Submitted:
01 November 2024
Posted:
04 November 2024
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Results and Discussion
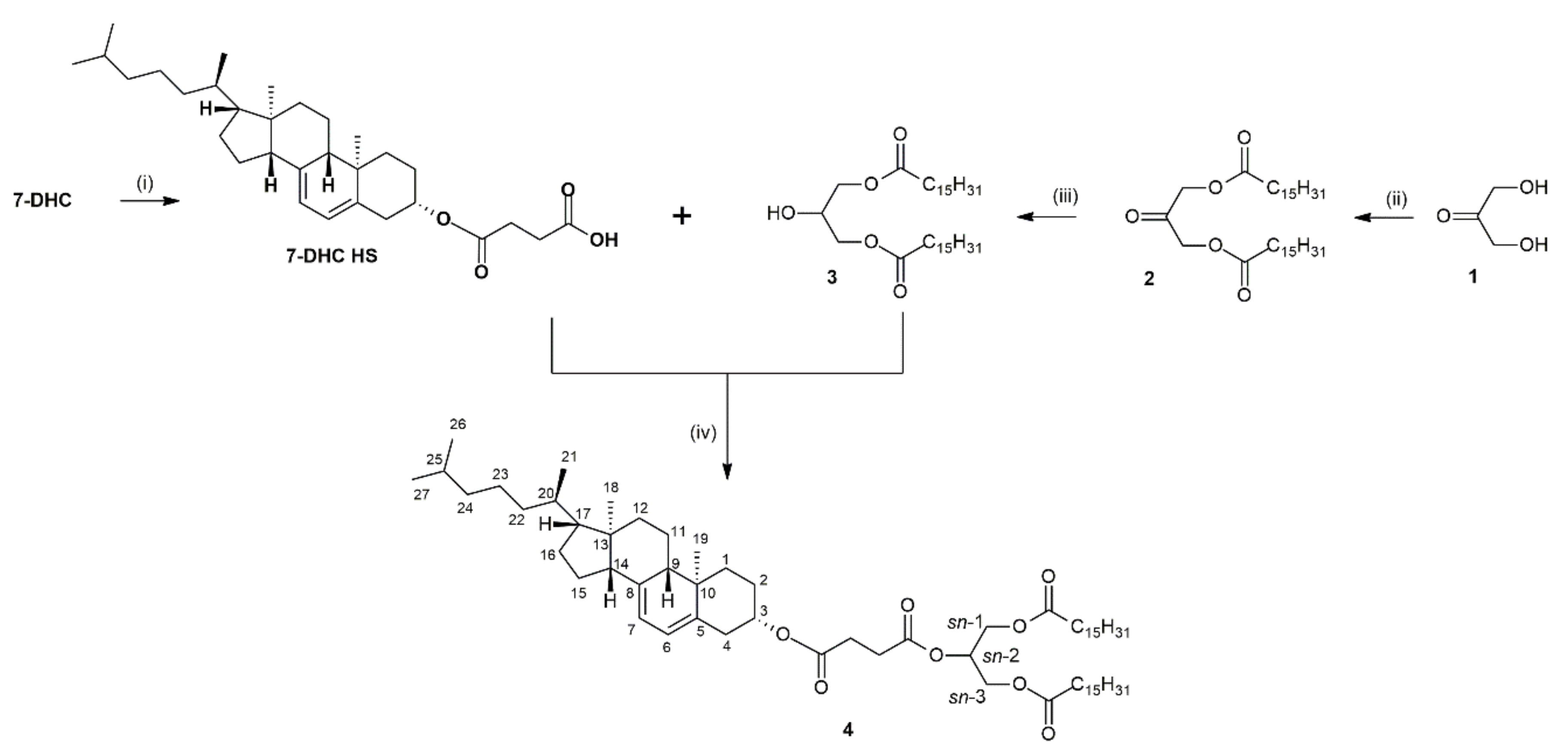
2.1. Synthesis of 7-Dehydrocholesterol Hemisuccinate
2.2. Synthesis of 1,3-Dipalmitoyl-2-(7-dehydrocholestyrylsuccinoyl)glycerol
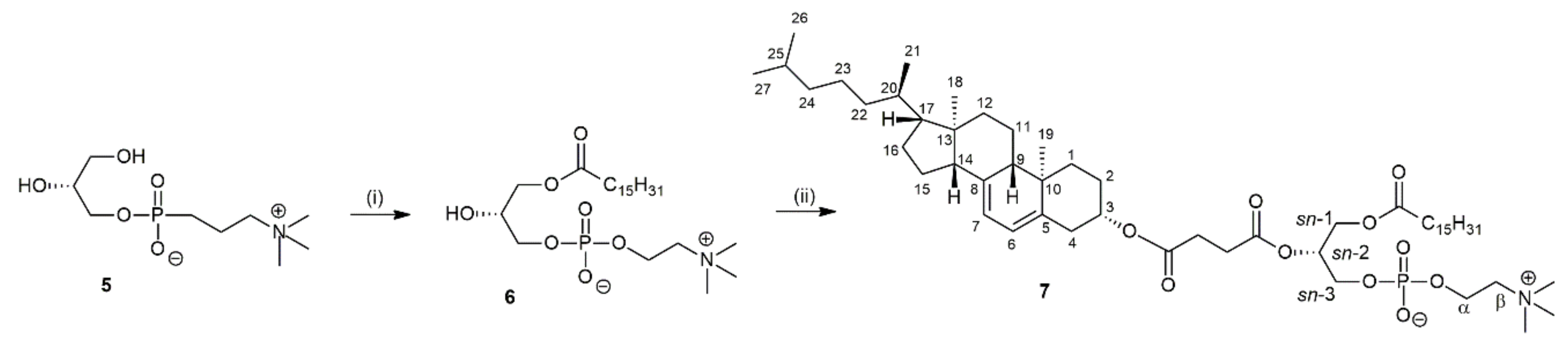
2.3. Synthesis of 1-Palmitoyl-2-(7-dehydrocholesterylsuccinoyl)-sn-glycero-3-phosphocholine (7)
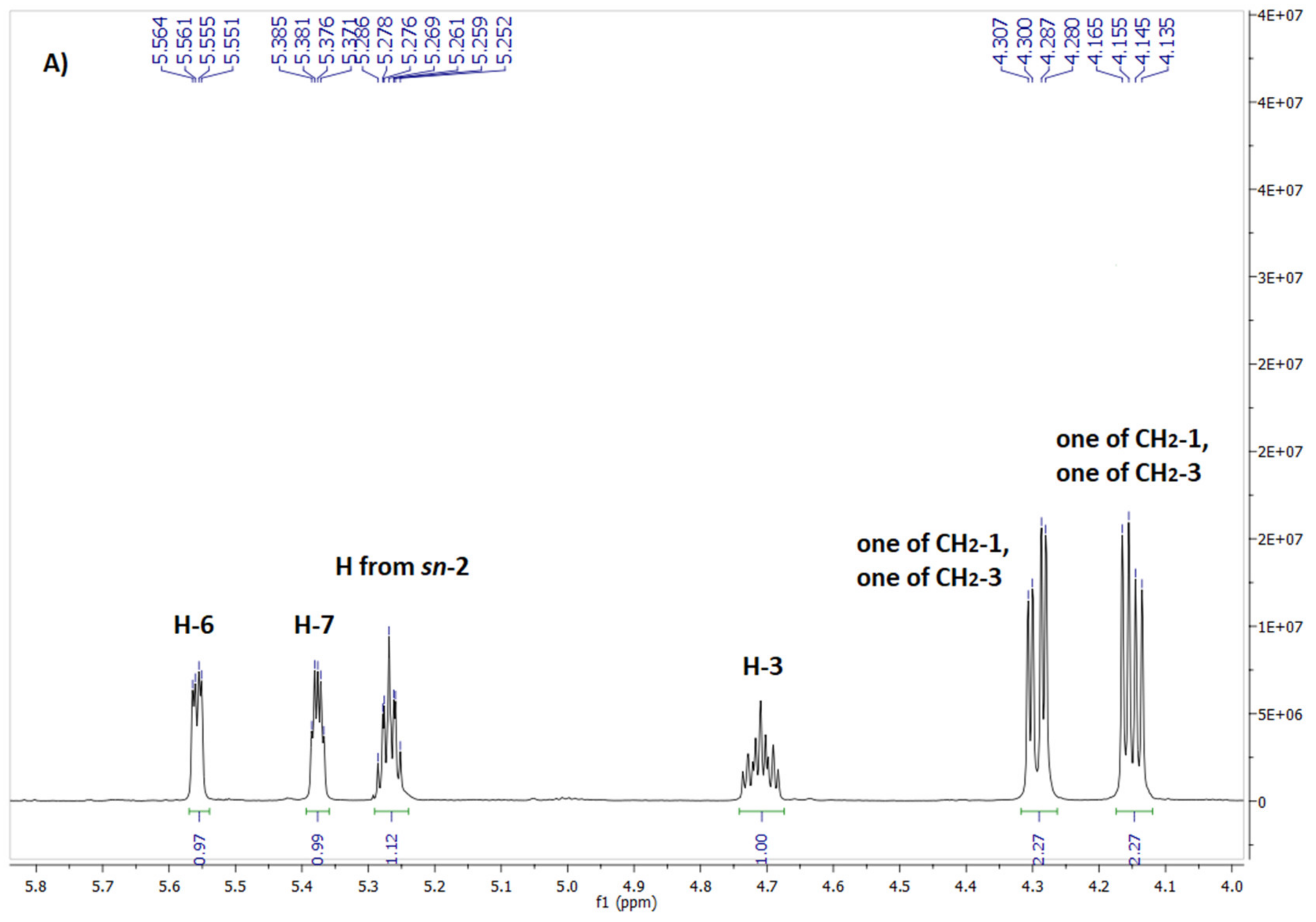
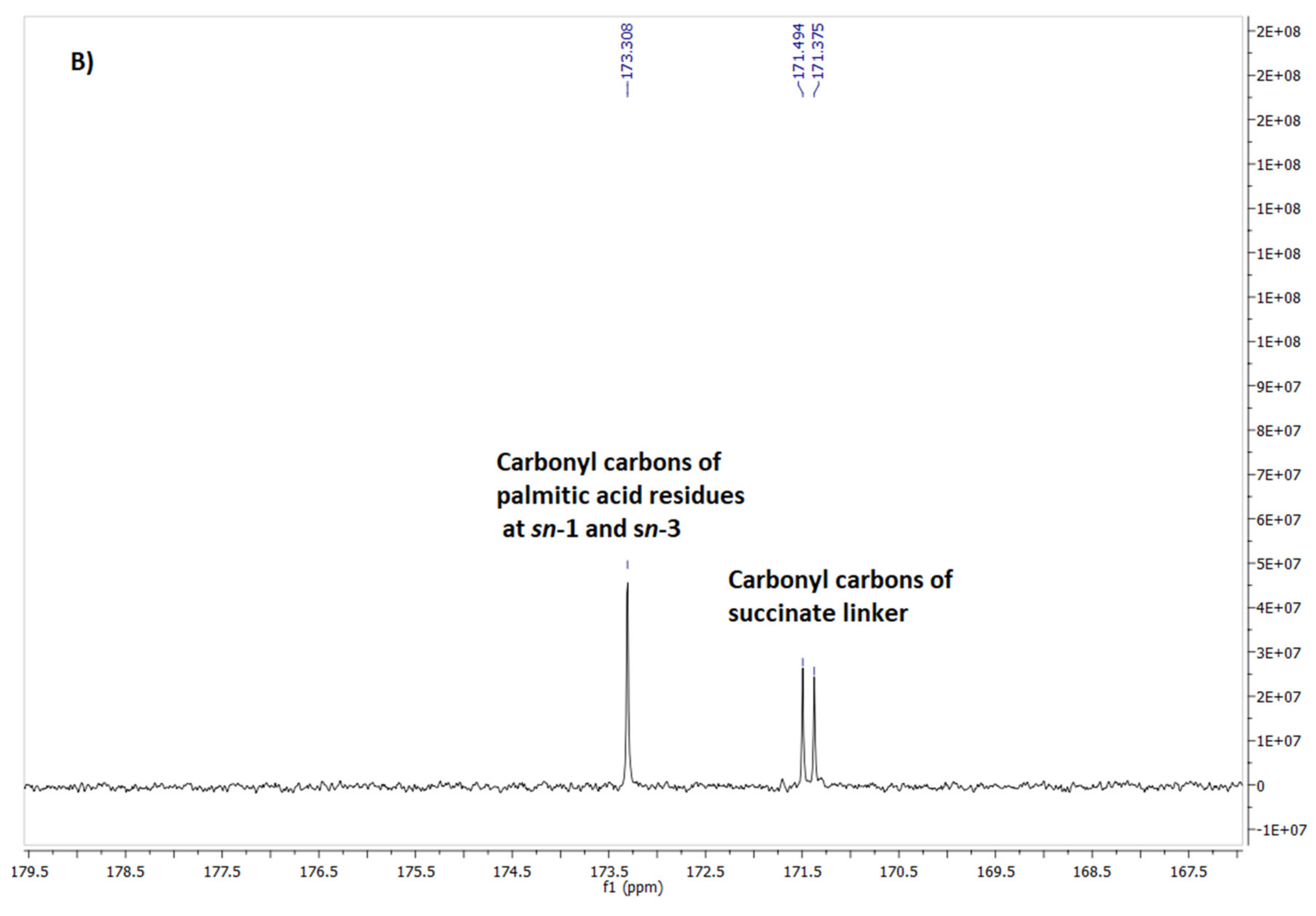
2.4. Spectroscopic Identification of the Final Products 4 and 7
4. Materials and Methods
4.1. Chemicals
4.2. Analytical Methods
4.3. Preparation of 7-Dehydrocholesteryl Hemisuccinate (7-DHC HS)
4.4. Synthesis of Target Acylglycerol with 7-Dehydrocholesterol Residue
4.4.1. Preparation of 1,3-Dipalmitoyloxypropan-2-one (2)
4.4.2. Preparation of 1,3-Dipalmitoylglycerol (3)
4.4.3. Preparation of 1,3-Dipalmitoyl-2-(7-dehydrocholesterylsuccinoyl)glycerol (4)
4.5. Synthesis of Glycerophosholipid with 7-Dehydrocholesterol Residue
4.5.1. Preparation of 1-Palmitoyl-sn-glycero-3-phosphocholine (6)
4.5.2. Preparation of 1-Palmitoyl-2-(7-dehydrocholesterylsuccinoyl)-sn-glycero-3-phosphocholine (7)
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Bhattarai, H.K.; Shrestha, S.; Rokka, K.; Shakya, R. Vitamin D, Calcium, Parathyroid Hormone, and Sex Steroids in Bone Health and Effects of Aging. J. Osteoporos. 2020, 2020, 9324505. [Google Scholar] [CrossRef] [PubMed]
- Wintermeyer, E.; Ihle, C.; Ehnert, S.; Stöckle, U.; Ochs, G.; de Zwart, P.; Flesch, I.; Bahrs, C.; Nussler, A.K. Crucial role of vitamin D in the musculoskeletal system. Nutrients 2016, 8, 319. [Google Scholar] [CrossRef] [PubMed]
- Deluca, H.F.; Cantorna, M.T. Vitamin D: its role and uses in immunology 1. FASEB J. 2001, 15, 2579–2585. [Google Scholar] [CrossRef] [PubMed]
- Ghaseminejad-Raeini, A.; Ghaderi, A.; Sharafi, A.; Nematollahi-Sani, B.; Moossavi, M.; Derakhshani, A.; Sarab, G.A. Immunomodulatory actions of vitamin D in various immune-related disorders: a comprehensive review. Front. Immunol. 2023, 14, 1–16. [Google Scholar] [CrossRef]
- Chiang, K.-C.; C. Chen, T. The Anti-cancer Actions of Vitamin D. Anticancer. Agents Med. Chem. 2012, 13, 126–139. [CrossRef]
- Deeb, K.K.; Trump, D.L.; Johnson, C.S. Vitamin D signalling pathways in cancer: Potential for anticancer therapeutics. Nat. Rev. Cancer 2007, 7, 684–700. [Google Scholar] [CrossRef]
- Harinarayan, C.V. Vitamin D and diabetes mellitus. Hormones 2014, 13, 163–181. [Google Scholar] [CrossRef]
- Wang, T.J.; Pencina, M.J.; Booth, S.L.; Jacques, P.F.; Ingelsson, E.; Lanier, K.; Benjamin, E.J.; D’Agostino, R.B.; Wolf, M.; Vasan, R.S. Vitamin D deficiency and risk of cardiovascular disease. Circulation 2008, 117, 503–511. [Google Scholar] [CrossRef]
- Sîrbe, C.; Rednic, S.; Grama, A.; Pop, T.L. An Update on the Effects of Vitamin D on the Immune System and Autoimmune Diseases. Int. J. Mol. Sci. 2022, 23, 9784. [Google Scholar] [CrossRef]
- Seraphin, G.; Rieger, S.; Hewison, M.; Capobianco, E.; Lisse, T.S. The impact of vitamin D on cancer: A mini review. J. Steroid Biochem. Mol. Biol. 2023, 231, 106308. [Google Scholar] [CrossRef]
- Matthias Wacker and; Michaela F. Holick Sunlight and Vitamin D - A global perspective for health. Dermatoendocrinol. 2013, 5, 211–217. [CrossRef]
- Glowka, E.; Stasiak, J.; Lulek, J. Drug delivery systems for vitamin D supplementation and therapy. Pharmaceutics 2019, 11, 347. [Google Scholar] [CrossRef] [PubMed]
- Duchow, E.G.; Sibilska-Kaminski, I.K.; Plum, L.A.; DeLuca, H.F. Vitamin D esters are the major form of vitamin D produced by UV irradiation in mice. Photochem. Photobiol. Sci. 2022, 21, 1399–1404. [Google Scholar] [CrossRef] [PubMed]
- Redasani, V.K.; Bari, S.B. Synthesis and evaluation of glyceride prodrugs of naproxen. Open J. Med. Chem. 2013, 3, 87–92. [Google Scholar] [CrossRef]
- Khan, M.S.Y.; Akhter, M. Synthesis, pharmacological activity and hydrolytic behavior of glyceride prodrugs of ibuprofen. Eur. J. Med. Chem. 2005, 40, 371–376. [Google Scholar] [CrossRef]
- Khan, M.S.Y.; Akhter, M. Glyceride derivatives as potential prodrugs: Synthesis, biological activity and kinetic studies of glyceride derivatives of mefenamic acid. Pharmazie 2005, 60, 110–114. [Google Scholar]
- Lalanne, M.; Paci, A.; Andrieux, K.; Dereuddre-Bosquet, N.; Clayette, P.; Deroussent, A.; Ré, M.; Vassal, G.; Couvreur, P.; Desmaële, D. Synthesis and biological evaluation of two glycerolipidic prodrugs of didanosine for direct lymphatic delivery against HIV. Bioorganic Med. Chem. Lett. 2007, 17, 2237–2240. [Google Scholar] [CrossRef]
- Garzon-Aburbeh, A.; Poupaert, J.H.; Claesen, M.; Dumont, P.; Atassi, G. 1,3-Dipalmitoylglycerol Ester of Chlorambucil as a Lymphotropic, Orally Administrable Antineoplastic Agent. J. Med. Chem. 1983, 26, 1200–1203. [Google Scholar] [CrossRef]
- Dhaneshwar, S.; Tewari, K.; Joshi, S.; Godbole, D.; Ghosh, P. Diglyceride prodrug strategy for enhancing the bioavailability of norfloxacin. Chem. Phys. Lipids 2011, 164, 307–313. [Google Scholar] [CrossRef]
- Hu, L.; Quach, T.; Han, S.; Lim, S.F.; Yadav, P.; Senyschyn, D.; Trevaskis, N.L.; Simpson, J.S.; Porter, C.J.H. Glyceride-Mimetic Prodrugs Incorporating Self-Immolative Spacers Promote Lymphatic Transport, Avoid First-Pass Metabolism, and Enhance Oral Bioavailability. Angew. Chemie - Int. Ed. 2016, 55, 13700–13705. [Google Scholar] [CrossRef]
- Dvir, E.; Friedman, J.E.; Lee, J.Y.; Koh, J.Y.; Younis, F.; Raz, S.; Shapiro, I.; Hoffman, A.; Dahan, A.; Rosenberg, G.; et al. A novel phospholipid derivative of indomethacin, DP-155 [mixture of 1-steroyl and 1-palmitoyl-2-{4-[1-(p-chlorobenzoyl)-5-methoxy-2-methyl-3- indolyl acetamido]butanoyl}-sn-glycero-3-phosophatidyl choline], shows superior safety and similar efficacy in re. J. Pharmacol. Exp. Ther. 2006, 318, 1248–1256. [Google Scholar] [CrossRef] [PubMed]
- Kłobucki, M.; Urbaniak, A.; Grudniewska, A.; Kocbach, B.; Maciejewska, G.; Kiełbowicz, G.; Ugorski, M.; Wawrzeńczyk, C. Syntheses and cytotoxicity of phosphatidylcholines containing ibuprofen or naproxen moieties. Sci. Rep. 2019, 9. [Google Scholar] [CrossRef] [PubMed]
- Dahan, A.; Duvdevani, R.; Shapiro, I.; Elmann, A.; Finkelstein, E.; Hoffman, A. The oral absorption of phospholipid prodrugs: In vivo and in vitro mechanistic investigation of trafficking of a lecithin-valproic acid conjugate following oral administration. J. Control. Release 2008, 126, 1–9. [Google Scholar] [CrossRef]
- Zhou, H.F.; Yan, H.; Senpan, A.; Wickline, S.A.; Pan, D.; Lanza, G.M.; Pham, C.T.N. Suppression of inflammation in a mouse model of rheumatoid arthritis using targeted lipase-labile fumagillin prodrug nanoparticles. Biomaterials 2012, 33, 8632–8640. [Google Scholar] [CrossRef]
- Rudzińska, M.; Grudniewska, A.; Chojnacka, A.; Gładkowski, W.; Maciejewska, G.; Olejnik, A.; Kowalska, K. Distigmasterol-Modified Acylglycerols as New Structured Lipids—Synthesis, Identification and Cytotoxicity. Molecules 2021, 26, 6837. [Google Scholar] [CrossRef]
- Gładkowski, W.; Włoch, A.; Pruchnik, H.; Chojnacka, A.; Grudniewska, A.; Wysota, A.; Dunal, A.; Castro, D.R.; Rudzińska, M. Acylglycerols of Myristic Acid as New Candidates for Effective Stigmasterol Delivery—Design, Synthesis, and the Influence on Physicochemical Properties of Liposomes. Molecules 2022, 27, 3406. [Google Scholar] [CrossRef]
- Gładkowski, W.; Chojnacka, A.; Włoch, A.; Pruchnik, H.; Grudniewska, A.; Dunal, A.; Dudek, A.; Maciejewska, G.; Rudzińska, M. Conjugates of 1,3- and 1,2-Acylglycerols with Stigmasterol: Synthesis, NMR Characterization, and Impact on Lipid Bilayers. Chempluschem 2023, 88, e202300161. [Google Scholar] [CrossRef]
- Huang, Z.; Szoka, F.C. Sterol-modified phospholipids: Cholesterol and phospholipid chimeras with improved biomembrane properties. J. Am. Chem. Soc. 2008, 130, 15702–15712. [Google Scholar] [CrossRef]
- Huang, Z.; Jaafari, M.R.; Szoka, F.C. Disterolphospholipids: Nonexchangeable lipids and their application to liposomal drug delivery. Angew. Chemie - Int. Ed. 2009, 48, 4146–4149. [Google Scholar] [CrossRef]
- Kłobucki, M.; Grudniewska, A.; Smuga, D.A.; Smuga, M.; Jarosz, J.; Wietrzyk, J.; Maciejewska, G.; Wawrzeńczyk, C. Syntheses and antiproliferative activities of novel phosphatidylcholines containing dehydroepiandrosterone moieties. Steroids 2017, 118, 109–118. [Google Scholar] [CrossRef]
- Tian, N.N.; Li, C.; Tian, N.; Zhou, Q.X.; Hou, Y.J.; Zhang, B.W.; Wang, X.S. Syntheses of 7-dehydrocholesterol peroxides and their improved anticancer activity and selectivity over ergosterol peroxide. New J. Chem. 2017, 41, 14843–14846. [Google Scholar] [CrossRef]
- Bentley, P.H.; McCrae, W. An Efficient Synthesis of Symmetrical 1,3-Diglycerides. J. Org. Chem. 1970, 35, 2082–2083. [Google Scholar] [CrossRef]
- Cockman, S.J.; Joll, C.A.; Mortimer, B.C.; Redgrave, T.G.; Stick, R.V. The Synthesis of Some Esters of Glycerol with Special Attention to the Problem of Acyl Migration. Aust. J. Chem. 1990, 43, 2093–2097. [Google Scholar] [CrossRef]
- Niezgoda, N.; Mituła, P.; Kempińska, K.; Wietrzyk, J.; Wawrzeńczyk, C. Synthesis of phosphatidylcholine with conjugated linoleic acid and studies on its cytotoxic activity. Aust. J. Chem. 2013, 66, 354–361. [Google Scholar] [CrossRef]
- Tordini, F.; Bencini, A.; Bruschi, M.; De Gioia, L.; Zampella, G.; Fantucci, P. Theoretical study of hydration of cyanamide and carbodiimide. J. Phys. Chem. A 2003, 107, 1188–1196. [Google Scholar] [CrossRef]
- Wilson, W.K.; Sumpter, R.M.; Warren, J.J.; Rogers, P.S.; Ruan, B.; Schroepfer, G.J. Analysis of unsaturated C27 sterols by nuclear magnetic resonance spectroscopy. J. Lipid Res. 1996, 37, 1529–1555. [Google Scholar] [CrossRef]
- Plückthun, A.; Dennis, E.A. Acyl and Phosphoryl Migration in Lysophospholipids: Importance in Phospholipid Synthesis and Phospholipase Specificity. Biochemistry 1982, 21, 1743–1750. [Google Scholar] [CrossRef]
- Niezgoda, N.; Gliszczy, A.; Gładkowski, W.; Chojnacka, A. Lipase-catalyzed enrichment of egg yolk phosphatidylcholine with conjugated linoleic acid. J. Mol. Catal. B Enzym. 2016, 123, 14–22. [Google Scholar] [CrossRef]
- Wang, T.; Cheng, J.; Wang, N.; Zhang, X.; Jiang, L.; Yu, D.; Wang, L. Study on the stability of intermediates in the process of enzymatic hydrolysis of phosphatidic acid by phospholipase A1. Lwt 2021, 142, 111015. [Google Scholar] [CrossRef]
- Guo, Y.; Wang, N.; Wang, D.; Luo, S.; Zhang, H.; Yu, D.; Wang, L.; Elfalleh, W.; Liao, C. Preparation of vacuum-assisted conjugated linoleic acid phospholipids under nitrogen: Mechanism of acyl migration of lysophospholipids. Food Chem. 2024, 436, 137680. [Google Scholar] [CrossRef]
- Zhang, K.; Zhang, L.; Qu, L.; Wang, X.; Liu, Y. Mechanism and effective factors of acyl migration of lysophospholipid. Chinese J. Org. Chem. 2014, 34, 2529–2536. [Google Scholar] [CrossRef]
- Kiełbowicz, G.; Smuga, D.; Gładkowski, W.; Chojnacka, A.; Wawrzeńczyk, C. An LC method for the analysis of phosphatidylcholine hydrolysis products and its application to the monitoring of the acyl migration process. Talanta 2012, 94, 22–29. [Google Scholar] [CrossRef] [PubMed]
- Papangelis, A.; Ulven, T. Synthesis of Lysophosphatidylcholine and Mixed Phosphatidylcholine. J. Org. Chem. 2022, 87, 8194–8197. [Google Scholar] [CrossRef] [PubMed]
- Sugasini, D.; Subbaiah, P.V. Rate of acyl migration in lysophosphatidylcholine (LPC) is dependent upon the nature of the acyl group. Greater stability of sn-2 docosahexaenoyl LPC compared to the more saturated LPC species. PLoS ONE 2017, 12, 1–13. [Google Scholar] [CrossRef]
- Schlenk, H.; Lamp, B.G.; Wallace DeHaas, B. Syntheses of Derivatives of Dihydroxyacetone and of Glycerides. J. Am. Chem. Soc. 1952, 74, 2550–2552. [Google Scholar] [CrossRef]
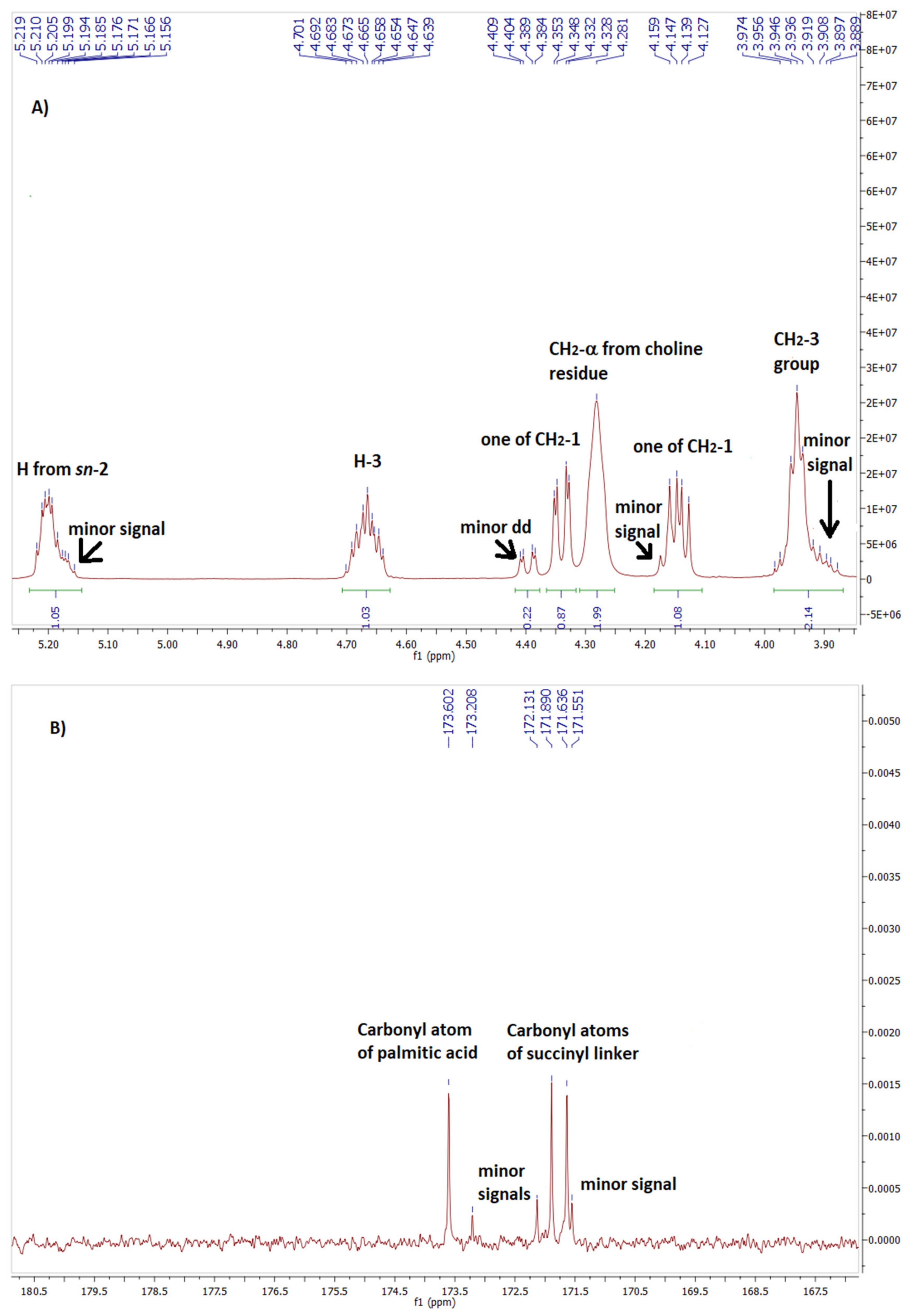
| Proton or carbon | Chemical shift [ppm] | |||
|---|---|---|---|---|
| Major signals on the spectrum of phospholipid 7 | 1-OA-2-DHEA- PChol3 |
Minor signals on the spectrum of phospholipid 7 | 1-DHEA-2-OA- PChol3 |
|
| one of the proton from CH2 at sn-1 | 4.14 | 4.14 | Overlapped with 4.14 | 4.14 |
| one of the proton from CH2 at sn-1 | 4.34 | 4.36 | 4.40 | 4.39 |
| H at sn-2 | 5.20 | 5.20 | 5.17 | 5.18 |
| carbonyl carbons from hemisuccinate linker | 171.64 and 171.89 | 171.63 and 171.69 | 171.55 and 172.13 | 171.55 and 171.95 |
| carbonyl carbon from fatty acid residue | 173.60 | 173.61 | 173.21 | 173.16 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





