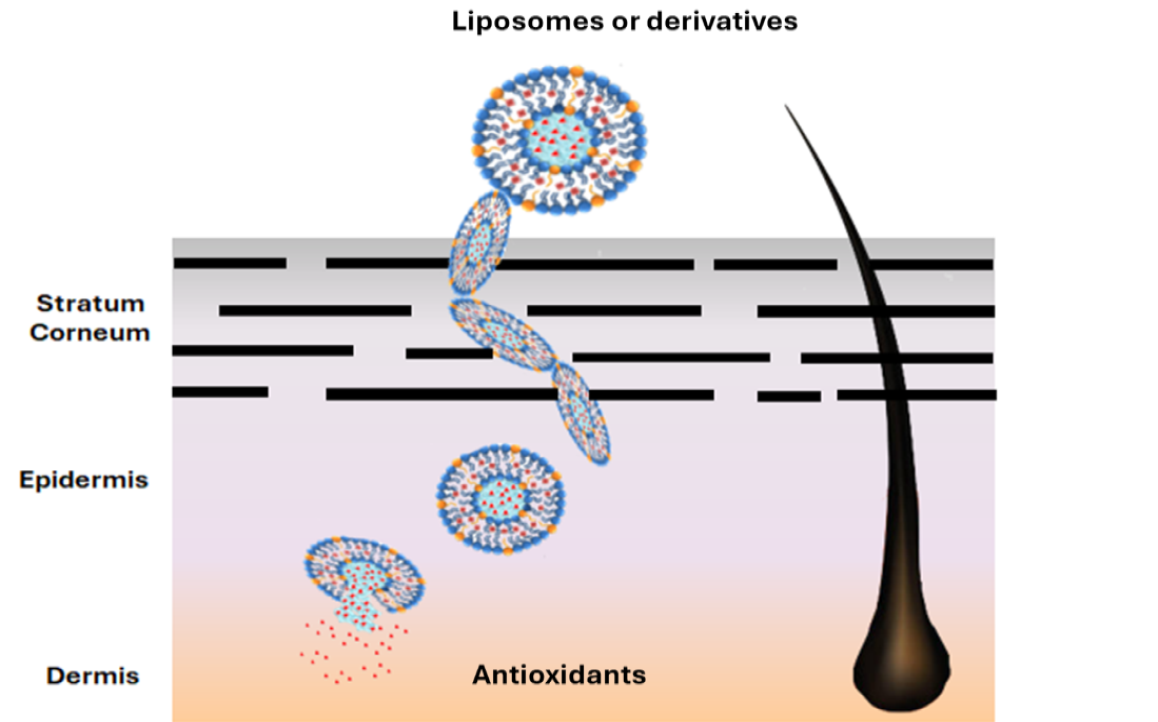
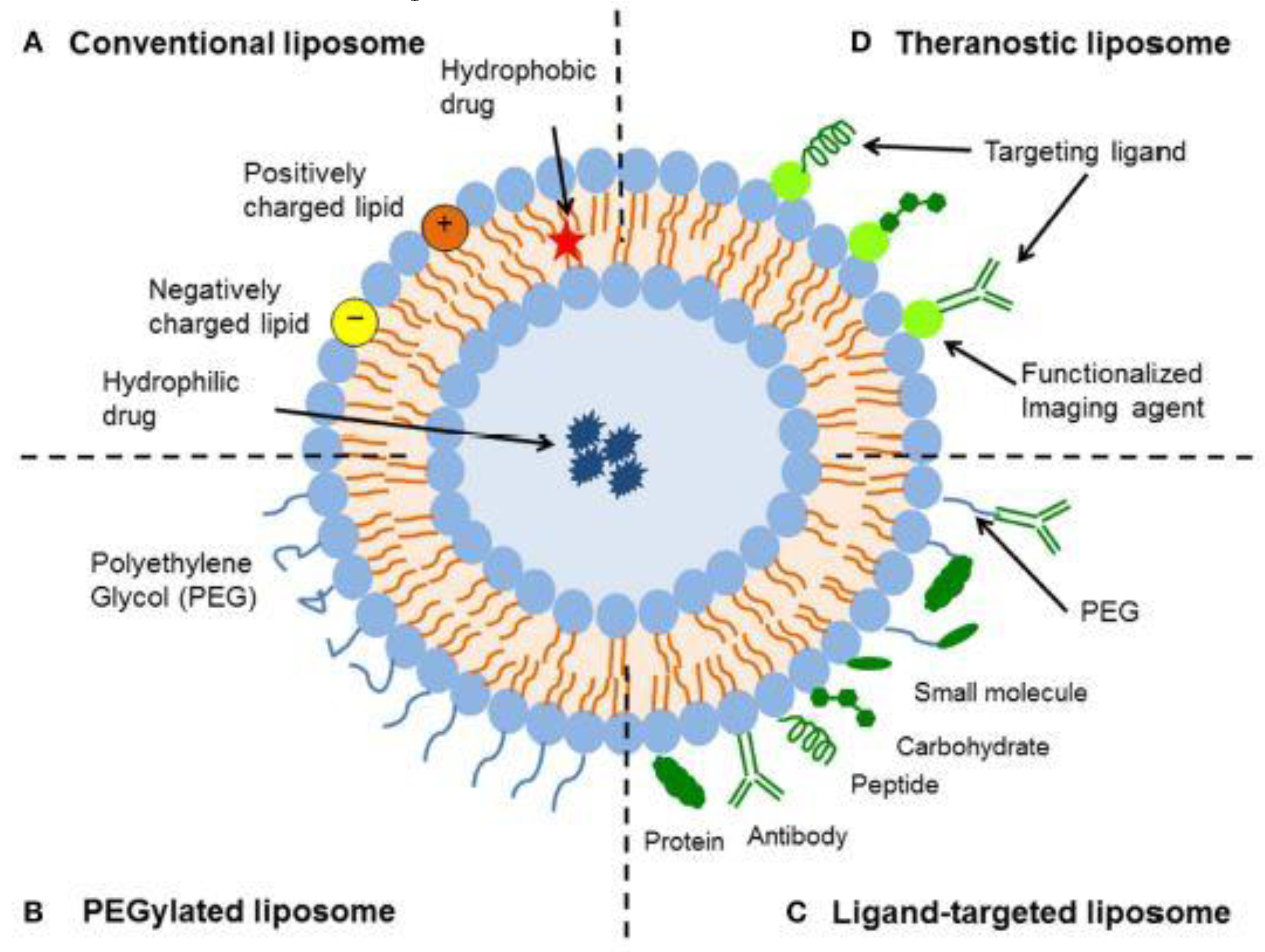
A natural vesicle is a small, membrane-bound cavity. Liposome vesicles are formed by cell's membrane phospholipids and can vary in size from nanometers to micrometers. These sacs play crucial roles in transporting, storing, and processing various substances within the cells. Since Bangham's discovery of the possibility to produce synthetic liposomes in laboratory [
14], their use as drug delivery systems has expanded significantly. Initially formed only by natural phospholipids (PL), such as dipalmitoyl phosphatidylcholine (lecithin or DPPC), phosphatidylethanolamine (cephalin) and phosphatidylserine derived by soybean, egg, and milk, they have also been produced later with synthetic or semisynthetic molecules. More recent formulations envisaged the use of organic solvents (ethosomes) or surfactants (transethosomes) to enhance the vesicle deformability. On the other hand, the use of polyethylene glycol bound to the membrane to produce stealth liposomes permitted to avoid immunity system recognition. Specific proteins can also be added to the outer layer to enhance the cell specificity and facilitate the phagocytosis (actively targeted liposomes) [
17]. Their biocompatibility and biodegradability have made them a popular choice in nanomedicine [
18,
19]. This has proven to be extremely useful in anticancer therapies with common drugs [
20] and is paving the way for the pharmacological application of natural bioactive molecules that were previously overlooked due to their low solubility and poor pharmacokinetics [
21].
2.1.1. Liposomes
Synthetic liposomes (LPS) can be produced with various methodologies and specific reviews are available dealing with this subject [
39,
40,
41]. LPS are composed of one or multiple bilayer membranes with variable diameters, made up of natural or synthetic phospholipids [
42]. The phospholipids used to prepare these vesicles influence their physico-chemical properties. Liposomes formed by saturated phospholipids have higher gel-liquid crystalline phase transition temperature, are more rigid and better retain drugs in their bilayer. One potential limitation of these vesicles is their excessive stability, which can lead to a reduced release of the active principle trapped inside. On the contrary, those LPS mainly formed by unsaturated phospholipids, have opposed properties. So, the proper design of a vesicle nanocarrier should be based on a suitable choice of a proper mixture of phospholipids to obtain a balance between stability and drug release [
43]. LPS can carry hydrophilic or hydrophobic molecules within their core or bilayer, making them versatile carriers for improving drug efficacy and reducing side effects [
44]. The efficiency of the active principle's absorption can be measured by calculating the encapsulation efficiency (EE), which is the percentage of the active principle that remains in the vesicles compared to the amount initially present in the solution. A schematic view of several kinds of liposomes is shown in
Figure 2.
Since the initial use of LPS as drug delivery system, some drawbacks have been envisaged and many efforts have been pursued to solve them. To enhance the bilayer stability versus mechanical and chemical stress, additives as cholesterol or vitamin E were added. Cholesterol can modulate membrane rigidity by reducing the interactions between saturated chains and enhancing those between unsaturated ones [
45]. More recently surfactants were also used to balance membrane stability and deformability [
28]. Moreover, as vesicles can reassemble together, charged phospholipids were preferred in order to keep vesicles away from each other by electrostatic repulsion [
46]. The surface potential of vesicles is influenced by the choice of phospholipid head groups in the bilayer and the presence of a drug with a specific charge. The ζ potential (ZP), typically determined by microelectrophoresis, provides insight into this parameter. In particular, anionic LPS are easily phagocytized by macrophages and can penetrate cells by endocytosis, making this two aspects particularly useful in drug delivery through the skin [
44].
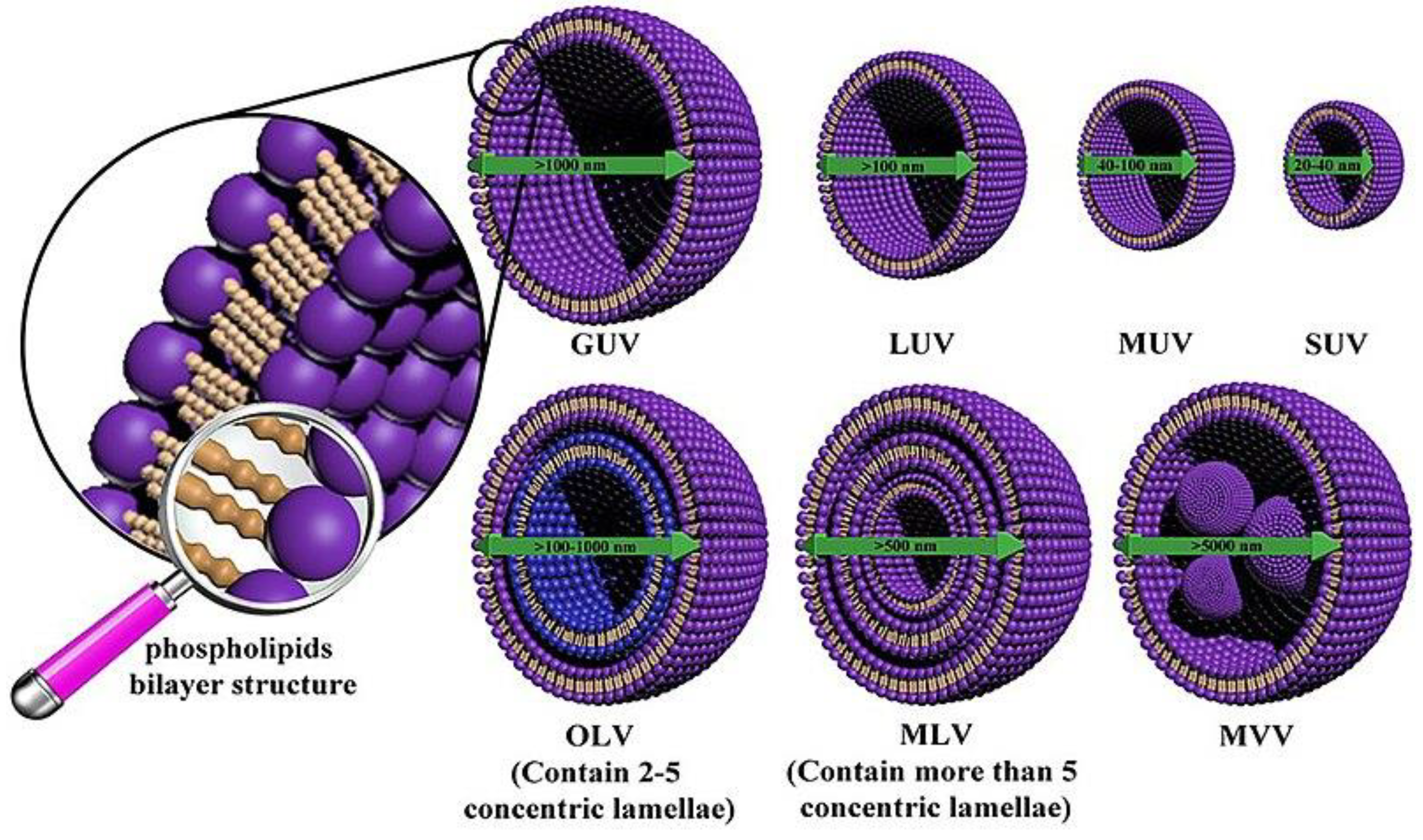
The vesicle size is another important parameter to control during LPS preparation. See for instance
Figure 3. Vesicles with a mean diameter (MD) in the range of 20-40 nm, known as small unilamellar vesicles (SUV), can evade immune system attacks, resulting in longer clearance times. In contrast, only vesicles smaller than 100 nm, referred to as medium unilamellar vesicles (MUV), can prolong their circulation in the blood and be easily absorbed by cells [
18].
In the case of dermal drug delivery, even larger vesicles (LUV) can be used, provided they can penetrate the SC. It is also important to recall that in loaded LPS the chemo-physical properties of the entrapped organic compounds can alter the dimensions of the vesicles, especially if the molecules interact with the bilayer. In some case vesicles dimensions were enlarged by the loosening of the interaction between phospholipids due to the intercalation of the drug molecule [
23,
28], while the opposite effect is detected with other drugs [
13,
30]. A parameter that represents the distribution of the vesicle size is the polydispersity index (PDI). It characterizes the distribution of molecular mass among the vesicles in the sample and provides insight into the heterogeneity of the vesicles' volume. Determined by Dynamic Light Scattering (DLS), a PDI lower than 0.1-0.2 indicates a narrow size distribution and a more uniform population of liposomes. The uniformity is often desirable for consistent drug delivery and predictable behavior in biological systems. Techniques generally used to enhance the liposomal size homogeneity are sonication during mixing operation [
23] or filtration of liposomes through filters smaller than their previous average diameter [
25].
Many liposomal formulations have been used to deliver antioxidants, either pure or derived by complex natural extracts. Bavarsad et al. [
22] developed liposomal formulations of pure quercetin for treating skin pressure ulcers. They employed a fusion method of phospholipid bilayers in the presence of 0.01-0.04 g/ml quercetin buffer solutions, without the use of organic solvents. The small unilamellar vesicles (SUV) produced had a homogeneous particle size with a MD of 10-16 nm, demonstrated good encapsulation efficiency (EE) of 65-78%, and were sufficiently viscous to be directly applied to the affected areas. This delivery system showed a high efficiency of retention (RE) into the skin (RE 46-51%) and a good level of penetration ratio (retention/penetration ratio 7.1-9.0), indicating that for the most part quercetin LPS were trapped in the skin. The LPS-loaded active ingredient regulated vascular circulation and necrosis, reducing inflammation and shortening recovery time for pressure ulcers. With minimal systemic absorption, the liposomal formulation had fewer side effects compared to phenytoin cream, which was used as the reference drug.
An example of plant extracts delivery has been brought up using
Moringa Oleifera leaves [
13]. Rich in carotenoids, glucosinolates, vitamins, amino acids, and many phenolic antioxidants,
Moringa Oleifera is well known for its anti-microbial, anticholesterolemic and anti-inflammatory properties. In the skin, this plant extract helps to maintain collagen and hydration level, thus enhancing defense against ageing and photo-damage. The hydroethanolic extracts of the
Moringa Oleifera leaves were encapsulated in LPS formed by DPPC and cholesterol (4:1 ratio). The presence of the extracts lowered the diameter of the LPS from the size of a giant unilamellar vesicle (GUV, 736±100 nm MD) to a large one (LUV, 154±1 nm MD). The loaded vesicles possessed a low polydispersity index (PDI 0.17±0.01), a weak positive charge (ZP +6.6±0.1), and a good retention rate (RR >80% at 35°C for 6 h). The antioxidant activity (AA), determined by the DPPH assay, of the pure extract (AA 85.8 ± 1.5%) proved to be similar to that of the reference, gallic acid (AA 87 ± 2%), while the liposomal formulation was less active (AA 70.0 ± 1.0%). Additionally, the antioxidant effect of liposomes, determined by ORAC experiments, diminished faster (60 minutes instead of 120 minutes for the pure extract). A gel formulation with propylene glycol, hydroxyethyl cellulose and glycerin was used to analyze effects on skin. Results demonstrated that even if the free hydroethanolic extracts had greater antioxidant properties and could better enhance hydration, the liposomal formulation was more effective at preventing transepidermal water loss.
Another interesting application of liposomal delivery was the preparation of vesicles loaded with berry extract from
Myrtus Communis [
23]. This mediterranean bush is well known for the presence of antioxidants in its berries. HPLC-DAD analyses detected high concentration of anthocyanins, galloyl derivatives and flavonoids in the macerated ethanolic extract (1:1 w/v). The dry extracts were used to prepare the liposomes using fat-free soybean phospholipids (70% phosphatidylcholine) in PBS solution under sonication cycles. This very simple method produced liposomes of 102±5.6 nm MD, slightly bigger than the corresponding empty vesicles, but similar in PDI (0,22 ±0.02) and ZP (-10.00 ±0.08 mV). The research group determined the entrapment efficiency (EE) of each main antioxidant of the myrtle extract: the EE ranges from 71.4% of Myricetin-3-O-galactoside to over 95% of most of the anthocyanins. The antioxidant activity of the loaded liposomes, measured by DPPH analysis, was only slightly lower (326±17 μg Trolox Equivalent/ml) than that of the free myrtle extract (344±22 μg TE/ml). The reducing power remained unchanged, as confirmed by FRAP analysis, with values of 1831±70 and 1867±32 μg Fe²⁺ equivalents/mL for the loaded liposomes and myrtle solution, respectively. The 3-(4,5-Dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) assay on cell viability demonstrated the low cell cytotoxicity of myrtle extracts and the even higher vitality of cells treated with loaded liposomes vs control cells. Furthermore, anti ROS-activity experiments made on fibroblasts exposed to AAPH radical generator showed that liposomes, loaded with increasing quantity of myrtle extracts (from 0.1 to 10 μg), significatively reduced the oxidative stress, even better than the pure myrtle extract after 24 hours. Unfortunately, no direct test on skin were performed by authors even with this encouraging results.
Permana, Rahman et al. [
24] investigated the use of liposomes to enhance the antioxidant activity, permeability and skin retention of propolis extracts (PEs). The more efficient extraction (75% v/v of ethanol/water, PE75) in term of total polyphenolic content (TPC 179.3±9.3 mg/ g PE) and total flavonoid concentration (TFC 277.2± 3.4 mg/ gPE) turned to be the more antioxidant either as radical scavenger capacity (DPPH IC
50 43.2± 3.0) or as lipid peroxidation inhibition (LP, 65.3±3.4 μg/ml). These PEs are rich in caffeic acid (maximum 4.15±0.01 mg/g in PE75), quercetin (0.72±0.01 mg/g in PE75) and kaempferol (2.62±0.01 in PE75), as confirmed by HPLC analyses. They were encapsulated into liposomal vesicles using different amount of phosphatidylcholine (PC) applying the thin-layer hydration technique with subsequent solvent evaporation. Higher concentrations of phospholipids, increasing the viscosity of the solution, generally produced larger vesicles due to the higher surface tension. On the other hand, the use of a lower weight ratio of PE/phosphatidylcholine combined with a longer duration of homogenization or bath sonication allowed for a reduction in liposomal size from 620±54 to 248±22 nm of diameter, with PDI and ZP remained constant. As the major interaction of the three main antioxidants depend on hydrogen bonds with the bilayer either as acceptor or donor, the EE seemed to depend on their hydrogen bond capacity. Quercetin exhibited better encapsulation (maximum 77±6%) than kaempferol or caffeic acid (maximum 45±4% and 55±5% respectively), the extent of EE being also dependent on the size of the LPS and the time of homogenization and sonication. The obtained vesicles enhanced the water solubility of caffeic acid, kaempferol, and quercetin by 5-fold, 11-fold, and 12-fold, respectively. LPS were dissolved in a hydrogel (Carbomer 940 and glycerol in water), in order to analyze their potentiality as skin formulation. Antioxidant analysis confirmed that their antioxidant activity either as radical scavenger (DPPH IC
50 48± 5) or as lipid peroxidation inhibitor (LP, 68 ± 5 μg/ml) remained similar to that of hydrogel containing simple propolis extract. On the other hand, tests in vitro on mouse skin, showed that the use of LPS-hydrogel enhanced the skin permeation and retention of all antioxidants by 4- to 8-fold compared to the hydrogel containing PEs. This was ascribed to the poor absorption of the free antioxidants into the skin, and the opposite effect of phospholipidic bilayer that facilitate the penetration into the stratum corneum. This hydrogel formulation containing propolis extract also acted as photoprotector against UVA and UVB aggression, as stated by the measured sun protection factor of 17± 2.
The combined use of liposomes and other carriers, such as cyclodextrin (CD), has been designed in pharmaceutical applications to enhance stability of loaded drugs and modulate their release (Modulatory Liposomal Controlled Release, MCLR) [
47].
Gardikis et al. [
25] analyzed the use of liposomes and CD to enhance the adsorption of propolis antioxidants and study their biochemical activity in skin. In their research they used micronized propolis extracted in presence of CD. The LPS mixed with the CD/propolis extract, produced large micelles that were made smaller through membrane filtration (0.22 mm membrane pore size). Using 10% w/w propolis concentration, the obtained liposomes showed an initial MD of 255±36 nm, decent PDI (0.366±0.051) and good ZP (-21±5 mV). This preparation gave higher EE (84±4%) with respect to previous works, thanks to the presence of CD. The TPC and the antioxidant activity determined by Folin-Ciocalteu's method and DPPH assay, respectively, evidenced a high polyphenols concentration of 100.0± 0.7 mg gallic acid eq/ml and a significant antioxidant activity of 33.7±0.2mM Trolox equivalent. However, both parameters decreased over time or when vesicles were stored at temperature higher than 6°C. The liposomal formulation was also studied in vitro on human skin cell (HaCaT) by biochemical and genetic tests. LPS enhanced skin cells metabolism enhancing ATP levels, and protected cells against UVB lowering photoaging. The upregulation of various genes connected with cell proliferation (AQP3), antiaging (TNFα), cell defense against pathogens (IL4) and immune protection (ITGB2) confirmed the beneficial effect of the propolis and the efficacy of the liposomal carrier in enhancing the absorbance of the active principles into the skin. Finally, a cosmetic formulation (1% LPS) proved that the loaded liposomes were not irritating for human skin cells (MTT on reconstructed skin model), opening to possible marketing application.
Oskoueiana, Karimi and coworkers produced nanoliposomes of Owhadi pistachio hull extract (PHE). The aim was to enhance the stability and applicability of the antioxidant mixture containing gallic acid, rutin, quercetin, myricetin, catechin and pyrogallol [
26]. The extract containing the higher quantity of polyphenols (TPC 52±g GAE/100g dry extract) was used in the liposomal encapsulation. The LPS were formed by mixing hot water solution of lecithin and ethanolic organic extract and then sonicating. Despite being sufficiently small (MD 96.3 ± 7.2 nm) and exhibiting a negative charge (ZP -48.0 ± 0.8 mV), the vesicles demonstrated a low entrapment efficiency (EE of 38.4 ± 2.7%). As indicated by the MTT assay, the liposomal formulations maintained high cell viability in murine hepatocytes, at extract concentrations up to 50 μg/ml. The antioxidant activity was confirmed by DPPH assay, even if LPS radical scavenging capacity was lower (IC
50 12.6±2.9 μg/L) in comparison with gallic acid reference (IC
50 8.8±0.6 μg/L). The LPS were also able to up-regulate the genes of endogenous antioxidant enzymes in murine hepatocytes: SOD (+3.2 fold change), CAT (+2.1), GPX (+1.9), and Nrf2 (+2.7). However, this effect was not as large as that observed with gallic acid, which showed fold changes of +3.8, +2.2, +2.8, and +3.3, respectively, for the same enzymes. Furthermore, the PHE liposomal formulation demonstrated activity against melanogenesis: it inhibited tyrosinase activity in mushroom cells (IC50 21.8 ± 4.3 μg/ml), although to a lesser extent than the kojic acid reference (16.2 ± 4.3 μg/ml). Additionally, it reduced melanin production and partially suppressed the expression of tyrosinase, MTIF, TRP-1 and TRP-2 genes in melanoma cells. These data confirmed the potential use of this extract in skin whitening processes.
Recently, the problem of scalability and industrial production, with all the challenges that this involves, has been raised from various groups of research. Caddeo, Pucci and coworkers [
27] analyzed the preparation of vesicle loaded with resveratrol from a commercial liposomal preparation to check the possibility of an easy-to-handle formulation for skin application. The Pronanosome LIPO-N (PLIPO-N) by Nanovex Biotechnologies SL (Llanera, Spain) is a commercial powder of dry LPS. The easy preparation of the liposomal carrier, through an overnight mixing of resveratrol, Tween 80 surfactant, and PLIPO-N in water, followed by sonication, produced highly homogeneous nanovesicles (PDI 0.19) having MD (80 nm) and ZP (-25 mV) statistically consistent with the same regenerated empty liposomes. The loading of the resveratrol was high (83%) and long-term tests confirmed the stability of the physico-chemical properties of the vesicles for more than three months. DPPH (321.0 ± 7.0 μg of Trolox equivalents/ml) and FRAP (8.1 ± 0.7 mg of Fe
2+ equivalents/ml) assay proved that resveratrol antioxidant activity was maintained even in the liposomal formulation. More surprisingly, while resveratrol ethanolic solution lowered 3T3-L1 cell viability up to 20 % at 10 μg maximum concentration, the loaded LPS proved to be less toxic with a 55% viability in the same condition. The ability of resveratrol to protect fibroblasts from ROS injuries was analyzed by dichlorofluorescein (DCF) assay in which the fluorescent radical DCF was produced by a ROS activator (AAPH 250 μM). In the DCF experiments, the fluorescence lowered as resveratrol concentration increased much faster than with the liposomal formulation. On the other hand, microscope images confirmed that, using free resveratrol, the cell mortality increased so much to invalidate the fluorescence data. Instead, the ROS scavenging capacity of liposomal resveratrol did not produce cell death at the higher concentration thus supporting the usefulness of the liposomal formulation.
Surfactants, which are amphiphilic, generally are used to stabilize the vesicles, reduce their diameter and increase their deformability by lowering the energy required to modify membrane curvature. For this reason, adding surfactant to liposomal formulation could be useful for a dermal formulation. In the case of naringenin, another antioxidant that is poorly soluble in water but shows high potential as anti-inflammatory agent and regulator of fibroblast and melanoma cell growth, Lowry et al. [
28] analyzed its incorporation into liposomal formulations containing different percentage of Tween 20 surfactant. The positioning of naringenin in the bilayer increased the diameter of the vesicles. The so formed loaded liposomes had a MD of 392 ± 32 nm that decrease to 235 ± 9 nm in presence of 10% Tween 20. The vesicles resulted neutral or slightly negative (ZP from 4.12 to -022 mV) but with a PDI of 0.3%. The EE of the active principle was also decreased by Tween 20, from 90.8±4.6% to 64.3±5.6% at 10% Tween 20 concentration. It was supposed that the surfactant competed with lipophilic naringenin for a position within the membrane. As the authors expected, the elasticity of the different liposomal formulations increased in presence of a higher percentage of surfactant as evidenced by a decrease in the deformability index (DI) from 80.7 ± 2.0% (without Tween 20) to 59.2 ± 4.4% (with 10% Tween 20). Gel derived by hydroxyethyl cellulose (HEC) and hydroxypropyl methylcellulose (HPMC) were added to naringenin and to the loaded liposomal formulations to compare the release of the active principle using side-by-side diffusion cell experiments. The results showed that the liposomes released naringenin faster than the solution, and the release rate increased as concentration of Tween 20 reached 10%. On the other hand, the comparison of the formulations containing either loaded liposomes, with 2% Tween 20 or without, and one cellulose gel evidenced that HMPC gel slowed the release of the naringenin better than HEC gel even in presence of the surfactant that enhanced the process in both formulations. The study of the uptake of naringenin by HaCat and HDFa cells, using sodium 3'–(1–phenylamino)–carbonyl–3,4–tetrazolium (XTT) assay with a fluorescent dye, confirmed the ability of gel liposomes to penetrate the cells, probably, by ionic interaction with cell membrane. These data illustrate the importance of both the structure of the vesicles and the formulation used for the delivery system in achieving a synergistic effect on the transport and adsorption of the active principle.
2.1.2. Ethosomes and Transethosomes
In the last years the use of vesicles has been further increased by the development of liposomal derivatives with higher membrane mobility and deformability. This kind of vesicles are particularly useful in dermal treatments. Ethosomes (ETH) are soft, malleable structure composed of phospholipids, ethanol (20-45%), and water, classified as ultra deformable vesicles [
48]. They penetrate deeper layers and increase drug permeation compared to traditional LPS. Their flexible structure and ability to enhance skin penetration, is due to ethanol ability to perturb the organization of SC lipids. The deformability index (DI) can parametrize the deformability of these vesicles, with a lower DI corresponding to a high degree of deformability, and it can be determined by analyzing the ethosomes’ diameter and weight before and after extrusion through membrane filters with pores smaller than the average diameter of the vesicles under examination. The deformability of ETH make them a valuable tool for transdermal drug delivery in pharmaceutical and cosmetic formulations. Furthermore, ethosomes are particularly advantageous for delivering drugs with poor water solubility or high molecular weight.
Sguizzato and coworker [
29] prepared ETH containing Coenzyme Q10 (CoQ10) to enhance the bioavailability of this important antioxidant in the skin. CoQ10 is insoluble in the most common solvents and poorly in ethanol (0.3 mg/ml). The preparation of ETH from a solution of soybeans PC and CoQ10 in ethanolic solution was performed at room temperature and enhanced the solubility of the antioxidant to 1 mg/ml, permitting a 98% adsorption into the vesicles. This procedure resulted in better performance if compared to that obtained with solid lipid nanoparticles or simple LPS which permitted an adsorption of only 73% and 89% respectively [
49]. Considering EE parameter, the more stable vesicles obtained were those derived by a 0.9% w/w concentration of PC in ethanol, with a 254-271 nm MD, and a mixed unilamellar and multilamellar structure. The stability of CoQ10 inside the ETH in three months was higher that the free molecule or other kind of supramolecular preparations. ETH alone or with CoQ10 was found to be safe for cells in MTT and LDH experiments. The research group demonstrated the ability of loaded vesicles to penetrate fibroblast membranes, probably by endocytosis, and protect human fibroblast cells from H
2O
2 insults in ex-vivo experiments, as demonstrated by analysis of the production of 4-hydroxynonenale (4-HNE) from lipids. The same fluorescence experiments on reconstructed human epidermis, and TEM images confirmed ETH adsorption within the stratum corneum (SC) and even into the stratum basale, as well as their protective effect against hydrogen peroxide-induced lipid degradation, resulting in a 35% reduction in the production of 4-HNE.
To fully utilize the strong antioxidant properties of rutin, given its difficulty to go beyond the SC, the set-up of an effective delivery system is almost essential. To reach this goal, Rolim Baby et al. [
30] prepared ethosomes with PC from egg yolk (2% w/v), rutin (0.03%w/v) and an hydroalcoholic solution (20% ethanol). The vesicles produced were bigger (369.5±5.2 nm) than the empty one, with a lower ZP (-19.6±0.4 mV) and a higher PDI (0.43±0.01). In particular a PDI higher than 0.3 could be due either to the formation of vesicles agglomerates during the addition of the rutin ethanolic solution or to a more disperse geometry of the vesicles. In vivo tape-stripping tests on human skin demonstrated that rutin-loaded ETH were not irritating and that they penetrated the lower layers of the stratum corneum (SC) three times more than the rutin solution. Even if the antioxidant activity in vitro was confirmed for the ETH formulation, the
ex vivo DPPH assay either on the upper or deeper layer of the skin, did not evidenced relevant differences between the loaded ethosomal formulation, the free rutin solution and the control. The authors ascribed this negative result to the low concentration of rutin in the formulation.
Two years later the group of Paolino et al. [
31] succeeded in enhancing the rutin antioxidant efficacy respect to the simple rutin solution by studying a different ethosomal composition. The ETH were prepared with Phospholipon 90G
©, water and rutin ethanolic solutions, in the dark to avoid rutin photodegradation. The concentration of rutin solutions, from 0.5 to 4 mg/ml, into the ETH was inversely correlated with the size (MD from 110 ± 8 nm to 575 ± 6 nm) and also with the elasticity of the vesicles. However, DI values (from 3.5 to 10%) permitted a reasonable degree of membrane elasticity. Also, the ZP of the different ETH, varied as a function of the rutin adsorbed into the bilayer. In all cases, ranging from -22.4 to -29.5 mV, ZP values were consistently negative and low enough to ensure effective repulsion of the vesicles. Interestingly, in comparison with the other formulations, those ETH prepared with higher concentration of ethanol (40%w/v instead 30%w/v) produced smaller ZP (from -5.8 to -12.8 mV) and were discarded. The EE was dependent on different parameters: a higher concentration of rutin solution significantly enhanced the EE, while a higher ethanol content reduced it. On the other hand, rutin diminished the release of the active principle by enhancing bilayer rigidity. Considering the higher EE (67.5 ±5.2 %) and DI (3.5±0.5%) obtained, the best ethosome composition was found to be 4 mg of rutin dissolved in 30 ml of EtOH, 69 ml water and 1%w/v PL90G
©. In comparison with rutin solution, the experiments conducted with this ETH showed that the delivery system stabilized the antioxidant and strongly reduced its photodegradation in presence of UV light. MTT tests on cell lines subjected to H
2O
2 oxidative stress, showed that the ETH not only maintained cell viability, but also exerted antioxidant protection on the cells, unlike the pure compound. This protection depended on the concentration of rutin, and increased as the concentration of the active ingredient rose up to 100 μM. Finally, the anti-inflammatory effect of rutin ETH, conducted on conveniently irritated epidermis of human volunteers, confirmed the higher effect of the loaded vesicles in comparison with simple solution of the active principle. It should be noted that rutin properties counterbalanced the initial irritating effect of ethanol on the skin due to the hydroalcoholic preparation, while the erythema recovery, in presence of rutin ETHs, was more rapid and with better results after 4-5h.
In case of
Euphorbia Characia latex extract, ETH were conveniently used as skin penetration enhancer of the quercetin derivatives [
32]. This extract inhibited collagenase, hyaluronidase and elastase enzymes responsible of skin ageing. Suitable ETH were prepared to analyze its inhibitory capacity against tyrosinase, responsible of skin melanogenesis. The obtained vesicles were nanostructures (MD 101.1 ± 9.7nm, PDI 0.29 ± 0.07%) with high ZP (−70.6 ± 7.3 mV) and a good EE of 85-86% depending on the glycoside bound to quercetin. MTT experiments pointed out the safety of the vesicles, with cell viability >82% for empty and even higher for loaded ETH, while the DPPH assay confirmed the maintenance of high antioxidant activity of 94.8± 0.4%, in the liposomal formulation. The anti-melanogenic effect of tyrosinase, determined by zymography on cells treated with the L-DOPA enzyme activator, confirmed a high level of inhibition from the plant extract, which was further enhanced by the loaded ETH. All of these properties, along with the long-term stability of the prepared ETH, confirm their potential for delivering plant extract bioactive compounds through the skin, even though no specific experiments were conducted on skin cells in this case.
Another recent application of nano delivery systems is based on the use of two or more components together for a synergistic effect. This is the case of the work of Ferrara et al. [
33] that used either ETH of curcumin (Cur) and piperine (Pip) to check their capacity to reduce the skin damages derived by environmental pollution. While Cur is well known as an powerful antioxidant, antibacterial and antiviral with effects against inflammation, and various pathologies [
50], Pip shares this properties but it also increases the bioavailability of other molecules [
51] as well, by slowing down the cell efflux systems and enhancing skin cells permeation [
52]. In their research, Ferrara and coworkers compared the bulk production method with microfluidic technique for creating ETH. In the microfluidic approach, two syringes containing an ethanolic solution of phospholipids and distilled water were mixed at a cross-junction. The flow ratio of the two solutions was maintained at 2:1 (v/v), while varying total flow rates were applied. During flows mixing the LPS were formed with homogeneous size as a function of the total flow rates. Nevertheless, the bulk technique turned out to be more rapid (5 ml vs 0.36 ml volume of 200 nm diameter ETH), also considering a possible scaling up of the production. The ETH so prepared were multilamellar and showed an EE of 97 and 79% for Cur and Pip respectively. In a second step, the researchers compared the antioxidant effect of Cur and Pip solutions and their ETH either alone or together. The results evidence that the mixed ETH solutions enhance the drug efficacy of Cur in the FRAP assay. However, some drawbacks can be envisaged in the release profile of the two drugs. In comparison with the drug loaded in the vesicles, the percentage of drug released from ETH was 33% in case of Pip and 15% for Cur, lower than the values obtained by the corresponding drug solutions that were 57% and 24% respectively. In the permeability tests, Cur and Pip behaved differently. Pip was faster in diffusing through the synthetic membrane (STRAT-M®) that simulate the SC, either in solution or in loaded ETH, the second being slower. Conversely, Cur in ETH showed a partition coefficient higher than the Cur solution even if both had a longer lag time than the Pip systems. If compared to those of Pip, Cur either in solution and in ETH also showed low permeability coefficient. Nevertheless, the Cur ETH system seemed to be more efficient than the corresponding solution in providing a prolonged protective effect. Additionally, the combined use of Cur ETH and Pip ETH, further enhanced this behavior against oxidative stress in skin submitted to diesel engine exhausts.
Transethosomes (TETH) are a further variation of ethosomes containing, together with ethanol, a surfactant that works as SC permeation enhancer, particularly useful for dermal delivery [
53].
To study the antioxidant activity of mangiferin, a glucitol xanthone present in many plants and fruits (papaya, mango), Valacchi, Esposito et al., considering its higher solubility in ethanol (0.20 mg/ml) respect to water (0.11 mg/ml), prepared ETH and TETH as nano delivery systems [
34]. They analyzed the different loaded vesicles in their physico-chemical characteristics, antioxidant activity, and anti-inflammatory properties on skin keratinocytes. The ETH were formed mixing PC and ethanol with mangiferin, enhancing its solubility to 3.3 mg/ml thanks to PC presence. TETH were obtained adding, to the same ingredients, different quantity of surfactants (0.1-0.6%) such as hydrophilic polysorbate (Twin 20, Twin 80), lipophilic sorbitane monoleate (SP20, SP 80), or ionic dimethyldidodecylammonium bromide (DDAB). Depending on the surfactant used, the vesicle size and zeta potential (ZP) varied, while the PDI was always less than 0.2, indicating good homogeneity in vesicle volumes. In general, a higher concentration of surfactant produced a lowering of the diameter and zeta potential. Except in case of DDAB that gave highly positively charged vesicles, the ZP were always negative. As already stated, stability tests confirmed that the presence of a low ZPs was detrimental to the stability of the vesicles, which could fuse over time due to the low repulsion. For these reasons the research group chose to continue the study with mangiferin (0.1%w/w), only on the vesicles containing either Twin 80 (MD 169.3 ± 0.46, ZP−28.29 ± 0.4) or DDAB (MD 86.41 ± 1.09, ZP 84.08 ± 0.6) as surfactant. However, in MTT tests, while ETH+TW80 produced low cytotoxicity that increased with mangiferin concentration, either empty or loaded DDAB ethosomes showed high toxicity and were discarded from further experiments. This is probably because the positive charged vesicles tented to destabilize the keratinocytes, leading to their lysis. The diffusion test, conducted with Franz cell using a nylon membrane, confirmed a lower diffusion capacity for both formulations compared to a simple Mangiferin solution. However, TETH showed better performance compared to ETH, because the surfactant most likely facilitated the release of the active principle from the vesicles. Finally, exposure to cigarette smoke was used to test the antioxidant and anti-inflammatory effect of the vesicles on HaCaT cells. The results demonstrated that both ETH and TETH permitted mangiferin to penetrate cells (TEM images) and to lower the inflammation of exposed keratinocytes as further demonstrated by the reduction of OH-1 and IL-6 inflammatory enzyme expression.
Subsequently, the same research group studied the anti-melanoma activity of different concentrations of pure quercetin loaded onto either ETH or TETH [
35]. Building on previous results, only Twin 80 was used as surfactant. Both kinds of the vesicles were multilamellar with similar diameters dependent on the PC and surfactant concentration but with generally uniform dispersity index. Quercetin, standing in the bilayer, produced a loosening of the membrane and an enlargement of vesicles diameter while Twin 80 seemed to enhance vesicles stability (ETH MD 258 ± 21nm, TETH MD 240 ± 21nm).
The entrapment capacity of TETH was determined to be slightly higher than that of ETH, with a percentage of 59.2% and 56.4% respectively, while antioxidant capacity on lipid proved to be almost equal for the two delivery systems (3.16 μmol TE/g vs 3.26 μmol TE/g) but lower than free quercetin solution (3.82 ± 0.05 μmol TE/g). In in vitro release test (IVRT) on lipophilic polytetrafluoroethylene (PTFE) membrane, which is more similar to human skin, the release rate remained constant over time (zero-order kinetics), confirming the ability of the vesicles to control and prolong the release of the active principle. In vitro permeation test (IVPT) confirmed that transethosomes possessed a better capacity in letting quercetin penetrate into the SC respect to ETH (4-fold lower), and quercetin solution (10-fold lower). The MTT experiments, conducted on both HT144 melanoma and HaCaT cells, demonstrated low cytotoxicity at quercetin concentrations below 5 µg/ml. The subsequent wound healing assay, performed at 2.5μg/ml quercetin concentration, evidenced a lower capacity of TETH and ETH to favor wound resolution in both the cell lines. This has been ascribed to the capacity of active principle to inhibit ROS production so lowering the natural response of cell to repair the tissue damage, partially activated by ROS as second messenger.
2.1.3. Niosomes
A different improvement in the use of synthetic vesicles has been the development of nonionic surfactant bilayer (alkyl ethers, alkylated sugars, polyethylene fatty acid esters, etc.) called niosomes (NIS). Niosomes exhibit higher chemical stability compared to LPS, and allowed for better control of shape and size, prolonged drug release and improved bioavailability, making them a preferred option in many delivery systems. Additionally, the cost-effectiveness and scalability of niosome production further contribute to their advantages over conventional LPS [
54,
55].
Al Saqr and coworkers [
36] prepared NIS to study the skin adsorption of hydroxytyrosol, an antioxidant present in olive oil with various benefits, including anti-inflammatory, anti-platelet aggregation, and anti-UV-B DNA damage properties, among others [
56]. Vesicles containing different percentages of Span 60 surfactant (27.6-45%), lecithin (0-15%), ethanol (40-45%), and buffered water (0-9%), along with cholesterol (5% w/w) and hydroxytyrosol (1% w/w), were prepared. Span 60 was chosen as surfactant for its higher transition temperature (53-57°C) and its high EE. Different percentage of lecithin and surfactant produced vesicles with similar small diameters (MD around 1 nm), high negative charge (ZP between -41.2 and -56.4 mV) and high entrapment efficiency (all EE > 92%). In the TEM images, the niosomes used in gel formulation, were found to consist of non-aggregated, spherical-shaped vesicles. Niosomes release capacity was determined and compared with either ethanolic or PBS aqueous solutions of hydroxytyrosol. In vitro results confirmed the slower releasing properties of niosomes related to Span 60/water concentration rate and to the surfactant capacity to load hydroxytyrosol into the bilayer. In vivo permeation and retention experiments were performed using human cadaver skin. Results confirmed the higher ability of niosomes to transport hydroxytyrosol through the SC and let it penetrate into skin cells in comparison with antioxidant solutions. Therefore, the authors attributed this result to the ability of Span 60 to deconstruct the SC. NIS water concentration was also important as it could be seen by the lower hydroxytyrosol transdermal permeation and retention from niosomes prepared without water. Analogously, higher concentration of lecithin lowered the delivery of the active principle.
The group led by Jingyuan Wen investigated the optimal niosome formulation for delivering epigallocatechin gallate (EGCG) into skin cells using mathematical methodologies, together with statistical optimization [
37]. They analyzed the effects of the concentration of EGCG, surfactant (Twin 40 or Span 60) and dihexadecyl phosphate (DCP), cholesterol/surfactant ratio, buffer amount (PBS solution + 10% EtOH, 7.4pH) and hydration time on the encapsulation efficiency. Different batches of niosomes were prepared, and their encapsulation efficiency (EE) was measured. A multivariable equation in which EE is the dependent variable was developed. The ANOVA statistics on the effects of these variables on the EE confirmed the importance of both the amount of antioxidant and the cholesterol/surfactant ratio in achieving higher EE. The subsequent experimental data confirmed that the niosomes containing 1.4 mg of EGCG and with a cholesterol/surfactant ratio of 0.9 had the best EE, which was 53.05 ± 4.46%. These niosomes were spherical, as shown in the TEM images, and had a MD of 235.4 ± 15.6 nm, a negative ZP of −45.20 ± 0.03 mV and a good PDI of 0.267 ± 0.053. Studies on the release of EGCG from loaded NIS, using Franz cell, evidenced a longer release time (35% after 3 h, 73% after 21 h) in comparison with the of EGCG solution (2h). Also, the deposition of EGCG from NIS onto the skin, after both 12 (69.0 ± 13.87 µmg/cm
2) and 24 hours (54.38 ± 8.86 µmg/cm
2), was proven twice as effective compared to the EGCG solution. Experiments with Fluorescein 5(6)-isothiocyanate (FITC) solution free or loaded in the same NIS demonstrated that the molecule was absorbed deeper in the human SC when released from the vesicles compared to the solution. The antioxidant activity of EGCG-loaded NIS was evaluated by measuring malondialdehyde (MDA) production in UV-stressed fetal bovine cells. Cells treated with EGCG-loaded NIS exhibited significantly lower MDA levels (0.80 ± 0.33 μmol/L/mg protein) compared to those treated with EGCG solution (2.08 ± 0.33 μmol/L/mg protein), confirming the enhanced protective effect of the loaded antioxidant. This was attributed to the activation of glutathione peroxidase (GPX) production by EGCG release in the treated cells. Notably, the cellular uptake of NIS requires energy, suggesting a time- and concentration-dependent mechanism mediated by endocytosis.