1. Introduction
This study aimed to investigate the validity of an in vivo porcine gastric vessel bleed model for evaluating hemostatic powder device efficacy.
Upper gastrointestinal bleeding (UGIB) is a prevalent clinical condition associated with considerable morbidity and mortality. Conventional endoscopic therapies, including thermocoagulation, injection therapy, and mechanical clipping procedures have been widely employed to achieve hemostasis and prevent adverse outcomes [
1]. However, the efficacy of these therapies may be limited in certain cases, demanding the innovation of alternative therapeutic modalities. Hemostatic powders are materials that can function through a combination of mechanisms to control bleeding [
2]. These powders can be sprayed endoscopically through a catheter using pressurized gas. One of the major advantages of using endoscopically applied hemostatic powder over standard hemostatic therapies is the ability to rapidly cover large areas of bleeding, even in difficult-to-access regions. The utilization of hemostatic powders within the gastrointestinal tract, first introduced in the early 2000s, has emerged as a promising adjunctive endoscopic therapy for the management of gastrointestinal bleeds.
Advancements in medical products that employ endoscopic therapies to target gastric ulcer bleeds have explored various hemostatic powder formulations and corresponding delivery devices. To evaluate the hemostatic efficacy of these different product prototypes, various preclinical endoscopic bleed models have been developed. One preclinical model, the porcine gastric ulcer model, mimics diffuse, low- pressure (Forrest 1b (F1b) classification) ulcer bleeds [
3,
4,
5]. Conversely, the Giday et al. model simulates high-pressure, pulsatile, spurting (Forrest 1a (F1a) classification) bleeds [
6]. Given the level of bleed severity, the Giday et al. model simulates a clinical “challenge condition”. Accordingly, the Giday et al. model is widely regarded as a “gold standard” preclinical F1a bleed model, used for safety and efficacy testing for regulatory submission of endoscopically applied hemostatic powders.
Giday et al. were the first to implement such a model to test hemostatic powder device efficacy and since then, subsequent studies have adopted analogous models [
7]. In the 2011 Giday et al. study, a single pulsatile bleed was created by inserting a gastroepiploic artery segment through a 1-cm gastrotomy and lacerating the artery using a needle knife. The study consisted of 5 treatment animals receiving the hemostatic powder under investigation (TC-325). Giday et al. report that an additional 5 animals were assigned to a sham control group. Animals in this group underwent the gastric bleed procedure and received no endoscopic treatment. 5/5 treatment animals achieved acute hemostasis, while 0/5 sham control animals achieved acute hemostasis. With a clear distinction between both groups, Giday et al. determined that “TC-325 is safe and highly effective in achieving hemostasis in an anticoagulated severe arterial gastrointestinal bleeding animal model” [
6].
Giday et al. then conducted a 2013 GLP study to evaluate the safety and efficacy of Hemospray™ (Cook Medical, Winston-Salem, NC, USA) by employing the same vessel bleed model used in the 2011 Giday et al. study [
8]. The results indicated that 6/6 treatment animals achieved acute hemostasis. The exclusion of a control group was justified by citing the sham control results from the 2011 Giday et al. study [
6]. In a 2021 study, Ali-Mohamad et al. evaluated a self-propelling thrombin powder (SPTP) for managing severe upper gastrointestinal bleeding [
9]. The study reproduced the vessel bleed model used in these prior studies. However, each animal provided up to 3 treatment sites as the gastroepiploic vascular bundle was pulled through separate gastrotomies along the greater curvature of the stomach. 12/12 treatment sites achieved acute hemostasis. Ali-Mohamad et al. expressed that future studies require a comparative control group that receives no powder.
The objective of this investigation was to determine if the established Giday et al. vessel bleed model is suitable for testing endoscopically delivered hemostatic materials. Upon closer examination, multiple factors regarding the validity of this model for hemostatic agent efficacy testing applications came into question.
The primary concern that prompted the reevaluation of the Giday et al. model is the nature of the sham control, i.e., the negative (no powder) control required for accurate evaluation and comparison to the endoscopically applied test powders. The Giday et al. vessel bleed model study describes the use of a sham control where the gastroepiploic artery is punctured and allowed to bleed without hemostatic intervention. However, this sham control study failed to test the impact of the ancillary administration device which pressurizes the gastric lumen. Although other study designs include a control group where an animal is allowed to bleed during the entirety of a defined observation time, to our knowledge, there have been no studies published to date that describe the incorporation of a true sham control, i.e., one in which gas is endoscopically delivered through the application catheter without the presence of the test material.
Additionally, the current state of this vessel bleed model does not incorporate quantitative measurement of intragastric pressures. Following the foundational laws of fluid mechanics, a pressure differential is what drives flow. Since the Giday et al. model is an endoscopic bleed model that involves insufflation and therapeutic application utilizing gas, intragastric pressure fundamentally plays a role in blood flow. Thereis also the inherent variability of insufflation pressures and bleed volumes achieved during such endoscopic procedures, which alter pressure, and thus, blood flow rate.
Another issue that warrants review of this model is the subjective nature of bleed scoring that is used to measure bleed severity or to determine if/when blood flow slows/stops. Considering that this model is used to test hemostatic powders that effectively cover a bleed site, the ability to accurately determine if there are changes in bleed severity e.g., to differentiate between a fully stopped vs. a very slow, oozing bleed is not always visually clear when concealed beneath the applied material. Hemostasis occurring due to artifactual events related to the model, that are independent of powder function, would similarly be obscured. Lastly, a quantitative measurement of blood flow rate, which allows accurate determination of hemostasis, is lacking.
When comparing the gastric vessel bleed model vs. a true ulcer bleed model, it should also be noted that the interaction between the test material and the surgically implanted vessel does not simulate therapeutic application in the true disease state, i.e., the hemostatic material-mucosal/submucosal tissue interaction that would occur in an ulcer bleed scenario. The vessel bleed model may prove adequate for testing the material application by devices in an endoscopic training environment and any mechanical mechanisms of acute hemostasis in the case of F1a bleeds. However, the physiological relevance of the vessel bleed model for testing hemostatic powder efficacy in terms of material exposure to ulcer bleeds, ulcer tissue, and/or any biological hemostatic mechanisms of action that may be at play should be considered.
Lastly, one remaining challenge with using a porcine bleed model, in general, is that pigs are hypercoagulative [
4,
10,
11,
12]. Bleeding is difficult to induce in swine during endoscopic procedures due to higher coagulation and platelet aggregation levels [
13,
14]. For upper GI ulcer bleed models, pigs are rendered ‘bleeders’ equivalent to non-medicated humans by pretreatment with anticoagulant and antiplatelet drugs which are administered before and during the endoscopic procedure [
4]. Thus, any other confounding factors referenced above which may potentially affect the data or even lead to false positive hemostasis using the Giday et al. vessel bleed model, may be exacerbated by the inherent tendency toward hypercoagulation. This alone provides rationale to confirm that in this model, bleeds induced in pigs are not stopping on their own, due to platelet plugging or coagulation, when no hemostatic material is applied (sham control). Additionally, the hypercoagulative nature of pigs and the required dosing of anti- coagulative and anti-platelet therapies should still be considered, if attempting to apply the Giday et al. vessel bleed model for chronic studies and the evaluation of hemostatic therapies for the prevention of re-bleeds.
Consideration of these factors and their potential impact on the Giday et al. vessel bleed model for evaluating the efficacy of endoscopic hemostatic therapies was the basis for this investigation. Testing the effect of endoscopically delivered gas via the application catheter without the accompanying test hemostatic material allowed for critical evaluation of the vessel bleed model and underscored the requirement for the incorporation of appropriate sham controls when employing this model in the future.
2. Materials and Methods
This study was overseen by the CBSET Inc., Contract Research Organization’s (Lexington, MA) Institutional Animal Care and Use Committee (IACUC) and conformed to the “Guide for the Care and Use of Laboratory Animals”. CBSET, Inc. is accredited by AAALAC International and is registered with the U.S. Department of Agriculture.
A procedure was performed in 10 Yorkshire swine (40-50kg; Sus scrofa domesticus), where CO2 was sprayed into the gastric lumen without the presence of a hemostatic powder.
Surgical Preparation and Peri-Operative Anesthesia Maintenance
Animals were fed a liquid diet daily beginning three days before anesthetic induction. The liquid diet consisted of pig chow (#5084 Laboratory Porcine Diet Grower), full-fat yogurt, a high-calorie supplemental drink, whey protein, and amino acid powder. All animals received their last feeding ~20-24 hours before the procedure.
A tiletamine-zolazepam (4-6 mg/kg) intramuscular injection was administered as a pre-anesthetic. An endotracheal tube was placed for ventilatory support and general inhalant anesthesia delivery. Animals were maintained under isoflurane inhalant anesthesia. An intravenous catheter was placed in a marginal ear vein for IV fluid and drug administration.
Vital signs including electrocardiogram (ECG), pulse-oximetry, temperature, respiratory rate, and blood pressure were monitored continuously at regular intervals during the procedure.
Blood Pressure and Activated Clotting Time Maintenance
A vascular introducer sheath was placed percutaneously into the right femoral artery for invasive blood pressure monitoring and blood sampling.
Heparin was administered intravenously (initial bolus: 50-150 IU/kg; maintenance CRI: 75-200 IU/kg/hr) to maintain an ACT between 170 seconds and 300 seconds (ACT measured using Hemochron Elite; ACT+ cartridge).
Mean arterial pressure was maintained between 75 and 85mmHg. Phenylephrine was administered intravenously (CRI: 0.25-20 mcg/kg/min) as needed to modulate the blood pressure.
In Vivo Forrest 1a Gastric Vessel Bleed Model Creation and Introduction of Pressure and Flow Measurement Devices (Figure S1)
The abdominal organs were exposed through a midline laparotomy and a ~3mm full-thickness defect was created in the gastric antrum. A foley catheter was inserted through the defect and secured within the gastric lumen by inflating the balloon oriented towards the catheter tip. The defect site was closed and cinched around the Foley catheter using a purse-string suturing pattern. The Foley catheter was attached to a pressure transducer and connected to a single analog channel on a PowerLab Instrument Interface unit to record intragastric pressure readings
Figure S1A).
Perivascular adipose tissue was carefully dissected away from the external surface of a 1-3 cm segment of the splenic arteriovenous bundle. The exposed vascular segment was positioned within the gastric lumen through an ~1cm gastrotomy. The vascular segment was secured within the stomach by suturing (3-0 Prolene) the gastrotomy margins closed using a continuous suturing pattern. Upon confirmation, an overstitch layer was sutured (3-0 Prolene) in place at the gastrotomy site using a Cushing suture pattern (
Figure S1B).
Perivascular flow probes were positioned both proximal and distal to the gastrotomy site and fixed to the external surface of the gastric wall. Each flow probe was connected to an analog channel on the PowerLab. The midline laparotomy was closed using standard surgical techniques (
Figure S1C).
Evaluating the Relationship Between Gastric Pressure and Vascular Blood Flow
An Olympus GIF-2TH180 gastroscope was advanced into the stomach. Animals with flow probe implants underwent a series of insufflation and desufflation cycles (flow rate: 1.5 SLPM) before induction of a bleed (
Figure S2A) to determine if gastric pressures typically achieved in endoscopic procedures can reduce or stop blood flow through the implanted artery. Observations of blood flow cessation or degradation were documented. Pressure and flow data were digitally recorded.
A 5.5Fr Rx Needle knife was advanced down the gastroscope’s working channel. The electrosurgical tip was positioned close to the internal segment of the splenic artery. Using an en-face approach, the electrosurgical tip was activated and poked through the arterial wall.
The resulting bleed was scored as a Rating 4 (extreme spurting flow), Rating 3 (spurting flow), Rating 2 (moderate oozing flow), Rating 1 (slow oozing leakage), or Rating 0 (no active blood flow) bleed by the Sponsor Surgeon (
Figure S4). If the initial injury did not produce a severe bleed, additional punctures were created until a Rating 3 or Rating 4 was scored.
After a Forrest 1a bleed is induced in this model, the procedural steps that follow depend on the resulting bleed score (
Figure S2B). Note that in addition to obtaining bleed ratings, pressure and flow data were recorded when possible. Overall, this post-puncture procedure utilizes an average spray algorithm established for a prototype hemostatic device equipped with a compressed gas canister, in which a hemostatic agent is applied endoscopically by CO
2 (flow rate: 6 SLPM). Utilization of this device in this splenic artery gastric bleed model using the typical spray algorithm for application of a test agent, but in the absence of any test material, allows for investigation into the effect of clinically relevant intragastric pressures on bleed reduction or hemostasis in this model.
In general, the spray algorithm proceeded as follows; CO
2 was sprayed at an active bleed utilizing the prototype hemostatic powder spray device, and if not rated a ‘0’ (i.e., if the bleed has not stopped), additional CO
2 sprays were applied. Following a set sequence of sprays, the stomach was desufflated, and this spray algorithm was repeated. If the bleed stopped, and a bleed score of ‘0’ was achieved, the stomach was desufflated and the bleed was monitored for 10 minutes to confirm acute hemostasis. If the total study time persisted past 40 minutes beyond the initial puncture time without achieving hemostasis, the study was terminated. The average spray algorithm applied to this splenic artery gastric bleed model for measuring the hemostatic efficacy of a test agent was determined to be 8-6-6 (in succession) seconds. The average spray algorithm used to maintain low intragastric pressures, below an established ‘threshold’, was 4-2-2 seconds, as spraying CO
2 into the stomach for longer times yields gastric pressures above a determined threshold (
Figure S3).
3. Results
Study Outline
7/10 animals were included in this vessel bleed model pressure study; 4/7 animals underwent high- pressure cycles, and 3/7 animals underwent low-pressure pressure cycles following bleed initiation. Of the 3 excluded animals, 1/3 animals were discounted due to the inability to achieve an F1a bleed and failure to visualize the bleed condition. Two animals were excluded from the overall data set due to inadvertent severing of the vessel with the electrosurgical tip, which yielded severe and inoperable hemorrhages unrepresentative of clinical bleed cases.
Intragastric Pressure Attenuates Blood Flow Through the Sutured Vessel
To evaluate the relationship between gastric pressure and vascular flow, and to determine if gastric pressures typically achieved in endoscopic procedures can reduce or stop blood flow through the implanted artery in this model, a series of insufflation and desufflation cycles was first performed before induction of a bleed (
Figure S2A). This was conducted with a pressure sensor sutured within the stomach wall and flow probes attached to the vessel sutured into the stomach (
Figure S1). Delivering these cycles of air into the stomach showed that with increased intragastric pressure there is attenuated vessel blood flow (Figure 1A,B). Furthermore, flow magnitude degradation was observed when the flow was restored in the artery post-insufflation cycle. Before proceeding with the puncture (bleed) trial (Figure 1C), a wait time was allotted until the flow was fully restored and stabilized, as measured by the flow probes. A defect was formed by advancing a needle knife through the working channel of the gastroscope. Once confirmed that the resulting bleed was classified as Forrest 1a, pressure cycling was initiated using the pressure cycling pattern outlined in
Figure S2B. Notably, the initial 8-second spray consistently resulted in an immediate bleed interruption (bleed score “0”). Thus, the procedure that resulted consisted of cycles of the 8-second spray followed by desufflation, resulting in the observed 8-second periodicity. In addition, recurring spikes in pressure, which are attributed to CO
2 gas introduced via the spray device, repeatedly reached peak pressures above baseline (insufflation pressure). Attenuation of flow with increased pressure was also observed in this post-puncture (bleed) case. In addition, the amplitude of flow restoration never reached that of the initial flow rate when cycles of pressurization and de- pressurization ensued (Figure 1C). This relationship between pressure and flow was consistently observed across all these initial tests in which CO
2 spray cycles established for a prototype hemostatic device were applied. Blood flow through the vessel completely ceased until desufflation. 4/4 animals in the studyreached hemostasis within the time constraints placed on the bleed study conducted at these higherpressures.
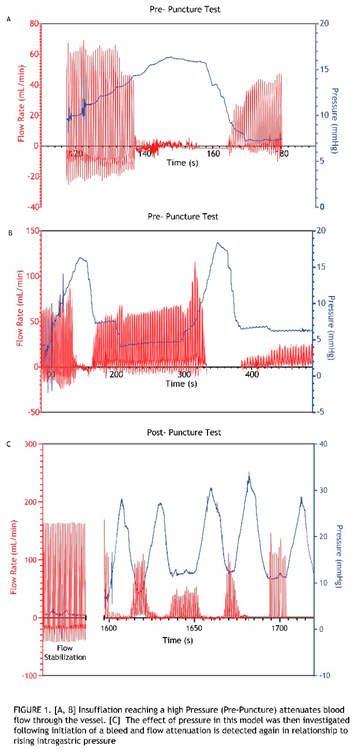
Low Intragastric Pressurization Cycles Impact Blood Flow
With the observation that pressure cycles representative of those used for spray device material application (pre- and post-puncture) completely attenuate flow, further studies were pursued to determine how blood flow is impacted if the pressurization cycle is conducted using shorter spray times, thereby achieving lower intragastric pressure (See
Figure S3). These studies were considered the “Low Pressure” studies and were carried out across 3 animals (Figure 2). Unlike the previous high-pressure cycles, which corresponded with longer spray times, these low-pressure cycles did not demonstrate complete flow stoppage, since bleeds persisted at maximum intragastric pressures, as denoted by the bleed rating scores (> 0) obtained with each pressurization cycle. However, regardless of incomplete flow attenuation at each cycle’s max pressure, flow degradation occurred over time with multiple low-pressure cycles. This degradation in flow with progressing low-pressure cycles was demonstrated by the trending drop-off of bleed rating scores. 3/3 of animals showed a decrease in bleed severity scoring with low-pressure cycling. 1/3 animals in the study reached hemostasis within the time constraints placed on the study at “low pressures”.

Hemostasis Can Be Achieved as a Function of Intragastric Pressure
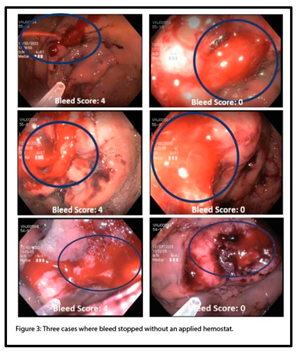
Hemostasis was defined as “achieved” in these experiments when, within 40 minutes of post-puncture insufflation cycles, the bleed ceased for 10 minutes following desufflation. In 7 total animals tested, 5 animals achieved hemostasis without any hemostatic application. Images depicting 3 separate cases in which hemostasis was achieved, as assessed by bleed ratings, are shown in Figure 3. Following puncture, bleeds were scored as “4” (F1a), and in these cases, bleeding subsequently ceased (bleed score “0”) after cycles of CO2 with no application of a hemostatic agent.
4. Discussion
The Giday et al. porcine gastric vessel bleed model, which is widely used to evaluate hemostatic products, is considered the “gold standard” in the field of endoscopy. However, the study described here demonstrates its shortcomings. Examination of a previously untested (control) condition provided for our comprehensive critical evaluation of the model. Multiple modes leading to potential false positive hemostasis were detected in this study, such that, if a candidate material were tested using this model, it could fail to function as an efficacious hemostat, yet seem successful in stopping bleeds. This poses a risk to the development of safe and effective endoscopic therapies and devices targeted to treat upper gastrointestinal bleeding.
One modification of the Giday et al. model employed in our study was the use of the splenic artery in place of the gastroepiploic artery. In our studies, and as reported in historical studies that used the Giday et al. model, gastroepiploic bleeds often yielded less aggressive Forrest 1b bleeds. Use of the splenic artery in this model predominantly yields aggressive bleeds, allowing for the evaluation of the porcine gastric vessel Forrest 1a bleed model. While this alteration of the model is noted, similar physiological responses occur and apply, regardless of the vessel used.
Figure 4 illustrates our hypothesis of what is occurring when pressurizing the stomach in this model. The splenic artery is sutured tightly within the stomach and flow persists without issue following a stabilization period (Figure 4A). When a hemostatic powder is applied, driven by pressurized air, the stomach wall expands, collapsing the artery (Figure 4B). This is similar to how a blood pressure cuff functions. A blood pressure cuff must exceed the pressure within the artery to occlude the vessel. When this happens, flow through the artery stops. As pressure is relieved from the cuff, flow restores slowly, and this flow restoration is what is detected when a blood pressure test is conducted. The same mechanism applies here when the intragastric pressure meets, and further exceeds, that of the splenic artery; the stomach collapses the sutured artery and thus “pinches” the artery. The stomach fundamentally acts as a pressure cuff to completely attenuate blood flow. The relationship between pressure and flow rate in the pre- puncture test as it corresponds to the open and occluded vessel states is illustrated below (Figure 4C).
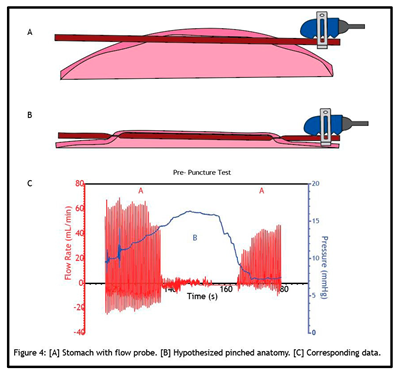
A vessel “pinch-off point”, i.e., the pressure at which blood flow through the vessel stops, was observed in every animal. We conclude that this pinch-off point has greater effects beyond just temporarily impeding blood flow. Impeding flow, through mechanical pinching and narrowing within an artery can lead to cascading events. This can result in a sustained decrease or even complete attenuation of flow. One of these events is vasospasm, the narrowing of arteries due to a persistent contraction of the blood vessels. We detected signs of vasospasm occurring on multiple occasions, as flow restoration was diminished after desufflating the stomach (Figures 1B,C, and 2). We conclude that mechanical manipulation of the artery can lead to its vasoconstriction, resulting in spasming and reduced blood flow. The effect of physical manipulation of the vessel as a function ofsurgical implantation, intragastric pressurization cycles, or importantly, the application of hemostatic agents that introduce gas into the system, on the flow status of bleeds is a primary concern associated with this vessel bleed model.
It could be argued that staying below the pressure pinch-off value would be sufficient to justify the continued use of this model. However, our “low-pressure” studies lend a different argument. These results showed that even though the vessel is never pinched due to pressure, the bleeding can still stop. Vasoconstriction that occurs in this model upon artery implantation and/or tissue puncture may be intensified by vasospasm as a function of repeated pressure cycling – even if only reaching pressures wellbelow any physical pinch-off point. This could explain the slowing and/or stopping of blood flow observed in the low-pressure tests.
Another potential explanation is the change in shear that occurs with altering the flow-pressure differential. Pressure drives fluid flow, and as the stomach pressure increases, the pressure differential between the bleed and the stomach decreases. This reduces the flow of blood from the vessel into the stomach. This reduction in flow in turn decreases shear stress experienced at the defect site. This can directly impact platelet activation and adhesion, especially in a hypercoagulative porcine model. A pierced vessel wall will attract platelets, but with high shear rates, platelets will have a difficult time adhering and plugging the bleed. Additionally, high shear rates trigger Nitric Oxide release by the endothelium, which prevents platelet adhesion. When these shear rates decrease, however, there is a reduction in Nitric Oxide synthesis and an increase in the activation of platelets. Thus, with a decreased pressure differential, reduced flow, and lower shear, platelets have a better chance of plugging, slowing, or stopping a bleed, and contributing to the signaling events that produce a full-fledged fibrin clot.
Even if we could keep platelets at bay using antiplatelet therapies while staying below a certain thresholdpressure in this model, other confounding factors still arise. One example detected within this model was adventitial encapsulation. The implanted splenic artery is composed of multiple layers of tissue (Figure 5A). Upon puncture for the creation of the test bleed in this model, all these layers are perforated. However, some bleeds were observed to stop, following extensive swelling of the artery (Figure 5B). This indicates that the outermost layer of the artery, the tunica adventitia, was shifting and encapsulating the bleed site, resulting in a bleed score of “0”, and achieving apparent hemostasis (Figure 5C). This artifactual event has likely occurred in a multitude of animal studies utilizing this model but had yet to be detected due to hemostatic application blocking the view of the test artery and determination of hemostasis using a subjective bleed scoring method.One last mode potentially leading to confounding data in this model was the impact of mucus. Figure 6 shows the formation of a substantial mucus plug over the defect site. This mucus plug was observed to both decrease and deflect the flow of the bleed. As a result, the bleed rating was skewed, leading to a false assumption of achieved hemostasis. This mucosal artifact would be easily overlooked when applying atest hemostatic agent in this model, which would obfuscate the defect site from view, with its hemostatic efficacy going unchallenged.


Interestingly, this model’s failure mode as a function of intragastric pressure was further reinforced by an observation made in one test animal. Acute hemostasis was achieved and maintained out to the defined 40-minute observation window. However, after an hour, the bleed reinitiated. There are a few different scenarios that might explain this occurrence. One might be that the pressure-induced mechanical manipulation of the vessel, which had stopped the bleed, could have been reversed by vasorelaxation that occurs over time, restoring the bleed. It is also possible that the bleed could have been reinstated after time if the platelet plug that had formed lost integrity with restoration of blood flow and increased shear. In addition, reinitiation of a bleed due to changing conditions following artifactual adventitial encapsulation-induced hemostasis, e.g. rupture of the adventitia, or changes in anti-thrombotic drug regimens over time also could have occurred. This unexplained re-bleed event, following achieved hemostasis, although occurring outside the boundary of the defined study, suggests yet another example of how inherent pressure-dependent effects may compromise this model.
This study not only demonstrates the limitations of this model but also underscores the complexity of animal models altogether and the importance of including proper sham controls. Through this study, with the discovery of multiple routes leading to false positive results, we have disqualified the current state of this model for efficacy testing of endoscopically applied hemostatic agents. Future iterations of this model should be vetted to avert the influence of the many confounding factors discussed in this paper, as well as any issues not yet discovered by way of this limited 7-animal study. Although investigation into alternative animal models for testing endoscopic bleed therapies was out of the scope of this study, the requirement for an appropriate sham control arm in any animal study became evident.
We acknowledge that a lot of the limitations in this model are challenging to work around. For example, having multiple cycles of insufflation is an inherent part of using this model for testing hemostatic powder, as insufflation is always required, and powder is applied using CO2/gas for such endoscopic procedures. If flow and pressure data could always be collected when using this model, it would be an improvement over its current state and over the subjective bleed scoring method used for assessing hemostasis. However, the combination of confounding factors, intragastric pressure, resulting vasospasm, and those factors that go uncontrolled in a complex in vivo model, e.g., adventitial encapsulation, are not easily remedied. Undeniably, these represent common clinical scenarios. However, these factors confound hemostasis models when the aim of the model is to measure hemostatic efficacy or determine a material’s mechanism of action, instead of the model being used solely as a training module to simulate real-life bleeds.
Furthermore, even if an endoscopic resection model that simulates diffuse, low severity (F1b) ulcer bleeds is used as an alternative to this vessel bleed model (which simulates aggressive (F1a) bleeds), the pressure effect would most likely also invalidate it for use. Although no true sham controls have yet been included in F1b bleed studies to date, and further inquiry into established endoscopic F1b bleed models is warranted, it can be rationalized that the intragastric pressure effect observed in the vessel bleed model would be exacerbated in an endoscopic model simulating diffuse capillary bleeds. Capillary intraluminal pressure is very low (~2mmHg), much lower than that of arteries. With minimal CO2 application, i.e., at pressures commonly achieved in any endoscopic procedure that involves insufflation, intragastric pressure would reach and further exceed the low intraluminal pressures of capillaries, causing the diffuse F1b ulcer bleeds to stop. Thus, the pressure effect identified using this vessel bleed model implicates established in vivo endoscopic bleed models used for efficacy testing of hemostats indicated for lower grade (F1b) bleeds.
Together, these multiple constraints underscore that there is an urgent need to develop more robust bleed models for testing endoscopic hemostatic devices, as no valid endoscopic bleed model currently exists. Benchtop studies using test methods developed to specifically parse out hemostatic properties can allow for mechanism of action studies, material characterization, and down selection, before testing hemostats In Vivo. In terms of animal models, we recommend future hemostatic testing of prototype therapies indicated for gastric bleeds be conducted outside of a pressurized environment and not directly atop arteries. While study criteria often dictate that evaluation of device performance is carried out in “clinically relevant environments”, sufficing to match the target anatomy does not ensure a scientifically sound in vivo model. Often, as demonstrated in this study, risk factors that lead to “clinical irrelevance” can still ensue. We propose that an appropriate substitute for this model could be a liver biopsy bleed or femoral bleed model for testing hemostatic agents [
15,
16,
17]. This would allow for a test environment that is not impacted by pressure or other confounding variables described throughout this paper. Additionally, in the case of the liver biopsy bleed model, this would provide for numerous test bleeds within one animal, reducing animal use. This is contrary to the gastric vessel bleed model, which allows for only one test bleed per porcine vessel.
In conclusion, this study was critical to our realization that the porcine gastric vessel bleed model, in its current state, cannot be used to accurately assess the safety and efficacy of endoscopic therapies targeting UGIB. This study also implicates risk in employing an F1b bleed model as an alternative, since intragastric pressure still plays a critical role. We recommend that alternate bleed models be established for continued product development of hemostatic agents.
Supplementary Materials
The following supporting information can be downloaded at the website of this paper posted on
Preprints.org.
Funding
This study was sponsored by Boston Scientific Corporation, Endoscopy.
Acknowledgments
we thank Boston Scientific Endoscopy Division, Boston Scientific Research and Technology Center, Boston Scientific Center for Biological Innovation, Boston Scientific Preclinical Operations and Study Management, Dennis Werner, Holly Coleman, Megan Fine, Chris Son, Joe Rausa, Robert Sears, Lauren Lydecker, Kevin Windheuser, Alex McLane, Alexandra Haugen, Kapil Gupta, and Brian Dunkin for their contributions to this work.
Conflicts of Interest
Jessica L. Grimsby, Matthew D. Szkolnicki, and Kevin A. Wood declare that they have full control of all primary data and agree to allow Gastroenterology Insights (MDPI) to review this data if requested. JessicaL. Grimsby declares ownership of Boston Scientific Corporation stock and earns profits from said stock. Matthew D. Szkolnicki and Kevin A. Wood declare that they have no conflicts of interest.
References
- Holster, I. L.; Kuipers, E. J. Management of acute nonvariceal upper gastrointestinal bleeding: current policies and future perspectives. World J Gastroenterol 2012, 18 (11), 1202-1207. From NLM Medlin. [CrossRef]
- Bustamante-Balen, M.; Plume, G. Role of hemostatic powders in the endoscopic management of gastrointestinal bleeding. World J Gastrointest Pathophysiol 2014, 5 (3), 284-292. From NLM PubMed-not-MEDLIN. [CrossRef]
- Ali-Mohamad, N.; Cau, M. F.; Zenova, V.; Baylis, J. R.; Beckett, A.; McFadden, A.; Donnellan, F.; Kastrup, C. J. Self-propelling thrombin powder enables hemostasis with no observable recurrent bleeding or thrombosis over 3 days in a porcine model of upper GI bleeding. Gastrointest Endosc 2023, 98 (2), 245-248. From NLM Medlin. [CrossRef]
- Camus, M.; Marteau, P.; Pocard, M.; Bal Dit Sollier, C.; Lavergne-Slove, A.; Thibault, A.; Lecleire, S.; Vienne, A.; Coffin, B.; Drouet, L.; et al. Validation of a live animal model for training in endoscopic hemostasis of upper gastrointestinal bleeding ulcers. Endoscopy 2013, 45 (6), 451-457. From NLM Medlin. [CrossRef]
- Kaehler, G.; Dutenhoefner, C.; Magdeburg, R. Endoscopic application of polysaccharide powder for hemostasis in anticoagulated pigs (with video). Gastrointest Endosc 2015, 82 (1), 161-163. From NLM Medlin. [CrossRef]
- Giday, S. A.; Kim, Y.; Krishnamurty, D. M.; Ducharme, R.; Liang, D. B.; Shin, E. J.; Dray, X.; Hutcheon, D.; Moskowitz, K.; Donatelli, G.; et al. Long-term randomized controlled trial of a novel nanopowder hemostatic agent (TC-325) for control of severe arterial upper gastrointestinal bleeding in a porcine model. Endoscopy 2011, 43 (4), 296-299. From NLM Medlin. [CrossRef]
- Holster, I. L.; van Beusekom, H. M.; Kuipers, E. J.; Leebeek, F. W.; de Maat, M. P.; Tjwa, E. T. Effects of a hemostatic powder hemospray on coagulation and clot formation. Endoscopy 2015, 47 (7), 638-645. From NLM Medlin. [CrossRef]
- Giday, S.; Van Alstine, W.; Van Vleet, J.; Ducharme, R.; Brandner, E.; Florea, M.; Johnston, K.; Negron- Garcia, J.; Ringenberger, K. Safety analysis of a hemostatic powder in a porcine model of acute severe gastric bleeding. Dig Dis Sci 2013, 58 (12), 3422-3428. From NLM Medlin. [CrossRef]
- Ali-Mohamad, N.; Cau, M.; Baylis, J.; Zenova, V.; Semple, H.; Beckett, A.; McFadden, A.; Donnellan, F.; Kastrup, C. Severe upper gastrointestinal bleeding is halted by endoscopically delivered self-propelling thrombin powder: A porcine pilot study. Endosc Int Open 2021, 9 (5), E693-E698. From NLM PubMed-not-MEDLIN. [CrossRef]
- Chen, V. K.; Marks, J. M.; Wong, R. C.; McGee, M. F.; Faulx, A. L.; Isenberg, G. A.; Schomisc, S. J.; Deng,C. X.; Ponsky, J. L.; Chak, A. Creation of an effective and reproducible nonsurvival porcine model that simulates actively bleeding peptic ulcers. Gastrointest Endosc 2008, 68 (3), 548-553. From NLM Medlin. [CrossRef]
- Chiu, P. W.; Hu, B.; Lau, J. Y.; Sun, L. C.; Sung, J. J.; Chung, S. S. Endoscopic plication of massively bleeding peptic ulcer by using the Eagle Claw VII device: a feasibility study in a porcine model. Gastrointest Endosc 2006, 63 (4), 681-685. From NLM Medlin. [CrossRef]
- Hu, B.; Chung, S. C.; Sun, L. C.; Lau, J. Y.; Kawashima, K.; Yamamoto, T.; Cotton, P. B.; Gostout, C. J.; Hawes, R. H.; Kalloo, A. N.; et al. Developing an animal model of massive ulcer bleeding for assessing endoscopic hemostatic devices. Endoscopy 2005, 37 (9), 847-851. From NLM Medlin. [CrossRef]
- Bowie, E. J.; Owen, C. A., Jr.; Zollman, P. E.; Thompson, J. H., Jr.; Fass, D. N. Tests of hemostasis in swine: normal values and values in pigs affected with von Willebrand's disease. Am J Vet Res 1973, 34 (11), 1405-1407. From NLM Medline.
- Roussi, J.; Andre, P.; Samama, M.; Pignaud, G.; Bonneau, M.; Laporte, A.; Drouet, L. Platelet functions and haemostasis parameters in pigs: absence of side effects of a procedure of general anaesthesia. Thromb Res 1996, 81 (3), 297-305. From NLM Medline. [CrossRef]
- Adams, G. L.; Manson, R. J.; Hasselblad, V.; Shaw, L. K.; Lawson, J. H. Acute in-vivo evaluation of bleeding with Gelfoam plus saline and Gelfoam plus human thrombin using a liver square lesion model in swine. J Thromb Thrombolysis 2009, 28 (1), 1-5. From NLM Medline. [CrossRef]
- Slezak, P.; Keibl, C.; Redl, H.; Labahn, D.; Gulle, H. An Efficacy Comparison of Two Hemostatic Agents in a Porcine Liver Bleeding Model: Gelatin/Thrombin Flowable Matrix versus Collagen/Thrombin Powder. J Invest Surg 2020, 33 (9), 828-838. From NLM Medline. [CrossRef]
- Vaezy, S.; Martin, R.; Yaziji, H.; Kaczkowski, P.; Keilman, G.; Carter, S.; Caps, M.; Chi, E. Y.; Bailey, M.; Crum, L. Hemostasis of punctured blood vessels using high-intensity focused ultrasound. Ultrasound Med Biol 1998, 24 (6), 903-910. From NLM Medline. [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).