1. Introduction
The carminic acid is a natural dye obtained from the female insects of Dactylopius coccus Costa, a parasite from nopal cactus, known also as cochineal grain.
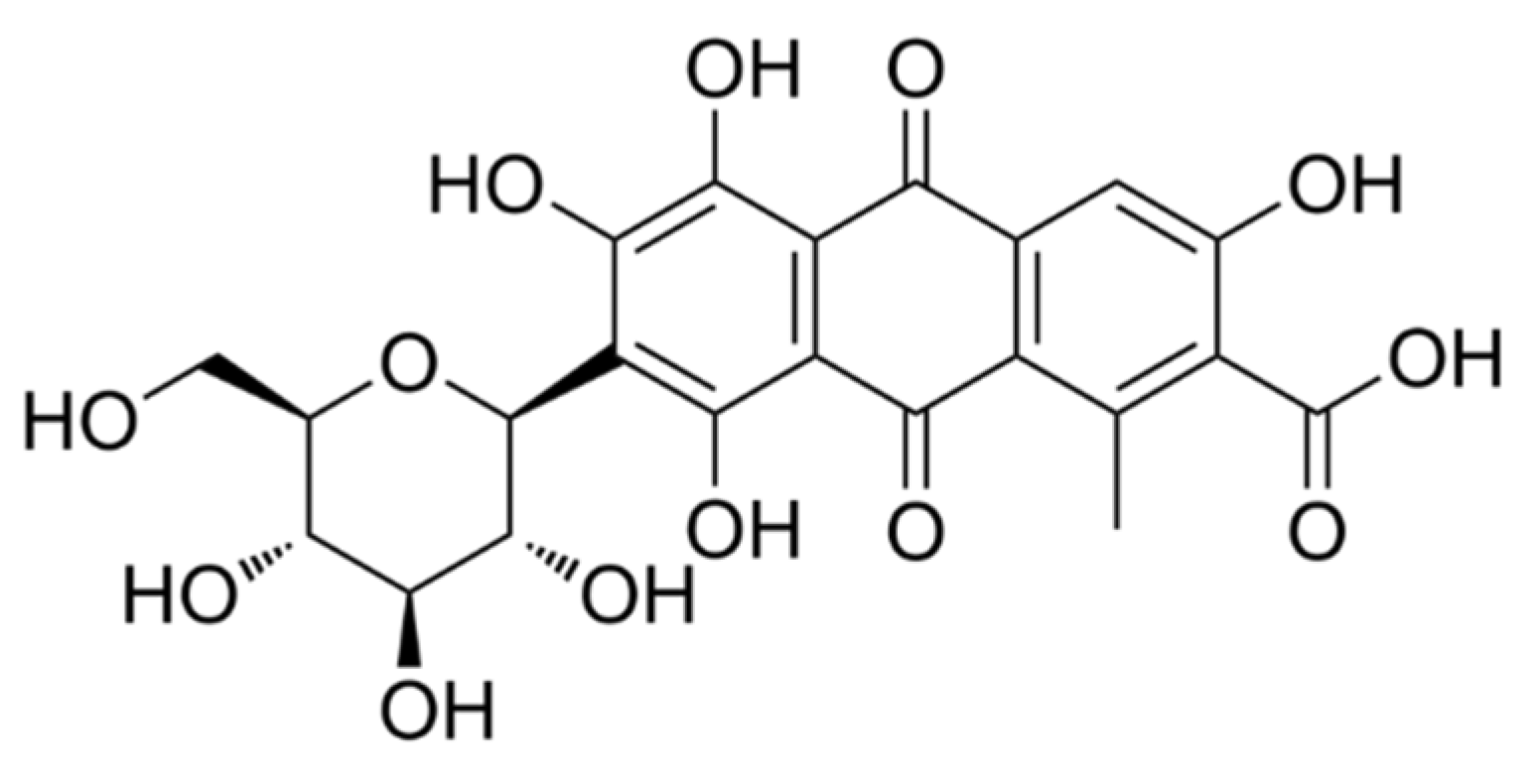
The molecular structure of carminic acid consists of an anthraquinone, a sugar residue and a carboxyl group, as shown in
Figure 1. This dye is an intense red pigment that produces other color schemes from orange to violet under varying pH conditions.
Currently, carmine dye is used at different industries from the food, pharmaceutical, cosmetic and textile sectors, particularly for handcraft [
1,
2]. Different extraction methods have been developed and used to obtain this pigment at industrial level [
3]. However, the major drawbacks shown by these conventional methods are low yields, high energy consumption, low selectivity and efficiency that produce low-quality products, the use of solvents, acids, and alkalis, also the extended processing times that generate more pollution and higher production costs [
4,
5]. To solve these problems at the different extraction stages, green extraction methods are emergently implemented, which involve new inventions, design of extraction processes and solvents application to eliminate the use and generation of dangerous substances. These methods enhance and ensure high quality extraction, some of these include emerging techniques such as ultrasound and microwave [
6,
7].
Ultrasound extraction is based on cavitation defined as the formation, growth, and collapse of microbubbles in the extraction solvent pushing to the processing material, which promotes a higher contact and enhance the mass transfer by the solvent moving inward and outward from the material [
8,
9]. The extraction assisted by microwave is based on the distribution of microwave energy on the material to be processed, penetrating inside, and interacting with the polar components to generate heat, acting directly on the molecules by ionic conduction and dipolar rotation, which allows selective heating of samples as a function of its dielectric constant [
10,
11].
Therefore, the aim of this work was establishing the optimal extraction conditions by ultrasound and microwave techniques, to enhance production yields and efficiency, lowering extraction times specifically of carminic acid.
To evaluate such conditions, a surface response methodology was applied based on a mathematical model for extraction optimizations. It was used a Box-Behnken design to optimize diverse parameters such as solvent amount, processing time and temperature in order to evaluate different variables and interaction effects [
12,
13]. The quantification of cochineal pigments was made by UV spectrophotometry, and the assessment of the carminic acid molecule was performed by FTIR, verifying that it remained unaffected after these processes.
2. Results
2.1. Cochineal Extraction Yield Obtained by the Conventional Method
Cochineal pigment was obtained by the conventional method (MC), further described at the Materials and Methods section, from which it was obtained a pigment yield of 31.9 % within 30 min, with an efficiency of 3.5 mg/ml.
2.2. Fractional Factorial Design
In the present study the effect of three factors was investigated: temperature, irradiation time and solvent volume, and the parameters condition (levels) was based on the knowledge acquired previously. The
Table 1 shows a 2K Factorial design (FD) using temperature (X1), irradiation time (X2) and solvent volume (X3) as independent variables, which were coded at two levels and central point (+1, 0, -1) to determine the ultrasonic and microwave carminic acid extraction conditions, and to compare the performance between conventional and ultrasonication and microwave methods.
Three samples were analyzed for each experimental design. The results of dependent variables were compared through an analysis of variance (ANOVA), from which a linear mathematical model was obtained:
Where Y is the predicted response according to the model (that determines the conditions for carminic acid extraction by microwaves and ultrasonication), β
0 is the intersection term, β
1 is the indicator coefficient of linear effect in response to temperature, β
2 is the indicator coefficient of linear effect in response to irradiation time, β
3 is related to solvent volume, and β
1,2,3 is the response coefficient of the interaction effect between temperature, irradiation time and solvent volume. All calculated coefficients are presented at
Table 2.
2.3. Statistical Analysis
The experimental design allowed us to identify the significative factors that had an influence on the yield of the extractions. It was performed an ANOVA test for each response (Table 3), and the obtained results indicate that carminic acid yields obtained by ultrasound showed a significant difference in the solvent and temperature interactions (P < 0.05). For the case of ANOVA analysis of microwave extractions, results indicate there is a significant difference only at the solvent factor (P < 0.05).
2.4. Analysis of Response Surface Plots
Extraction assisted by ultrasound
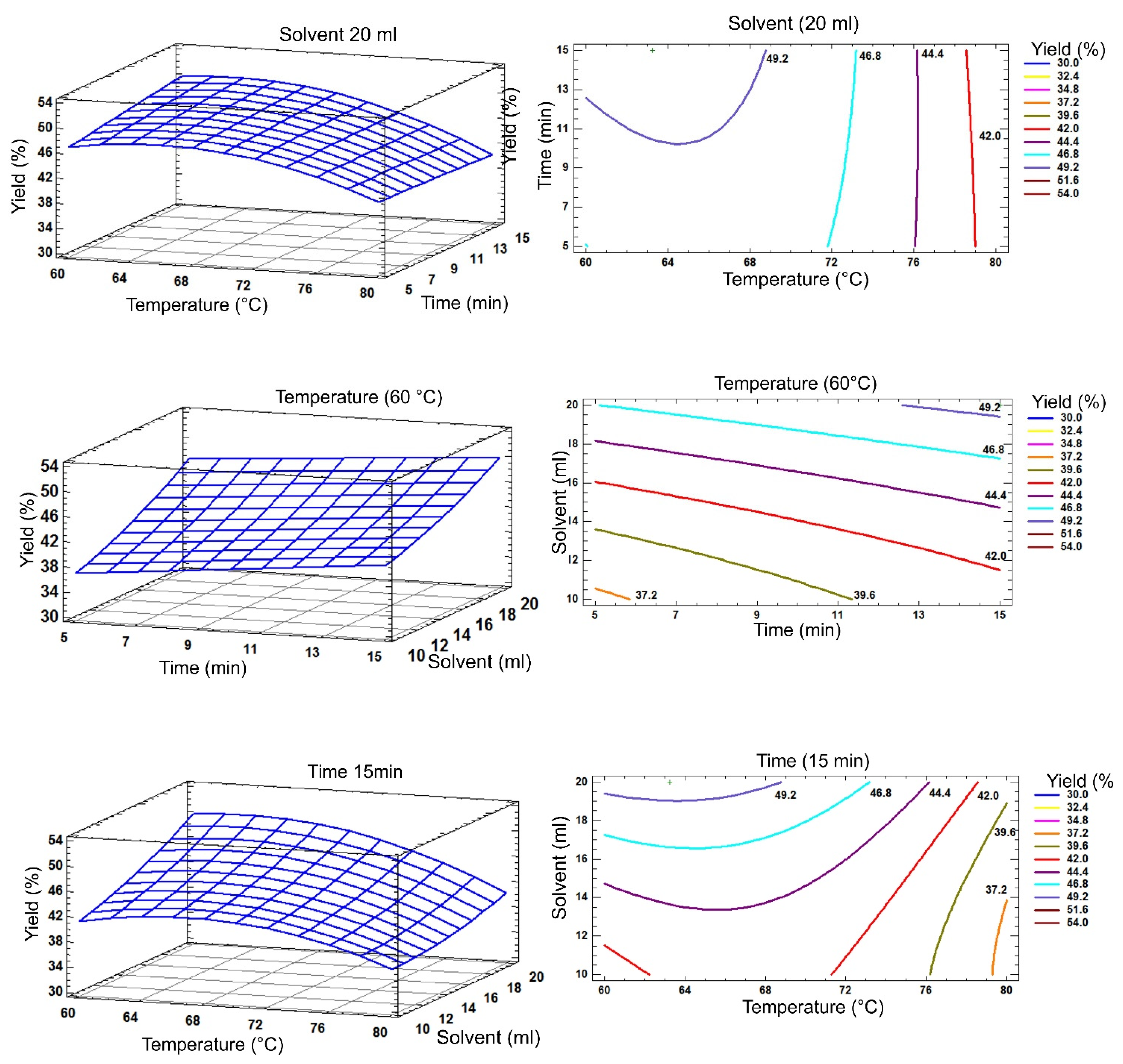
Figure 2 shows the response surface and surface contour plots of the yields obtained by the extraction assisted by ultrasound (EAU). In
Figure 2(b) it is observed that at 60°C for 15 min it is obtained a yield of 49.2% with a solvent ratio of 1:20 g/ml. Then, the surface contour plot indicates that it is possible to obtain this yield ranging from 13 min at 60°C until 15 min at 69 °C, at the same solvent ratio (1:20 g/mL). These results reflect an enhanced extraction yield that was 6.8% higher than the reported by Borges et al. [
4] with the Pressurized Liquid Extraction technique (PLE: 42.4%), and 9.8 % higher than the reported with the Supercritical Fluid Extraction (SFE: 39.4%).
The average levels (Box- Benhken): 70 °C, 10 min and 15 mL solvent were considered, from which the estimated response has a change with a minor modification in the experimental factors. The optimal variables were obtained for the EAU method, predicting that the optimal temperature is 63.2 °C, at 15.0 min, with a sample: solvent ratio of 1:20 g/mL, reaching a yield of 50.3% with an increase of 1.1% from the estimated value.
Extraction assisted by microwave
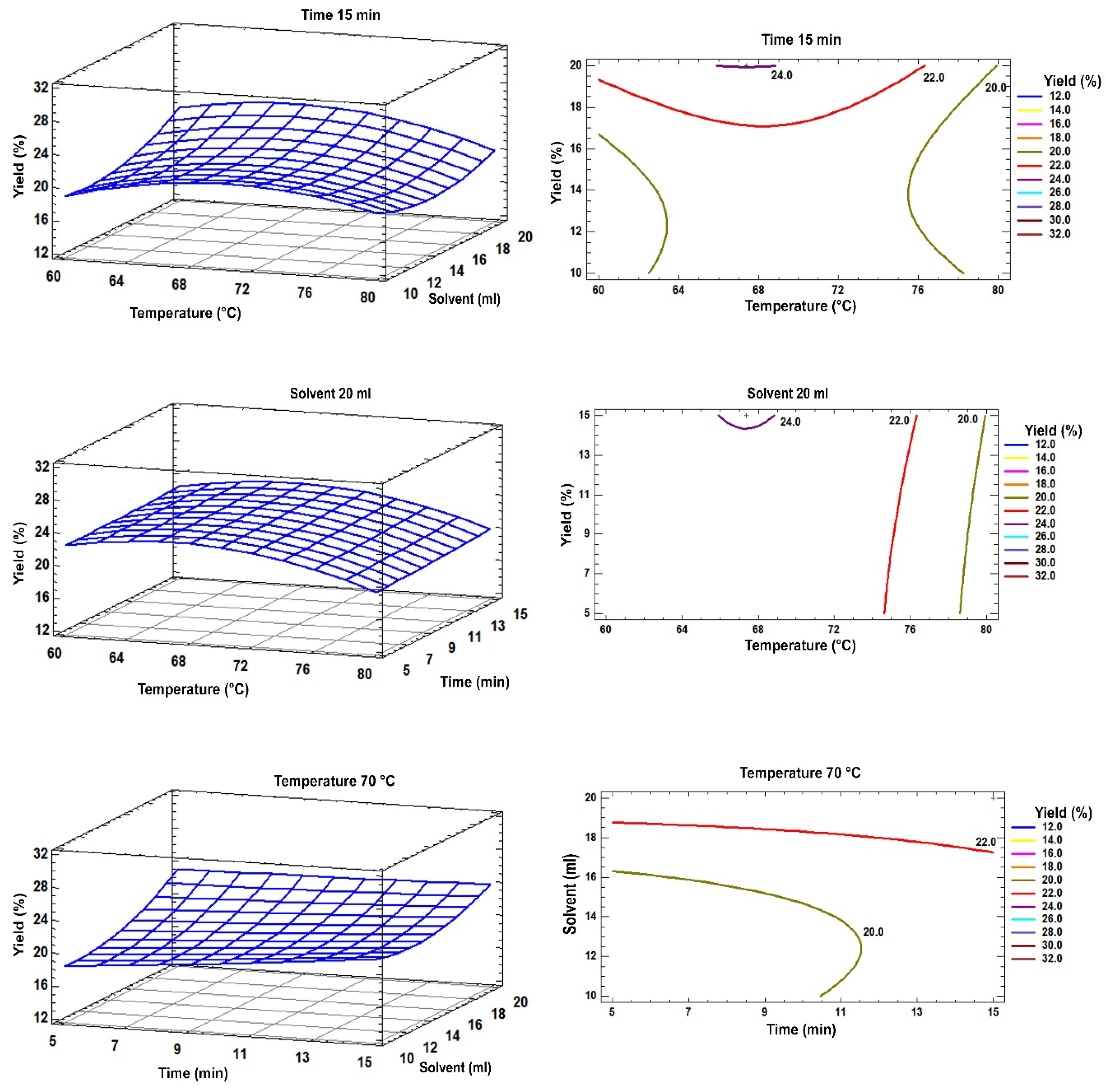
The analysis of variance performed for the obtained results from the extraction assisted by microwave (EAM), indicates there is only a significant difference for the solvent factor (P < 0.05).
The response surface plots estimated for EAM present the highest yield of 41% obtained at 60 °C, 15 min with a solvent ratio of 1:20 g/mL. The surface contour plots clearly show the variation of yield (
Figure 3), showing 1.2% lower yield than the obtained by ELP and 1.6% higher than the reported by Borges et al. by SFE [
4].
2.5. Comparison Between the Conventional Method and Green Extraction Techniques
The
Table 4 shows the comparison between the yields obtained when using a conventional method for carminic acid extraction, and the efficiencies and yields shown by green extraction techniques. Conventional extraction resulted in a net yield of 17.8 % with a carminic acid efficiency of 2.0 mg/min. The extraction by ultrasound presented the best yields than the green methods reported by Borges [
4], obtaining 42.4 % with PLE and 39.4 % with SFE method.
2.6. Infrared Spectra of Carminic Acid Extracts
A spectral analysis at the infrared region was carried out using a standard commercial control of carminic acid, which was compared to the obtained by Cañamares et al. 2006 [
18] and the extracts obtained by the proposed EAU and EAM methods.
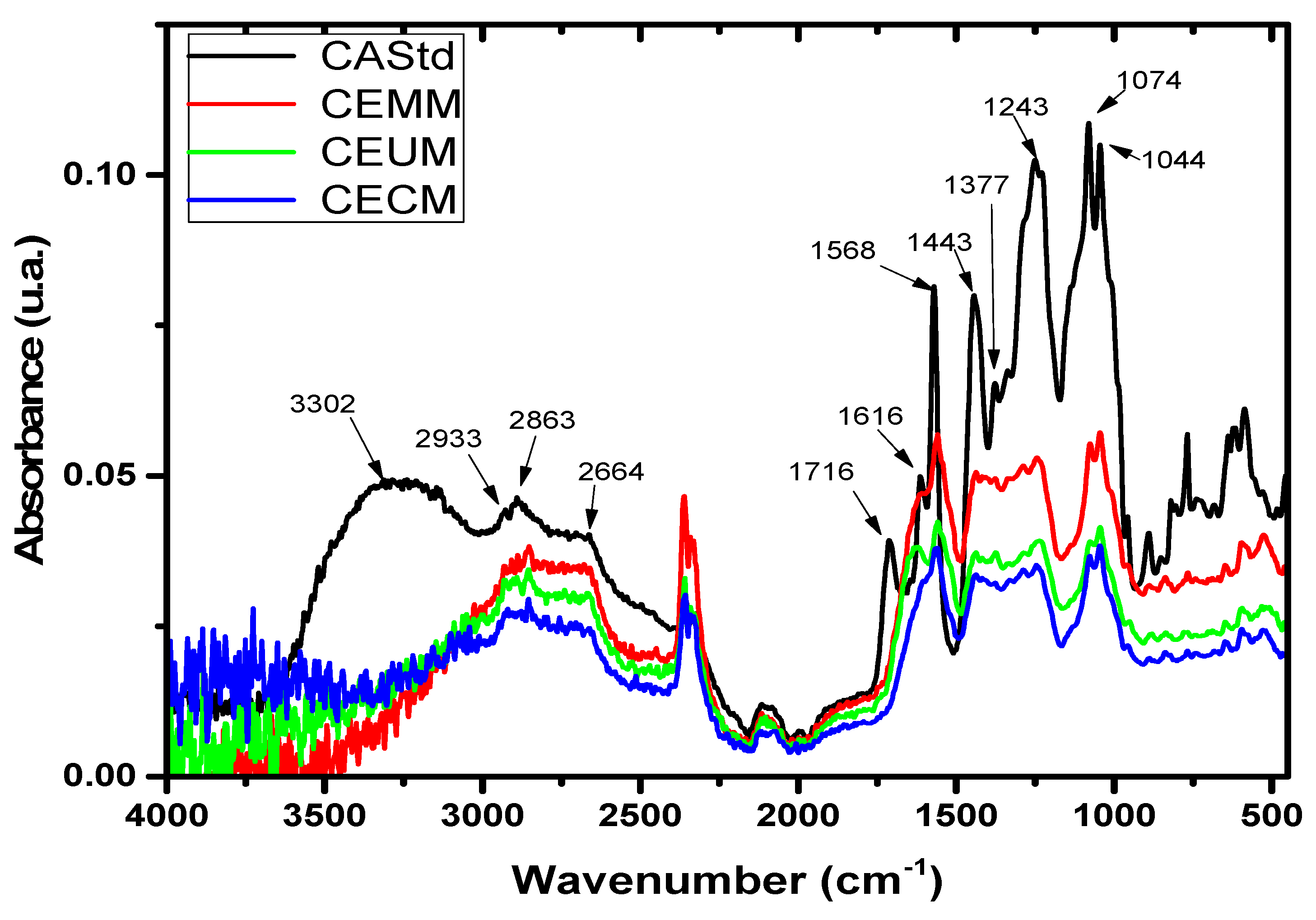
The
Figure 4 shows the comparison of IR spectra, where the characteristic bands attributable to carminic acid are observed and compared to the reported by Cañamares et al., which are also shown at
Table 5, mainly related to absorption peaks related to anthraquinones (1568 y 1273cm
-1), sugar residues (2933, 1074 y 1044cm
-1) and carboxyl groups (3302, 1616 y 1377cm
-1). The extraction methods proposed in this work also correlate to the bands assigned to these functional groups presents from the standard controls and the carminic acid signals reported by Cañamares, suggesting that we are obtaining carminic acid pigments using EAU and EAM methods.
The peaks observed at 1616, and 1243 cm-1 are related to C=O and C=C groups from the aromatic ring of anthraquinones that are mixed and show variations, depending on each extraction method. This was corroborated by UV-vis spectroscopy that shows the presence of carminic acid isomers and additional acids.
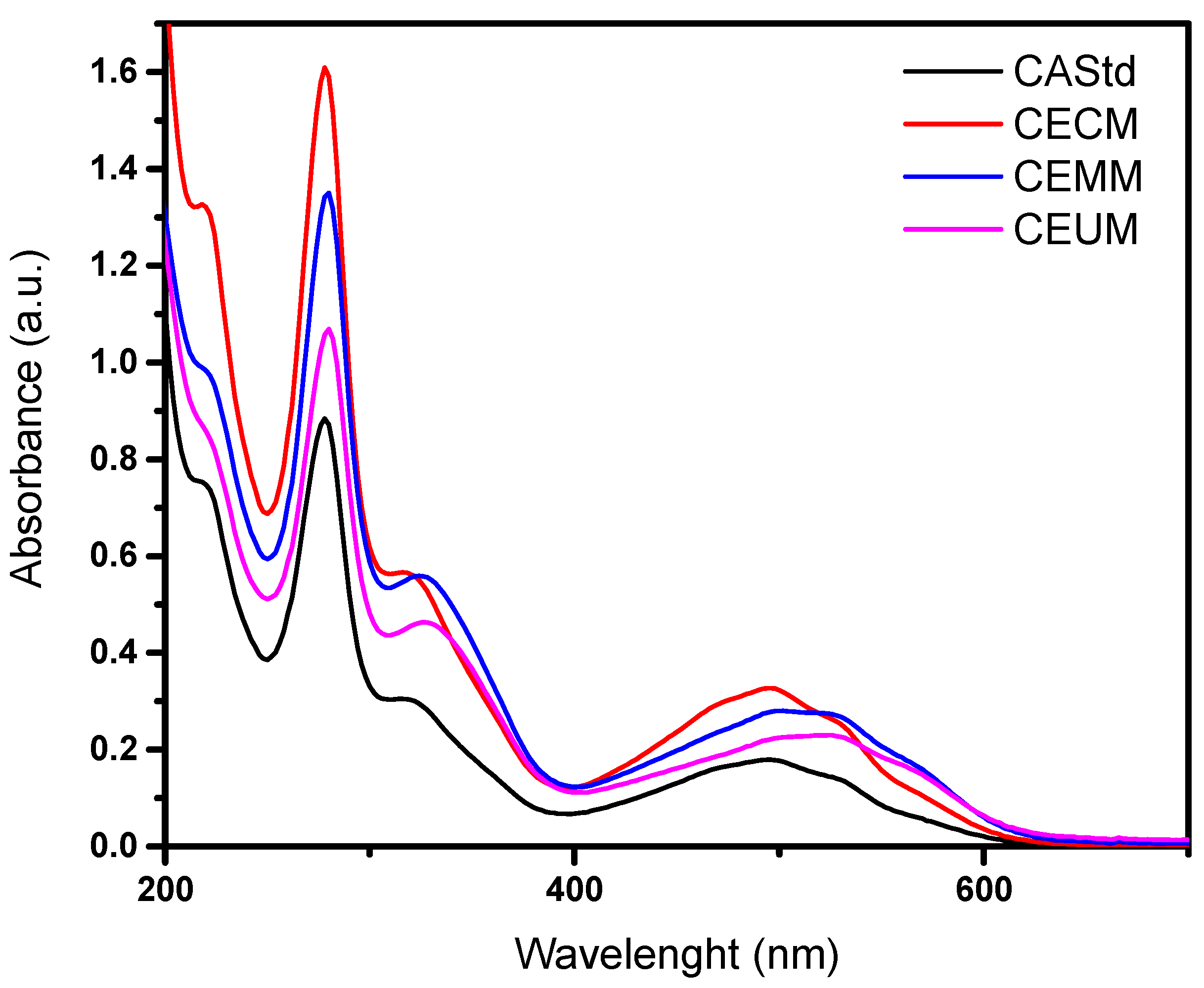
The
Figure 5 shows the UV-Vis spectra of the cochineal extracts obtained from the different methods, comparing them to the standard spectra. The commercial carminic acid standard showed a spectrum with characteristic absorption peaks at 275, 311, 495 y 530nm, and the extract obtained by the conventional method showed highly similar peaks. A slight difference was perceived for EAU and EAM methods showing bands at 276, 328, 527 and 562 nm, this variation could be attributed to the obtention as well of additional isomers from carminic acid, also flavokermesic and kermesic acid, which have been reported by Gonzales et al. 2002.
3. Discussion
The carminic acid extraction assisted by ultrasound and microwave as green technologies provide advantages over the conventional extraction method, since they do not modify the molecular composition, obtaining higher yields, efficiencies, and shorter processing times.
The use of EAU produced extraction yields of 47% that compared to the methodology reported by Borges et al. [
4], it seems to be the best and more effective for the extraction of carminic acid. It must be mentioned that EAU and conventional methods used water as the extraction media, but EAU uses cycles of low- and high-pressure waves over the sonicated liquid. Within the low-pressure waves cycle, the generated bubbles implode violently with the high-pressure waves, hence they can break materials in tiny particles, which allows the reduction of particles size, increasing the contact area to the solvent and the produced cavitation [
15]. Cavitations are formed over the surface of cochineal specimens that implode forming ‘’hot spots’’, with an effective temperature estimated within the range between 4500-5000 K. Considering these values, the pressure during collapses can be inferred through the van der Waals equation, to be approximately of 1700 atm [
16]. This released energy results in a more efficient extraction but is not enough for causing a bond breaking within the carminic acid molecule.
On the other hand, the use of microwave extraction (EAM) showed an extract yield of 41% and carminic extract yield of 18 %. Although the obtained yield was slightly higher than the reported by pressurized liquids, the extraction efficiency was clearly higher with 27.3%.
This increase in efficiency is related to the dissipation factor that determines the ability of a substance for converting the electromagnetic energy to heat. In this case, the used solvent was water, with a dissipation factor of 0.123, which is considered as regular to absorb microwaves. The cochineal insect also has a certain amount of moisture along with proteins, carbohydrates and lipids which absorb microwaves, promoting the extraction of carminic acid. As explained before, green methods were based on the improvement of efficiency over traditional methods by physical action over the medium. This also helps to preserve the structure of carminic acid even at elevated temperatures. Despite it is reported that ultrasound can generate new species during nanosecond lapses, but this only happens in presence of ionic compounds [
17]. Although there are many speculations about the microwave effects over molecules, these could not be structurally modified or induce a chemical reaction. The explanation is based on the microwave photon, which corresponds to the frequency used in the heating systems based on microwaves which have an approximate energy near to 0.00001 eV (2.45 GHz, 12.22 cm). According to these values, the photon of microwaves is not energetic enough to break hydrogen bonds, and it is smaller than the Brownian movement, being unable to induce chemical reactions.
From the analysis of FTIR spectra from carminic acid samples obtained by EAU and EAM, there were observed characteristic peaks related to the functional groups of the molecule, as reported by Camañares et al. [
18], showing slight shifts in the wavenumber that could be related to additional components present in the extracts. However, the typical absorption peaks were identified from the standard spectrum, suggesting that the molecule was not modified at its chemical structure. For the case presented by Borges et al. [
4], the molecule was not affected by the extraction methods PLE and SFE. Moreover, they achieved high extraction yields, and the extraction time was reduced by 6.
On the other hand, the work reported by Guo et al. [
19] described a Box -Behnken experimental design, where the time and solvent ratio were included as factors for the two techniques: microwaves and ultrasound. Since both extraction methodologies are based on distinct physical phenomena, we detected the need to establish experimental design by separate, including intervals of the variables related to each of the technologies. Guo and co-workers reported an extract yield of 95%, achieving this after 10 repeated extractions combining these methods; however, they did not report if the molecule structure of carminic acid was preserved.
The advantages of green technologies like EAU and EAM are diverse, because they can highly increase the yields and efficiencies of extraction, lowering energy consumption. This can be achieved by having a significant decrease of processing times, reduction of the solvent volume to have an eco-friendly treatment [
20], which allows a decrease in the general costs, which constitute the most desirable aspects of all extraction processes, obtaining as well high yields of the interest compounds. In addition, the cavitation effect can promote the removal of biological pollutants, an essential factor for the commercialization of this pigment [
21].
The present work has elucidated the optimal variables for the extraction of carminic acid by EAU using water as the only solvent; however, it is necessary to perform scaling experiments at industrial level for verifying the obtained results. It is important to mention that the most relevant challenges of green technologies require the involvement of industries that assume the commitment of modifying their extraction procedures, replacing harmful and pollutant materials [
22]. It would greatly benefit to the environment that extraction industries could implement the green technologies proposed in this work.
For this purpose, it is suggested the further implementation of pilot-scale experiments for assessing the profitability of carminic acid extraction by ultrasonication.
It will be important to apply this technology for the processing of wild-type cochineal grain from
Dactylopius genre, which is abundant in Mexico; however, it is underestimated because of its low content of carminic acid so it is considered a plague [
23], in contrast to the fine grain. Finally, one important perspective is to evaluate how is the effect of ultrasonication on the allergenic proteins (high and low density) that has the carminic acid, since it has been reported before that ultrasonication treatment can decrease the allergenicity, antioxidant capability and protein digestibility of shrimp [
24].
4. Materials and Methods
4.1. Cochineal Specimen Data
Cochineal insects Dactylopius coccus C. were provided by the Asociación Rojas Tepale, located at Santa María Zacatepec, a community from Juan C. Bonilla, in Puebla, México.
4.2. Carminic acid Determination
The contents of carminic acid present in the cochineal grain was made by the method established by the Food Chemical Codex [
25].
4.3. Carminic Acid Extraction by the Conventional Method
The conventional method was taken from Borges [
4], which uses water as the only extraction medium. The cochineal grain was mixed with water solvent in a ratio of 1:15 g/mL, and it was boiled without stirring for 30 min, then the mixture was decanted, and the supernatant was filtered. The precipitate was extracted twice, and the extracts were mixed and preserved under refrigeration.
4.4. Extraction Assisted by Ultrasound (EAU)
A Cole Parmer® ultrasonication equipment (Model CP 505) was employed with a 1/4 microtip, 500 Watts and a 20 Khz frequency. Distilled water was used as a solvent in different ratios with the ground cochineal grain (1:10, 1:15 and 1:20 g/mL). All samples were placed in a jacketed tube coupled to a cooling system, to regulate temperature. The testing temperatures were 60, 70 and 80 ºC, whereas the ultrasound exposure times were 5, 10 and 15 min.
4.6. Extraction Assisted by Microwave (EAM)
The cochineal grain was previously ground and placed in vials in a ratio of 1:10, 1:15 and 1:20 g/mL with deionized water. The vials were kept in peek jackets within a circular tray of the Anthon Paar microwave equipment (Synthos 3000, 1200 W). The used temperatures were similar to the EAU extraction, and also the exposure times.
4.7. Carminic Acid Analysis by FTIR Spectroscopy
To verify that the carminic acid molecules from the obtained solid extracts were not affected during the followed processes by the EAU, EAM and Conventional Method (CM). The cochineal extracts as well as the carminic acid standard (Sigma Aldrich) were measured using a FTIR spectrometer (Bruker Vertex 70 model) in the Attenuated Total Reflectance mode (ATR module with diamond crystal), with a scanning from 4000 to 400cm-1 and a resolution of 4cm-1.
4.8. Analysis and Quantification of Carminic Acid by UV-vis Spectroscopy
To analyze and ensure the presence of carminic acid from the obtained extracts, we performed UV-Vis spectroscopy to identify the characteristic absorption bands of carminic acid. The equipment used was a Thermo Model Evolution 600 spectrometer, scanning from 190 to 700nm with 1-nm resolution. To perform a quantitative analysis, a calibration curve was prepared with carminic acid standards at different concentrations starting from 5, 25, 35, 50, 65, 75, 85 until 100 ppm. The quantification of carminic acid from samples was determined by measuring their absorbance at a wavelength of 494 nm [
4].
4.9. Experimental Design and Statistical Analysis
To optimize the extraction methods, it was applied the Box-Behnken response surface design with three factors and three levels for each factor: a low level (-1), medium level (0) and high level (1). The three established independent variables were: Temperature (X1), solvent volume (X2) and time (X3); and the response variable was the yield of carminic acid. The
Table 1 represents the range of independent variables and their levels: high, low, and central points.
The experiment consisted of 15 trials with three replicates (Table 2). Treatments were generated with Statgraphics Centurion XVI software.
Corroboration of optimal variables
The optimal variables were obtained from an estimation of the extraction and the carminic acid yields. Therefore, experimental runs were carried out with the optimal variables obtained from EAU and EAM, using the Statgraphics software for a quantitative verification of the yields of extracts and carminic acid samples.
5. Conclusions
In the present work we conclude that using an extraction method assisted by ultrasound we were able to obtain high yields of extract (47%) and carminic acid (26.3%), with 67.34 °C temperature, 20 ml of solvent and a 15 min processing time as the optimized conditions. These parameters are lower compared to those used in conventional methods. Furthermore, the carminic acid molecule remained unaltered when subjected to cavitation, highlighting that ultrasound-assisted extraction is an excellent green technology for obtaining carminic acid from cochineal grains.
6. Patents
This work was based on two patent applications: MX/a/2021/015175 y Mx/a/2021/015176
Author Contributions
Conceptualization, Gabriel Ríos-Cortés and Ada María Ríos-Cortes; Formal analysis, Pedro Antonio López; Funding acquisition, Oxana Lazo Zamalloa; Investigation, Rogelio Reyes Perez; Methodology, Rogelio Reyes Perez and Juanita Pérez-Hernández; Project administration, Ada María Ríos-Cortes; Resources, Minerva Rosas-Morales; Software, Juanita Pérez-Hernández and Pedro Antonio López; Supervision, Gabriel Ríos-Cortés; Validation, Valentín López-Gayou; Writing—original draft, Gabriel Ríos-Cortés; Writing—review & editing, Miguel Ángel Plascencia-Espinosa and Ada María Ríos-Cortes.
Funding
The authors acknowledge the funding received from the Instituto Politécnico Nacional and The Instituto Nacional de México, Campus Orizaba, Ver.
Institutional Review Board Statement
Not applicable
Informed Consent Statement
Not applicable
Data Availability Statement
Not applicable
Acknowledgments
The authors acknowledge to the Instituto Politécnico Nacional for the grant and funding received for the development of this work, and also acknowledge to the Instituto Tecnológico Nacional de México Campus Orizaba and to the Asociación Yapaltic S.P.R.L. for their important collaborative work during this research.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Gonzalez, Monica & Méndez-Gallegos, Santiago De Jesús & Carnero, Aurelio & Lobo, Gloria & Afonso, Ana. (2002). Optimizing Conditions for the Extraction of Pigments in Cochineals (Dactylopius coccus Costa) Using Response Surface Methodology. Journal of agricultural and food chemistry. 50. 6968-74. [CrossRef]
- Nambela, Lutamyo, Liberato Venant Haule, and Quintino Mgani. 2020. “A Review on Source, Chemistry, Green Synthesis and Application of Textile Colorants.” Journal of Cleaner Production 246: 119036. [CrossRef]
- Pérez Q. 2014. Estudio Técnico para la implementación de una planta procesadora de cochinilla para la obtención del carmín. Thesis. Pontificia Universidad Católica del Perú. Lima, Perú.
- Borges, M. E., Tejera, R. L., Díaz, L., Esparza, P., & Ibáñez, E. (2012). Natural dyes extraction from cochineal (Dactylopius coccus). New extraction methods. Food Chemistry, 132(4), 1855–1860. [CrossRef]
- Ferreyra-Suarez, D., Paredes-Vargas, L., Jafari, S. M., García-Depraect, O., & Castro-Muñoz, R. (2024). Extraction pathways and purification strategies towards carminic acid as natural-based food colorant: A comprehensive review. Advances in Colloid and Interface Science, 323(October 2023). [CrossRef]
- Chemat, F., Vian, M. A., & Cravotto, G. (2012). Green extraction of natural products: Concept and principles. International Journal of Molecular Sciences, 13(7), 8615–8627. [CrossRef]
- Cristianini, M., & Guillén Sánchez, J. S. (2020). Extraction of bioactive compounds from purple corn using emerging technologies: A review. Journal of Food Science, 85(4), 862–869. [CrossRef]
- Bouloumpasi, E., Skendi, A., Christaki, S., Biliaderis, C. C., & Irakli, M. (2024). Optimizing conditions for the recovery of lignans from sesame cake using three green extraction methods: Microwave-, ultrasound- and accelerated-assisted solvent extraction. Industrial Crops and Products, 207(P2), 117770. [CrossRef]
- Biswas, R., Sarkar, A., Alam, M., Roy, M., & Mahdi Hasan, M. M. (2023). Microwave and ultrasound-assisted extraction of bioactive compounds from Papaya: A sustainable green process. Ultrasonics Sonochemistry, 101(May), 106677. [CrossRef]
- Sharma, M., & Dash, K. K. (2022). Microwave and ultrasound assisted extraction of phytocompounds from black jamun pulp: Kinetic and thermodynamics characteristics. Innovative Food Science and Emerging Technologies, 75(December 2021), 102913. [CrossRef]
- Chan, C. H., Yusoff, R., Ngoh, G. C., & Kung, F. W. L. (2011). Microwave-assisted extractions of active ingredients from plants. Journal of Chromatography A, 1218(37), 6213–6225. [CrossRef]
- Bansod, S. P., Parikh, J. K., & Sarangi, P. K. (2023). Pineapple peel waste valorization for extraction of bio-active compounds and protein: Microwave assisted method and Box Behnken design optimization. Environmental Research, 221(October 2022), 115237. [CrossRef]
- Muruganandam L., Krishna, A., Reddy, J., & Nirmala, G. S. (2017). Optimization studies on extraction of phytocomponents from betel leaves. Resource-Efficient Technologies, 3(4), 385–393. [CrossRef]
- Centeno, M. M. (2003). Extracción, estabilización y evaluaciones analíticas del carmín. Tesis de Maestría. Instituto Politécnico Nacional, México. (justificación: información amplia sobre métodos ocupados por industria que por lo regular son secretos industriales).
- Azuola, R., & Vargas Aguilar, P. (2007). Extracción de sustancias asistida por ultrasonido (EUA). Tecnología En Marcha, 20(4), 1. ISSN: 0379-3962.
- Cravotto, C. y Cintas, P. (2006). Power ultrasound in organic synthesis: moving cavitational chemistry from academia to innovative and large-scale applications. Chemical Society. Reviews, 35: 180-196.
- Zhang, W., Boateng, I. D., Zhang, W., Jia, S., Wang, T., & Huang, L. (2022). Effect of ultrasound-assisted ionic liquid pretreatment on the structure and interfacial properties of soy protein isolate. Process Biochemistry, 115(May 2021), 160–168. [CrossRef]
- Cañamares, M. V., García-ramos, J. V., Domingo, C. y Sanchez-Cortes, S. (2006). Surface-enhanced Raman scattering study of the anthraquinone red pigment carminic acid. Vibrational Spectroscopy, 40, 161–167.
- Guo, Yuan & Zheng, Hua & Zhang, Hong & Li-Yi, Ma & Han, Juan & Li, Kun. (2012). Optimization of Combined Microwave-Ultrasonic Wave Extraction of Cochineal Dye by Response Surface Methodology. Applied Mechanics and Materials. 161. 82-87. 10.4028/www.scientific.net/AMM.161.82.
- Anastas, P., Bastlett, L., Kirchoff, M., y Williamson T. (2000). The role of catalysis in the design, development, and implementation of green chemistry. Catalysis Today, 55, 11 -22.
- Robles-Azuna, L. E., & Ochoa-Martínez, L. A. (2012). Disponible en: http://www.redalyc.org/articulo.oa?id=81325441002. Revista Iberoamericana de Tecnología Postcosecha, 13(2), 109–122.
- Pájaro Nerlis Paola, & Olivero Jesús. (2011). Química Verde: Un nuevo reto. Ciencia e Ingeniería Neogranadina, 21(2), 169–182. http://www.redalyc.org/articulo.oa?id=91123440009.
- Flores-Hernández, Arnoldo, Murillo-Amador, Bernardo, Rueda-Puente, Edgar Omar, Salazar-Torres, José Cruz, García-Her nández, José Luis, & Troyo-Diéguez, Enrique. (2006). Reproducción de cochinilla silvestre Dactylopius opuntiae (Homóptera: Dactylopiidae). Revista mexicana de biodiversidad, 77(1), 97-102. Recuperado en 18 de diciembre de 2023, de http://www.scielo.org.mx/scielo.php?script=sci_arttext&pid=S1870-34532006000100011&lng=es&tlng=es.
- Dong, X., Wang, J., & Raghavan, V. (2020). Effects of high-intensity ultrasound processing on the physiochemical and allergenic properties of shrimp. Innovative Food Science and Emerging Technologies, 65(July), 102441. [CrossRef]
-
Food Chemicals Codex. 1981. Third Edition. National Research Council. Washington, DC: The National Academies Press. [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).