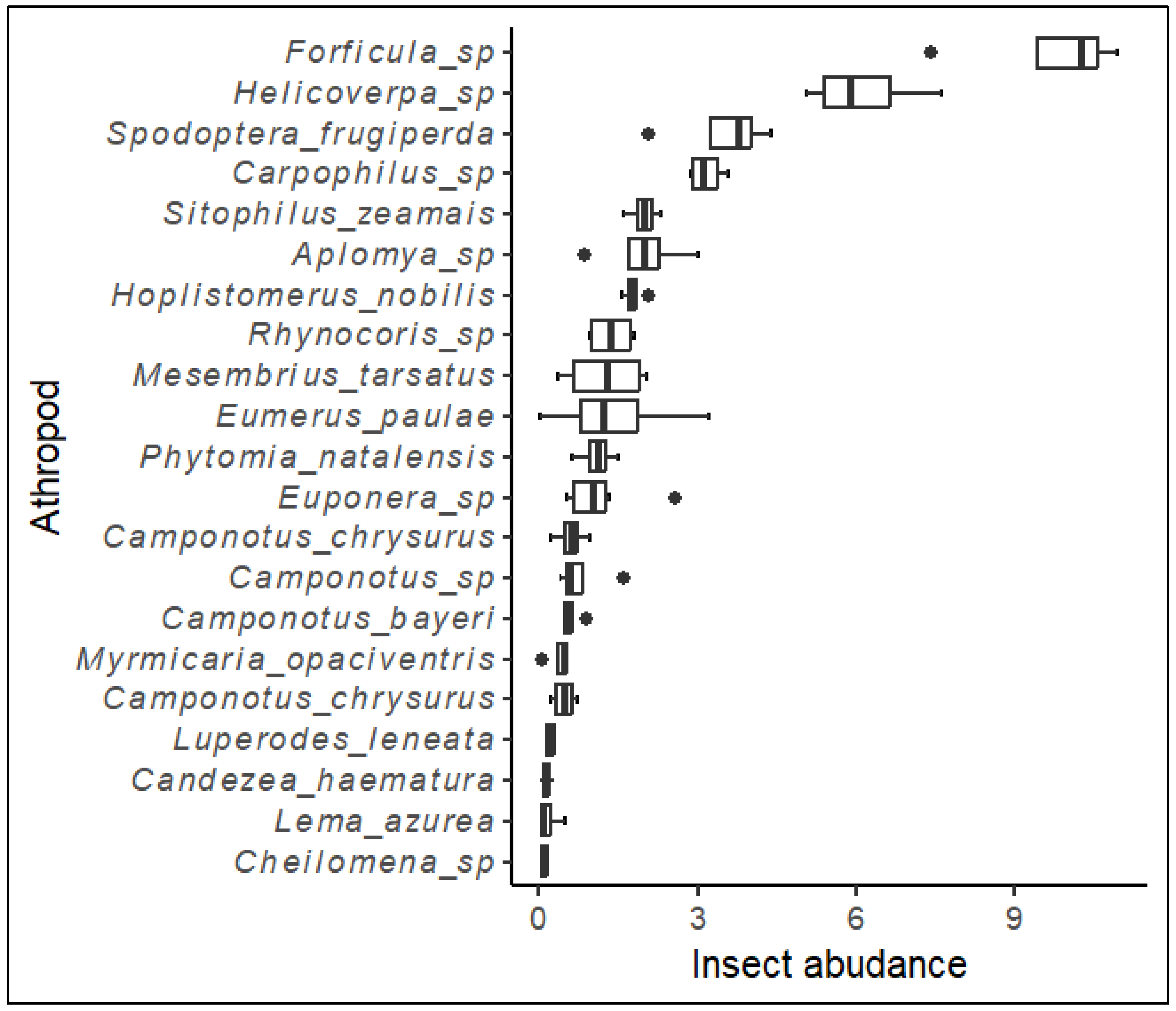
Among the arthropods, the most frequently observed taxa, in descending order, were
Forficula spp.,
Helicoverpa zea,
Spodoptera frugiperda,
Carpophilus spp.,
Sitophilus zeamais, Aplomya sp., among others (
Figure 3). Four insect taxa, namely
Sitophilus zeamais,
Carpophilus spp.,
Forficula spp., and
Camponotus spp., were identified from the list of insects analysed for fungal spore load on their exoskeleton (
Table 3. These four taxa exhibited a significant number of mycotoxigenic fungi spores on their bodies. The predominant fungal genera isolated from the insects captured were
Aspergillus and
Fusarium. The site of collection significantly influenced the
Aspergillus spore load on
S. zeamais (P=0.004),
Carpophilus spp. (P=0.009) and
Camponotus spp. (P=0.034) and the
Fusarium spore load on
Forficula spp. (P=0.008). The season influenced the
Aspergillus spore load on
S. zeamais (P=0.004),
Carpophilus spp. (P=0.015); and
Forficula spp
. (P=0.009) and
Fusarium on
S. zeamais (P=0.035) and
Forficula spp. (P=0.007)
. The maize weevil (
S. zeamais) harboured the highest
Aspergillus spore load (125.8), whereas the sugar ants (
Camponotus spp.) had the lowest no. of
Aspergillus spores (5.0) on their exoskeleton. Similarly,
S. zeamais harboured a very high
Fusarium spore load (176.1) on their exoskeleton, while the
Carpophilus spp. had the lowest CFUs (11.4) on their exoskeleton. The site and seasons significantly influenced the
Aspergillus and
Fusarium recovery from the insects' exoskeleton.
Aspergillus load was highest in Makueni during the long rain-cropping season, whereas
Fusarium was higher during the short rain season than during the long rain-cropping season. The main
Aspergillus species isolated were
A. flavus,
A.
minisclerotigenes, A. japonicus, and
A. niger, whereas all the
Fusarium specimens were identified as
Fusarium verticillioides.
3.3. Mechanism of Fungal Spores Spread by Coleopterans
The highest prevalence of
Aspergillus infestation was observed in
S. zeamais specimens (52.5%), followed by
Carpophilus dimidiatus (27.1%) and
Forficula spp. (26%). In contrast,
C. dimidiatus was more infested with
F. verticilloides (35%), followed by
S. zeamais and
Forficula sp. at 29.5% and 23.2% respectively (
Table 4). In both fungal species, the elytra exhibited the greatest prevalence of spores, followed by the head, gut, and faeces in descending order.
3.4. Maize and Companion Crop Yield Parameters
The cropping systems significantly influenced the percent grain spoilage (F= 6.65, df=3, P< 0.001) and the aflatoxin levels in maize kernels (F= 8.97, df=3, P< 0.001). Maize yield within a site did not differ significantly. However, the yields were significantly different from one season (F=10.55, df=1, P=0.03) and from location to location (F=3.49, df=1, <0.001). The highest maize yields (in kg/ha) were observed in the maize monoculture, while the lowest yields were recorded in the maize-bean intercrop (
Table 5). The intercrop yields were found to be comparable to one another. The short rain season yielded a higher crop than the long rain season, and the yields in Kisumu were three times higher than in Makueni.
Kernel spoilage differed significantly between the sites (F=3.10, df=1, <0.001) and cropping systems (F=6.64, df=3, P=0.045). The highest grain spoilage was observed in the maize monocultures, while the lowest was observed in the push-pull cropping system. The kernel spoilage was recorded highest in Makueni while the lowest record came from Kisumu. The 100 seed weight was found to be highest during the long rain season and differed significantly (F=15.3, df=1, P< 0.001) from that in the short rain season. The highest levels of aflatoxin contamination were observed in Makueni during the long rain season and in the maize monocrop with levels influenced by several factors including site, season, and cropping system. Bean yields were highest in Kisumu and during the long rain season. The cropping system did not influence the bean yields.
4. Discussion
The ongoing effects of climate change have resulted in increased insect damage to maize crops, as well as the proliferation of mycotoxigenic fungal infestations, which have subsequently led to the contamination of maize intended for human consumption and animal feed with mycotoxins. Here the field study in two regions of Kenya investigated the impact of different maize-legume cropping systems on the arthropod taxa most prevalent on maize at flowering and grain-filling stages, the arthropods that cause the most damage, those that could potentially disperse mycotoxigenic fungi on pre-harvest maize, and the aflatoxin contamination of the grain.
4.1. FAW Damage
Desmodium in push-pull technology significantly reduced the abundance of
Spodoptera frugiperda pest insects and the crop damage. It is known that
Desmodium reduces Lepidopteran pests when intercropped with cereals [5, 12, 32]. However, the mechanism of the management strategy is still under debate. It is not clear whether the
Desmodium repels the pests or intercepts and kills them. Intercropping can interrupt the visual orientation of pests to their hosts. It can also interrupt olfactory host-finding mechanisms with volatile chemical compounds [
33].
4.2. Microbial Recovery from Insects Captured from Maize
Aspergillus and Fusarium were the mycotoxigenic fungi recovered from the insects trapped in the two regions studied. Although arthropod spore dispersal in pre-harvest maize has not been studied in Kenya before, studies of fungal contamination of maize in farms, markets, and farm stores have been reported [34, 35, 36]. Aspergillus, Fusarium, and Penicillium were isolated at varying levels in these studies. Thus, the isolation of similar fungal species in and on beetles captured from the same areas in the field poses a risk to humans and animals that rely on maize for food and feed. Aspergillus and Fusarium are harmful pathogens of maize that produce secondary metabolites and toxins under favourable conditions. Toxigenic species and strains of the two fungi isolated from the insects are potential producers of toxic secondary metabolites (aflatoxins and fumonisins) [37, 38]. In this study, maize kernels had mean total aflatoxin levels of 4.9 and 1.9 ppb in Makueni and Kisumu counties, respectively. Although this is below the maximum allowable limit of 10ppb, it still poses a threat of chronic aflatoxicosis [39, 40].
Mode of Dispersal of Aspergillus and Fusarium Spores in Pre-Harvest Maize
In the present study, many insects carried viable
Aspergillus and
Fusarium spores on their elytra and their head. In contrast, only few of the insects had viable spores in the gut or faeces. The high number of spores on the exoskeleton suggests that the dispersal of mycotoxigenic fungi is primarily passive and is in agreement with the findings of [41, 42] who, although studying fungal dispersal in stored grain, concluded that weevils played a role in the dispersal of
Aspergillus and that the dispersal was primarily passive. A greenhouse experiment in Kenya [
43] showed that both
S. zeamais and
C. dimidiatus increased
A. flavus and aflatoxin contamination in pre-harvest maize. Among the sap beetles, many individuals had viable spores in the gut and the faeces. The high number of spores in the guts of sap beetles may be due to possible fungivory or accidental ingestion of fungal propagules when feeding on plant material (endozoochory) [
44]. Many species of sap beetles in the Nitidulidae are herbivores or fungivores that are attracted to damaged maize plants or plants with exposed kernels. There they feed on fungi that develop on the exudates from plant wounds or directly on the kernels [
45]. Zoochory in maize has been studied in relation to ear rot by
F. verticillioides [
46], and the authors reported that the rootworm enhanced ear rot. However, they did not investigate the mechanisms of interaction.
4.3. Maize and Companion Crop Yields
Maize yields were higher in the short rain season than in the long rain season. The average yields for both locations were 4.9 and 5.8 tons per ha in the short and long rain seasons, respectively. The difference in yield is attributed to the climatic conditions during the trial periods. The long rain season was heavy, particularly in Kisumu, and could have led to a reduction in yields due to flooding and soil leaching. Although rain is generally good for maize growth, too much rainfall can cause nitrogen to leach out of nutrient-poor soils, leading to a negative feedback and lower yields [
47].
Kisumu had an average maize yield of 8 tonnes per ha compared to 2.7 tonnes in Makueni. The considerable variance in yield is attributed to the difference in climatic conditions in the two regions. Kisumu usually receives more favourable rainfall than Makueni. During the trial period, Kisumu received 1714 mm of rainfall compared to 828 mm in Makueni. According to [
48], estimates of climate change show a trend towards lower maize yields in some locations, with temperature increases above certain thresholds contributing to severe yield losses. Rainfall accounts for 44% of the variance in maize yields [
49].
In terms of cropping systems, maize monoculture produced the highest yields. Higher yields in monocultures may be attributed to the lack of intraspecific competition for resources. This finding is in line with Pierre et al. [
5], who indicated that the yield advantages of monocultures over maize-bean intercropping are due to interspecific competition between cereal/legume species for nutrients, space, water, and light. The competition for resources between maize and the intercrops may result in decreased yields of maize [
50]. The maize-legume intercrops were comparable in terms of maize yield. Although their maize yields were lower than that in the monocrops, the yields of the companion crops (beans and fodder) would supplement the farmers's total yield. However, bean and
Desmodium yields behaved similarly to the maize yields, suggesting that they were equally affected by the weather and the agro-ecological sites in the same way as maize. Beans are the third most important crop and a source of dietary protein [
51].The fact that the intercropping did not significantly reduce the maize yields is therefore beneficial to farmers, who can easily meet their dietary requirements by adding a protein source without compromising their maize yields. Similarly, by using the push-pull technology, the farmer can easily get fodder for his cows while preserving his food source.
4.4. Grain Spoilage, Fungal Infestation and Mycotoxin Concentration
Grain spoilage was higher in Makueni than in Kisumu. The higher grain spoilage in Makueni can be attributed to the high level of Fall armyworm damage. In Makueni, the Fall armyworm damage was so severe that the incidence was 100% in both leaves and cobs. Cob damage was lowest in the push-pull treatment, with the lowest grain spoilage. Push-pull cropping is known to reduce the damage caused by Fall armyworms to maize [12, 52]. The present study shows, that the push-pull technology effectively reduces FAW damage and subsequent aflatoxin contamination in maize. Although the FAW larvae did not carry Aspergillus spores, we hypothesize that the heavy damage of the ears in Makueni, coupled with the drought situation and availability of the aflatoxigenic Aspergillus species, enhanced the aflatoxin levels in maize.
Grain spoilage was lower in maize-bean intercrops and in maize-bean intercrops with
Trichoderma. The reduced damage in the intercrops echoed the findings by [
53], who, while studying the effect of maize-bean intercropping, reported that the intercrops had a lower Fall armyworm infestation than the maize monocrops. Aflatoxin contamination was also lower in kernels from maize-legume intercrops than in those from maize monocrops due to less severe damage caused by the herbivorous lepidopterans and less subsequent infestation by
Aspergillus species.
Among the counties, maize from Makueni had a higher aflatoxin contamination than that from Kisumu. This can be attributed to the high prevalence of Aspergillus species known to synthesize aflatoxins in Makueni [34, 35] and drought stress on maize at flowering.
5. Conclusions and Recommendations
In the present study, maize-legume intercrops and push-pull technology enhanced general insect abundance. At the same time, the intercrops reduced pest damage to maize crops, resulting in a decline in aflatoxin contamination in maize. Although the maize yield was lower in the intercrops, the bean grain yield in the maize-bean intercrop and the fodder in the push-pull cropping system quickly compensated for the loss.
This study shows that maize weevils and sap beetles passively spread Aspergillus and Fusarium spores on pre-harvest maize. Spore loads varied between species, with weevils carrying more spores on their bodies than the other insects. The fact that maize weevils infested with mycotoxigenic fungi start infesting maize right in the field (before harvest) is a concern because when the crop is harvested, there is a chance that either the weevils will spread to neighbouring fields or get into the farm stores. This cycle would perpetuate more ear rot in the field or fungal contamination of the stored grain, potentially increasing the levels of mycotoxins in grain for food and feed. It is recommended that further studies on plant-insect-mycotoxigenic fungi interactions are undertaken in the wake of climate change, which increases the abundance and diversity of pests.
CRediT authorship contribution statement
Conceptualization, G.R.W.B,T.M and J.M.; Methodology, G.R, J.M, MW,W.B.; Validation, G.R,T.M, and E.P.; Formal Analysis, E.P, G.R.; Investigation, G.R, M.W.; Resources, W.B. and T.M; Data Curation, G.R.and TM; Writing – Original Draft Preparation, G.R. and E.P; Writing – Review & Editing, T.M, J.M,W.B.; Supervision, W.B, J.M, M.W, T.M.; Project Administration, T.M.; Funding Acquisition, W.B and T.M.”,.
Data availability
The data supporting this study's findings are available from the corresponding author upon written request. Voucher specimens were preserved at the Kenya Agricultural and Livestock Research Organization.
Submission declaration
The work described has not been published previously (except as a PhD academic thesis by the corresponding author) and is not under consideration for publication elsewhere. All authors have approved the publication, and if accepted, it will not be published elsewhere in the same form, in English, or in any other language, including electronically, without the written consent of the copyright holder.
Funding
The authors thank the Federal Ministry of Food and Agriculture (BMEL) based on a decision of the Parliament of the Federal Republic of Germany via the Federal Office for Agriculture and Food (BLE) for their financial support of this work [2816PROC12].
Acknowledgments
We extend our sincere thanks to the Kenya Agricultural and Livestock Research Organization for their generous support, which allowed us to utilize their field and laboratory facilities. Their commitment to advancing agricultural research is truly commendable. We thank the AflaZ consortium for continuous support and fruitful discussions.
Declaration of competing interest
The authors of the manuscript “A Polyphasic Characterization of Toxigenic Aspergillus Species Isolated from Carpophilus dimidiatus and Sitophilus zeamais” report no conflicts of interest.
References
- Mucheru-Muna, M.; Pieter, P.; Mugendi, D.; Kung’u, J.; Jayne Mugwe, J.; Merckx, R.; Vanlauwe, B. A staggered maize–legume intercrop arrangement robustly increases crop yields and economic returns in the highlands of Central Kenya. Field Crops Res. 2010, 15, 132–139. [Google Scholar] [CrossRef]
- Foyer, C. H., Lam, H.-M., Nguyen, H. T., Siddique, K. H. M., Varshney, R. K., Colmer, T. D., Cowling, W., Bramley, H., Mori, T. A., Hodgson, J. M.; et al. Neglecting legumes has compromised human health and sustainable food production. Nat. Plants 2016, 2, Article 8. [Google Scholar] [CrossRef]
- Yimer, T., Abera, G., Beyene, S., & Rasche, F. Optimizing maize–bean cropping systems for sustainable intensification in southern Ethiopia. J. Agron. 2022, 114, 3283–3296. [Google Scholar] [CrossRef]
- Raza, M. A., Khalid, M. H. B., Xia, Z., Ling, F., Khan, İ., Hassan, M. J., Ahmed, M., Ansar, M., Chen, Y. K., Fan, Y., Yang, F., & Yang, W. Effect of planting patterns on yield, nutrient accumulation and distribution in maize and soybean under relay intercropping systems. Sci. Rep. 2019, 9. [Google Scholar] [CrossRef]
- Pierre, J. F., Latournerie-Moreno, L., Garruña, R., Jacobsen, K. L., Laboski, C. A. M., Us-Santamaría, R., & Ruiz-Sánchez, E. Effect of maize–legume intercropping on maize physio-agronomic parameters and beneficial insect abundance. Sustainability 2022, 14, Article 19. [Google Scholar] [CrossRef]
- Maitra, S., Shankar, T., & Banerjee, P. Potential and advantages of maize-legume intercropping system. Maize - Production and Use 2020. [Google Scholar] [CrossRef]
- Li, L., Duan, R., Li, R., Zou, Y., Liu, J., Chen, F., & Xing, G. Impacts of corn intercropping with soybean, peanut, and millet through different planting patterns on population dynamics and community diversity of insects under fertilizer reduction. Front. Plant Sci. 2022, 13. [Google Scholar] [CrossRef]
- Brandmeier, J., Reininghaus, H., & Scherber, C. Multispecies crop mixtures increase insect biodiversity in an intercropping experiment. Ecol. solut. Evid. 2023, 4. [Google Scholar] [CrossRef]
- Midega, C. , Bruce T., Pickett J., Pittchar J., Murage A., & Khan Z.. Climate-adapted companion cropping increases agricultural productivity in East Africa. Field Crops Research 2015;180:118-125. [CrossRef]
- Mutyambai, D. . Push-pull cropping system soil legacy alter maize metabolism and fall armyworm, Spodoptera frugiperda (Lepidoptera: Noctuidae) resistance through tritrophic interactions. 2023. [CrossRef]
- Owuor, M. J. , Midega, C. A. O., Obonyo, M., & Khan, Z. R. Impact of companion cropping on incidence and severity of maize ear rots and mycotoxins in Western Kenya. African Journal of Agricultural Research, 2018, 13(41), 2224-2231.
- Njeru, N. K. , Midega, C. A. O., Muthomi, J. W., & Wagacha, J. M. Impact of push–pull cropping system on pest management and occurrence of ear rots and mycotoxin contamination of maize in western Kenya. Plant Pathol. 2020; 69, 1644–1654. [Google Scholar] [CrossRef]
- Yao, X. , Guo, H., Zhang, K., Zhao, M., Ruan, J., & Chen, J. Trichoderma and its role in biological control of plant fungal and nematode disease. Frontiers in Microbiology, 2023, 14. [CrossRef]
- Kifle, M. , Yobo, K. , & Laing, M. Biocontrol of Aspergillus flavus in groundnut using Trichoderma harzianum stain kd. Journal of Plant Diseases and Protection, 2016, 124, 1–6. [Google Scholar] [CrossRef]
- Madbouly, A. K. , Rashad, Y. M., Ibrahim, M. I. M., & Elazab, N. T. Biodegradation of Aflatoxin B1 in Maize Grains and Suppression of Its Biosynthesis-Related Genes Using Endophytic Trichoderma harzianum AYM3. Journal of Fungi, 2023, 9(2), Article 2. [CrossRef]
- Widstrom, N. W. The role of insects and other plant pests in aflatoxin contamination of corn, cotton, and peanuts—A review. J. Environ. Qual. 1979, 8(1), 5–11. [Google Scholar] [CrossRef]
- Magagnoli, S. , Lanzoni, A., Masetti, A., Depalo, L., Albertini, M., Ferrari, R., Spadola, G., Degola, F., Restivo, F.M. and Burgio, G. Sustainability of strategies for Ostrinia nubilalis management in Northern Italy: Potential impact on beneficial arthropods and aflatoxin contamination in years with different meteorological conditions. Crop Prot. 2020; 142, 105529. [Google Scholar] [CrossRef]
- Rashid, G. , Kisangiri, M., & Mbega, E. R. Development of optical-based and imaging technology detection, diagnosis, and prevention of aflatoxin contamination on maize crop. International Journal of Advances in Scientific Research and Engineering, 2020; 08. [Google Scholar] [CrossRef]
- Skendžić, S. , Zovko, M., Živković, I. P., Lešić, V., & Lemić, D. The impact of climate change on agricultural insect pests. Insects 2021, 12(5), 440. [Google Scholar] [CrossRef]
- Kamara, A. Y. , Tofa, A. I., Ademulegun, T. D., Solomon, R., Shehu, H., Kamai, N., & Omoigui, L. O. (2017). Maize–soybean intercropping for sustainable intensification of cereal–legume cropping systems in Northern Nigeria. Exp. Agric. 2017, 55(1). [CrossRef]
- Assefa, A. , Tana, T., Dechassa, N., Dessalgn, Y., Tesfaye, K., & Wortmann, C. S. Maize-common bean/lupine intercrop productivity and profitability in maize-based cropping system of Northwestern Ethiopia. Ethiop. J. Sci. Technol. 2016; 9, 69. [Google Scholar] [CrossRef]
- Lancashire, P.D. ; H. Bleiholder; P. Langeluddecke; R. Stauss; T. van den Boom; E. Weber; A. Witzen-Berger. A uniform decimal code for growth stages of crops and weeds. Ann. Biol. 1991; 119, 561–601. [Google Scholar]
- Picker, M. , Griffiths, C., & Weaving, A. (2019). Field Guide to Insects of South Africa. Struik Nature, Penguin Random House South Africa 2019.
- Ojumoola, A. , Omoloye, A., & Thomas, K. Maize Farmers’ Knowledge and Management of Fall Armyworm (Spodoptera frupigerda) in Southwest Nigeria. Journal of Agricultural Extension, 2022, 26, 38–51. [Google Scholar] [CrossRef]
- Yamoah, E. , Jones, E. E., Weld, R. J., Suckling, D. M., Waipara, N., Bourdôt, G. W., Hee, A. K. W., & Stewart, A. Microbial population and diversity on the exoskeletons of four insect species associated with gorse (Ulex europaeus L.). Aust. J. Entomol. 2008; 47, 370–379. [Google Scholar] [CrossRef]
- Awad, M. F. , Albogami, B., Mwabvu, T., Hassan, M. M., Baazeem, A., Hassan, M. M., & Elsharkawy, M. M. Identification and biodiversity patterns of Aspergillus species isolated from some soil invertebrates at high altitudes using morphological characteristics and phylogenetic analyses. PeerJ, 2023, 11, e15035. [CrossRef]
- Lunde, L. F. , Boddy, L., Sverdrup-Thygeson, A., Jacobsen, R. M., Kauserud, H., & Birkemoe, T. Beetles provide directed dispersal of viable spores of a keystone wood decay fungus. Fungal Ecol. 2023; 63, 101232. [Google Scholar] [CrossRef]
- Klich, M.A. Identification of Common Aspergillus Species. Centreal Bureau Voor Schimmel Culture, AD Utrecht, Netherland, 2002, pp: 116.
- Leslie, J.F. and Summerell, B.A. The Fusarium Laboratory Manual. Hoboken: Blackwell Publishing 2006.
- Cardwell, K. F. , Kling, J. G., Maziya-Dixon, B., and Bosque-Pérez, N. A. Interactions between Fusarium verticillioides, Aspergillus flavus, and insect infestation in four maize genotypes in lowland Africa. Phytopathol. 2020; 90, 276–284. [Google Scholar]
- Hygiena. https://www.hygiena.com/wp-content/uploads/2021/02/Helica-Total-Aflatoxin-Low-Matrix-ELISA-Kit-Insert.pdf assessed 30/3/2023.
- Erdei, A. L. , David, A. B., Savvidou, E. C., Džemedžionaitė, V., Chakravarthy, A., Molnár, B. P., & Dekker, T. The push-pull intercrop desmodium does not repel, but intercepts and kills pests. eLife, 2024; 13. [Google Scholar] [CrossRef]
- López, M. and Liburd, O. E. (2023). Effects of intercropping marigold, cowpea and an insecticidal soap on whiteflies and aphids in organic squash. J. Appl, Entomol. 2023, 147, 452–463. [Google Scholar] [CrossRef]
- Kagot, V. , Boevre, M., Saeger, S., Moretti, A., Mwamuye, M., & Okoth, S. Incidence of toxigenic Aspergillus and Fusarium species occurring in maize kernels from Kenyan households. World Mycotoxin J. 2022, 15, 1–10. [Google Scholar] [CrossRef]
- Maina, A. W. , Wagacha, J. M., Mwaura, F. B., Muthomi, J. W., & Woloshuk, C. P. Assessment of farmers maize production practices and effect of triple-layer hermetic storage on the population of Fusarium spp. and fumonisin contamination. World J. Agric. Res. 2017; 5, Article 1. [Google Scholar] [CrossRef]
- Okoth, S. , De Boevre, M., Vidal, A., Diana Di Mavungu, J., Landschoot, S., Kyallo, M., Njuguna, J., Harvey, J., & De Saeger, S. Genetic and toxigenic variability within Aspergillus flavus population isolated from maize in two diverse environments in Kenya. Front. Microbiol.2018, 9, 3389. [Google Scholar]
- Li, J. , Does, H. C. v. d., Borkovich, K. A., Coleman, J. J., Daboussi, M., Pietro, A. D. & Rep, M. Comparative genomics reveals mobile pathogenicity chromosomes in Fusarium. Nature 2010, 464(7287), 367–373. [Google Scholar] [CrossRef]
- Youssef, F. S. and Singab, A. N. B. An updated review on the secondary metabolites and biological activities of Aspergillus ruber and Aspergillus flavus and exploring the cytotoxic potential of their isolated compounds using virtual screening. Evidence-Based Complementary and Alternative Medicine, 2021, 1-11. [CrossRef]
- Mutegi, C. , Cotty, P. J., & Bandyopadhyay, R. Prevalence and mitigation of aflatoxins in Kenya (1960-to date). World Mycotoxin J. 2018, 11, 341-357. [CrossRef]
- Okayo, R. , Andika, D., Dida, M., K'Otuto, G., & Gichimu, B. Morphological and molecular characterization of toxigenic Aspergillus flavus from groundnut kernels in Kenya. Int. J. Microbiol. 2020, 1–10. [Google Scholar] [CrossRef]
- Betti, J.A. , Phillips, T. & Smalley, E.B. Effect of maize weevils (Coleoptera: Curculionidae) on the production of aflatoxin B1 by Aspergillus flavus in stored corn. J. Econ. Entomol. 1995; 88, 1776–1782. [Google Scholar]
- Bhusal, K. , & Khanal, D. Role of maize weevil, Sitophilus zeamais Motsch. On the spread of Aspergillus, section flavi in different Nepalese maize varieties. Advances in Agriculture, 2019; e7584056. [Google Scholar] [CrossRef]
- Riungu, G. , Muthomi, J. W., Buechs, W., Wagacha, J. M., Philip, E. S., & Meiners, T. The role of maize sap beetles (Coleoptera: Nitidulidae) and maize weevils (Coleoptera: Curculionidae) in the spread of Aspergillus flavus in pre-harvest maize in Kenya. J. Econ. Entomol. 2024; toae2017. [Google Scholar] [CrossRef]
- Mannino, M.C. , Huarte-Bonnet C., Davyt-Colo B., Pedrini N. Is the Insect cuticle the only entry gate for fungal infection? Insights into alternative modes of action of entomopathogenic Fungi. J. Fungi 2019, 5, 33. [Google Scholar] [CrossRef]
- Meissle, M. , Naranjo, S. E., & Romeis, J. Does the growing of Bt. maize change the abundance or ecological function of non-target animals compared to the growing of non-GM maize? A systematic review. Environmental Evidence, 2022; 11. [Google Scholar] [CrossRef]
- Kurtz, B. , Karlovsky P., Vidal S. Interaction between Western corn rootworm (Coleoptera: Chrysomelidae) larvae and root-infecting Fusarium verticillioides. Environ. Entomol. 2010; 39, 1532–1538. [Google Scholar] [CrossRef]
- Ray, D. K. , Gerber, J., MacDonald, G. K., & West, P. Climate variation explains a third of global crop yield variability. Nat. Commun. 2015; 6. [Google Scholar] [CrossRef]
- Adhikari, U. , Nejadhashemi, A. P., & Woznicki, S. A. Climate change and eastern Africa: a review of impact on major crops. Food and Energy Security, 2015; 4, 110–132. [Google Scholar] [CrossRef]
- Lukali, A. , Osima, S. E., Lou, Y., & Kai, K. Assessing the impacts of climate change and variability on Maize (Zea mays) yield over Tanzania. Atmospheric and Climate Sciences, 2021, 11, 569–588. [Google Scholar] [CrossRef]
- Dudwal, M. , Singh, R. P., Verma, B. L., & Choudhary, B. Effects of different maize–soybean intercropping patterns on yield attributes, yield, and B: C ratio. International Journal of Plant & Soil Science, 2021; 33, 51–58. [Google Scholar] [CrossRef]
- Ritho, A. W, Sila D. N, Ndungu Z. W. Nutritional and Antinutritional Characteristics of Two Biofortified Bean Varieties Grown in Kenya. Curr Res Nutr Food Sci 2023; 11(2). doi. [CrossRef]
- Guera, O. G. M. , Castrejón-Ayala, F., Robledo, N., Jiménez-Pérez, A., Le, V., Díaz-Viera, M. A., & Silva, J. A. Geostatistical analysis of Fall armyworm damage and edaphoclimatic conditions of a mosaic of agroecosystems predominated by push-pull systems. Chilean Journal of Agricultural Research, 2023; 83, 14–30. [Google Scholar] [CrossRef]
- Sisay, B. , Subramanian, S., Weldon, C. W., Krüger, K., Torto, B., & Tamiru, A. Responses of the fall armyworm (Spodoptera frugiperda) to different host plants: implications for its management strategy. Pest Management Science 2022, 79(2), 845-856. [CrossRef]
Figure 1.
Location of experimental sites in Kibos, Kisumu County, and Kambi Mawe, Makueni County.
Figure 1.
Location of experimental sites in Kibos, Kisumu County, and Kambi Mawe, Makueni County.
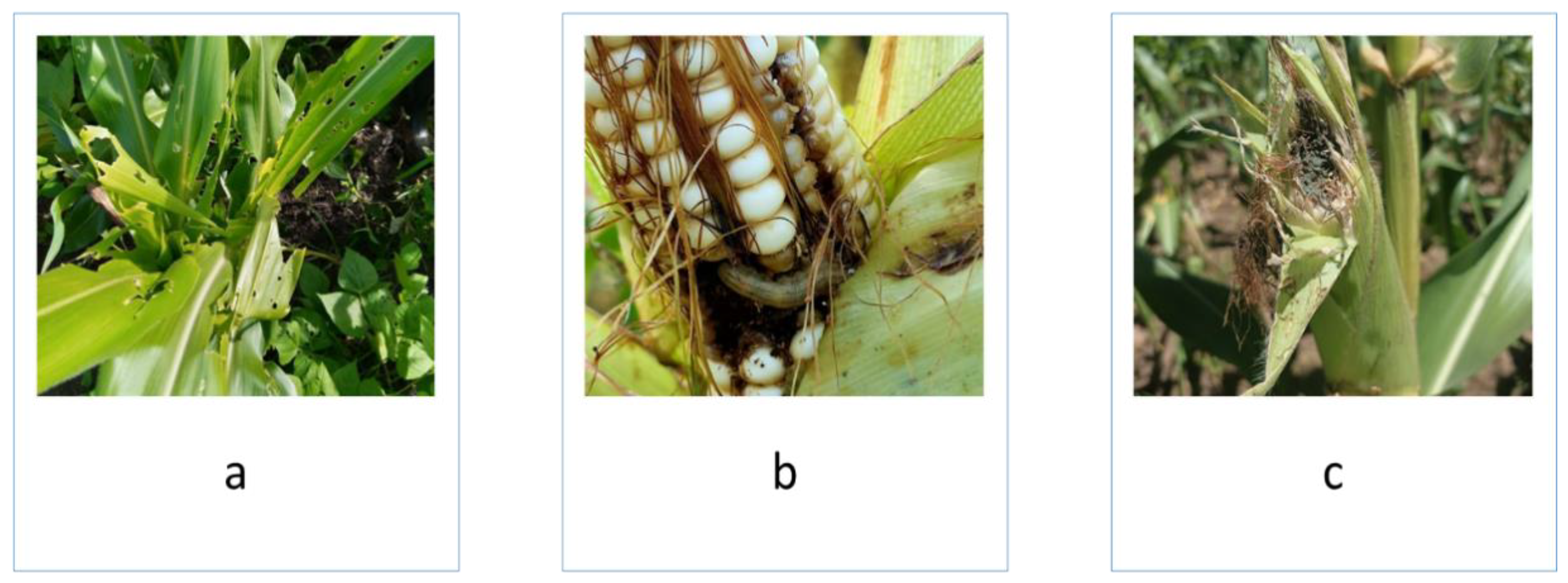
Figure 2.
Fall armyworm damage on a) maize foliage, b) maize ears, and c) moulds on damaged maize ears.
Figure 2.
Fall armyworm damage on a) maize foliage, b) maize ears, and c) moulds on damaged maize ears.
Figure 3.
Whisker plots of the mean abundance of the most prevalent arthropod taxa (Individuals/plant per sampling event) in the two seasons in Kisumu and Makueni. The dark line represents the median value, the whiskers represent the minimum and maximum values, and the dots represent the outliers.
Figure 3.
Whisker plots of the mean abundance of the most prevalent arthropod taxa (Individuals/plant per sampling event) in the two seasons in Kisumu and Makueni. The dark line represents the median value, the whiskers represent the minimum and maximum values, and the dots represent the outliers.
| p |
Season |
Cropping system |
Percent FAW damage incidence on foliage |
FAW severity on foliage (1-5) |
Percent FAW damage incidence on cobs |
| Kisumu |
Long rain |
Maize monocrop |
60.7±2.6a |
3.1±0.1 |
20.0±5.8a |
| Maize/bean |
47.0±4.9a |
2.0±0.1 |
20.0±0.0a |
| Maize/bean/Trichoderma
|
43.7±7.8ab |
2.0±0.1 |
23.3±6.7a |
| Push-pull |
31.3±5.0b |
1.2±0.2 |
6.7±6.7a |
|
MeanP value
|
45.7±3.90.030
|
2.08±0.2-
|
17.5±3.10.22
|
| Short rain |
Maize monocrop |
78.3±10.8a |
3.1±0.1 |
40.0±5.8a |
| Maize/bean |
49.7±4.5b |
2.1±0.0 |
33.3±3.3ab |
| Maize/bean/Trichoderma
|
41.7±8.3b |
2.0±0.0 |
23.3±3.3b |
| Push-pull |
45.0±4.9b |
1.4±0.1 |
26.7±3.3b |
|
MeanP value
|
53.7±5.50.033
|
2.1±0.2-
|
30.8±2.60.034
|
| Makueni |
Long rain |
Maize monocrop |
100.0±0.0a |
3.8±0.2 |
100.0±0.0a |
| Maize/bean |
100.0±0.0a |
3.2±0.2 |
100.0±0.0a |
| Maize/bean/Trichoderma
|
100.0±0.0a |
3.3±0.3 |
100.0±0.0a |
| Push-pull |
43.3±8.8b |
2.3±0.2 |
56.7±21.9b |
|
MeanP value
|
85.8±7.6<0.001
|
3.14±0.2-
|
89.2±7.3<0.001
|
| Short rain |
Maize monocrop |
62.0±15.3a |
3.4±0.5 |
100.0±0.0a |
| Maize/bean |
56.7±3.4a |
3.0±0.0 |
100.0±0.0a |
| Maize/bean/Trichoderma
|
42.3±5.5a |
3.0±0.0 |
100.0±0.0a |
| Push-pull |
42.7±7.8a |
2.8±0.2 |
78.7±10.7b |
|
MeanP value
|
50.9±4.70.37
|
3.0±0.1-
|
94.7±3.60.053
|
Table 1.
Maize-legume intercrop varieties, spacing, and planting seasons of the on-station trials in Kibos, Kisumu County, and Kambi ya Mawe in Makueni County.
Table 1.
Maize-legume intercrop varieties, spacing, and planting seasons of the on-station trials in Kibos, Kisumu County, and Kambi ya Mawe in Makueni County.
| County |
Maize variety and spacing (interrow x Intra row) |
Bean variety |
Year of trial |
Annual rainfall |
| Kisumu |
DK 8031
0.75 m x 0.3 m |
GLP 2 |
Long season
(March -Aug, 2021)
Short season (Sept-January 2021) |
1714 mm |
| Makueni |
DK 8031
0.9 m x 0.3 m |
KAT B1 |
Long season
(March -Aug, 2021)
Short season (Sept-January 2021) |
828mm |
Table 2.
Percent of Fall armyworm (FAW) incidence (mean +SE) on maize foliage and cobs and the severity of FAW damage to maize in different maize/legume intercropping systems (1 = low to 5 = high) in Kisumu and Makueni in the long rain and short rain season.
Table 2.
Percent of Fall armyworm (FAW) incidence (mean +SE) on maize foliage and cobs and the severity of FAW damage to maize in different maize/legume intercropping systems (1 = low to 5 = high) in Kisumu and Makueni in the long rain and short rain season.
| p |
Season |
Cropping system |
Percent FAW damage incidence on foliage |
FAW severity on foliage (1-5) |
Percent FAW damage incidence on cobs |
| Kisumu |
Long rain |
Maize monocrop |
60.7±2.6a |
3.1±0.1 |
20.0±5.8a |
| Maize/bean |
47.0±4.9a |
2.0±0.1 |
20.0±0.0a |
| Maize/bean/Trichoderma
|
43.7±7.8ab |
2.0±0.1 |
23.3±6.7a |
| Push-pull |
31.3±5.0b |
1.2±0.2 |
6.7±6.7a |
Mean
P value
|
45.7±3.9
0.030
|
2.08±0.2
-
|
17.5±3.1
0.22
|
| Short rain |
Maize monocrop |
78.3±10.8a |
3.1±0.1 |
40.0±5.8a |
| Maize/bean |
49.7±4.5b |
2.1±0.0 |
33.3±3.3ab |
| Maize/bean/Trichoderma
|
41.7±8.3b |
2.0±0.0 |
23.3±3.3b |
| Push-pull |
45.0±4.9b |
1.4±0.1 |
26.7±3.3b |
Mean
P value
|
53.7±5.5
0.033
|
2.1±0.2
-
|
30.8±2.6
0.034
|
| Makueni |
Long rain |
Maize monocrop |
100.0±0.0a |
3.8±0.2 |
100.0±0.0a |
| Maize/bean |
100.0±0.0a |
3.2±0.2 |
100.0±0.0a |
| Maize/bean/Trichoderma
|
100.0±0.0a |
3.3±0.3 |
100.0±0.0a |
| Push-pull |
43.3±8.8b |
2.3±0.2 |
56.7±21.9b |
Mean
P value
|
85.8±7.6
<0.001
|
3.14±0.2
-
|
89.2±7.3
<0.001
|
| Short rain |
Maize monocrop |
62.0±15.3a |
3.4±0.5 |
100.0±0.0a |
| Maize/bean |
56.7±3.4a |
3.0±0.0 |
100.0±0.0a |
| Maize/bean/Trichoderma
|
42.3±5.5a |
3.0±0.0 |
100.0±0.0a |
| Push-pull |
42.7±7.8a |
2.8±0.2 |
78.7±10.7b |
Mean
P value
|
50.9±4.7
0.37
|
3.0±0.1
-
|
94.7±3.6
0.053
|
Table 3.
Effects of site, season, and treatment on the mean ±SE) fungal contamination (colony forming units = CFU) of maize weevils (Sitophilus zeamais), sap beetles (Carpophilus spp.), earwigs (Forficula spp.), and sugar ants (Camponotus spp.) captured from Makueni and Kisumu.
Table 3.
Effects of site, season, and treatment on the mean ±SE) fungal contamination (colony forming units = CFU) of maize weevils (Sitophilus zeamais), sap beetles (Carpophilus spp.), earwigs (Forficula spp.), and sugar ants (Camponotus spp.) captured from Makueni and Kisumu.
| Variable |
|
Fungal genera (CFU/insect) |
| Aspergillus |
Fusarium |
| Sitophilus zeamais |
Carpophilus spp. |
Forficula spp. |
Camponotus spp. |
Sitophilus zeamais |
Carpophilus spp. |
Forficula spp. |
Camponotus spp. |
| Site |
|
|
|
|
|
|
|
|
| Makueni |
116.5±37.7a |
46.4±19.0a |
36.9±16.5a |
0.5±0.5b |
47.3±29.9a |
4.2±4.2a |
0.4±0.4b |
0.0±0.0a |
| Kisumu |
15.8±10.8b |
0.8±0.8b |
14.6±5.6a |
3.5±1.5a |
60.5±20.2a |
0.0±0.0a |
43.3±5.8a |
5.8±5.8a |
| Tukey HSD 0.05 |
66.8 |
33.3 |
33.9 |
2.7 |
93.5 |
6.03 |
30.9 |
11.1 |
| Season |
|
|
|
|
|
|
|
|
| Long rain |
129.7±37.5a |
45.5±19.1a |
49.1±16.3a |
2.6±1.4a |
3.5±1.4b |
0.0±0.0a |
0.0±0.0b |
0.0±0.0a |
| Short rain |
2.6±0.9b |
1.7±1.1b |
2.3±1.7b |
1.4±0.8a |
104.4±41.0a |
4.2±3.0a |
43.8±18.0a |
5.8±5.8a |
| Tukey HSD 0.05 |
66.9 |
33.3 |
33.9 |
2.7 |
93.5 |
6.03 |
30.87 |
11.9 |
| Cropping system |
|
|
|
|
|
|
|
| Sole maize |
90.0a |
24.2a |
25.0a |
5.8a |
25.0a |
5.3a |
35.8a |
0.0a |
| Maize/bean |
89.3a |
31.1a |
33.8a |
1.3ab |
34.7a |
0.0a |
20.3a |
0.0a |
| Maze/ bean /T |
40.0a |
0.0a |
31.3a |
0.8ab |
52.8a |
3.1a |
31.4a |
11.7a |
| Push-pull |
45.5a |
39.2a |
12.8a |
0.0b |
103.3a |
0.0a |
0.0a |
0.0a |
| Tukey HSD 0.05 |
125.8 |
62.7 |
63.8 |
5.0 |
176.1 |
11.4 |
58.1 |
22.4 |
Table 4.
Viable Aspergillus and Fusarium spores from faecal matter and different body parts of earwigs, maize weevils, and sap beetles. The numbers in the brackets represent the percentage of individuals harbouring fungal spores at the respective body part. N=20, spores from twenty individuals per taxon were determined.
Table 4.
Viable Aspergillus and Fusarium spores from faecal matter and different body parts of earwigs, maize weevils, and sap beetles. The numbers in the brackets represent the percentage of individuals harbouring fungal spores at the respective body part. N=20, spores from twenty individuals per taxon were determined.
| Fungi |
Insect |
Number of Aspergillus & Fusarium spores on body part (mean±SE) |
|
% total infected individuals |
| Faeces |
Elytra |
Head |
Gut |
| Aspergillus |
Forficula spp. |
0.33±0.33 |
(1.65) |
5.00±2.89 |
(25.00) |
0.67±0.60 |
(3.35) |
0.00±0.00 |
(0.00) |
26.00 |
| S. zeamais |
0.00±0.00 |
(0.00) |
9.33±3.28 |
(46.65) |
6.67±3.53 |
(33.35) |
3.33±1.76 |
(16.65) |
52.50 |
| C. dimidiatus |
1.33±0.88 |
(6.65) |
3.67±2.03 |
(18.35) |
5.33±2.03 |
(26.65) |
3.33±2.03 |
(16.65) |
27.10 |
| Mean |
0.56±0.34 |
(2.76) |
6.00±1.63 |
(30.00) |
4.22±1.50 |
(21.11) |
2.22±0.95 |
(11.10) |
35.2 |
| F. verticilloides |
Forficula spp. |
1.33±1.33 |
(0.65) |
2.67±0.67 |
(13.35) |
3.33±0.88 |
(16.65) |
0.67±0.67 |
(3.35) |
23.20 |
| S. zeamais |
0.00±0.00 |
(0.00) |
5.33±0.67 |
(26.65) |
4.33±0.88 |
(21.65) |
0.33±0.33 |
(1.65) |
29.50 |
| C. dimidiatus |
0.67±0.67 |
(3.35) |
5.33±0.88 |
(26.65) |
4.67±0.33 |
(23.35) |
2.67±1.45 |
(13.35) |
35.00 |
| Mean |
0.67±0.47 |
(1.33) |
4.44±0.58 |
(22.21) |
4.11±0.42 |
(20.55) |
1.22±0.60 |
(6.11) |
29.23 |
Table 5.
Effects of site, season, and treatment on maize grain yield, grain spoilage, bean yield, Desmodium yield, and aflatoxin content.
Table 5.
Effects of site, season, and treatment on maize grain yield, grain spoilage, bean yield, Desmodium yield, and aflatoxin content.
| Variable |
Seasons |
Treatment |
Counties |
| Makueni |
Kisumu |
| Maize yield (kg/ka) |
Long rain season |
Maize monocrop |
2937.5±406.4a |
7924.7±196.4a |
| Maize/bean |
2158.3±61.7a |
6933.7±346.1a |
| Maize/bean/Trichoderma
|
2639.2±434.6a |
7322.3±649.0a |
| Push-pull |
2276.7±64.3a |
7062.0±575.2a |
| Short rain season |
Maize monocrop |
3396.0±462.4a |
10150.0±1175.8a |
| Maize/bean |
2520.0±72.0a |
7516.7±183.3a |
| Maize/bean/Trichoderma
|
3054.0±276.9a |
8983.3±799.1a |
| Push-pull |
2644.0±220.2a |
8433.3±1322.0a |
| Percent spoilt grain |
Long rain season |
Maize monocrop |
41.3±6.9a |
4.2±1.6ab |
| Maize/bean |
37.2±1.7ab |
3.5±1.7b |
| Maize/bean/Trichoderma
|
24.3±2.5ab |
7.0±1.3a |
| Push-pull |
21.5±5.8b |
2.2±0.3b |
| Short rain season |
Maize monocrop |
37.3±1.3a |
5.0±2.9a |
| Maize/bean |
29.7±2.9a |
4.2±0.8a |
| Maize/bean/Trichoderma
|
25.3±5.0a |
0.8±0.8a |
| Push-pull |
26.0±2.7a |
1.7±0.8a |
| 100 kernel weight( g) |
Long rain season |
Maize monocrop |
25.3±1.3a |
32.9±1.1a |
| Maize/bean |
25.4±1.1a |
29.8±0.4a |
| Maize/bean/Trichoderma
|
28.2±2.7a |
31.3±1.2a |
| Push-pull |
28.0±2.8a |
29.9±0.4a |
| Short rain season |
Maize monocrop |
27.4±2.0a |
23.9±2.2a |
| Maize/bean |
26.3±0.6a |
23.3±1.7a |
| Maize/bean/Trichoderma
|
28.0±1.5a |
25.6±1.8a |
| Push-pull |
27.2±0.1a |
22.7±2.2a |
| Bean yield (kg/ha) |
Long rain season |
Maize monocrop |
0.0±0.0 |
0.0±0.0 |
| Maize/bean |
249.3±20.4 |
388.5±22.7 |
| Maize/bean/Trichoderma
|
239.3±22.4 |
384.8±27.9 |
| Push-pull |
0.0±0.0 |
0.0±0.0 |
| Short rain season |
Maize monocrop |
0.0±0.0 |
0.0±0.0 |
| Maize/bean |
122.0±23.2 |
360.5±14.5 |
| Maize/bean/Trichoderma
|
128.3±2.0 |
322.7±28.8 |
| Push-pull |
0.0±0.0 |
0.0±0.0 |
| Desmodium (kg/ha) |
Long rain season |
Maize monocrop |
0.0±0.0 |
0.0±0.0 |
| Maize/bean |
0.0±0.0 |
0.0±0.0 |
| Maize/bean/Trichoderma
|
0.0±0.0 |
0.0±0.0 |
| Push-pull |
1983±308.7 |
4991.7±162.6 |
| Short rain season |
Maize monocrop |
0.0±0.0 |
0.0±0.0 |
| Maize/bean |
0.0±0.0 |
0.0±0.0 |
| Maize/bean/Trichoderma
|
0.0±0.0 |
0.0±0.0 |
| Push-pull |
916.7±78.8 |
3675±322.5.6 |
| |
|
|
|
| Aflatoxins (ppm) |
Long rain season |
Maize monocrop |
10.6±0.3a |
<LoD |
| Maize/bean |
10.4±0.3a |
<LoD |
| Maize/bean/Trichoderma
|
10.7±0.4a |
<LoD |
| Push-pull |
6.6±0.7b |
<LoD |
| Short rain season |
Maize monocrop |
4.7±0.1ba |
<LoD |
| Maize/bean |
3.9±0.9a |
<LoD |
| Maize/bean/Trichoderma
|
2.6±0.5a |
<LoD |
| Push-pull |
3.0±0.8a |
<LoD |
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).