Submitted:
29 October 2024
Posted:
30 October 2024
You are already at the latest version
Abstract
Keywords:
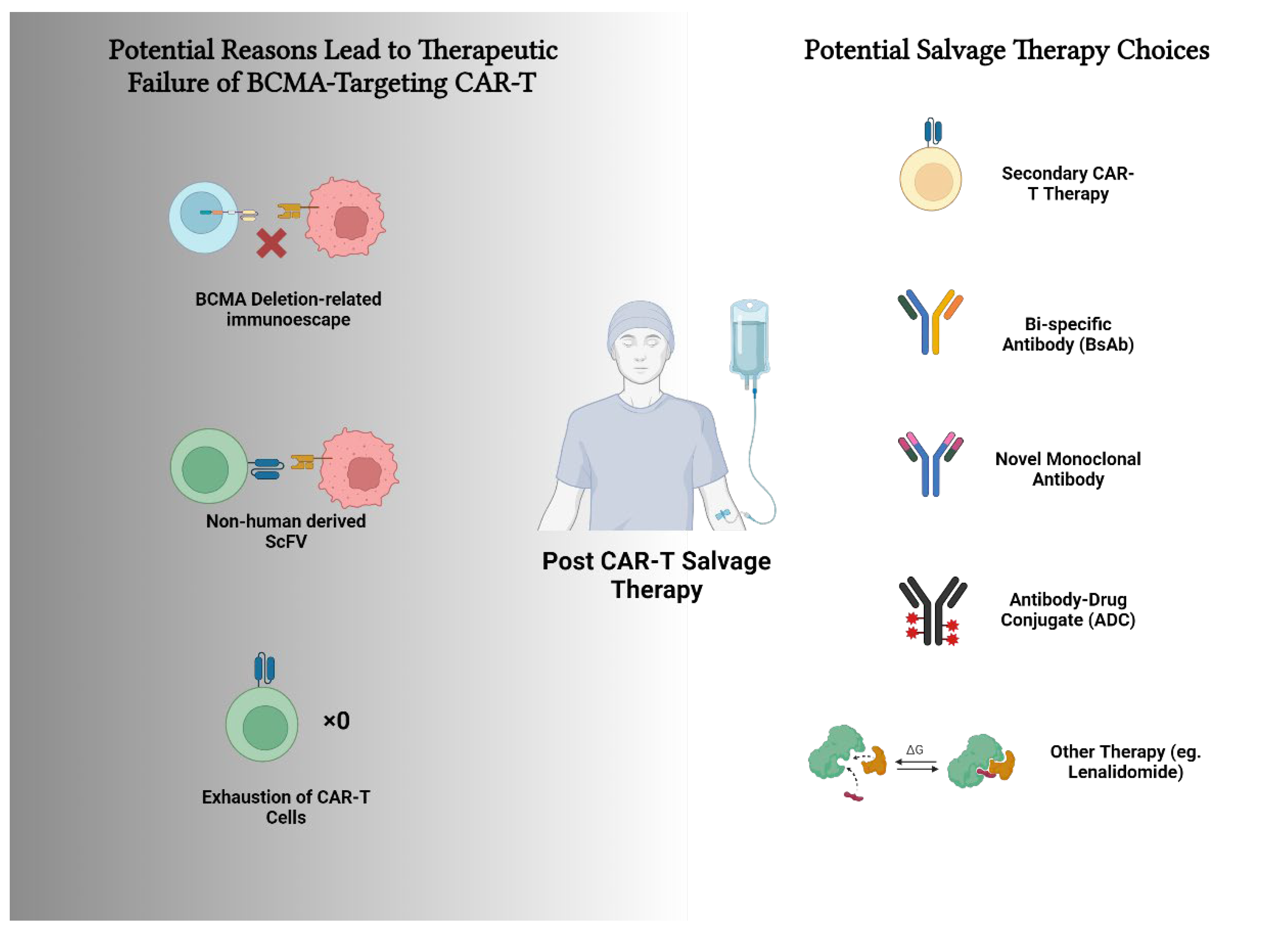
Visual Abstract
1. Introduction
2. Literature Review
2.1. Current Landscape of CAR-T Cell Therapy towards Multiple Myeloma
2.2. Potential Factors Contributing to CAR-T Therapy Failure
2.3. Salvage Therapies
2.3.1. Secondary CAR-T Cell Therapy
2.3.2. Bispecific Antibodies
2.3.3. Novel Monoclonal Antibodies (PD-1/PD-L1)
2.3.4. Antibody-Drug Conjugates (ADCs)
2.3.5. Other Treatments
2.4. Recommended Strategies
2.4.2. Exploring CAR-T Cells with Different Targets and Origins
2.4.3. Rapid-Manufactured CAR-T
2.4.4. Sequential Selection of T-Cell Redirecting Therapies
2.4.5. Combination Therapy
2.4.6. Maintenance Therapy
3. Conclusions
Conflict of Interests
Acknowledgments
References
- Xia, C.; Dong, X.; Li, H.; Cao, M.; Sun, D.; He, S.; Yang, F.; Yan, X.; Zhang, S.; Li, N.; et al. Cancer statistics in China and United States, 2022: profiles, trends, and determinants. Chin Med J (Engl) 2022, 135, 584–590. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.K.; Callander, N.S.; Adekola, K.; Anderson, L.D., Jr.; Baljevic, M.; Baz, R.; Campagnaro, E.; Castillo, J.J.; Costello, C.; D'Angelo, C.; et al. Multiple Myeloma, Version 2.2024, NCCN Clinical Practice Guidelines in Oncology. J Natl Compr Canc Netw 2023, 21, 1281–1301. [Google Scholar] [CrossRef] [PubMed]
- Hu, D.; Chen, L.; Yan, D.; Dong, W.; Chen, M.; Niu, S.; Wang, S.; Zhang, J.; Nie, X.; Fang, Y. Effectiveness and safety of anti-BCMA chimeric antigen receptor T-cell treatment in relapsed/refractory multiple myeloma: a comprehensive review and meta-analysis of prospective clinical trials. Front Pharmacol 2023, 14, 1149138. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Wei, R.; Guo, S.; Min, C.; Zhong, X.; Huang, H.; Cheng, Z. An alternative fully human anti-BCMA CAR-T shows response for relapsed or refractory multiple myeloma with anti-BCMA CAR-T exposures previously. Cancer Gene Ther 2024, 31, 420–426. [Google Scholar] [CrossRef]
- Keller, A.L.; Parzych, S.E.; Reiman, L.T.; Walker, Z.J.; Forsberg, P.A.; Sherbenou, D.W. BCMAxCD3 Bispecific Antibody Elranatamab Is Effective in Patient Myeloma Relapsed after BCMA CAR-T. Blood 2023, 142, 4684. [Google Scholar] [CrossRef]
- Reyes, K.R.; Liu, Y.-C.; Huang, C.-Y.; Banerjee, R.; Martin, T.; Shah, N.; Wong, S.W.; Wolf, J.L.; Arora, S.; Chung, A. Clinical Outcomes and Salvage Therapies in Patients with Relapsed/Refractory Multiple Myeloma Following Progression on BCMA-Targeted CAR-T Therapy. Blood 2022, 140, 617–619. [Google Scholar] [CrossRef]
- Van Oekelen, O.; Nath, K.; Mouhieddine, T.H.; Farzana, T.; Aleman, A.; Melnekoff, D.T.; Ghodke-Puranik, Y.; Shah, G.L.; Lesokhin, A.; Giralt, S.; et al. Interventions and outcomes of patients with multiple myeloma receiving salvage therapy after BCMA-directed CAR T therapy. Blood 2023, 141, 756–765. [Google Scholar] [CrossRef]
- Liu, R.; Yang, R.; Xu, X.; Zhao, W.; Wang, F.; Zhang, W.; Lei, B.; Yang, R.; Wang, Y.; He, A.; et al. Outcomes in patients with multiple myeloma receiving salvage treatment after BCMA-specific CAR-T therapy: A retrospective analysis of LEGEND-2. Br J Haematol 2024. [Google Scholar] [CrossRef]
- Snyder, J.; Bellman, P.; Alsaddi, Z.; Alkharabsheh, O.; Paul, B.; Hashmi, H.; Lutfi, F.; Ahmed, N.; Mahmoudjafari, Z.; Abdallah, A.-O.; et al. Patient Outcomes Following First and Second Exposure to BCMA-Directed Therapies Including CAR-T Cell Therapy in Relapsed/Refractory Multiple Myeloma. Transplantation and Cellular Therapy 2024, 30, S394–S395. [Google Scholar] [CrossRef]
- Bernabei, L.; Garfall, A.L.; Melenhorst, J.J.; Lacey, S.F.; Stadtmauer, E.A.; Vogl, D.T.; Gonzalez, V.; Plesa, G.; Young, R.M.; Waxman, A.; et al. PD-1 Inhibitor Combinations As Salvage Therapy for Relapsed/Refractory Multiple Myeloma (MM) Patients Progressing after Bcma-Directed CAR T Cells. Blood 2018, 132, 1973–1973. [Google Scholar] [CrossRef]
- Chung, A.; Huang, C.-Y.; Martin, T.; Wolf, J.L.; Wong, S.W.; Shah, N. Phase II Study of Pembrolizumab in Multiple Myeloma Patients Relapsing after or Refractory to Anti-BCMA CAR-T Therapies. Blood 2022, 140, 12651–12652. [Google Scholar] [CrossRef]
- Jakubowiak, A.J.; Anguille, S.; Karlin, L.; Chari, A.; Schinke, C.; Rasche, L.; San-Miguel, J.; Campagna, M.; Hilder, B.W.; Masterson, T.J.; et al. Updated Results of Talquetamab, a GPRC5D×CD3 Bispecific Antibody, in Patients with Relapsed/Refractory Multiple Myeloma with Prior Exposure to T-Cell Redirecting Therapies: Results of the Phase 1/2 MonumenTAL-1 Study. Blood 2023, 142, 3377. [Google Scholar] [CrossRef]
- Grajales-Cruz, A.F.; Castaneda, O.; Hansen, D.K.; Vazquez-Martinez, M.A.; Blue, B.; Khadka, S.; Liu, H.; Ochoa-Bayona, J.L.; Freeman, C.L.L.; Locke, F.L.; et al. Teclistamab Induces Favorable Responses in Patients with Relapsed and Refractory Multiple Myeloma after Prior BCMA-Directed Therapy. Blood 2023, 142, 3351. [Google Scholar] [CrossRef]
- Ferreri, C.J.; Hildebrandt, M.A.T.; Hashmi, H.; Shune, L.O.; McGuirk, J.P.; Sborov, D.W.; Wagner, C.B.; Kocoglu, M.H.; Rapoport, A.; Atrash, S.; et al. Real-world experience of patients with multiple myeloma receiving ide-cel after a prior BCMA-targeted therapy. Blood Cancer J 2023, 13, 117. [Google Scholar] [CrossRef]
- Popat, R.; Zweegman, S.; Cavet, J.; Yong, K.; Lee, L.; Faulkner, J.; Kotsopoulou, E.; Al-Hajj, M.; Thomas, S.; Cordoba, S.P.; et al. Phase 1 First-in-Human Study of AUTO2, the First Chimeric Antigen Receptor (CAR) T Cell Targeting APRIL for Patients with Relapsed/Refractory Multiple Myeloma (RRMM). Blood 2019, 134, 3112–3112. [Google Scholar] [CrossRef]
- Reyes, K.R.; Liu, Y.C.; Huang, C.Y.; Banerjee, R.; Martin, T.; Wong, S.W.; Wolf, J.L.; Arora, S.; Shah, N.; Chari, A.; et al. Salvage therapies including retreatment with BCMA-directed approaches after BCMA CAR-T relapses for multiple myeloma. Blood Adv 2024, 8, 2207–2216. [Google Scholar] [CrossRef]
- Smith, E.L.; Harrington, K.; Staehr, M.; Masakayan, R.; Jones, J.; Long, T.J.; Ng, K.Y.; Ghoddusi, M.; Purdon, T.J.; Wang, X.; et al. GPRC5D is a target for the immunotherapy of multiple myeloma with rationally designed CAR T cells. Sci Transl Med 2019, 11. [Google Scholar] [CrossRef]
- Zhang, X.; Zhang, H.; Lan, H.; Wu, J.; Xiao, Y. CAR-T cell therapy in multiple myeloma: Current limitations and potential strategies. Front Immunol 2023, 14, 1101495. [Google Scholar] [CrossRef]
- Xu, J.; Wang, B.Y.; Yu, S.H.; Chen, S.J.; Yang, S.S.; Liu, R.; Chen, L.J.; Hou, J.; Chen, Z.; Zhao, W.H.; et al. Long-term remission and survival in patients with relapsed or refractory multiple myeloma after treatment with LCAR-B38M CAR T cells: 5-year follow-up of the LEGEND-2 trial. J Hematol Oncol 2024, 17, 23. [Google Scholar] [CrossRef]
- Lin, Y.; Raje, N.S.; Berdeja, J.G.; Siegel, D.S.; Jagannath, S.; Madduri, D.; Liedtke, M.; Rosenblatt, J.; Maus, M.V.; Massaro, M.; et al. Idecabtagene vicleucel for relapsed and refractory multiple myeloma: post hoc 18-month follow-up of a phase 1 trial. Nat Med 2023, 29, 2286–2294. [Google Scholar] [CrossRef]
- Li, C.; Wang, D.; Song, Y.; Huang, H.; Li, J.; Chen, B.; Liu, J.; Dong, Y.; Hu, K.; Liu, P.; et al. CT103A, a novel fully human BCMA-targeting CAR-T cells, in patients with relapsed/refractory multiple myeloma: Updated results of phase 1b/2 study (FUMANBA-1). Journal of Clinical Oncology 2023, 41, 8025–8025. [Google Scholar] [CrossRef]
- Fu, C.; Chen, W.; Cai, Z.; Yan, L.; Wang, H.; Shang, J.; Wu, Y.; Yan, S.; Gao, W.; Shi, X.; et al. Three-Year Follow-up on Efficacy and Safety Results from Phase 1 Lummicar Study 1 of Zevorcabtagene Autoleucel in Chinese Patients with Relapsed or Refractory Multiple Myeloma. Blood 2023, 142, 4845–4845. [Google Scholar] [CrossRef]
- Hashmi, H.; Hansen, D.K.; Peres, L.C.; Puglianini, O.C.; Freeman, C.; De Avila, G.; Sidana, S.; Shune, L.; Sborov, D.W.; Davis, J.; et al. Factors associated with refractoriness or early progression after idecabtagene vicleucel in patients with relapsed/ refractory multiple myeloma: US Myeloma Immunotherapy Consortium real world experience. Haematologica 2024, 109, 1514–1524. [Google Scholar] [CrossRef] [PubMed]
- Huang, H.; Hu, Y.; Zhang, M.; Wei, G.; Zhou, L.; Fu, S.; Feng, J.; Hong, R.; Cui, J.; Huang, S.; et al. OriCAR-017, a novel GPRC5D-targeting CAR-T, in patients with relapsed/refractory multiple myeloma: Long term follow-up results of phase 1 study (POLARIS). 2024, 42, 7511–7511. [CrossRef]
- Xia, J.; Li, H.; Yan, Z.; Zhou, D.; Wang, Y.; Qi, Y.; Cao, J.; Li, D.; Cheng, H.; Sang, W.; et al. Anti-G Protein-Coupled Receptor, Class C Group 5 Member D Chimeric Antigen Receptor T Cells in Patients With Relapsed or Refractory Multiple Myeloma: A Single-Arm, Phase Ⅱ Trial. J Clin Oncol 2023, 41, 2583–2593. [Google Scholar] [CrossRef]
- Mei, H.; Li, C.; Jiang, H.; Zhao, X.; Huang, Z.; Jin, D.; Guo, T.; Kou, H.; Liu, L.; Tang, L.; et al. A bispecific CAR-T cell therapy targeting BCMA and CD38 in relapsed or refractory multiple myeloma. J Hematol Oncol 2021, 14, 161. [Google Scholar] [CrossRef]
- Li, C.; Xu, J.; Luo, W.; Liao, D.; Xie, W.; Wei, Q.; Zhang, Y.; Wang, X.; Wu, Z.; Kang, Y.; et al. Bispecific CS1-BCMA CAR-T cells are clinically active in relapsed or refractory multiple myeloma. Leukemia 2024, 38, 149–159. [Google Scholar] [CrossRef]
- Du, J.; Fu, W.-J.; Jiang, H.; Dong, B.; Gao, L.; Liu, L.; Ge, J.; He, A.; Li, L.; Lu, J.J.J.C.O. Updated results of a phase I, open-label study of BCMA/CD19 dual-targeting fast CAR-T GC012F for patients with relapsed/refractory multiple myeloma (RRMM). 2023, 41, 8005.
- Da Via, M.C.; Dietrich, O.; Truger, M.; Arampatzi, P.; Duell, J.; Heidemeier, A.; Zhou, X.; Danhof, S.; Kraus, S.; Chatterjee, M.; et al. Homozygous BCMA gene deletion in response to anti-BCMA CAR T cells in a patient with multiple myeloma. Nat Med 2021, 27, 616–619. [Google Scholar] [CrossRef]
- Perica, K.; Nataraj, S.; Sjojstrand, M.L.; Nagel, K.; Pavkovic, E.; Payson, E.; Cuenca, A.; Patel, A.; Chung, D.; Mailankody, S.; et al. Low Target Antigen Expression Mediates Resistance to BCMA CAR T Cell Therapy. Blood 2023, 142, 2124. [Google Scholar] [CrossRef]
- Mailankody, S.; Devlin, S.M.; Landa, J.; Nath, K.; Diamonte, C.; Carstens, E.J.; Russo, D.; Auclair, R.; Fitzgerald, L.; Cadzin, B.; et al. GPRC5D-Targeted CAR T Cells for Myeloma. N Engl J Med 2022, 387, 1196–1206. [Google Scholar] [CrossRef]
- Zhang, M.; Wei, G.; Zhou, L.; Zhou, J.; Chen, S.; Zhang, W.; Wang, D.; Luo, X.; Cui, J.; Huang, S.; et al. GPRC5D CAR T cells (OriCAR-017) in patients with relapsed or refractory multiple myeloma (POLARIS): a first-in-human, single-centre, single-arm, phase 1 trial. Lancet Haematol 2023, 10, e107–e116. [Google Scholar] [CrossRef]
- Caillot, L.; Sleiman, E.; Lafon, I.; Chretien, M.-L.; Gueneau, P.; Payssot, A.; Pedri, R.; Lakomy, D.; Bailly, F.; Guy, J.; et al. Early Chimeric Antigen Receptor T Cell Expansion Is Associated with Prolonged Progression-Free Survival for Patients with Relapsed/Refractory Multiple Myeloma Treated with Ide-Cel: A Retrospective Monocentric Study. Transplantation and Cellular Therapy 2024. [Google Scholar] [CrossRef] [PubMed]
- Lam, N.; Trinklein, N.D.; Buelow, B.; Patterson, G.H.; Ojha, N.; Kochenderfer, J.N. Anti-BCMA chimeric antigen receptors with fully human heavy-chain-only antigen recognition domains. Nat Commun 2020, 11, 283. [Google Scholar] [CrossRef] [PubMed]
- Zelle-Rieser, C.; Thangavadivel, S.; Biedermann, R.; Brunner, A.; Stoitzner, P.; Willenbacher, E.; Greil, R.; Johrer, K. T cells in multiple myeloma display features of exhaustion and senescence at the tumor site. J Hematol Oncol 2016, 9, 116. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez-Marquez, P.; Calleja-Cervantes, M.E.; Serrano, G.; Oliver-Caldes, A.; Palacios-Berraquero, M.L.; Martin-Mallo, A.; Calvino, C.; Espanol-Rego, M.; Ceballos, C.; Lozano, T.; et al. CAR density influences antitumoral efficacy of BCMA CAR T cells and correlates with clinical outcome. Sci Adv 2022, 8, eabo0514. [Google Scholar] [CrossRef]
- Gandhi, U.H.; Cornell, R.F.; Lakshman, A.; Gahvari, Z.J.; McGehee, E.; Jagosky, M.H.; Gupta, R.; Varnado, W.; Fiala, M.A.; Chhabra, S.; et al. Outcomes of patients with multiple myeloma refractory to CD38-targeted monoclonal antibody therapy. Leukemia 2019, 33, 2266–2275. [Google Scholar] [CrossRef]
- Chinese Medical Doctor Association, H.B.; Chinese Society of Hematology, C.M.A. [The Chinese consensus for the CAR-T cell therapy in multiple myeloma (2022 version)]. Zhonghua Xue Ye Xue Za Zhi 2022, 43, 265–271. [Google Scholar] [CrossRef]
- Subramanian, N.G.; Garcia Pleitez, H.; Pasvolsky, O.; Kalariya, N.; Patel, K.K.; Ferreri, C.J. Clinical outcomes of patients with multiple myeloma following disease progression after BCMA-targeted CAR T-cell therapy. Journal of Clinical Oncology 2023, 41, e20002–e20002. [Google Scholar] [CrossRef]
- Brudno, J.N.; Maric, I.; Hartman, S.D.; Rose, J.J.; Wang, M.; Lam, N.; Stetler-Stevenson, M.; Salem, D.; Yuan, C.; Pavletic, S.; et al. T Cells Genetically Modified to Express an Anti-B-Cell Maturation Antigen Chimeric Antigen Receptor Cause Remissions of Poor-Prognosis Relapsed Multiple Myeloma. J Clin Oncol 2018, 36, 2267–2280. [Google Scholar] [CrossRef] [PubMed]
- Guoxing, Z.; CHENG, Z.; Runhong, W.; Yi, W.; Lei, F.; Qiuling, M.; Xianhui, L. Efficacy and safety of anti-B cell maturation antigen chimeric antigen receptor T-cell for retreatment of relapsed/refractory multiple myeloma. Journal of Leukemia Lymphoma 2022, 229–234. [Google Scholar] [CrossRef]
- Bal, S.; Htut, M.; Nadeem, O.; Anderson, L.D.; Koçoğlu, H.; Gregory, T.; Rossi, A.C.; Martin, T.; Egan, D.N.; Costa, L.; et al. BMS-986393 (CC-95266), a G Protein-Coupled Receptor Class C Group 5 Member D (GPRC5D)-Targeted Chimeric Antigen Receptor (CAR) T-Cell Therapy for Relapsed/Refractory Multiple Myeloma (RRMM): Updated Results from a Phase 1 Study. Blood 2023, 142, 219. [Google Scholar] [CrossRef]
- Shi, M.; Wang, J.; Huang, H.; Liu, D.; Cheng, H.; Wang, X.; Chen, W.; Yan, Z.; Sang, W.; Qi, K.; et al. Bispecific CAR T cell therapy targeting BCMA and CD19 in relapsed/refractory multiple myeloma: a phase I/II trial. Nat Commun 2024, 15, 3371. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Wang, X.; Xu, J.; Liu, J.; Mei, H. Treatment of multiple myeloma: What is the impact on T-cell function? Ther Adv Hematol 2024, 15, 20406207241245194. [Google Scholar] [CrossRef] [PubMed]
- Metelo, A.M.; Jozwik, A.; Luong, L.A.; Dominey-Foy, D.; Graham, C.; Attwood, C.; Inam, S.; Dunlop, A.; Sanchez, K.; Cuthill, K.; et al. Allogeneic Anti-BCMA CAR T Cells Are Superior to Multiple Myeloma-derived CAR T Cells in Preclinical Studies and May Be Combined with Gamma Secretase Inhibitors. Cancer Res Commun 2022, 2, 158–171. [Google Scholar] [CrossRef] [PubMed]
- Dholaria, B.; Shune, L.; Kin, A.; McArthur, K.; Eskew, J.D.; Martin, C.E.; Haag, S.; McCaigue, J.; Namini, H.; DePrimo, S.; et al. Abstract CT071: Clinical activity of P-BCMA-ALLO1, a B-cell maturation antigen (BCMA) targeted allogeneic chimeric antigen receptor T-cell (CAR-T) therapy, in relapsed refractory multiple myeloma (RRMM) patients (pts) following progression on prior BCMA targeting therapy. Cancer Research %J Cancer Research 2024, 84, CT071–CT071. [Google Scholar] [CrossRef]
- P. Moreau, A.L. P. Moreau, A.L. Garfall, N. van de Donk, H. Nahi, J.F. San-Miguel, A. Oriol, A.K. Nooka, T. Martin, L. Rosinol, A. Chari, L. Karlin, L. Benboubker, M.V. Mateos, N. Bahlis, R. Popat, B. Besemer, J. Martinez-Lopez, S. Sidana, M. Delforge, L. Pei, D. Trancucci, R. Verona, S. Girgis, S.X.W. Lin, Y. Olyslager, M. Jaffe, C. Uhlar, T. Stephenson, R. Van Rampelbergh, A. Banerjee, J.D. Goldberg, R. Kobos, A. Krishnan, and S.Z. Usmani, Teclistamab in Relapsed or Refractory Multiple Myeloma. N Engl J Med 387 (2022) 495-505.
- Chari, M.C. Minnema, J.G. Berdeja, A. Oriol, N. van de Donk, P. Rodriguez-Otero, E. Askari, M.V. Mateos, L.J. Costa, J. Caers, R. Verona, S. Girgis, S. Yang, R.B. Goldsmith, X. Yao, K. Pillarisetti, B.W. Hilder, J. Russell, J.D. Goldberg, and A. Krishnan, Talquetamab, a T-Cell-Redirecting GPRC5D Bispecific Antibody for Multiple Myeloma. N Engl J Med 387 (2022) 2232-2244.
- A.M. Lesokhin, M.H. A.M. Lesokhin, M.H. Tomasson, B. Arnulf, N.J. Bahlis, H. Miles Prince, R. Niesvizky, P. Rodriotaguez-Otero, J. Martinez-Lopez, G. Koehne, C. Touzeau, Y. Jethava, H. Quach, J. Depaus, H. Yokoyama, A.E. Gabayan, D.A. Stevens, A.K. Nooka, S. Manier, N. Raje, S. Iida, M.S. Raab, E. Searle, E. Leip, S.T. Sullivan, U. Conte, M. Elmeliegy, A. Czibere, A. Viqueira, and M. Mohty, Elranatamab in relapsed or refractory multiple myeloma: phase 2 MagnetisMM-3 trial results. Nat Med 29 (2023) 2259-2267.
- Varshavsky-Yanovsky, A.; Jethava, Y.; Stevens, D.A.; Nooka, A.K.; Stiff, P.J.; Perez-Cruz, I.; Leip, E.; Lesokhin, A. Efficacy and Safety of Elranatamab in Black Patients with Relapsed/Refractory Multiple Myeloma (RRMM): A Subgroup Analysis of the Magnetismm Studies. Blood 2023, 142, 3333. [Google Scholar] [CrossRef]
- Trudel, S.; Cohen, A.D.; Krishnan, A.Y.; Fonseca, R.; Spencer, A.; Berdeja, J.G.; Lesokhin, A.; Forsberg, P.A.; Laubach, J.P.; Costa, L.J.; et al. Cevostamab Monotherapy Continues to Show Clinically Meaningful Activity and Manageable Safety in Patients with Heavily Pre-Treated Relapsed/Refractory Multiple Myeloma (RRMM): Updated Results from an Ongoing Phase I Study. Blood 2021, 138, 157–157. [Google Scholar] [CrossRef]
- Schinke, C.D.; Touzeau, C.; Minnema, M.C.; van de Donk, N.W.; Rodríguez-Otero, P.; Mateos, M.-V.; Rasche, L.; Ye, J.C.; Vishwamitra, D.; Ma, X. Pivotal phase 2 MonumenTAL-1 results of talquetamab (tal), a GPRC5DxCD3 bispecific antibody (BsAb), for relapsed/refractory multiple myeloma (RRMM). 2023.
- Mouhieddine, T.H.; Van Oekelen, O.; Melnekoff, D.T.; Li, J.; Ghodke-Puranik, Y.; Lancman, G.; Thibaud, S.; Pan, D.; Rajeeve, S.; Agte, S.; et al. Sequencing T-cell redirection therapies leads to deep and durable responses in patients with relapsed/refractory myeloma. Blood Adv 2023, 7, 1056–1064. [Google Scholar] [CrossRef]
- Banerjee, R.; Lynch, R.C.; Wu, Q.V.; Simon, S.; Ujjani, C.S.; Till, B.G.; Wuliji, N.; Gausman, D.; Dizon, J.; Kwok, M.L.; et al. Post-CAR-T Checkpoint Inhibition Can Result in Durable Responses in a Minority of Patients with Multiple Myeloma (MM) or Non-Hodgkin's Lymphoma (NHL): Results of a Phase 2 Study of Nivolumab after CAR-T Failure. Transplantation and Cellular Therapy 2024, 30, S399–S400. [Google Scholar] [CrossRef]
- Vaxman, I.; Abeykoon, J.; Dispenzieri, A.; Kumar, S.K.; Buadi, F.; Lacy, M.Q.; Dingli, D.; Hwa, Y.; Fonder, A.; Hobbs, M.; et al. "Real-life" data of the efficacy and safety of belantamab mafodotin in relapsed multiple myeloma-the Mayo Clinic experience. Blood Cancer J 2021, 11, 196. [Google Scholar] [CrossRef]
- Gazeau, N.; Beauvais, D.; Yakoub-Agha, I.; Mitra, S.; Campbell, T.B.; Facon, T.; Manier, S. Effective anti-BCMA retreatment in multiple myeloma. Blood Adv 2021, 5, 3016–3020. [Google Scholar] [CrossRef]
- Agte, S.; Elnaggar, M.; Adamopolous, C.; Melnekoff, D.; Adleman, A.; Kappes, K.; Restrepo, P.; Oekelen, O.V.; Leshchenko, V.; Poulikakos, P.I.; et al. P-090: BRAF V600E multiple myeloma patient salvaged with triple MAPK inhibition after CAR T relapse. Clinical Lymphoma Myeloma and Leukemia 2021, 21, S88. [Google Scholar] [CrossRef]
- Qian, Y.; Qian, Z.; Zhao, X.; Pan, W.; Wei, X.; Meng, H.; Yang, L.; Xiao, H. Successful Treatment of Relapsed/Refractory Extramedullary Multiple Myeloma With Anti-BCMA CAR-T Cell Therapy Followed by Haploidentical Hematopoietic Stem Cell Transplantation: A Case Report and a Review of the Contemporary Literature. Front Med (Lausanne) 2021, 8, 649824. [Google Scholar] [CrossRef] [PubMed]
- Garfall, A.L.; Dancy, E.K.; Cohen, A.D.; Hwang, W.T.; Fraietta, J.A.; Davis, M.M.; Levine, B.L.; Siegel, D.L.; Stadtmauer, E.A.; Vogl, D.T.; et al. T-cell phenotypes associated with effective CAR T-cell therapy in postinduction vs relapsed multiple myeloma. Blood Adv 2019, 3, 2812–2815. [Google Scholar] [CrossRef]
- Dima, D.; Ullah, F.; Mazzoni, S.; Williams, L.; Faiman, B.; Kurkowski, A.; Chaulagain, C.; Raza, S.; Samaras, C.; Valent, J.; et al. Management of Relapsed-Refractory Multiple Myeloma in the Era of Advanced Therapies: Evidence-Based Recommendations for Routine Clinical Practice. Cancers (Basel) 2023, 15. [Google Scholar] [CrossRef]
- Abecassis, A.; Roders, N.; Fayon, M.; Choisy, C.; Nelson, E.; Harel, S.; Royer, B.; Villesuzanne, C.; Talbot, A.; Garrick, D.; et al. CAR-T cells derived from multiple myeloma patients at diagnosis have improved cytotoxic functions compared to those produced at relapse or following daratumumab treatment. EJHaem 2022, 3, 970–974. [Google Scholar] [CrossRef]
- Rodriguez-Otero, P.; Ailawadhi, S.; Arnulf, B.; Patel, K.; Cavo, M.; Nooka, A.K.; Manier, S.; Callander, N.; Costa, L.J.; Vij, R.; et al. Ide-cel or Standard Regimens in Relapsed and Refractory Multiple Myeloma. N Engl J Med 2023, 388, 1002–1014. [Google Scholar] [CrossRef]
- Wu, J.F.; Dhakal, B. BCMA-targeted CAR-T cell therapies in relapsed and/or refractory multiple myeloma: latest updates from 2023 ASCO Annual Meeting. J Hematol Oncol 2023, 16, 86. [Google Scholar] [CrossRef] [PubMed]
- Berdeja, J.G.; Madduri, D.; Usmani, S.Z.; Jakubowiak, A.; Agha, M.; Cohen, A.D.; Stewart, A.K.; Hari, P.; Htut, M.; Lesokhin, A.; et al. Ciltacabtagene autoleucel, a B-cell maturation antigen-directed chimeric antigen receptor T-cell therapy in patients with relapsed or refractory multiple myeloma (CARTITUDE-1): a phase 1b/2 open-label study. Lancet 2021, 398, 314–324. [Google Scholar] [CrossRef]
- Lin, Q.; Zhao, J.; Song, Y.; Liu, D. Recent updates on CAR T clinical trials for multiple myeloma. Mol Cancer 2019, 18, 154. [Google Scholar] [CrossRef]
- Ahmed, N.; Wesson, W.; Mushtaq, M.U.; Bansal, R.; AbdelHakim, H.; Bromert, S.; Appenfeller, A.; Ghazal, B.A.; Singh, A.; Abhyankar, S.; et al. "Waitlist mortality" is high for myeloma patients with limited access to BCMA therapy. Front Oncol 2023, 13, 1206715. [Google Scholar] [CrossRef]
- Afrough, A.; Hashmi, H.; Hansen, D.K.; Sidana, S.; Ahn, C.; Dima, D.; Freeman, C.L.; Castaneda Puglianini, O.A.; Kocoglu, M.H.; Atrash, S.; et al. Impact of bridging therapy (BT) on outcome of relapsed refractory multiple myeloma (RRMM) with Ide-cel CAR T-cell therapy: Real-world experience from the US myeloma CAR T consortium. Journal of Clinical Oncology 2023, 41, 8013–8013. [Google Scholar] [CrossRef]
- Sperling, A.S.; Derman, B.A.; Nikiforow, S.; Im, S.-Y.; Ikegawa, S.; Prabhala, R.H.; Rodriguez, D.H.; Li, Y.; Quinn, D.S.; Pearson, D.; et al. Updated phase I study results of PHE885, a T-Charge manufactured BCMA-directed CAR-T cell therapy, for patients (pts) with r/r multiple myeloma (RRMM). 2023, 41, 8004–8004. [CrossRef]
- Costa, L.J.; Kumar, S.K.; Atrash, S.; Liedtke, M.; Kaur, G.; Derman, B.A.; Bergsagel, P.L.; Mailankody, S.; McCarthy, P.L.; Limones, J.; et al. Results from the First Phase 1 Clinical Study of the B-Cell Maturation Antigen (BCMA) Nex T Chimeric Antigen Receptor (CAR) T Cell Therapy CC-98633/BMS-986354 in Patients (pts) with Relapsed/Refractory Multiple Myeloma (RRMM). Blood 2022, 140, 1360–1362. [Google Scholar] [CrossRef]
- Liu, H.; Wang, T.; Yang, Y.; Feng, R.; Li, J.; Zhang, C.; Bai, J.; Ding, Y.; Liu, G.; Wu, F.; et al. Phase I Study of a BCMA-Directed CAR-T Cell Therapy for Relapsed/Refractory Multiple Myeloma Manufactured in <3 Days Using the High-Performance Platform. Blood 2022, 140, 10307–10308. [Google Scholar] [CrossRef]
- Choi, T.; Kang, Y. Chimeric antigen receptor (CAR) T-cell therapy for multiple myeloma. Pharmacol Ther 2022, 232, 108007. [Google Scholar] [CrossRef] [PubMed]
- Kegyes, D.; Constantinescu, C.; Vrancken, L.; Rasche, L.; Gregoire, C.; Tigu, B.; Gulei, D.; Dima, D.; Tanase, A.; Einsele, H.; et al. Patient selection for CAR T or BiTE therapy in multiple myeloma: Which treatment for each patient? J Hematol Oncol 2022, 15, 78. [Google Scholar] [CrossRef]
- Dima, D.; Rashid, A.; Davis, J.A.; Shune, L.; Abdallah, A.O.; Li, H.; DeJarnette, S.; Khouri, J.; Williams, L.; Hashmi, H.; et al. Efficacy and safety of idecabtagene vicleucel in patients with relapsed-refractory multiple myeloma not meeting the KarMMa-1 trial eligibility criteria: A real-world multicentre study. Br J Haematol 2024, 204, 1293–1299. [Google Scholar] [CrossRef]
- Cohen, A.D.; Mateos, M.V.; Cohen, Y.C.; Rodriguez-Otero, P.; Paiva, B.; van de Donk, N.; Martin, T.; Suvannasankha, A.; De Braganca, K.C.; Corsale, C.; et al. Efficacy and safety of cilta-cel in patients with progressive multiple myeloma after exposure to other BCMA-targeting agents. Blood 2023, 141, 219–230. [Google Scholar] [CrossRef]
- Wang, X.; Walter, M.; Urak, R.; Weng, L.; Huynh, C.; Lim, L.; Wong, C.W.; Chang, W.C.; Thomas, S.H.; Sanchez, J.F.; et al. Lenalidomide Enhances the Function of CS1 Chimeric Antigen Receptor-Redirected T Cells Against Multiple Myeloma. Clin Cancer Res 2018, 24, 106–119. [Google Scholar] [CrossRef]
- Works, M.; Soni, N.; Hauskins, C.; Sierra, C.; Baturevych, A.; Jones, J.C.; Curtis, W.; Carlson, P.; Johnstone, T.G.; Kugler, D.; et al. Anti-B-cell Maturation Antigen Chimeric Antigen Receptor T cell Function against Multiple Myeloma Is Enhanced in the Presence of Lenalidomide. Mol Cancer Ther 2019, 18, 2246–2257. [Google Scholar] [CrossRef]
- Zhao, G.; Wei, R.; Feng, L.; Wu, Y.; He, F.; Xiao, M.; Cheng, Z. Lenalidomide enhances the efficacy of anti-BCMA CAR-T treatment in relapsed/refractory multiple myeloma: a case report and revies of the literature. Cancer Immunol Immunother 2022, 71, 39–44. [Google Scholar] [CrossRef]
- Pont, M.J.; Hill, T.; Cole, G.O.; Abbott, J.J.; Kelliher, J.; Salter, A.I.; Hudecek, M.; Comstock, M.L.; Rajan, A.; Patel, B.K.R.; et al. gamma-Secretase inhibition increases efficacy of BCMA-specific chimeric antigen receptor T cells in multiple myeloma. Blood 2019, 134, 1585–1597. [Google Scholar] [CrossRef] [PubMed]
- Cowan, A.J.; Pont, M.J.; Sather, B.D.; Turtle, C.J.; Till, B.G.; Libby, E.N., 3rd; Coffey, D.G.; Tuazon, S.A.; Wood, B.; Gooley, T.; et al. gamma-Secretase inhibitor in combination with BCMA chimeric antigen receptor T-cell immunotherapy for individuals with relapsed or refractory multiple myeloma: a phase 1, first-in-human trial. Lancet Oncol 2023, 24, 811–822. [Google Scholar] [CrossRef] [PubMed]
- Aleman, A.; Kogan-Zajdman, A.; Upadhyaya, B.; Van Oekelen, O.; Chen, L.; Leshchenko, V.; Jagannath, S.; Parekh, S. P-175 Improving Anti-BCMA CAR-T functionality with novel immunomodulatory agent Iberdomide (CC220) in Multiple Myeloma. Clinical Lymphoma Myeloma and Leukemia 2023, 23, S131–S132. [Google Scholar] [CrossRef]
- Hassan, H.; Samur, M.K.; Cohen, A.D.; Moreau, P.; Rodríguez Otero, P.; Kumar, S.K.; Mateos, M.V.; Avigan, D.; Sperling, A.S.; Nadeem, O.; et al. Heterogeneity in Access and Toxicity Management of Commercially Available BCMA-Directed CAR-T and Bispecific T-Cell Engager Therapy Among the International Myeloma Community. Blood 2023, 142, 7248–7248. [Google Scholar] [CrossRef]
- Hashmi, H.; Kumar, A.; Kharfan-Dabaja, M.A.; Munshi, P.N.; Inamoto, Y.; DeFilipp, Z.M.; Dholaria, B.; Jain, T.; Perales, M.A.; Carpenter, P.A.; et al. ASTCT Committee on Practice Guidelines Survey on Evaluation & Management of Relapsed Refractory Multiple Myeloma after Failure of Chimeric Antigen Receptor T-Cell Therapy. Transplant Cell Ther 2024. [Google Scholar] [CrossRef]
- Deng, J.; Lin, Y.; Zhao, D.; Tong, C.; Chang, A.H.; Chen, W.; Gao, W. Case report: Plasma cell leukemia secondary to multiple myeloma successfully treated with anti-BCMA CAR-T cell therapy. Front Oncol 2022, 12, 901266. [Google Scholar] [CrossRef]
- Arnulf, B.; Kerre, T.; Agha, M.E.; Delforge, M.; Braunschweig, I.; Shah, N.; Richard, S.; Alsina, M.; Einsele, H.; Mistry, P.; et al. Efficacy and safety of ciltacabtagene autoleucel ± lenalidomide maintenance in newly diagnosed multiple myeloma with suboptimal response to frontline autologous stem cell transplant: CARTITUDE-2 cohort D. 2024, 42, 7505–7505. [CrossRef]
| Trial Code/Number | CAR-T Product | CAR-T Target | Median Follow-up (months) | Efficacy | Safety | |||
|---|---|---|---|---|---|---|---|---|
| ORR | Median PFS (months) | Median OS (months) | CRS | ICANS | ||||
| LEGEND-2 (NCT03090659) |
LCAR-B38M [19] | BCMA | 65.4 | 87.8% | 18 | 55.8 | - | - |
| CRB401 (NCT02658929) |
bb2121 [20] | BCMA | 18.1 (1.5-44.5) | 75.8% | 8.8 (95% CI, 5.9-11.9) | 34.2 (95% CI, 19.2-NE) | 75.8% | - |
| FUMANBA-1 (NCT05066646) |
CT103A [21] | BCMA | 13.8 (0.4-27.2) | 96% | NR | - | - | - |
| LUMMICAR-1 (NCT03975907) |
CT053 [22] | BCMA | 37.7 (14.8-44.2) | 100% | 25 | NR | 92.9% | 0 |
| POLARIS (NCT05016778) |
OriCAR-017 [24] | GPRC5D | >24 (all patients) | 100% | 11.37 (95% CI, 5.93-18.00) | NR | 100% | 0 |
| ChiCTR2100048888 | anti-GPRC5D CAR-T [25] | GPRC5D | 5.2 (3.2-8.9) | 91% | NR | NR | 76% | 6% |
| ChiCTR1800018143 | BM38 CAR-T [26] | BCMA & CD38 | 9.0 (0.5-18.5) | 87% | 17.2 (95% CI, 7.5-26.8) | NR | 87% | - |
| NCT04662099 | CS1-BCMA CAR-T [27] | CS1 & BCMA | 246 days (55-547) | 81% | 9.0 (95% CI, 2.1-NR) | NR | 38% | 0 |
| NCT04236011 & NCT04182581 | GC012F [28] | BCMA & CD19 | 30.7 (14.6-43.6) | 93.1% | 38.0 (95% CI, 11.8-NE) | - | 86.2% | 0 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





