Submitted:
24 October 2024
Posted:
24 October 2024
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Materials and Methods
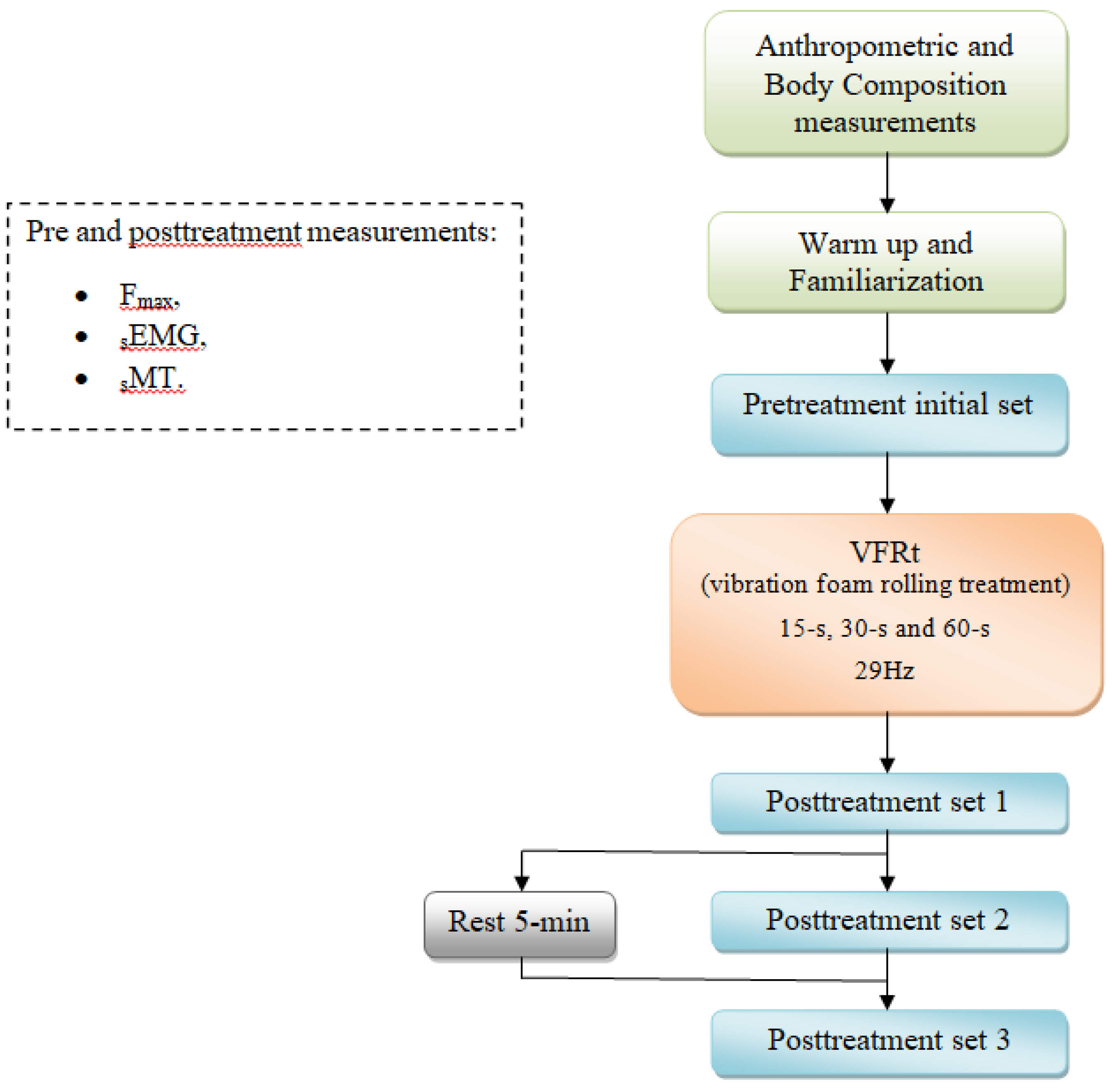
2.1. Experimental Approach to the Problem
2.2. Subjects
2.3. Procedures
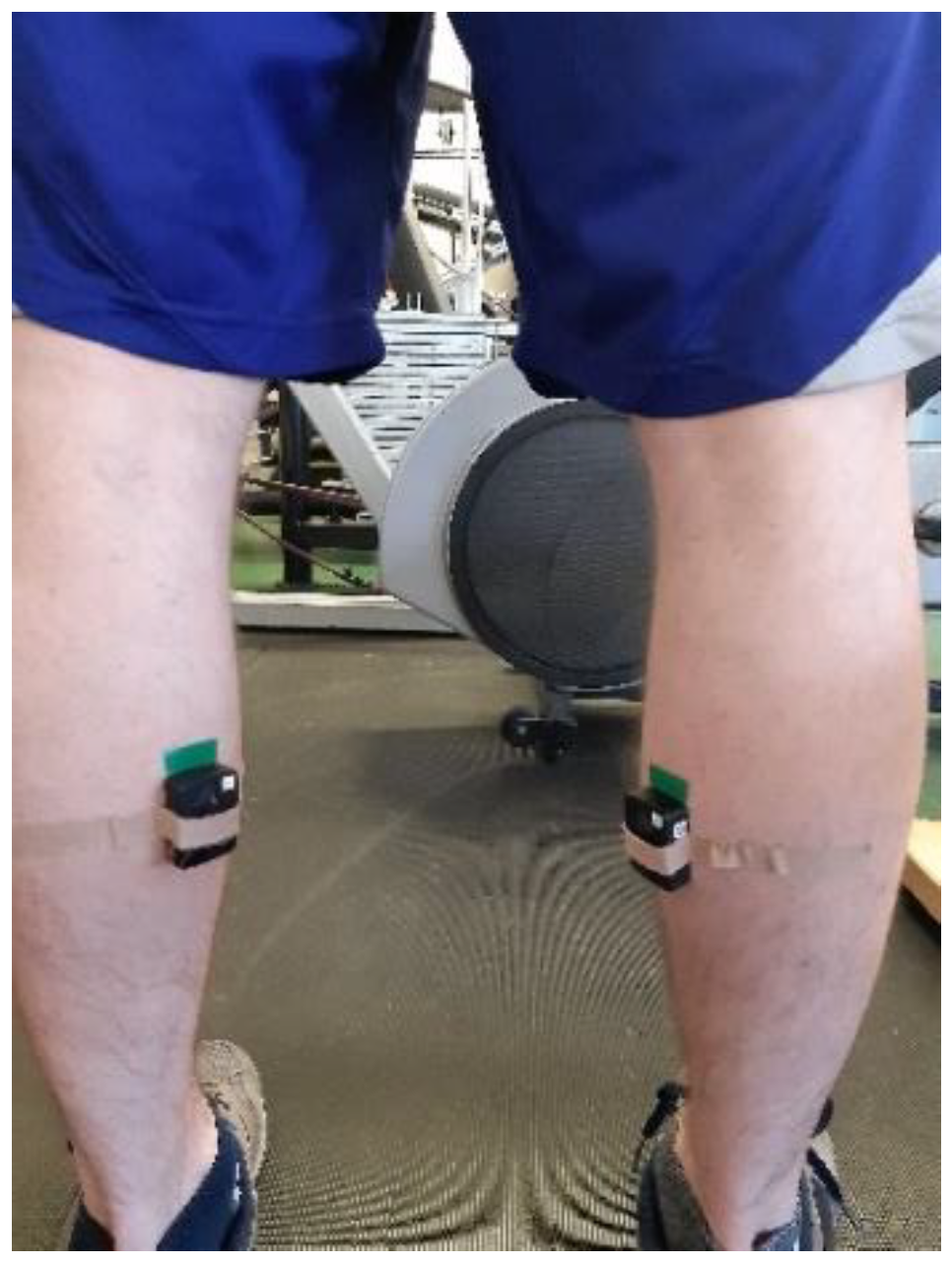
2.4. Electromyography and Temperature Outputs from MVIC Heel Rise
2.5. VFRt Protocol
2.6. Variables
2.7. Statistical Analysis
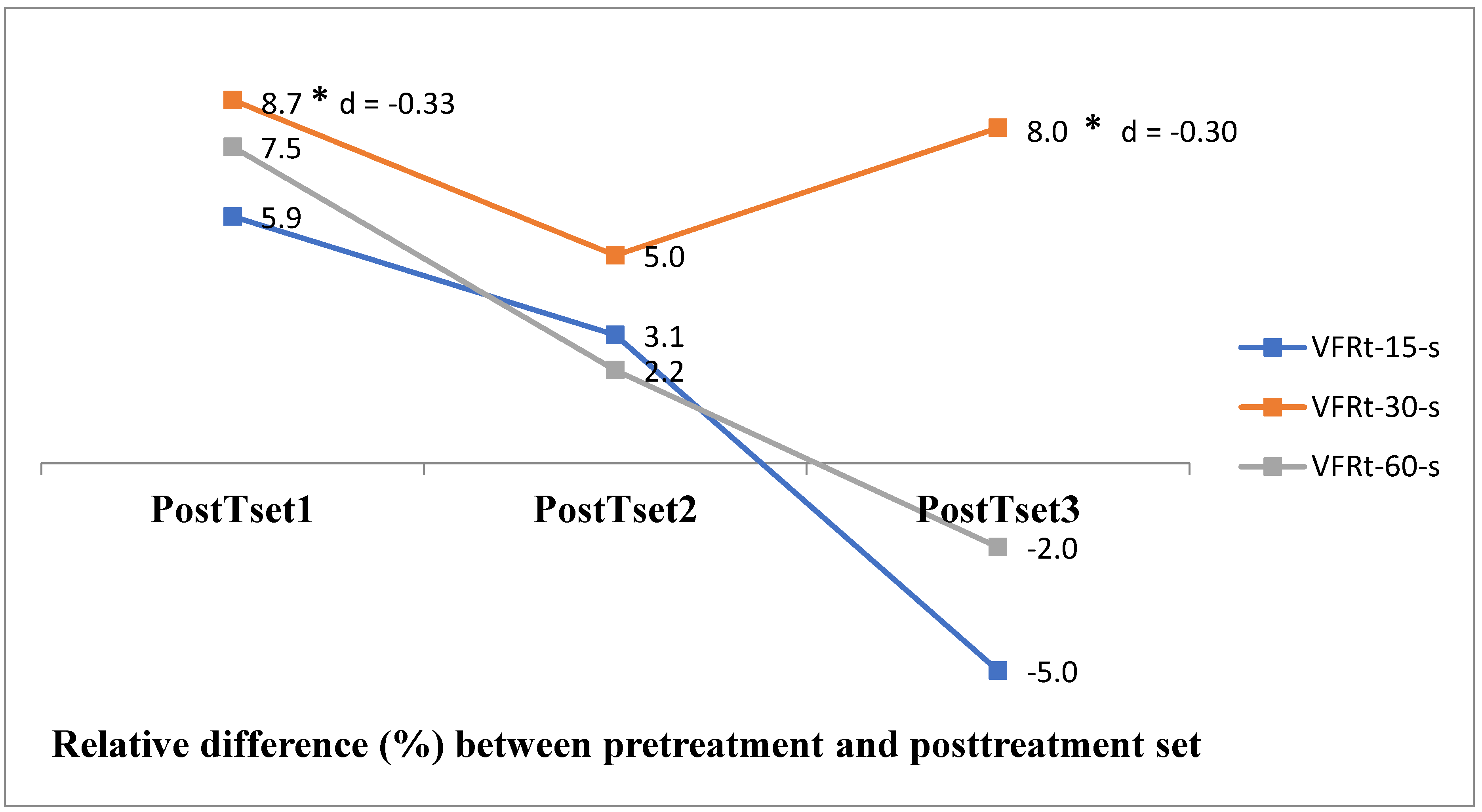
3. Results
4. Discussion
5. Conclusions
Author Contributions
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Murray, A.; Jones, T.; Horobeanu, C.; Turner, P.; Sproule, J. Sixty Seconds of Foam Rolling Does Not Affect Functional Flexibility or Change Muscle Temperature in Adolescent Athletes. Int J Sports Phys Ther, 2016, 11, 765–776. [Google Scholar] [PubMed]
- Wiewelhove, T.; Döweling, A.; Schneider, C.; Hottenrott, L.; Meyer, T.; Kellmann, M.; Pfeiffer, M.; Ferrauti, A. A meta-analysis of the effects of foam rolling on performance and recovery. Front Physiol 2019. [Google Scholar] [CrossRef] [PubMed]
- Cavanaugh, T.; Döweling, A.; Young, D.; Quigley, J.; Hodgson, D. , Whitten, H.; Behm, G. An acute session of roller massage prolongs voluntary torque development and diminishes evoked pain. Eur. J Appl. Physiol, 2017, 117, 109–117. [Google Scholar] [CrossRef] [PubMed]
- Borisavljević, A.; Kukić, F.; Janković, G.; Ćosić, M.; Dopsaj, M. Acute effects of vibration foam rolling on the explosive strength properties of the plantar flexors during maximal isometric contraction. IES 2023. [Google Scholar] [CrossRef]
- Esmaeilzadeh, S.; Akpinar, M.; Polat, S.; Yildiz, A.; Oral, A. The effects of two different frequencies of whole-body vibration on knee extensors strength in healthy young volunteers: a randomized trial. J Musculoskelet Neuronal Interact, 2015, 15, 333–340. [Google Scholar]
- Silva, E.; Nubi, M.; Vaamonde, D.; Fernandez, J.; Garcia-Manso, M.; Lancho, L. Effects of different frequencies of whole body vibration on muscular performance. Biol. Sport, 2006, 23, 124–132. [Google Scholar]
- Luo, J.; McNamara, B.; Moran, K. The Use of Vibration Training to Enhance Muscle Strength and Power. Sports medicine (Auckland, N.Z.), 2005, 35. [Google Scholar] [CrossRef]
- Ranatunga, W.; Sharpe, B.; Turnbull, B. Contractions of a human skeletal muscle at different temperatures. J Physiol, 1987, 390, 383–395. [Google Scholar] [CrossRef]
- Okamoto, T.; Masuhara, M.; Ikuta, K. Acute effects of self-myofascial release using a foam roller on arterial function. J Strength Cond Res, 2014, 28, 69–73. [Google Scholar] [CrossRef] [PubMed]
- Kerschan-Schindl, K.; Grampp, S.; Henk, C.; Resch, H.; Preisinger, E.; Fialka-Moser, V.; Imhof, H. Whole-body vibration exercise leads to alterations in muscle blood volume. Clin Physiol, 2001, 21, 377–382. [Google Scholar] [CrossRef] [PubMed]
- Schroeder, J.; Wilke, J.; Hollander, K. Effects of Foam Rolling Duration on Tissue Stiffness and Perfusion: A Randomized Cross-Over Trial. J Sports Sci Med, 2021, 20, 626–634. [Google Scholar] [CrossRef] [PubMed]
- Halperin, I.; Aboodarda, J.; Button, C.; Andersen, L. , Behm, G. Roller massager improves range of motion of plantar flexor muscles without subsequent decreases in force parameters. Int J Sports Phys Ther, 2014, 9, 92–102. [Google Scholar] [PubMed]
- Bradbury-Squires, J.; Noftall, C.; Sullivan, M.; Behm, G.; Power, E.; Button, C. Roller-massager application to the quadriceps and knee-joint range of motion and neuromuscular efficiency during a lunge. J Athl Train, 2015, 50, 133–140. [Google Scholar] [CrossRef] [PubMed]
- Reiner, M.; Glashüttner, C.; Bernsteiner, D.; Tilp, M.; Guilhem, G.; Morales-Artacho, A.; Konrad, A. A comparison of foam rolling and vibration foam rolling on the quadriceps muscle function and mechanical properties. Eur J Appl Physiol, 2021, 121, 1461–1471. [Google Scholar] [CrossRef] [PubMed]
- Phillips, J.; Diggin, D.; King, L.; Sforzo, A. Effect of Varying Self-myofascial Release Duration on Subsequent Athletic Performance. J Strength Cond Res, 2021, 35, 746–753. [Google Scholar] [CrossRef]
- Dos Anjos, F.; Pinto, P.; Cerone, L.; Gazzoni, M.; Vieira, M. Is the attenuation effect on the ankle muscles activity from the EMG biofeedback generalized to - or compensated by - other lower limb muscles during standing? J Electromyogr Kinesiol, 2022, 67. [Google Scholar] [CrossRef]
- Dopsaj, M.; Kukić, F.; Đorđević-Nikić, M.; Koropanovski, N.; Radovanović, D.; Miljuš, D.; Subošić, D.; Tomanić, M.; Dopsaj, V. Indicators of Absolute and Relative Changes in Skeletal Muscle Mass during Adulthood and Ageing. Int J Environ Res Public Health, 2020, 17. [Google Scholar] [CrossRef] [PubMed]
- Williams, R. The Declaration of Helsinki and public health. Bull World Health Organ, 2008, 86, 650–652. [Google Scholar] [CrossRef]
- Majstorović, N.; Nešić, G.; Grbić, V.; Savić, Z.; Živković, M.; Aničić, Z.; Marković, S.; Dopsaj, M. Reliability of a simple novel field test for the measurement of plantar flexor muscle strength. Rev. Bras. Med. Esporte, 2021, 27. [Google Scholar] [CrossRef]
- Hermens, H.; Freriks, B.; Disselhorst-Klug, C.; Rau, G. Development of recommendations for SEMG sensors and sensor placement procedures. J Electromyogr Kinesiol, 2000, 10. [Google Scholar] [CrossRef]
- Sullivan, M.; Feinn, R. Using Effect Size—or Why the P Value Is Not Enough. J Grad Med Educ, 2012, 4. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, M.; Sato, S.; Kiyono, R.; Yoshida, R.; Murakami, Y.; Yasaka, K.; Yahata, K.; Konrad, A. Acute Effect of Vibration Roller With and Without Rolling on Various Parts of the Plantar Flexor Muscle. Front Physiol, 2021, 12. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, M.; Sato, S.; Kiyono, R.; Yoshida, R.; Yasaka, K.; Yahata, K.; Konrad, A. Comparison Between Foam Rolling With and Without Vibration on Passive and Active Plantar Flexor Muscle Properties. J Strength Cond Res, 2021, 1. [Google Scholar] [CrossRef] [PubMed]
- Lee, L.; Chu, H.; Lyu, J.; Chang, D.; Chang, J. Comparison of vibration rolling, nonvibration rolling, and static stretching as a warm-up exercise on flexibility, joint proprioception, muscle strength, and balance in young adults. J Sports Sci, 2018, 36, 2575–2582. [Google Scholar] [CrossRef] [PubMed]
- Arroyo-Morales, M.; Olea, N.; Martínez, M.; Hidalgo-Lozano, A.; Ruiz-Rodríguez, C. , Díaz-Rodríguez, L. Psychophysiological effects of massage-myofascial release after exercise: a randomized sham-control study. J Altern Complement Med, 2008, 14, 1223–1229. [Google Scholar] [CrossRef] [PubMed]
- Rodrigues, P.; Trajano, S.; Stewart, B.; Minett, M. Potential role of passively increased muscle temperature on contractile function. Eur J Appl Physiol, 2022, 122. [Google Scholar] [CrossRef] [PubMed]
- Pearson, J. , Low, A.; Stöhr, E.; Kalsi, K.; Ali, L.; Barker, H.; González-Alonso, J. Hemodynamic responses to heat stress in the resting and exercising human leg: insight into the effect of temperature on skeletal muscle blood flow. Am J Physiol Regul Integr Comp Physiol, 2011, 300, 663–673. [Google Scholar] [CrossRef] [PubMed]
- Heinonen, I.; Brothers, M.; Kemppainen, J.; Knuuti, J.; Kalliokoski, K.; Crandall, G. Local heating, but not indirect whole body heating, increases human skeletal muscle blood flow. J Appl Physiol, 1985, 111, 818–824. [Google Scholar] [CrossRef]
- Hafen, S.; Abbott, K.; Bowden, J.; Lopiano, R.; Hancock, R.; Hyldahl, D. Daily heat treatment maintains mitochondrial function and attenuates atrophy in human skeletal muscle subjected to immobilization. J Appl Physiol, 1985, 127, 47–57. [Google Scholar] [CrossRef]
- Fan, W.; Evans, M. Exercise Mimetics: Impact on Health and Performance. Cell Metab, 2017, 25. [Google Scholar] [CrossRef]
- Hunt, P.; Minett, M.; Gibson, R.; Kerr, K. Stewart, B. Could Heat Therapy Be an Effective Treatment for Alzheimer's and Parkinson's Diseases? A Narrative Review. Front Physiol, 2019, 10. [Google Scholar] [CrossRef]
- Macgregor, L.; Fairweather, M.; Bennett, R.; Hunter, A. The Effect of Foam Rolling for Three Consecutive Days on Muscular Efficiency and Range of Motion. Sports Med Open, 2018, 4. [Google Scholar] [CrossRef] [PubMed]
- Chambers, J.; Cham, R. Slip-related muscle activation patterns in the stance leg during walking. Gait Posture, 2007, 25, 565–572. [Google Scholar] [CrossRef] [PubMed]
- Harwood, B.; Scherer, J.; Brown, E.; Cornett, M.; Kenno, A. , Jakobi, M. Neuromuscular responses of the plantar flexors to whole-body vibration. Scand J Med Sci Sports, 2017, 27, 1569–1575. [Google Scholar] [CrossRef] [PubMed]
- MacDonald, Z.; Penney, D.; Mullaley, E.; Cuconato, L. , Drake, D.; Behm, G.; Button, C. An acute bout of self-myofascial release increases range of motion without a subsequent decrease in muscle activation or force. J Strength Cond Res, 2013, 27, 812–821. [Google Scholar] [CrossRef]
- Nakamura, M.; Konrad, A.; Ryosuke, K.; Sato, S.; Yahata, K.; Yoshida, R.; Murakami, Y.; Sanuki, F.; Wilke, J. Sex Differences in the Mechanical and Neurophysiological Response to Roller Massage of the Plantar Flexors. J Sports Sci Med, 2021, 20, 665–671. [Google Scholar] [CrossRef]
- Martinez-Valdes, E.; Negro, F.; Falla, D.; De Nunzio, M.; Farina, D. Surface electromyographic amplitude does not identify differences in neural drive to synergistic muscles. J Appl Physiol, 2018, 124, 1071–1079. [Google Scholar] [CrossRef]
- Vieira, M.; Bisi, C.; Stagni, R.; Botter, A. Changes in tibialis anterior architecture affect the amplitude of surface electromyograms. J Neuroeng Rehabil, 2017, 14. [Google Scholar] [CrossRef]
- Basmajian, V. Muscles Alive—their functions revealed by electromyography. Postgrad Med J, 1963, 39, 162. [Google Scholar]
- Fukuda, Y.; Echeimberg, O.; Pompeu, E.; Lucareli, G.; Garbelotti, S.; Gimenes, O.; Apolinário, A. Root Mean Square Value of the Electromyographic Signal in the Isometric Torque of the Quadriceps, Hamstrings and Brachial Biceps Muscles in Female Subject. J App Res, 2010, 10, 32–39. [Google Scholar]


| Testing | Experimental session | ||||
| PreTset | Fmax _15-s | Fmax_30-s | Fmax_60-s | Wilks' Lambda | p |
| Mean ± SD | 3569 ± 373 | 3651 ± 484 | 3672 ± 620 | 0.93 | 0.57 |
| cV% | 10.4 | 13.2 | 16.8 | ||
| VFRt-15-s | VFRt-30-s | VFRt-60-s | |||
| PostTset1 | 3513 ± 366 | 3585 ± 441 | 3593 ± 571 | 0.94 | 0.61 |
| 10.4 | 12.3 | 15.9 | |||
| PostTset2 | 3509 ± 421 | 3560 ± 474 | 3574 ± 537 | 0.96 | 0.74 |
| 12.0 | 13.3 | 15.0 | |||
| PostTset3 | 3506 ± 368 | 3607 ± 557 | 3570 ± 588 | 0.94 | 0.63 |
| 10.5 | 15.4 | 16.4 | |||
| Wilks' Lambda | 0.94 | 0.85 | 0.84 | ||
| p | 0.82 | 0.48 | 0.46 | ||
| Testing | Experimental session | ||||
| sMT-bfr-GW | sMT_15-s | sMT_30-s | sMT_60-s | Wilks' Lambda | p |
| Mean ± SD | 35.62 ± 0.32 | 35.72 ± 0.49 | 35.81 ± 0.48 | 0.85 | 0.24 |
| cV% | 0.9 | 1.4 | 1.3 | ||
| sMT-aftr-GSW | 35.42 ± 0.61 | 35.80 ± 0.42 | 35.86 ± 0.40 | 0.48 | 0.21 |
| 1.7 | 1.2 | 1.1 | |||
| sMT-PreTset-1min | 35.82 ± 0.39 | 35.95 ± 0.22 | 35.91 ± 0.34 | 0.87 | 0.28 |
| 1.1 | 0.6 | 0.9 | |||
| sMT-PreTset-15min | 35.71 ± 0.33 | 35.54 ± 0.43 | 35.75 ± 0.33 | 0.82 | 0.16 |
| 0.9 | 1.2 | 0.9 | |||
| VFRt-15-s | VFRt-30-s | VFRt-60-s | |||
| sMT-PostTset1-1min | 35.65 ± 0.27 | 35.50 ± 0.42 | 35.72 ± 0.28 | 0.78 | 0.11 |
| 0.8 | 1.2 | 0.8 | |||
| sMT-PostTset1-5min | 35.63 ± 0.27 | 35.51 ± 0.41 | 35.71 ± 0.28 | 0.80 | 0.14 |
| 0.8 | 1.2 | 0.8 | |||
| Wilks' Lambda | 0.52 | 0.39 | 0.64 | ||
| p | 0.06 | 0.003* | 0.11 | ||
| *. The mean difference is significant at the 0.05 level. | |||||
| Pairwise Comparisons | ||||||
| sMT(°C) | Mean Diff. (I-J) | Std. Error | Sig.b | 95% Confidence Interval for Differenceb | ||
| Lower Bound | Upper Bound | |||||
| sMT-bfr-GW | sMT-aftr-GSW | -0.08 | 0.07 | 1.00 | -0.33 | 0.18 |
| sMT-PreTset-1min | -0.23 | 0.10 | 0.49 | -0.56 | 0.10 | |
| sMT-PreTset-15min | 0.18 | 0.12 | 1.00 | -0.23 | 0.59 | |
| sMT-PostTset1-1min | 0.22 | 0.09 | 0.34 | -0.08 | 0.51 | |
| sMT-PostTset1-5min | 0.21 | 0.09 | 0.33 | -0.07 | 0.50 | |
| sMT-aftr-GSW | sMT-PreTset-1min | -0.15 | 0.07 | 0.67 | -0.39 | 0.09 |
| sMT-PreTset-15min | 0.26 | 0.11 | 0.38 | -0.10 | 0.61 | |
| sMT-PostTset1-1min | 0.293* | 0.07 | 0.013* | 0.04 | 0.54 | |
| sMT-PostTset1-5min | 0.290* | 0.07 | 0.011* | 0.05 | 0.53 | |
| sMT-PreTset-1min | sMT-PreTset-15min | 0.410* | 0.09 | 0.004* | 0.10 | 0.72 |
| sMT-PostTset1-1min | 0.446* | 0.07 | 0.000* | 0.20 | 0.69 | |
| sMT-PostTset1-5min | 0.443* | 0.07 | 0.000* | 0.21 | 0.68 | |
| sMT-PreTset-15min | sMT-PostTset1-1min | 0.04 | 0.10 | 1.00 | -0.31 | 0.39 |
| sMT-PostTset1-5min | 0.03 | 0.10 | 1.00 | -0.32 | 0.38 | |
| sMT-PostTset1-1min | sMT-PostTset1-5min | 0.00 | 0.01 | 1.00 | -0.03 | 0.02 |
| Based on estimated marginal means | ||||||
| *. The mean difference is significant at the 0.05 level. | ||||||
| b. Adjustment for multiple comparisons: Bonferroni. | ||||||
| Testing | Experimental session | ||||
| PreTset | sEMG_15-s | sEMG_30-s | sEMG_60-s | Wilks' Lambda | p |
| Mean ± SD | 276.84 ± 84.74 | 255.35 ± 69.02 | 239.80 ± 76.66 | 0.75 | 0.18 |
| cV% | 30.6 | 27.0 | 32.0 | ||
| VFRt-15-s | VFRt-30-s | VFRt-60-s | |||
| PostTset1 | 293.13 ± 88.13 | 277.48 ± 62.79 | 257.90 ± 81.20 | 0.67 | 0.09 |
| 30.1 | 22.6 | 31.5 | |||
| PostTset2 | 285.29 ± 91.80 | 268.01 ± 67.37 | 245.13 ± 83.77 | 0.52 | 0.02* |
| 32.2 | 25.1 | 34.2 | |||
| PostTset3 | 263.14 ± 88.71 | 275.79 ± 65.44 | 234.00 ± 71.89 | 0.36 | 0.002* |
| 33.7 | 23.7 | 30.6 | |||
| Wilks' Lambda | 0.47 | 0.27 | 0.42 | ||
| p | 0.03* | 0.002* | 0.002* | ||
| *. The mean difference is significant at the 0.05 level. | |||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





