Submitted:
26 October 2024
Posted:
28 October 2024
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. The Main Features of Metabolism in Mammals
3. Types of Experiments and Information Regarding Metabolism Obtained from Animals
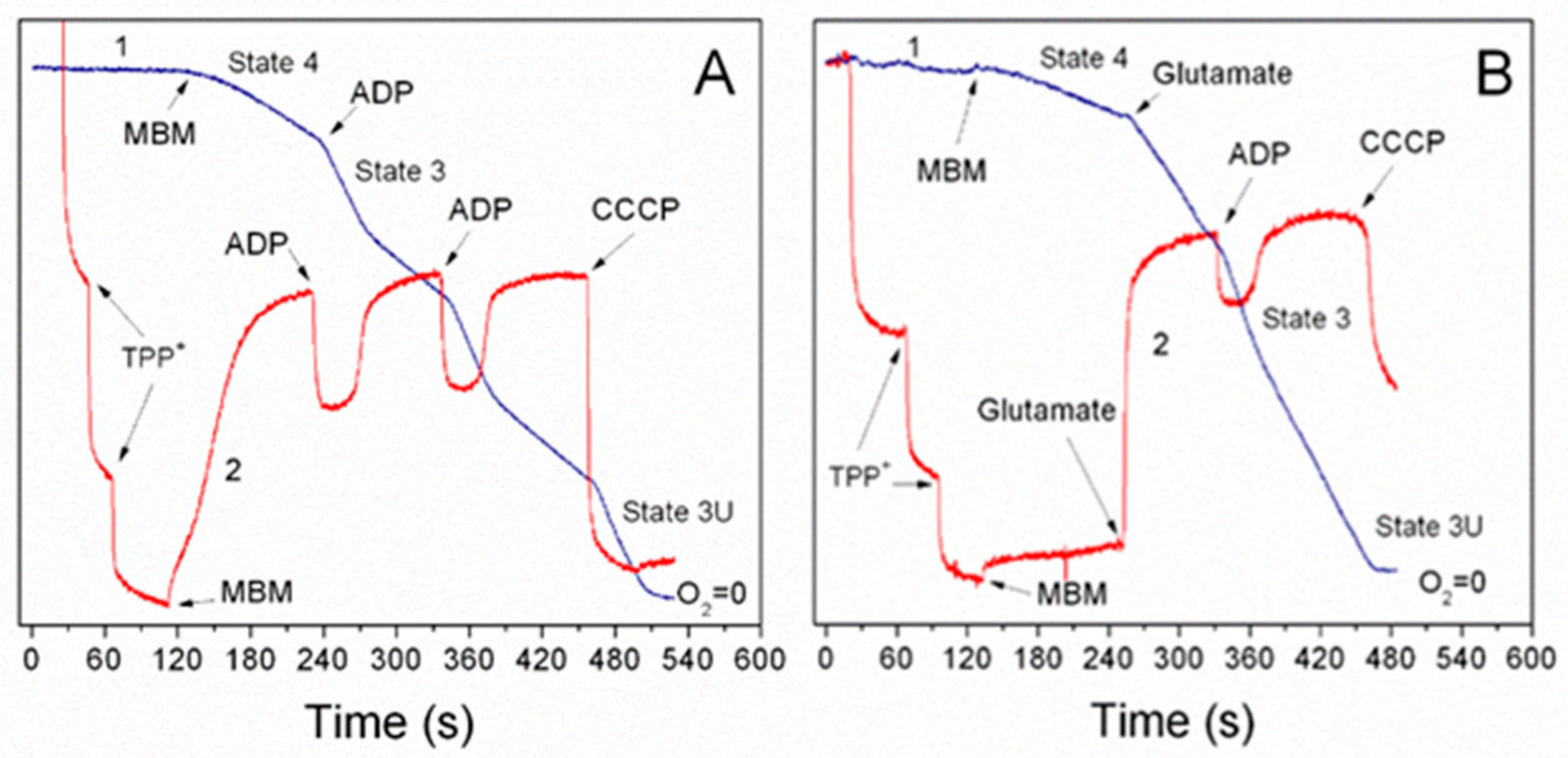
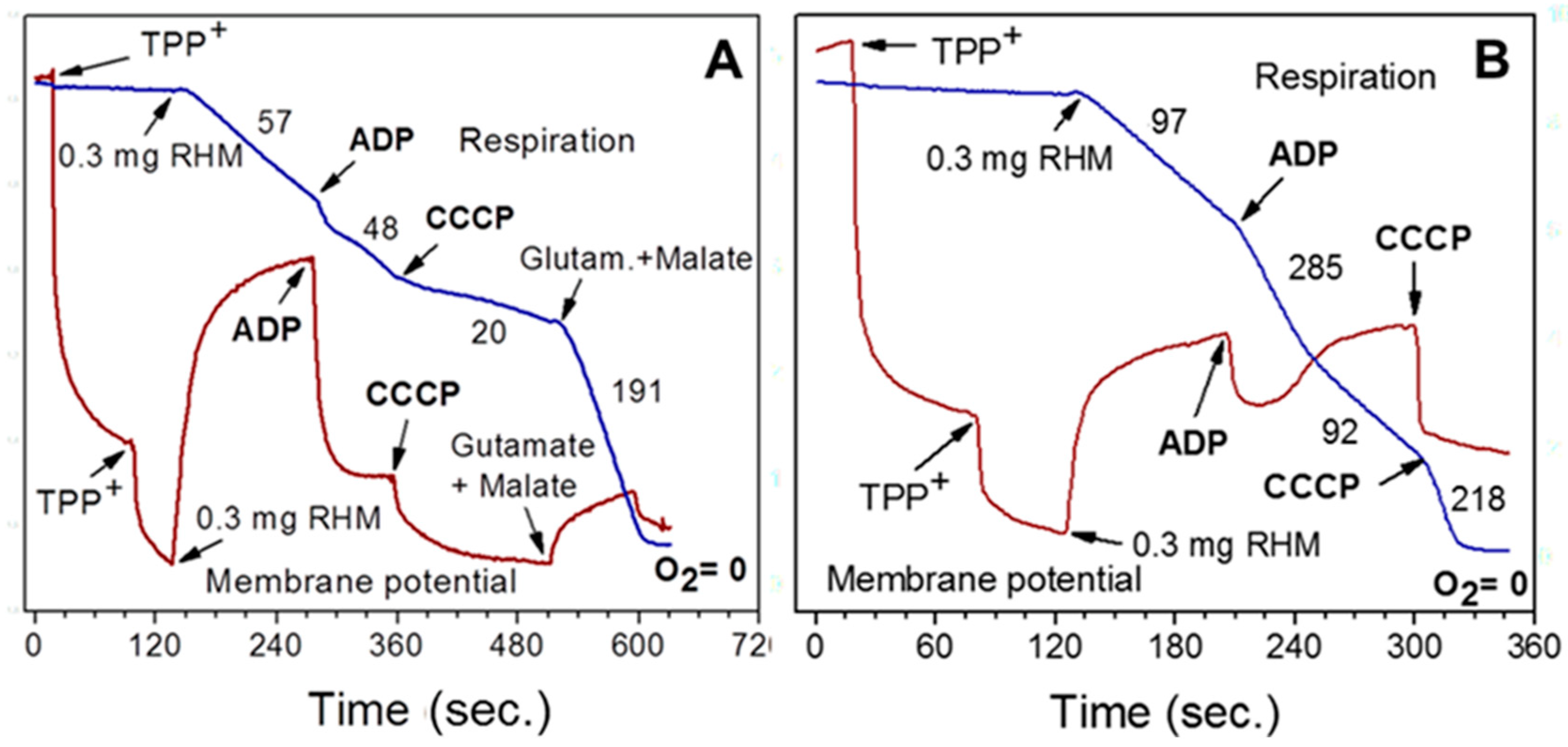
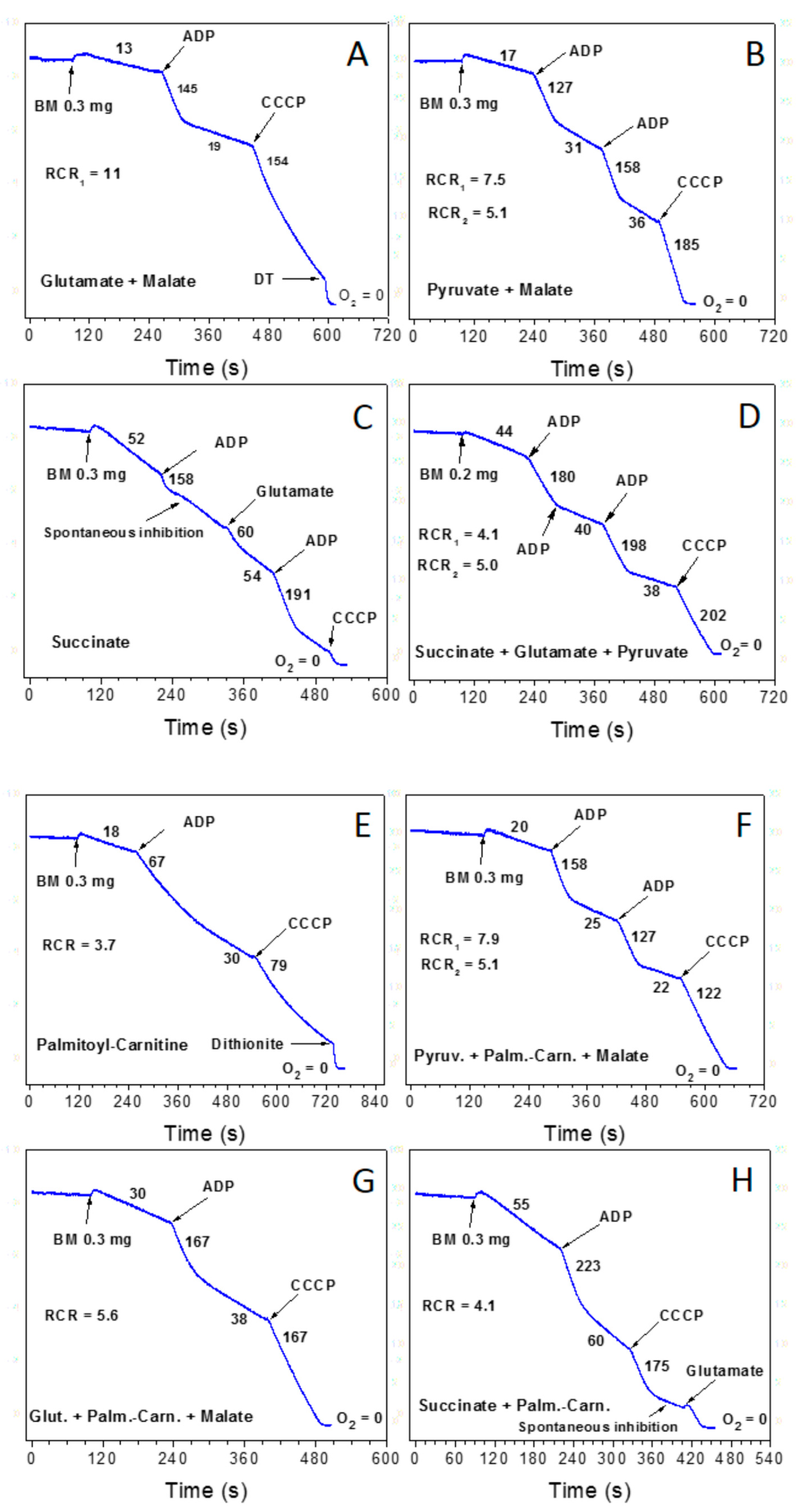
3.1. Importance of the Substrate Combinations for the In Vitro Experiments with Isolated Mitochondria
3.2. Endogenous Inhibition of Succinate Dehydrogenase (SDH)
4. Methodological Flaws That Negatively Impacted Metabolism Research
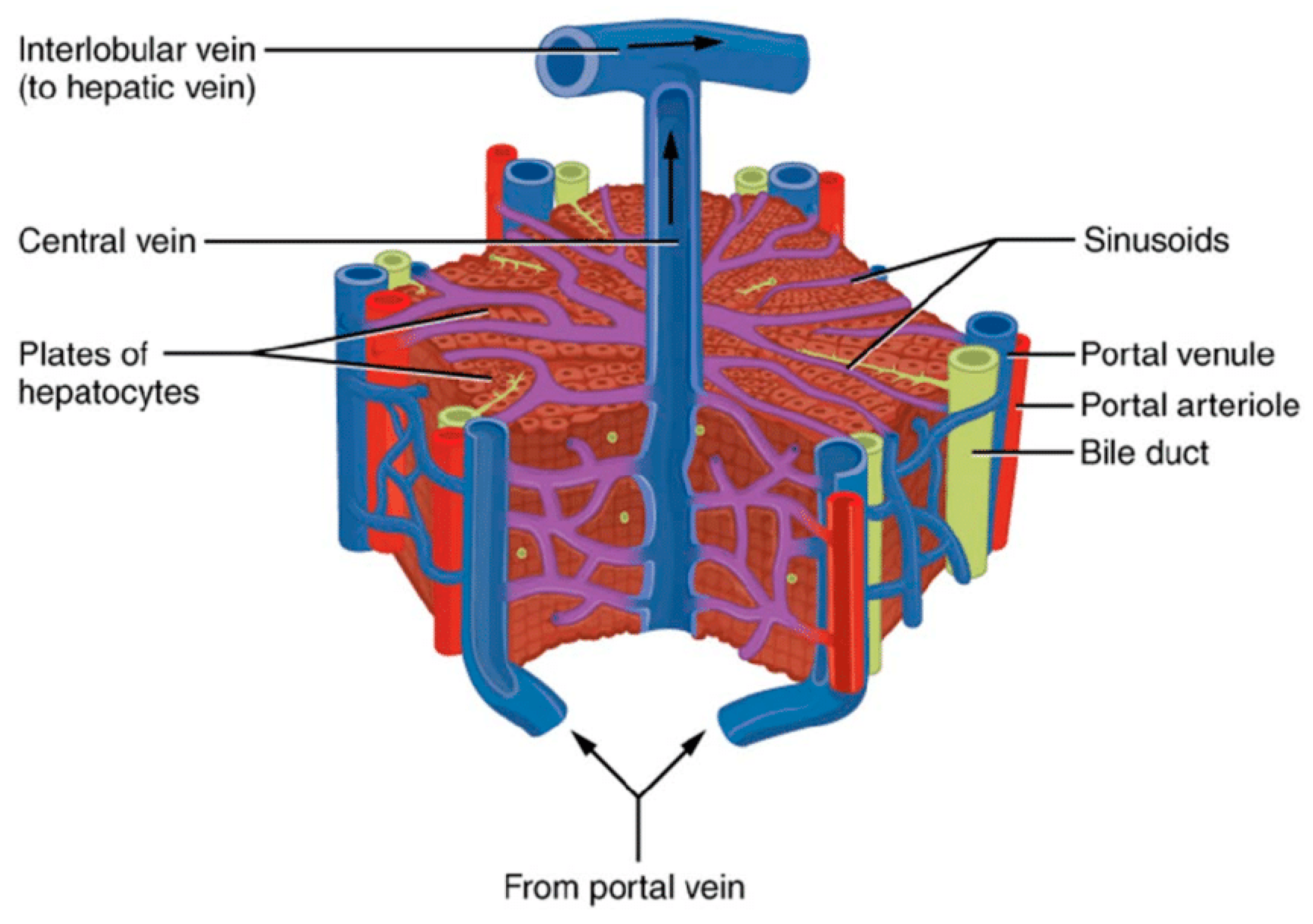
5. The Metabolic Properties of Liver Mitochondria Play Unique and Crucial Roles in the Liver's Metabolic States Distinct from Those of the Heart or Brain Mitochondria
5.1. The Absorption Period
5.2. The Postabsorptive or Fed Metabolic State
5.3. The Starved Metabolic State
5.4. Metabolic Liver Ischemia
6. Digestive Efficiency
7. Caloric and Metabolic Efficiencies of the Three Macronutrients.
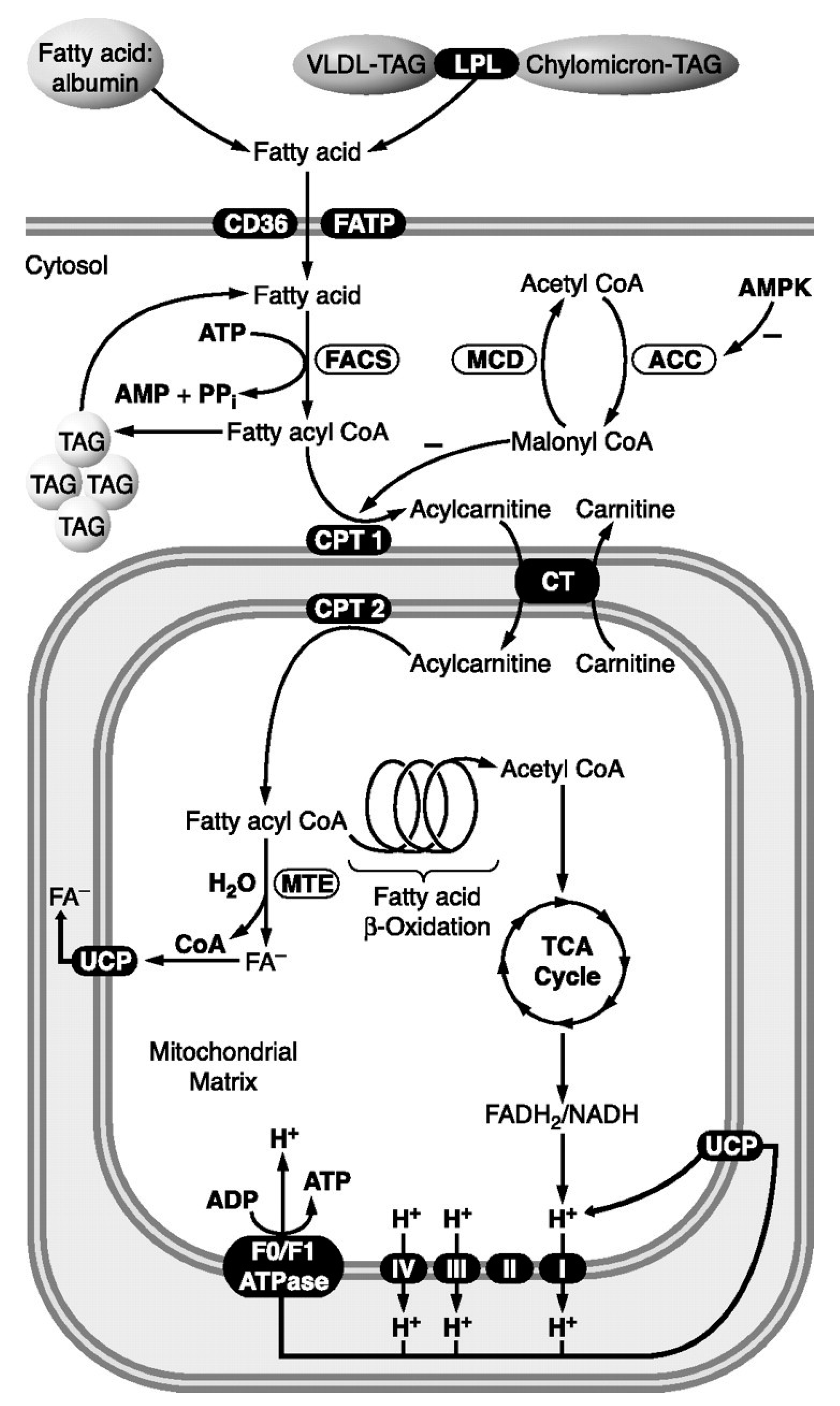
7.1. Structural and Functional Diversity of Fatty Acids
7.1.1. Long-Chain Fatty Acids (LCFAs).
7.1.2. Medium-Chain Fatty Acids (MCFAs)
7.1.3. Short-Chain Fatty Acids
7.1.4. Not Common Low Abundance Fatty Acids
7.1.5. Omega-oxidation of fatty acids in the endoplasmic reticulum primarily functions to hydroxylate and oxidize fatty acids to dicarboxylic acids to increase water solubility for excretion in the urine. This enzymatic conversion relies on the cytochrome P450 superfamily to catalyze this reaction between xenobiotic compounds and molecular oxygen [49]. Deficiencies in some enzymes of ω-fatty acid oxidation may result in their accumulation. Thus, up-regulation of ω-oxidation and increased serum and urine medium-chain dicarboxylic acids can diagnose certain deficiencies.
7.2. Amino acid reserves are practically absent and are formed continuously due to the digestion of food proteins, anaplerotic reactions in mitochondria, and degradation of proteins from the body’s dying cells.7.3. Carbohydrates
8. A Deeper Understanding of the Body’s Metabolism Requires Fundamental Paradigm Changes
8.1. Description of Old Paradigms.
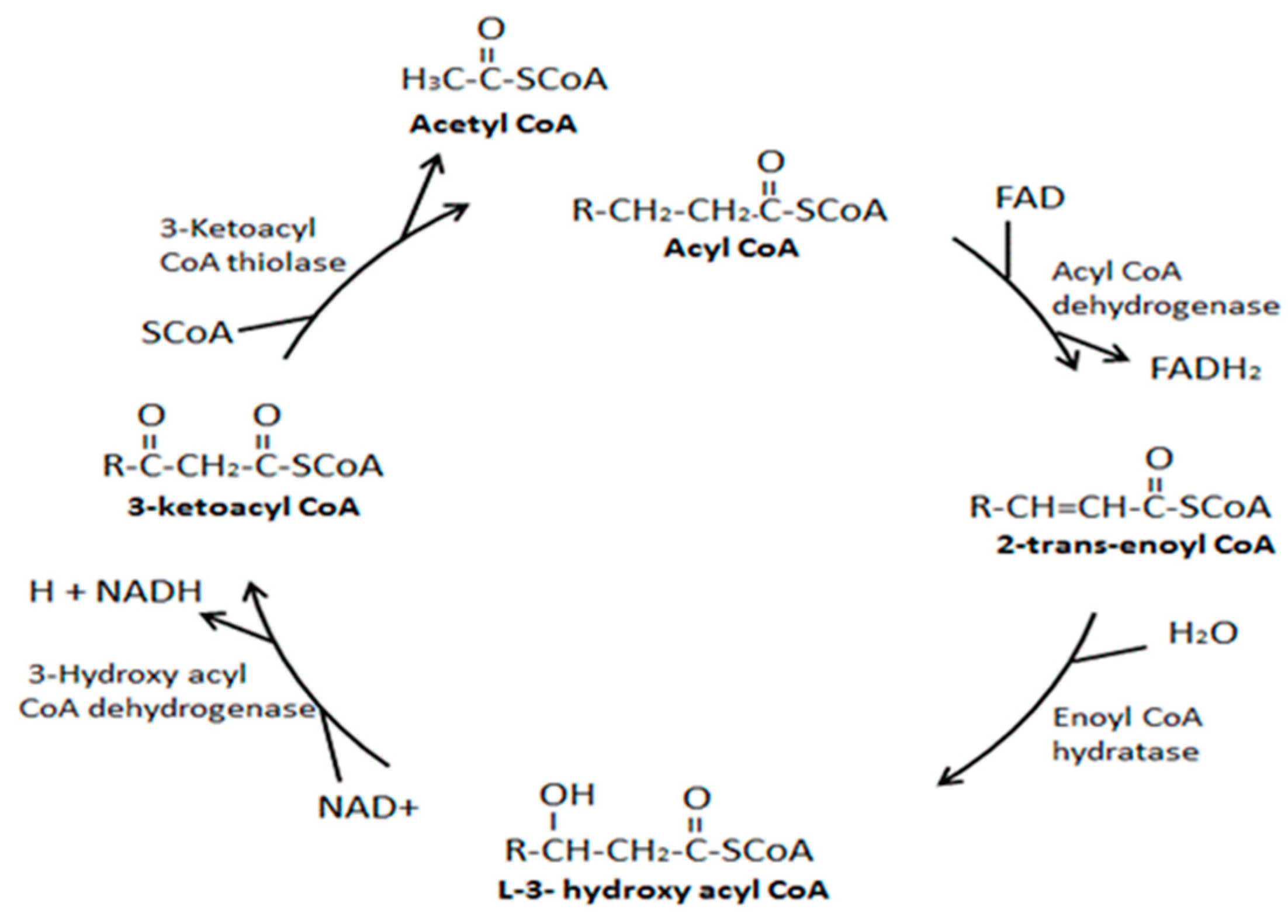
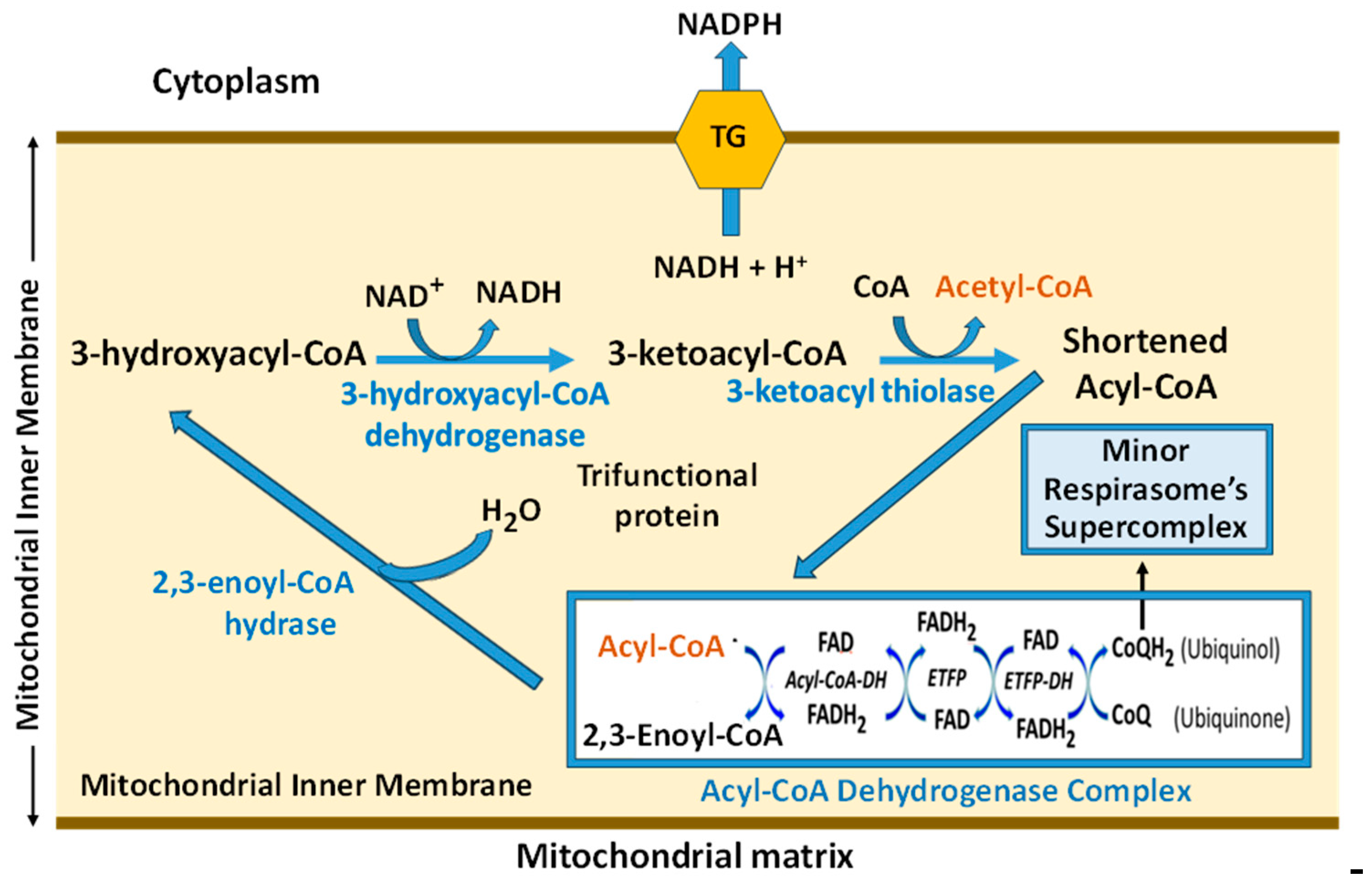
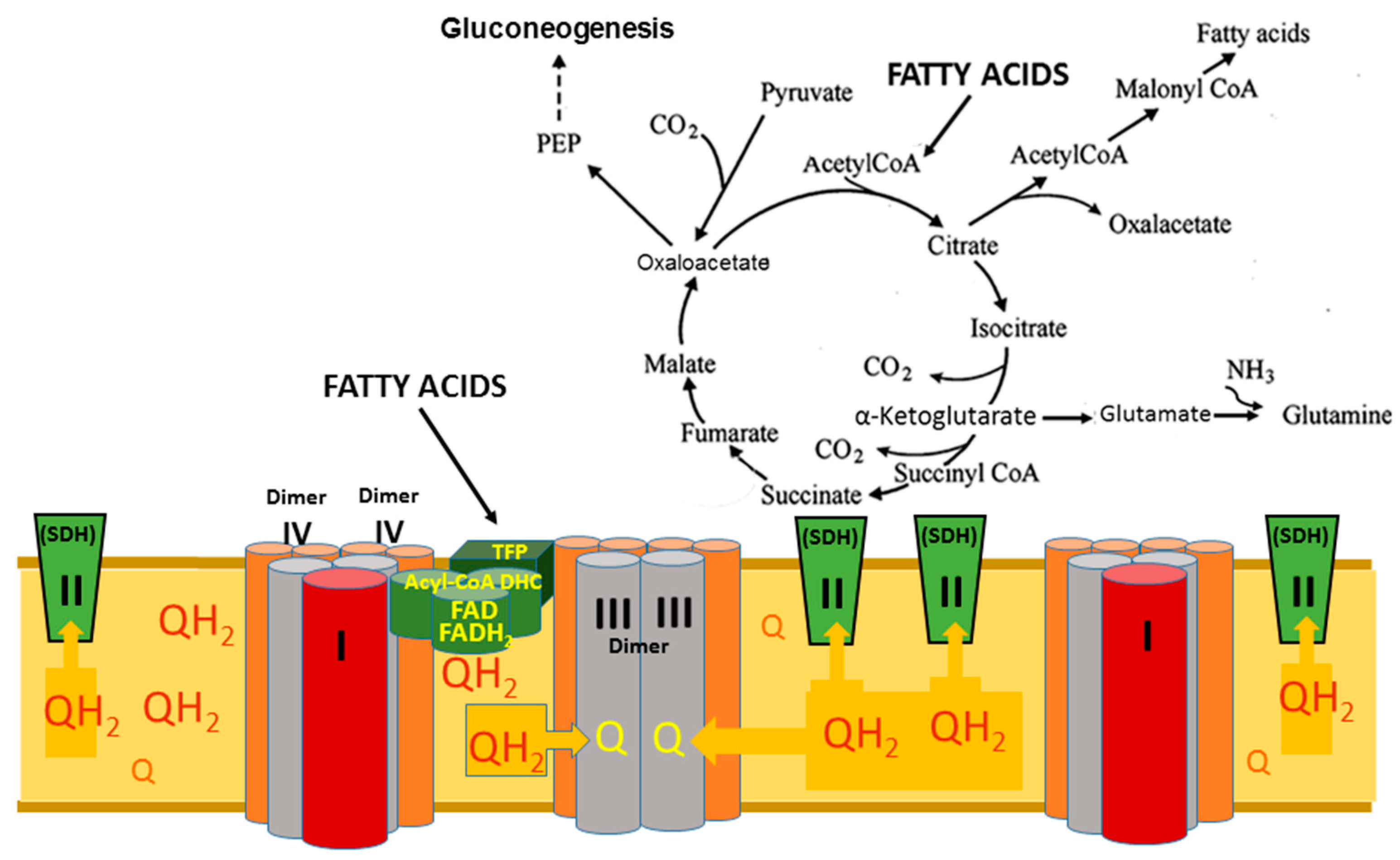
8.2. Superstructural Functional Organization of the Fatty Acid Metabolism
8.3. The Necessity of Substrates Alternative to Fatty Acids as Energy Sources
8.4. Metabolic Flexibility
9. Aerobic Glycolysis Products Lactate and Pyruvate Are Crucial Alternative Energy Sources
10. The Role of Fatty Acid Oxidation Products in Cell Synthesis, Signaling, and Homeostasis
11. Discussion and Final Remarks
12. Conclusion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Stanley, W.C.; Chandler, M.P. Energy metabolism in the normal and failing heart: potential for therapeutic interventions. Heart Failure Review 2002, 7.2, 115–130. [Google Scholar] [CrossRef] [PubMed]
- Wirthensohn, G.; Guder, W.G. Triacylglycerol metabolism in isolated rat kidney cortex tubules. Biochem. J. 1980, 186, 317–324. [Google Scholar] [CrossRef]
- Portincasa, P.L.; Bonfrate, M.; Vacca, M.; De Angelis, I. Farella, et al. Gut Microbiota and Short Chain Fatty Acids: Implications in Glucose Homeostasis. Int. J. Mol. Sci. 2022, 23, 1105. [Google Scholar] [CrossRef] [PubMed]
- McCay, C.M.; Crowell, M.F.; Maynard, L.A. The effect of retarded growth upon the length of life and upon the ultimate body size. J. Nutr. 1935, 10, 63–79. [Google Scholar] [CrossRef]
- Sohal, R.S.; Weindruch, R. Oxidative stress, caloric restriction, and aging. Science 1996, 273, 59–63. [Google Scholar] [CrossRef]
- Panov, A.V. Synergistic Oxidation of Fatty Acids, Glucose and Amino Acids Metabolites by Isolated Rat Heart Mitochondria. EC Cardiology. 2018, 5.1, 98–208. [Google Scholar]
- Panov, A.; Mayorov, V.I.; Dikalov, S. Metabolic syndrome and beta-oxidation of long-chain fatty acids in the brain, heart, and kidney Mitochondria. Int. J. Mol. Sci 2022, 23. [Google Scholar] [CrossRef]
- Panov, A.V.; Mayorov, V.I.; Dikalova, A.E.; Dikalov, S.I. Long-Chain and Medium-Chain Fatty Acids in Energy Metabolism of Murine Kidney Mitochondria. Int. J. Mol. Sci. 2023, 24, 379. [Google Scholar] [CrossRef] [PubMed]
- Panov, A.; Schonfeld, P.; Dikalov, D.; Hemendinger, R.; Bonkovsky, H.L.; Brooks, B.R. (2009). The Neuromediator Glutamate, through Specific Substrate Interactions, Enhances Mitochondrial ATP Production and Reactive Oxygen Species Generation in Nonsynaptic Brain Mitochondria. J. Biol. Chem. 2009, 284, 14448–14456. [Google Scholar] [CrossRef]
- Panov, A.; Orynbayeva, Z. Determination of mitochondrial metabolic phenotype through investigation of the intrinsic inhibition of succinate dehydrogenase. Analytical Biochemistry. 2018, 552, 30–37. [Google Scholar] [CrossRef]
- Panov, A.; Orynbayeva, Z.; Vavilin, V.; Lyakhovich, V. Fatty Acids in Energy Metabolism of the Central Nervous System. BioMed. Res. Intern 2014, Article ID 472459, 22 pages. [Google Scholar] [CrossRef]
- Panov, A.V. The Structure of the Cardiac Mitochondria Respirasome Is Adapted for the Oxidation of Fatty Acids. Int. J. Mol. Sci. 2024, 25, 2410. [Google Scholar] [CrossRef] [PubMed]
- Ballard, J.W.; Melvin, R.G.; Miller, J.T.; Katewa, S.D. Sex differences in survival and mitochondrial bioenergetics during aging in Drosophila. Aging Cell. 2007, 6, 699–708. [Google Scholar] [CrossRef] [PubMed]
- Vina, J.; Borras, C.; Gambini, J.; Sastre, J.; Pallardo, F.V. Why females live longer than males? Importance of the upregulation of longevity-associated genes by oestrogenic compounds. FEBS Lett. 2005, 579, 2541–2545. [Google Scholar] [CrossRef] [PubMed]
- Panov, A.V.; Darenskaya, M.A.; Dikalov, S.I.; Kolesnikov, S.I. Chapter 3. Metabolic syndrome as the first stage of eldership; the beginning of real aging. In: Gerontology, Ed. InTech, 2021, pp. 1–31.
- Panov, A. Practical Mitochondriology. Pitfalls and Problems in Studies of Mitochondria. 2015. ISBN 9781483963853, Lexington, KY. 236 pages.
- https: britishlivertrust.org.uk.
- Thompson, W. L. and T. Takebe. Human liver model systems in a dish. Dev. Growth Differ. 2021, 63, 47–58. [Google Scholar] [CrossRef]
- Hepatic Circulation. Physiology and Pathophysiology. W. Wayne Lautt. 2009. San Rafael (CA): Morgan & Claypool Life Sciences; 2009.
- Vavilin, V.A.; Filippova, S.N.; Panov, A.V.; Levandovsky, I.V. Mechanisms of disturbance of mitochondrial adenine nucleotide transport in the course of acute liver ischemia. Bulletin of Experimental Biology and Medicine 1980, 89, 444–447. [Google Scholar] [CrossRef]
- Brody T Nutritional Biochemistry (2nd ed.). Academic Press. 1999, 320. ISBN 978-0121348366.
- Panov, A.V.; Konstantinov, Yu.M.; Lyakhovich, V.V. The possible roles of palmitoyl-CoA in the regulation of the adenine nucleotides transport in mitochondria under different metabolic states. J. Bioenergetics 1975, 7, 75–85. [Google Scholar] [CrossRef]
- Panov, A.V.; Solov'ev, V.N.; Vavilin, V.A. Interstrain differences in organization of metabolic processes in the rat liver. I. The dynamics of changes in the contents of adenine nucleotides, glycogen and fatty acyl-CoAs in the course of short-term starvation in the livers of rats of Wistar, August and Wag strains. International J. Biochem. 1991, 23, 875–879. [Google Scholar]
- Rau, A.R. Biological scaling and physics. J. Biosci. 2002, 27, 475–478. [Google Scholar] [CrossRef]
- Wang, Z.; O'Connor, T.P.; Heshka, S.; Heymsfield, S.B. The reconstruction of Kleiber's law at the organ-tissue l-evel. J. Nutr. 2001, 131, 2967–2970. [Google Scholar] [CrossRef]
- Panov, A.V.; Filippova, S.N.; Lyakhovich, V.V. Adenine nucleotide translocase as a site of regulation by ADP of the rat liver mitochondrial permeability to protons and potassium ions. Archives Biochem. Biophys 1980, 199, 420–426. [Google Scholar] [CrossRef] [PubMed]
- Atkinson, D.E. The energy charge of the adenylate pool as a regulatory parameter. Interaction with feedback modifiers. Biochemistry, 1978, 7, 4030–3034. [Google Scholar] [CrossRef]
- Min, Y.; Ahn, D.; Truong, T.M.T.; Kim, M.; Heo, Y.; Jee, Y.; Son, Y.O.; Kang, I. Excessive sucrose exacerbates high fat diet-induced hepatic inflammation and fibrosis promoting osteoarthritis in mice model. J. Nutr. Biochem. 2023, 112, 109223. [Google Scholar] [CrossRef] [PubMed]
- Diaz-Aguila, Y.; Cuevas-Romero, E.; Castelan, E.F.; Martinez-Gomez, M.; Rodriguez-Antolin, J.; Nicolas-Toledo, L. Chronic stress and high sucrose intake cause distinctive morphometric effects in the adrenal glands of post-weaned rats. Biotech. Histochem. 2018, 93, 565–574. [Google Scholar] [CrossRef]
- Ugolev, A.M. Evolution of digestion and principles of function evolutions; Elements of modern functionalism. N.N. Iesuitova, Editor, Academy Science USSR, Division of Physiology, Leningrad: “Nauka”, 1985, 544 pages (Russian).
- Sensoy, I. A review on the food digestion in the digestive tract and the used in vitro models. Curr. Res. Food Sci. 2021, 4, 308–319. [Google Scholar] [CrossRef]
- Saladin, K.S. ; Human Anatomy, 5th Edition. 2017, McGraw-Hill Education, New York.
- Patricia JJ, Dhamoon AS. Physiology, Digestion. 2022 Sep 12. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2024 Jan–. [PubMed]
- Berezhnov, A.V.; “fed” otova, E.I.; Nenov, M.N.; Kasymov, V.A.; Pimenov, O.Y.; Dynnik, V.V. Dissecting Cellular Mechanisms of Long-Chain Acylcarnitines-Driven Cardiotoxicity: Disturbance of Calcium Homeostasis, Activation of Ca(2+)-Dependent Phospholipases, and Mitochondrial Energetics Collapse. Int. J. Mol. Sci. 2020, 21, 7461. [Google Scholar] [CrossRef] [PubMed]
- Panov, A.V.; Dikalov, S.I. Cardiolipin, Perhydroxyl Radicals and Lipid Peroxidation in Mitochondrial Dysfunctions and Aging. Oxid. Med. and Cellular Longevity 2020, 1323028. [Google Scholar] [CrossRef]
- Marten, B.; Pfeuffer, M.; Schrezenmeir, J. Medium-chain triglycerides. Int. Dairy J 2006, 16, 1374–1382. [Google Scholar] [CrossRef]
- Schonfeld, P.; Wojtczak, L. Short- and medium-chain fatty acids in energy metabolism: the cellular perspective. J. Lipid Res. 2016, 57, 943–954. [Google Scholar] [CrossRef]
- Aluko, R.E. Functional Foods and Nutraceuticals. 2012, Springer, New York. https://www.ncbi.nlm.nih.gov/pubmed/18511986. [CrossRef]
- Jadhav, H.B. Triglycerides of medium-chain fatty acids: a concise review. J Food Sci Technol. 2023, 60, 2143–2152. [Google Scholar] [CrossRef]
- Fan, L.; Zhu, X.; Chen, Q.; Huang, X.; Steinwandel, M.D.; Shrubsole, M.J.; Dai, Q. Dietary medium-chain fatty acids and risk of incident colorectal cancer in a predominantly low-income population: a report from the Southern Community Cohort Study. Am. J. Clin. Nutr. 2024, 119, 7–17. [Google Scholar] [CrossRef] [PubMed]
- Tan, J.; C. McKenzie, M. Potamitis, A. N. Thorburn, C. R. Mackay, and L. Macia. 2014. The role of short-chain fatty acids in health and disease. Adv. Immunol. 2014, 121, 91–119. [Google Scholar] [PubMed]
- Wong, J.M.; de Souza, R.; Kendall, C.W.; Emam, A.; Jenkins, D.J. Colonic health: fermentation and short chain fatty acids. J. Clin. Gastroenterol. 2006, 40, 235–243. [Google Scholar] [CrossRef]
- Kuksis, A. Biochemistry of Glycerolipids and Formation of Chylomicrons. In Christophe AB, DeVriese S (eds.). Fat Digestion and Absorption. 2000, The American Oil Chemists Society. p. 163. ISBN 978-1893997127.
- Beck, W.S.; Flavin, M. and Ochoa, S. Metabolism of propionic acid in animal tissues. III. Formation of succinate. J. Biol. Chem. 1957, 229, 997–1010. [Google Scholar] [CrossRef]
- Szrok-Jurga, S.; Czumaj, A.; Turyn, J.; Hebanowska, A.; Swierczynski, J.; Sledzinski, T.; Stelmanska, E. The Physiological and Pathological Role of Acyl-CoA Oxidation. Int. J. Mol. Sci 2023, 24. [Google Scholar] [CrossRef]
- Lopaschuk, G.D.; Ussher, J.R.; Folmes, C.D.; Jaswal, J.S.; Stanley, W.C. Myocardial fatty acid metabolism in health and disease. Physiol. Rev. 2010, 90, 207–258. [Google Scholar] [CrossRef] [PubMed]
- Schönfeld, P.; Reiser, G. Brain Lipotoxicity of Phytanic Acid and Very Long-chain Fatty Acids. Harmful Cellular/Mitochondrial Activities in Refsum Disease and X-Linked Adrenoleukodystrophy. Aging Dis. 2016, 7, 136–49. [Google Scholar] [CrossRef]
- Wanders, R.J.; Waterham, H.R.; Ferdinandusse, S. Metabolic Interplay between Peroxisomes and Other Subcellular Organelles Including Mitochondria and the Endoplasmic Reticulum. Front Cell. Dev. Biol. 2015, 3, 83. [Google Scholar] [CrossRef]
- Munro, A.W.; McLean, K.J.; Grant, J.L.; Makris, T.M. Structure and function of the cytochrome P450 peroxygenase enzymes. Biochem. Soc. Trans. 2018, 19, 183–196. [Google Scholar] [CrossRef]
- Kiens, B.; Roepstorff, C. Utilization of long-chain fatty acids in human skeletal muscle during exercise. Acta Physiol. Scand. 2003, 178, 391–396. [Google Scholar] [CrossRef]
- Nakrani, M.N.; Wineland, R.H.; Anjum, F. Physiology, Glucose Metabolism. 2023. StatPearls [Internet].
- Gerich, J.E.; Meyer, C.; Woerle, H.J.; Stumvoll, M. Renal gluconeogenesis. Its importance in human glucose homeostasis. Diabetes Care 2001, 24, 382–391. [Google Scholar] [CrossRef] [PubMed]
- Ebert, D.; Haller, R.G.; Walton, M.E. Energy contribution of octanoate to intact rat brain metabolism measured by13C nuclear magnetic resonance spectroscopy. J. Neurosci. 2003, 23, 5928–5935. [Google Scholar] [CrossRef] [PubMed]
- Houten, S.M.; Violante, S.; Ventura, F.V.; Wanders, R.J. The Biochemistry and Physiology of Mitochondrial Fatty Acid β-Oxidation and Its Genetic Disorders. Annu. Rev. Physiol. 2016, 78, 23–44. [Google Scholar] [CrossRef] [PubMed]
- de Carvalho, C.; Caramujo, M.J. The Various Roles of Fatty Acids. Molecules. 2018, 23, 2583. [Google Scholar] [CrossRef] [PubMed]
- Narayanan, B.; Xia, C.; McAndrew, R.; Shen, A.L.; Kim, J.P. Structural basis for expanded substrate specificities of human long chain acyl-CoA dehydrogenase and related acyl-CoA dehydrogenases. Sci. Rep. 2024, 2024 14, 12976. [Google Scholar] [CrossRef]
- Thorpe, C.; Kim, J.J. Structure and mechanism of action of the acyl-CoA dehydrogenases. FASEB J. 1995, 9, 718–725. [Google Scholar] [CrossRef]
- Battaile, K.P.; Molin-Case, J.; Paschke, R.; Wang, M.; Bennett, D.; et al. Crystal structure of rat short-chain acyl-CoA dehydrogenase complexed with acetoacetyl-CoA: comparison with other acyl-CoA dehydrogenases. J. Biol. Chem. 2002, 277, 12200–12207. [Google Scholar] [CrossRef]
- Eaton, S.; Bursby, T.; Middleton, B.; Pourfarzam, M.; Mills, K.; Johnson, A.W.; Bartlett, K. The mitochondrial trifunctional protein: centre of a beta-oxidation metabolon? Biochem. Soc. Trans. 2000, 28, 177–182. [Google Scholar] [CrossRef]
- Adeva-Andany, M.M.; Carneiro-Freire, N.; Seco-Filgueira, M.; Fernandez-Fernandez, C.; Mourino-Bayolo, D. Mitochondrial beta-oxidation of saturated fatty acids in humans. Mitochondrion. 2019, 46, 73–90. [Google Scholar] [CrossRef]
- Xia, C.; Fu, Z.; Battaile, K.P.; Kim, J.P. Crystal structure of human mitochondrial trifunctional protein, a fatty acid beta-oxidation metabolon. Proc. Natl. Acad. Sci. USA 2019, 116, 6069–6074. [Google Scholar] [CrossRef]
- Wang, Y.; Mohsen, A.W.; Mihalik, S.J.; Goetzman, E.S.; Vockley, J. Evidence for Physical Association of Mitochondrial Fatty Acid Oxidation and Oxidative Phosphorylation Complexes. J. Biol. Chem. 2010, 285, 29834–29841. [Google Scholar] [CrossRef] [PubMed]
- Brand, M.D. Mitochondrial generation of superoxide and hydrogen peroxide as the source of mitochondrial redox signaling. Free. Radic. Biol. Med. 2016, 100, 14–31. [Google Scholar] [CrossRef] [PubMed]
- Perevoshchikova, I.V.; Quinlan, C.L.; Orr, A.O.; Gerencser, A.A.; Brand, M.D. Sites of superoxide and hydrogen peroxide production during fatty acid oxidation in rat skeletal muscle mitochondria. Free Radic. Biol. Med. 2013, 61C, 298–309. [Google Scholar] [CrossRef] [PubMed]
- Shi, L.; Tu, B.P. "Acetyl-CoA and the regulation of metabolism: mechanisms and consequences. " Curr. Opin. Cell. Biol. 2015, 33, 125–131. [Google Scholar] [CrossRef]
- Wang, Y.; Yang, H.; Geerts, C.; Furtos, A.; Waters, P.; et al. The multiple facets of acetyl-CoA metabolism: Energetics, biosynthesis, regulation, acylation and inborn errors. Mol. Genet. Metab. 2023, 138, 106966. [Google Scholar] [CrossRef]
- Izzo, L.T.; Trefely, S.; Demetriadou, C.; Drummond, J.M.; Mizukami, T.; et al. Acetylcarnitine shuttling links mitochondrial metabolism to histone acetylation and lipogenesis. Sci. Adv. 2023, 9, eadf0115. [Google Scholar] [CrossRef]
- Gonzalez-Freire, M.; de Cabo, R.; Bernier, M.; Sollott, S.J.; Fabbri, E.; et al. Reconsidering the Role of Mitochondria in Aging. J. Gerontol. A. Biol. Sci. Med. Sci. 2015, 70, 1334–1342. [Google Scholar] [CrossRef]
- Schonfeld, P.; Wojtczak, A.B.; Geelen, M.J.H.; Kunz, W.; Wojtczak, L. On the mechanism of so-called uncoupling effect of medium and short-chain fatty acids. Biochim. Biophys. Acta, 1988, 936, 280–288. [Google Scholar] [CrossRef]
- Pellerin, L.; Magistretti, P.J. Glutamate uptake into astrocytes stimulates aerobic glycolysis: a mechanism coupling neuronal activity to glucose utilization. Proc. Natl. Acad. Sci. USA. 1994, 91, 10625–10629. [Google Scholar] [CrossRef]
- Pellerin, L.; Pellegri, G.; Bittar, P.G.; Charnay, Y. Bouras, et al. Evidence supporting the existence of an activity-dependent astrocyte-neuron lactate shuttle. Dev. Neurosci. 1998, 20, 291–299. [Google Scholar] [CrossRef]
- Storlien, L.; Oakes, N.D.; Kelley, D.E. Metabolic flexibility. Proc. Nutr. Soc. 2004, 63, 363–368. [Google Scholar] [CrossRef] [PubMed]
- Smith, R.L.; Soeters, M.R.; Wust, R.C.I.; Houtkooper, R.H. Metabolic Flexibility as an Adaptation to Energy Resources and Requirements in Health and Disease. Endocr. Rev. 2018, 39, 489–517. [Google Scholar] [CrossRef] [PubMed]
- Galgani, J.E.; Fernandez-Verdejo, R. Pathophysiological role of metabolic flexibility on metabolic health. Obes. Rev. 2021, 22, e13131. [Google Scholar] [CrossRef] [PubMed]
- Fernandez-Verdejo, R.; Galgani, J.E. Metabolic elasticity - a new trait associated with health? " Nat Rev. Endocrinol. 2023, 19, 689–690. [Google Scholar] [CrossRef]
- Goodpaster, B.H.; Sparks, L.M. Metabolic flexibility in health and disease. Cell Metab. 2017, 25, 1027–1036. [Google Scholar] [CrossRef] [PubMed]
- Brown-Borg, H.M.; Anderson, R.M. Metabolic adventures in aging research. Mol. Cell. Endocrinol. 2017, 455, 1–3. [Google Scholar] [CrossRef]
- Ahlborg, G.; Felig, P.; Hagenfeldt, L.; Hendler, R.; Wahren, J. Substrate turnover during prolonged exercise in man. Splanchnic and leg metabolism of glucose, free fatty acids, and amino acids. J. Clin. Invest. 1974, 53, 1080–1090. [Google Scholar] [CrossRef]
- Bergman, E.N. Energy contributions of volatile fatty acids from the gastrointestinal tract in various species. Physiol. Rev. 1990, 70, 567–590. [Google Scholar] [CrossRef]
- Hays, K.E.; Pfaffinger, J.M.; Ryznar, R. The interplay between gut microbiota, short-chain fatty acids, and implications for host health and disease. Gut Microbes. 2024, 16, 2393270. [Google Scholar] [CrossRef]
- Henderson, G.C.; Horning, M.A.; Wallis, G.A.; Brooks, G.A. Pyruvate metabolism in working human skeletal muscle. Am. J. Physiol. Endocrinol. Metab. 2007, 292, E366. [Google Scholar] [CrossRef]
- Schurr, A. Lactate: a major and crucial player in normal function of both muscle and brain. J. Physiol. 2008, 586, 2665–2666. [Google Scholar] [CrossRef] [PubMed]
- Brooks, G.A. Lactate as a fulcrum of metabolism. Redox Biol. 2020, 35, 101454. [Google Scholar] [CrossRef] [PubMed]
- Poole, D.C.; Rossiter, H.B.; Brooks, G.A.; Gladden, L.B. The anaerobic threshold: 50+ years of controversy. J. Physiol. 2021, 599, 737–767. [Google Scholar] [CrossRef] [PubMed]
- Brooks, G.A.; Curl, C.C.R.; Leija, R.G.; Osmond, A.D.; Duong, J.J.; Arevalo, J.A. Tracing the lactate shuttle to the mitochondrial reticulum. Exp. Mol. Med. 2022, 54, 1332–1347. [Google Scholar] [CrossRef] [PubMed]
- Gladden, L.B. Lactate metabolism: a new paradigm for the third millennium. J. Physiol. 2004, 558, 5–30. [Google Scholar] [CrossRef]
- Garcia, C.K.; Goldstein, J.L.; Pathak, R.K.; Anderson, R.G.; Brown, M.S. Molecular characterization of a membrane transporter for lactate, pyruvate, and other monocarboxylates: implications for the Cori cycle. Cell. 1994, 76, 865–873. [Google Scholar] [CrossRef] [PubMed]
- Bergman, B.C.; Tsvetkova, T.; Lowes, B.; Wolfel, E.E. Myocardial glucose and lactate metabolism during rest and atrial pacing in humans. J. Physiol. 2009, 587, 2087–2099. [Google Scholar] [CrossRef]
- Stanley, W.C.; Gertz, E.W.; Wisneski, J.A.; Neese, R.A.; Morris, D.L.; Brooks, G.A. Lactate extraction during net lactate release in legs of humans during exercise. J. Appl. Physiol. 1986, 60, 1116–1120. [Google Scholar] [CrossRef]
- Glenn, T.C.; Martin, N.A.; Horning, M.A.; McArthur, D.L.; Hovda, D.A.; et al. Lactate: brain fuel in human traumatic brain injury: a comparison with normal healthy control subjects. J. Neurotrauma. 2015, 32, 820–832. [Google Scholar] [CrossRef]
- Bergman, B.C.; Horning, M.A.; Casazza, G.A.; Wolfel, E.E.; Butterfield, G.E.; Brooks, G.A. Endurance training increases gluconeogenesis during rest and exercise in men. Am. J. Physiol. Endocrinol. Metab. 2000, 278, E244–E251. [Google Scholar] [CrossRef]
- Gerich, J.E.; Meyer, C.; Woerle, H.J.; Stumvol, l.M. Renal gluconeogenesis: its importance in human glucose homeostasis. Diabetes Care. 2001, 24, 382–391. [Google Scholar] [CrossRef] [PubMed]
- Donovan, C.M.; Brooks, G.A. Endurance training affects lactate clearance, not lactate production. Am. J. Physiol. 1983, 244, E83–E92. [Google Scholar] [CrossRef] [PubMed]
- Donovan, C.M.; Sumida, K.D. Training improves glucose homeostasis in rats during exercise via glucose production. Am. J. Physiol. 1990, 258, R770–R776. [Google Scholar] [CrossRef] [PubMed]
- Bergman, B.C.; Wolfel, E.E.; Butterfield, G.E.; Lopaschuk, G.D.; Casazza, G.A.; Horning, M.A.; Brooks, G.A. Active muscle and whole body lactate kinetics after endurance training in men. J. Appl. Physiol. 1999, 87, 1684–1696. [Google Scholar] [CrossRef]
- Pellerin, L.; Pellegri, G.; Bittar, P.G.; Charnay, Y.; Bouras; et al. Evidence supporting the existence of an activity-dependent astrocyte-neuron lactate shuttle. Dev. Neurosci. 1998, 20, 291–299. [Google Scholar] [CrossRef]
- Brooks, G.A.; Arevalo, J.A.; Osmond, A.D.; Leija, R.G.; Curl, C.C.; Tovar, A.P. Lactate in contemporary biology: a phoenix risen. J. Physiol. 2022, 600, 1229–1251. [Google Scholar] [CrossRef]
- Henderson, G.C.; Horning, M.A.; Lehman, S.L.; Wolfel, E.E.; Bergman, B.C.; Brooks, G.AS. (2004). "Pyruvate shuttling during rest and exercise before and after endurance training in men. J. Appl. Physiol. 1985, 97, 317–325. [Google Scholar] [CrossRef]
- Nath, S. Elucidating Events within the Black Box of Enzyme Catalysis in Energy Metabolism: Insights into the Molecular Mechanism of ATP Hydrolysis by F(1)-ATPase. Biomolecules. 2023, 13. [Google Scholar] [CrossRef] [PubMed]
- Rogatzki, M.J.; Ferguson, B.S.; Goodwin, M.L.; Gladden, L.B. Lactate is always the end product of glycolysis. Front. Neurosci. 2015, 9, 22. [Google Scholar] [CrossRef]
- Richardson, R.S.; Noyszewski, E.A.; Leigh, J.S.; Wagner, P.D. Lactate efflux from exercising human skeletal muscle: role of intracellular PO2. J. Appl. Physiol. 1998, 85, 627–634. [Google Scholar] [CrossRef]
- Prigogine, Ilya. Introduction to Thermodynamics of Irreversible Processes (Second ed.). 1961. New York: Interscience. OCLC 219682909.
- Roosterman, D.; Meyerhof, W.; Cottrell, G.S. Proton Transport Chains in Glucose Metabolism: Mind the Proton. Front. Neurosci. 2018, 12, 404. [Google Scholar] [CrossRef] [PubMed]
- Cassens, R.G.; Cooper, C.C. Red and white muscle. Adv. Food Res. 1971, 19, 1–74. [Google Scholar] [PubMed]
- Bender, T.; Martinou, J.C. The mitochondrial pyruvate carrier in health and disease: To carry or not to carry? Biochim. Biophys. Acta. 2016, 1863, 2436–2442. [Google Scholar] [CrossRef] [PubMed]
- van Meer, G.; Voelker, D.R.; Feigenson, G.W. Membrane lipids: where they are and how they behave. Nat Rev Mol Cell Biol. 2008, 9, 112–124. [Google Scholar] [CrossRef]
- Saini, R.K.; Keum, Y.S. Omega-3 and omega-6 polyunsaturated fatty acids: Dietary sources, metabolism, and significance - A review. Life Sci. 2018, 203, 255–267. [Google Scholar] [CrossRef]
- Kersten, S. The impact of fasting on adipose tissue metabolism. Biochim. Biophys. Acta. Mol. Cell. Biol. Lipids. 2023, 1868, 159262. [Google Scholar] [CrossRef]
- Pietrocola, F.; Galluzzi, L.; Bravo-San Pedro, J.M.; Madeo, F.; Kroemer, G. Acetyl coenzyme A: a central metabolite and second messenger. Cell. Metab. 2015, 21, 805–21. [Google Scholar] [CrossRef]
- Booker, S.J. Unraveling the pathway of lipoic acid biosynthesis. Chem Biol. 2004, 11, 10–12. [Google Scholar] [CrossRef]
- Secor, J.D.; Fligor, S.C.; Tsikis, S.T.; Yu, L.J.; Puder, M. Free Fatty Acid Receptors as Mediators and Therapeutic Targets in Liver Disease. Front. Physiol. 2021, 12, 656441. [Google Scholar] [CrossRef]
- Huang, L.; Gao, L.; Chen, C. Role of Medium-Chain Fatty Acids in Healthy Metabolism: A Clinical Perspective. Trends Endocrinol. Metab. 2021, 32, 351–366. [Google Scholar] [CrossRef]
- Crispo, F.; Condelli, V.; Lepore, S.; Notarangelo, T.; Sgambato, A.; et al. Metabolic Dysregulations and Epigenetics: A Bidirectional Interplay that Drives Tumor Progression. Cells. 2019, 30, 798. [Google Scholar] [CrossRef] [PubMed]
- Paul, S.; Ghosh, S.; Kumar, S. Tumor glycolysis, an essential sweet tooth of tumor cells. Semin. Cancer Biol. 2022, 86, 1216–1230. [Google Scholar] [CrossRef] [PubMed]
- Hardie, D. G. 100 years of the Warburg effect: a historical perspective. Endocr. Relat. Cancer. 2022, 29, T1–T13. [Google Scholar] [CrossRef] [PubMed]
- Schagger, H. Respiratory chain supercomplexes. IUBMB Life, 2001, 52, 119–128. [Google Scholar] [CrossRef]
- Schagger, H.; Pfeiffer, K. Supercomplexes in the respiratory chains of yeast and mammalian mitochondria. EMBO J. 2000, 19, 1777–1783. [Google Scholar] [CrossRef]
- Brooks, G.A.; Mercier, J. Balance of carbohydrate and lipid utilization during exercise: the "crossover" concept. J. Appl. Physiol. 1994, 76, 2253–2261. [Google Scholar] [CrossRef]
- Panov, A.; Dikalov, S.; Shalbuyeva, N.; Hemendinger, R.; Greenamyre, J.T.; Rosenfeld, J. Species- and tissue-specific relationships between mitochondrial permeability transition and generation of ROS in brain and liver mitochondria of rats and mice. Am. J. Physiol. Cell. Physiol. 2007, 292, C708–C718. [Google Scholar] [CrossRef]
- Chretien, D.; Benit, P.; Ha, H. H.; Keipert, S.; El-Khoury, R.; et al. Mitochondria are physiologically maintained at close to 50 degrees C. PLoS Biol. 2018, 16, e2003992. [Google Scholar] [CrossRef]
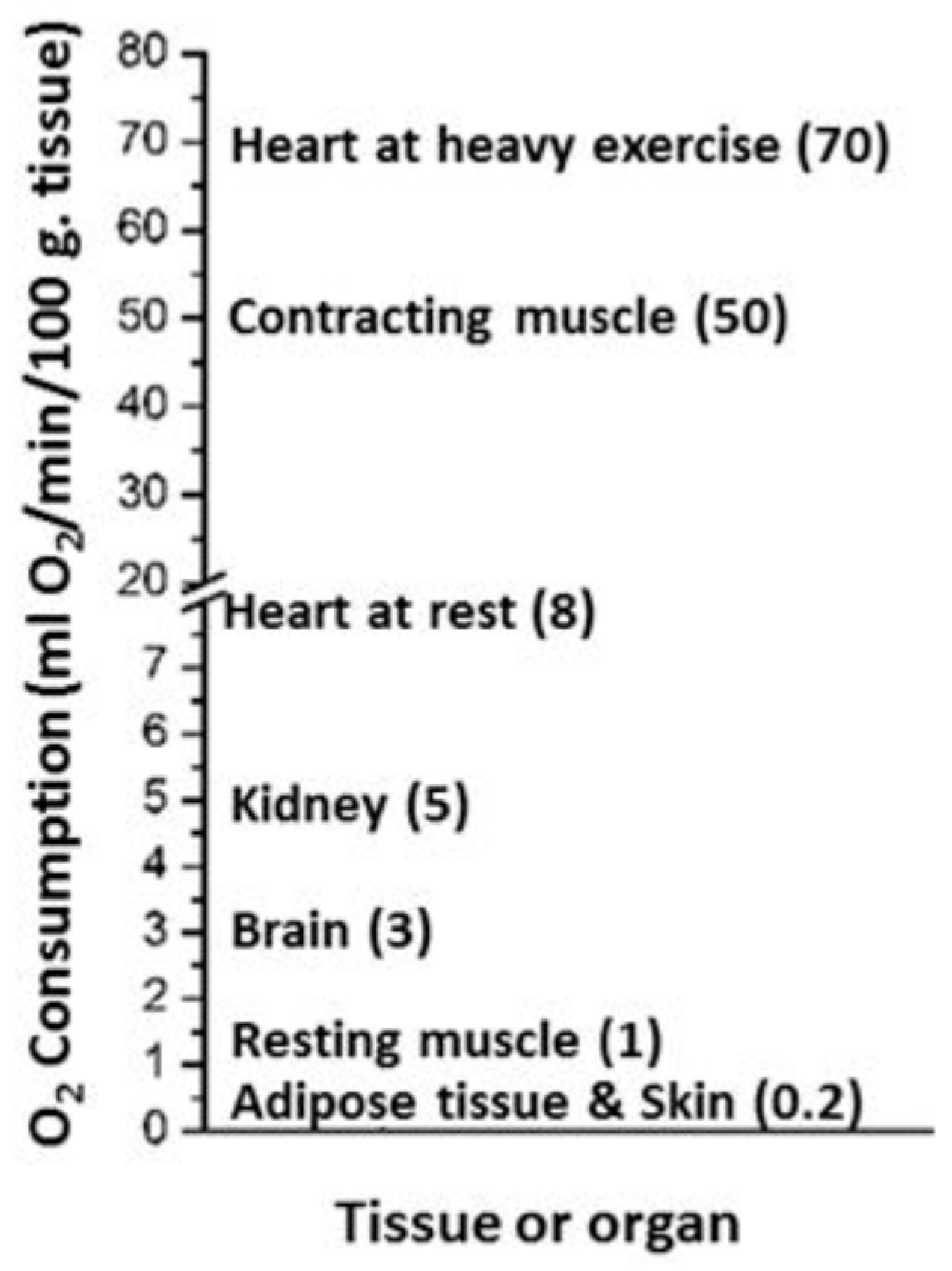
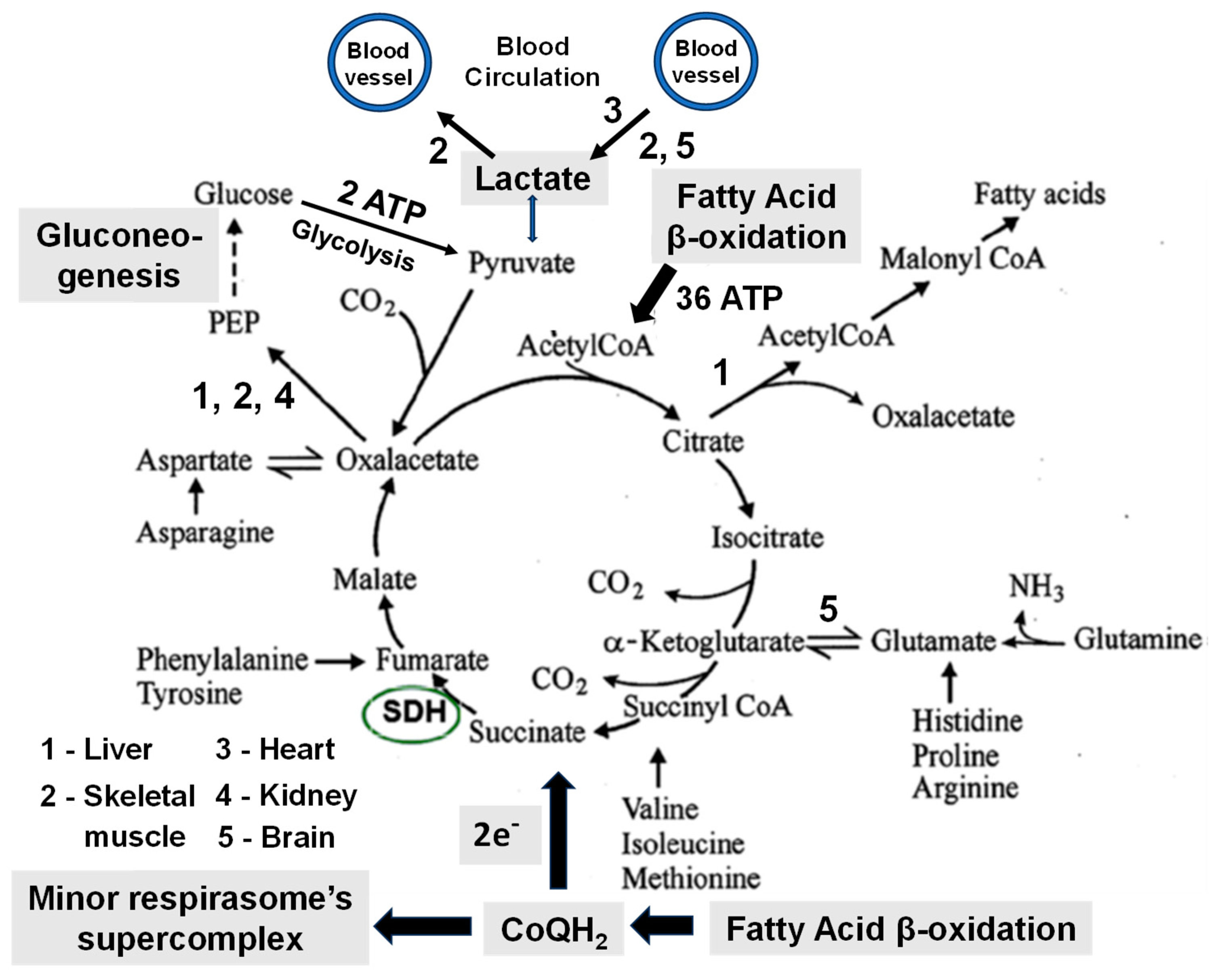
| Source of Energy (Caloric value– kcal/g) |
Storage amount (time of consumption) |
|---|---|
|
Carbohydrates: Blood glucose & Glycogen in the liver (CV = 3.81) Amino acids *(CV = 3.12) Acyl Fatty Acids (CV = 9.3) |
Total 4-5 grams (20-30 min) 100-120 grams (1-3 hrs.) Released during catabolism of food, damaged tissue proteins, and anaplerotic reactions. The content is highly dynamic. Fat storage kilograms, consumption duration in days |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).




