Submitted:
02 November 2024
Posted:
05 November 2024
You are already at the latest version
Abstract
There is growing interest in understanding the connection between concussions on physical and mental health acutely and, in retirement, on neurodegenerative diseases. In this study, we utilised a “high-impact trauma” (HIT) device to investigate the effects of multiple concussions on the motor activity, and lifespan of the adult female Drosophila melanogaster as well as reactive oxygen and nitrogen species (RONS) levels in the fly brain and body. We found that repetitive hits, while not having acute physical effects, significantly increased long-term mobility deficits, and shortened lifespan, and exacerbated oxidative stress in both the brain and body. Notably, the novel CONKA product (Withania somnifera, Curcuma longa, Melissa officinalis, Rhodiola rosea, Vaccinium myrtillus) demonstrated promising protective effects, including mitigation of motor deficits, extension of lifespan, and reduction of oxidative stress in both the brain and body of the flies. When evaluating the contributions of individual components within the CONKA formulation, Curcuma longa, despite extending lifespan, did not contribute to mobility improvement or oxidative stress amelioration. This suggests that the benefits of CONKA are largely driven by its other four components, which displayed all the positive effects evaluated. The exclusion of Curcuma longa may streamline the formulation without diminishing its brain and body effects following a history of repetitive concussions, although this would require further study to confirm. Oral bioavailability may be an issue with Curcuma longa. Taken together, the findings validate that Drosophila melanogaster is a suitable system to mimic and investigate the effects of repetitive concussions on bodies and brains and assess the effects of health products and drug therapies.
Keywords:
1. Introduction
2. Results
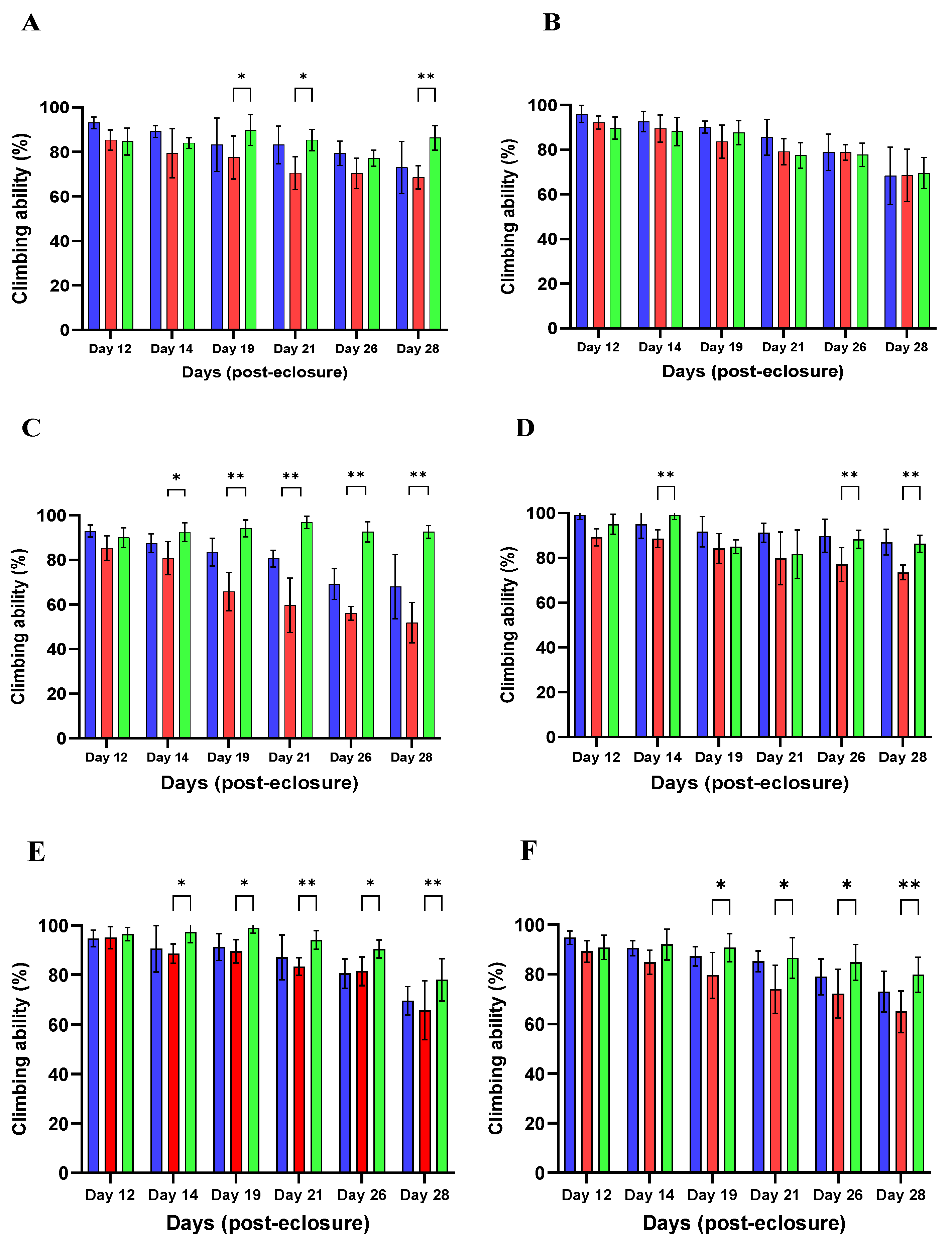
Motor Ability Assay
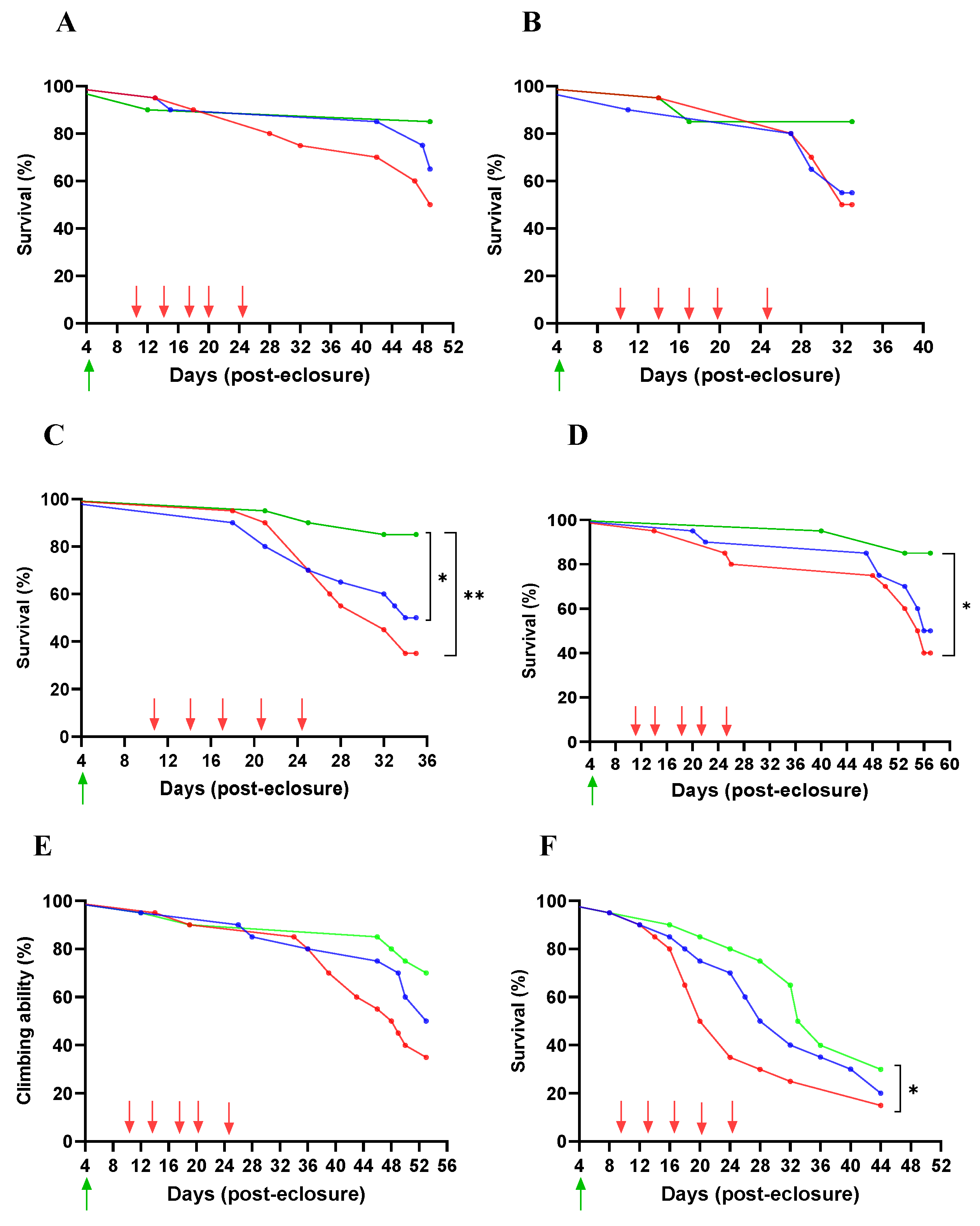
Lifespan
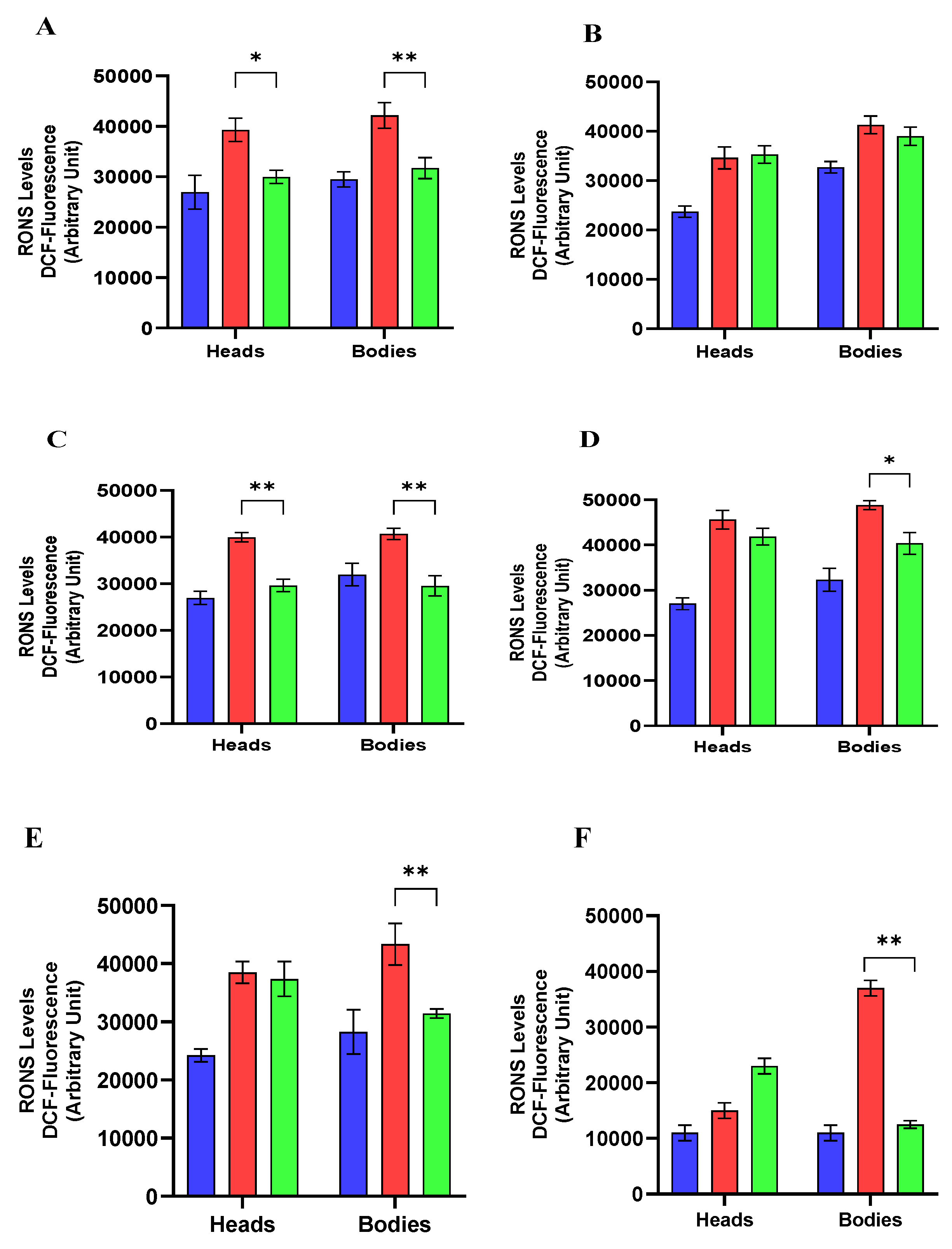
Reactive Oxygen and Nitrogen Species (RONS) Levels
3. Discussion
4. Methods and Materials
4.1. Plant Powders
4.2. Liquid Chromatography-Mass Spectrometry (LC-MS) Analysis Method
4.3. Water Extract
4.4. Flies Stocks and Culturing Conditions
4.5. Fly Concussion Model Induction
4.6. Product Concentration
4.7. Flies Treatment
4.8. Survival Rate (Lifespan Assay)
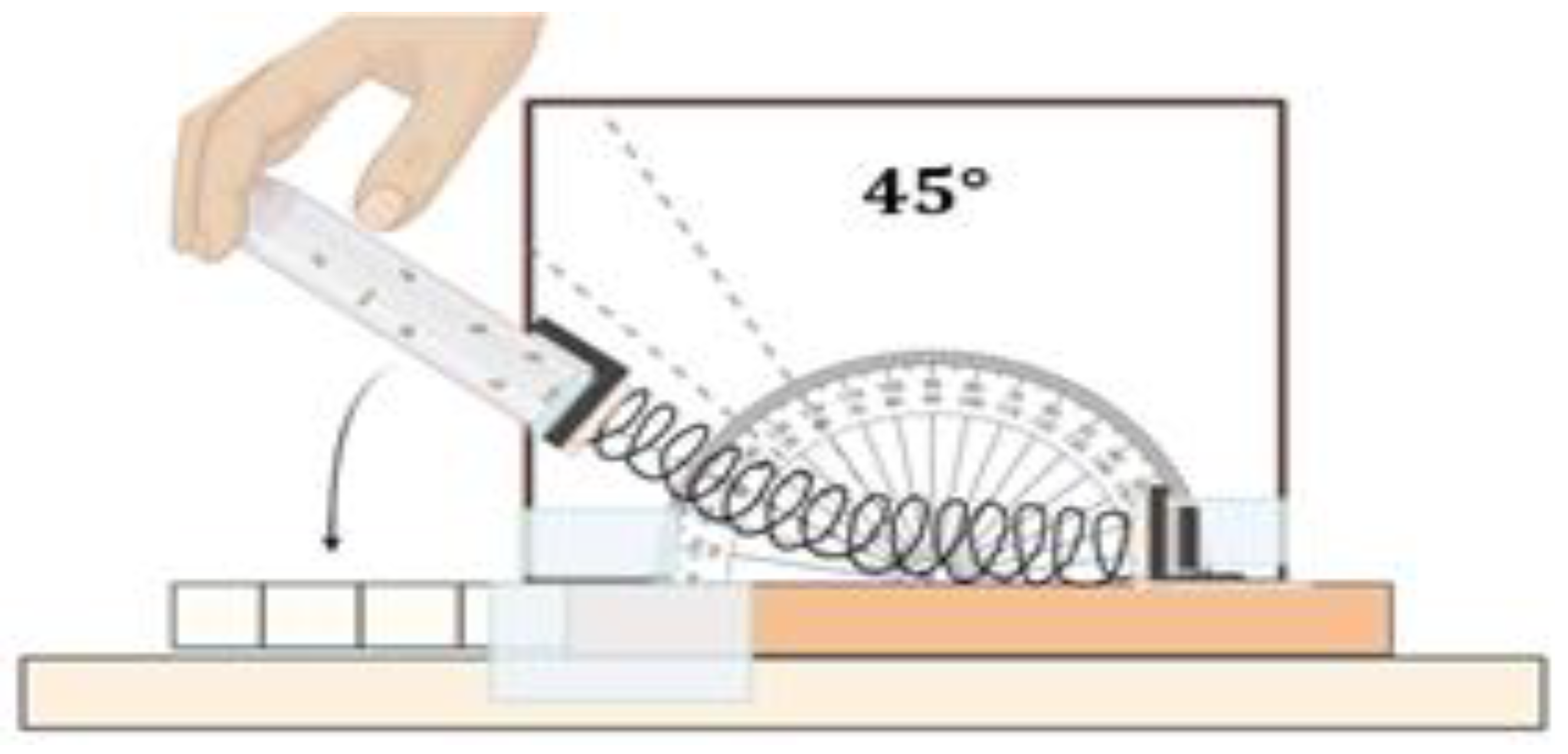
4.9. Climbing Assay
4.10. Biochemical Assays in Fly Brains and Bodies
4.11. Reactive Oxygen and Nitrogen Species (RONS) Levels Assay
4.12. Statistical Analyses
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Barekat, A.; Gonzalez, A.; Mauntz, R.E.; Kotzebue, R.W.; Molina, B.; El-Mecharrafie, N.; Conner, C.J.; Garza, S.; Melkani, G.C.; Joiner, W.J.; et al. Using Drosophila as an integrated model to study mild repetitive traumatic brain injury. Sci. Rep. 2016, 6, 25252–25252. [CrossRef]
- Martini, D.N.; Broglio, S.P. Long-term effects of sport concussion on cognitive and motor performance: A review. Int. J. Psychophysiol. 2018, 132, 25–30. [CrossRef]
- Hind, K.; Konerth, N.; Entwistle, I.; Hume, P.; Theadom, A.; Lewis, G.; King, D.; Goodbourn, T.; Bottiglieri, M.; Ferraces-Riegas, P.; et al. Mental Health and Wellbeing of Retired Elite and Amateur Rugby Players and Non-contact Athletes and Associations with Sports-Related Concussion: The UK Rugby Health Project. Sports Med. 2021, 52, 1419–1431. [CrossRef]
- Alanazi, N.; Fitzgerald, M.; Hume, P.; Hellewell, S.; Horncastle, A.; Anyaegbu, C.; Papini, M.G.; Hargreaves, N.; Halicki, M.; Entwistle, I.; et al. Concussion-Related Biomarker Variations in Retired Rugby Players and Implications for Neurodegenerative Disease Risk: The UK Rugby Health Study. Int. J. Mol. Sci. 2024, 25, 7811. [CrossRef]
- Katzenberger, R.J.; Loewen, C.A.; Wassarman, D.R.; Petersen, A.J.; Ganetzky, B.; Wassarman D.A. A Drosophila Model of Closed Head Traumatic Brain Injury. Proc. Natl. Acad. Sci. USA 2013, 110, E4152–E4159. [CrossRef]
- Swanson, L.C., Trujillo, E.A., Thiede, G.H., Katzenberger, R.J., Shishkova, E., Coon, J.J., Ganetzky, B. and Wassarman, D.A., 2020. Survival following traumatic brain injury in drosophila is increased by heterozygosity for a mutation of the NF-κB innate immune response transcription factor relish. Genetics, 216(4), pp.1117-1136.
- Freire, M.A.M.; Rocha, G.S.; Bittencourt, L.O.; Falcao, D.; Lima, R.R.; Cavalcanti, J.R.L.P. Cellular and Molecular Pathophysiology of Traumatic Brain Injury: What Have We Learned So Far?. Biology 2023, 12, 1139. [CrossRef]
- Jones, T.B.; Mackey, T.; Juba, A.N.; Amin, K.; Atyam, A.; McDole, M.; Yancy, J.; Thomas, T.C.; Buhlman, L.M. Mild traumatic brain injury in Drosophila melanogaster alters reactive oxygen and nitrogen species in a sex-dependent manner. Exp. Neurol. 2023, 372, 114621. [CrossRef]
- Pratte, M.A.; Nanavati, K.B.; Young, V.; Morley, C.P. An Alternative Treatment for Anxiety: A Systematic Review of Human Trial Results Reported for the Ayurvedic Herb Ashwagandha (Withania somnifera). J. Altern. Complement. Med. 2014, 20, 901–908. [CrossRef]
- Langade, D.; Kanchi, S.; Salve, J.; Debnath, K.; Ambegaokar, D. Efficacy and Safety of Ashwagandha (Withania somnifera) Root Extract in Insomnia and Anxiety: A Double-blind, Randomized, Placebo-controlled Study. Cureus 2019, 11, e5797. [CrossRef]
- Wankhede, S., Langade, D., Joshi, K., Sinha, S.R. and Bhattacharyya, S., 2015. Examining the effect of Withania somnifera supplementation on muscle strength and recovery: a randomized controlled trial. Journal of the International Society of Sports Nutrition, 12, pp.1-11.
- Gupta, M.; Kaur, G. Aqueous extract from the Withania somnifera leaves as a potential anti-neuroinflammatory agent: a mechanistic study. J. Neuroinflammation 2016, 13, 1–17. [CrossRef]
- Choudhary, D., Bhattacharyya, S. and Bose, S., 2017. Efficacy and safety of Ashwagandha (Withania somnifera (L.) Dunal) root extract in improving memory and cognitive functions. Journal of dietary supplements, 14(6), pp.599-612.
- Flores, G. Curcuma longa L. extract improves the cortical neural connectivity during the aging process. Neural Regen. Res. 2017, 12, 875–880. [CrossRef]
- Zhao, J.; Wu, J.-X.; Zhang, L.-Y.; Chen, Y.-L.; Yu, S.-S.; Zhao, Y. Curcumin pretreatment and post-treatment both improve the antioxidative ability of neurons with oxygen-glucose deprivation. Neural Regen. Res. 2015, 10, 481–9. [CrossRef]
- Wang, N.; Wang, Z.; Tootle, S.; Philip, T.; Zhao, Z. Curcumin promotes cardiac repair and ameliorates cardiac dysfunction following myocardial infarction. Br. J. Pharmacol. 2012, 167, 1550–1562. [CrossRef]
- Kennedy, D.; Scholey, A.B.; Tildesley, N.; Perry, E.; Wesnes, K. Modulation of mood and cognitive performance following acute administration of Melissa officinalis (lemon balm). Pharmacol. Biochem. Behav. 2002, 72, 953–964. [CrossRef]
- Kennedy, D.O.; Little, W.; Scholey, A.B. Attenuation of Laboratory-Induced Stress in Humans After Acute Administration of Melissa officinalis (Lemon Balm). Psychosom. Med. 2004, 66, 607–613. [CrossRef]
- Chindo, B.A.; Howes, M.-J.R.; Abuhamdah, S.; Yakubu, M.I.; Ayuba, G.I.; Battison, A.; Chazot, P.L. New Insights Into the Anticonvulsant Effects of Essential Oil From Melissa officinalis L. (Lemon Balm). Front. Pharmacol. 2021, 12. [CrossRef]
- Chindo, B.A.; Howes, M.-J.R.; Abuhamdah, S.; Mallam, D.; Micah, T.; Awotula, R.I.; Battison, R.; Chazot, P.L. Evaluation of the anti-nociceptive profile of essential oil from Melissa officinalis L. (lemon balm) in acute and chronic pain models. J. Ethnopharmacol. 2023, 321, 117500. [CrossRef]
- Birdane, Y.O., Buyukokuroglu, M.E., Birdane, F.M., Cemek, M. and Yavuz, H., 2007. Anti-inflammatory and antinociceptive effects of Melissa officinalis L. in rodents. Rev Med Vet, 158(02), pp.75-81.
- Darbinyan, V.; Kteyan, A.; Panossian, A.; Gabrielian, E.; Wikman, G.; Wagner, H. Rhodiola rosea in stress induced fatigue — A double blind cross-over study of a standardized extract SHR-5 with a repeated low-dose regimen on the mental performance of healthy physicians during night duty. Phytomedicine 2000, 7, 365–371. [CrossRef]
- Spasov, A.; Wikman, G.; Mandrikov, V.; Mironova, I.; Neumoin, V. A double-blind, placebo-controlled pilot study of the stimulating and adaptogenic effect of Rhodiola rosea SHR-5 extract on the fatigue of students caused by stress during an examination period with a repeated low-dose regimen. Phytomedicine 2000, 7, 85–89. [CrossRef]
- Edwards, D., Heufelder, A. and Zimmermann, A., 2012. Therapeutic Effects and Safety of Rhodiola rosea Extract WS® 1375 in Subjects with Life-stress Symptoms–Results of an Open-label Study. Phytotherapy Research, 26(8), pp.1220-1225.
- Olsson, E.M., von Schéele, B. and Panossian, A.G., 2009. A randomised, double-blind, placebo-controlled, parallel-group study of the standardised extract shr-5 of the roots of Rhodiola rosea in the treatment of subjects with stress-related fatigue. Planta medica, 75(02), pp.105-112.
- Bayazid, A.B.; Chun, E.M.; Al Mijan, M.; Park, S.H.; Moon, S.-K.; Lim, B.O. Anthocyanins profiling of bilberry ( Vaccinium myrtillus L.) extract that elucidates antioxidant and anti-inflammatory effects. Food Agric. Immunol. 2021, 32, 713–726. [CrossRef]
- Chu, W.K., Cheung, S.C., Lau, R.A. and Benzie, I.F., 2011. Bilberry (Vaccinium myrtillus L.). Herbal Medicine, 20115386, pp.55-71.
- Miyake, S.; Takahashi, N.; Sasaki, M.; Kobayashi, S.; Tsubota, K.; Ozawa, Y. Vision preservation during retinal inflammation by anthocyanin-rich bilberry extract: cellular and molecular mechanism. Mod. Pathol. 2012, 92, 102–109. [CrossRef]
- A English, E.; Chazot, P.L. Sports Post-Concussion Syndrome (PCS) Model in Drosophila melanogaster. Alzheimer's Dement. 2021, 17, e058148. [CrossRef]
- Jennings, B.H., 2011. Drosophila–a versatile model in biology & medicine. Materials today, 14(5), pp.190-195.
- Li, D., Li, F., Guttipatti, P. and Song, Y., 2018. A Drosophila in vivo injury model for studying neuroregeneration in the peripheral and central nervous system. Journal of Visualized Experiments: JoVE, (135).
- Jiang, Z.; Chazot, P.L.; Celebi, M.E.; Crookes, D.; Jiang, R. Social Behavioral Phenotyping of Drosophila With a 2D–3D Hybrid CNN Framework. IEEE Access 2019, 7, 67972–67982. [CrossRef]
- Nitta, Y.; Sugie, A. Studies of neurodegenerative diseases using Drosophila and the development of novel approaches for their analysis. Fly 2022, 16, 275–298. [CrossRef]
- Lateef, S.; Holman, A.; Carpenter, J.; James, J. Can Therapeutic Hypothermia Diminish the Impact of Traumatic Brain Injury in Drosophila melanogaster?. J. Exp. Neurosci. 2019, 13. [CrossRef]
- Anderson, E.N.; Gochenaur, L.; Singh, A.; Grant, R.; Patel, K.; Watkins, S.; Wu, J.Y.; Pandey, U.B. Traumatic injury induces stress granule formation and enhances motor dysfunctions in ALS/FTD models. Hum. Mol. Genet. 2018, 27, 1366–1381. [CrossRef]
- Jones, T.B.; Mackey, T.; Juba, A.N.; Amin, K.; Atyam, A.; McDole, M.; Yancy, J.; Thomas, T.C.; Buhlman, L.M. Mild traumatic brain injury in Drosophila melanogaster alters reactive oxygen and nitrogen species in a sex-dependent manner. Exp. Neurol. 2023, 372, 114621. [CrossRef]
- Sun, M. and Chen, L.L., 2017. A novel method to model chronic traumatic encephalopathy in Drosophila. Journal of visualized experiments: JoVE, (125).
- Shah, E.J.; Gurdziel, K.; Ruden, D.M. Drosophila Exhibit Divergent Sex-Based Responses in Transcription and Motor Function After Traumatic Brain Injury. Front. Neurol. 2020, 11, 511. [CrossRef]
- Behnke, J.A.; Ye, C.; Setty, A.; Moberg, K.H.; Zheng, J.Q. Repetitive mild head trauma induces activity mediated lifelong brain deficits in a novel Drosophila model. Sci. Rep. 2021, 11, 1–15. [CrossRef]
- Alphen, B.V., Stewart, S., Iwanaszko, M., Xu, F., Bang, E., Rozenfeld, S., Ramakrishnan, A., Itoh, T.Q., Braun, R.I. and Allada, R., 2018. Glial immune-related pathways as mediators of closed head TBI effects on behavior in Drosophila. BioRxiv, p.422535.
- Saikumar, J.; Byrns, C.N.; Hemphill, M.; Meaney, D.F.; Bonini, N.M. Dynamic neural and glial responses of a head-specific model for traumatic brain injury inDrosophila. Proc. Natl. Acad. Sci. 2020, 117, 17269–17277. [CrossRef]
- Liu, W.; Zhai, Y.; Heng, X.; Che, F.Y.; Chen, W.; Sun, D.; Zhai, G. Oral bioavailability of curcumin: problems and advancements. J. Drug Target. 2016, 24, 694–702. [CrossRef]
- Nichols, C.D., Becnel, J. and Pandey, U.B., 2012. Methods to assay Drosophila behavior. Journal of visualized experiments: JoVE, (61).
- Bartholomew, N.R.; Burdett, J.M.; VandenBrooks, J.M.; Quinlan, M.C.; Call, G.B. Impaired climbing and flight behaviour in Drosophila melanogaster following carbon dioxide anaesthesia. Sci. Rep. 2015, 5, 15298. [CrossRef]
- Ohiomokhare, S.; Olaolorun, F.; Ladagu, A.; Olopade, F.; Howes, M.-J.R.; Okello, E.; Olopade, J.; Chazot, P.L. The Pathopharmacological Interplay between Vanadium and Iron in Parkinson’s Disease Models. Int. J. Mol. Sci. 2020, 21, 6719. [CrossRef]
| No | Individual Components vs Total CONKA |
Climbing ability (%) Averages of six climbing days |
| 1 | Ashwagandha – Withania somnifera | 84% |
| 2 | Turmeric – Curcuma longa | 81% |
| 3 | Lemon balm – Melissa officinalis | 92% |
| 4 | Roseroot – Rhodiola rosea | 89% |
| 5 | Bilberry – Vaccinium myrtillus | 92% |
| Average total CONKA nutraceutical | 87% | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





