Submitted:
23 November 2024
Posted:
26 November 2024
You are already at the latest version
Abstract
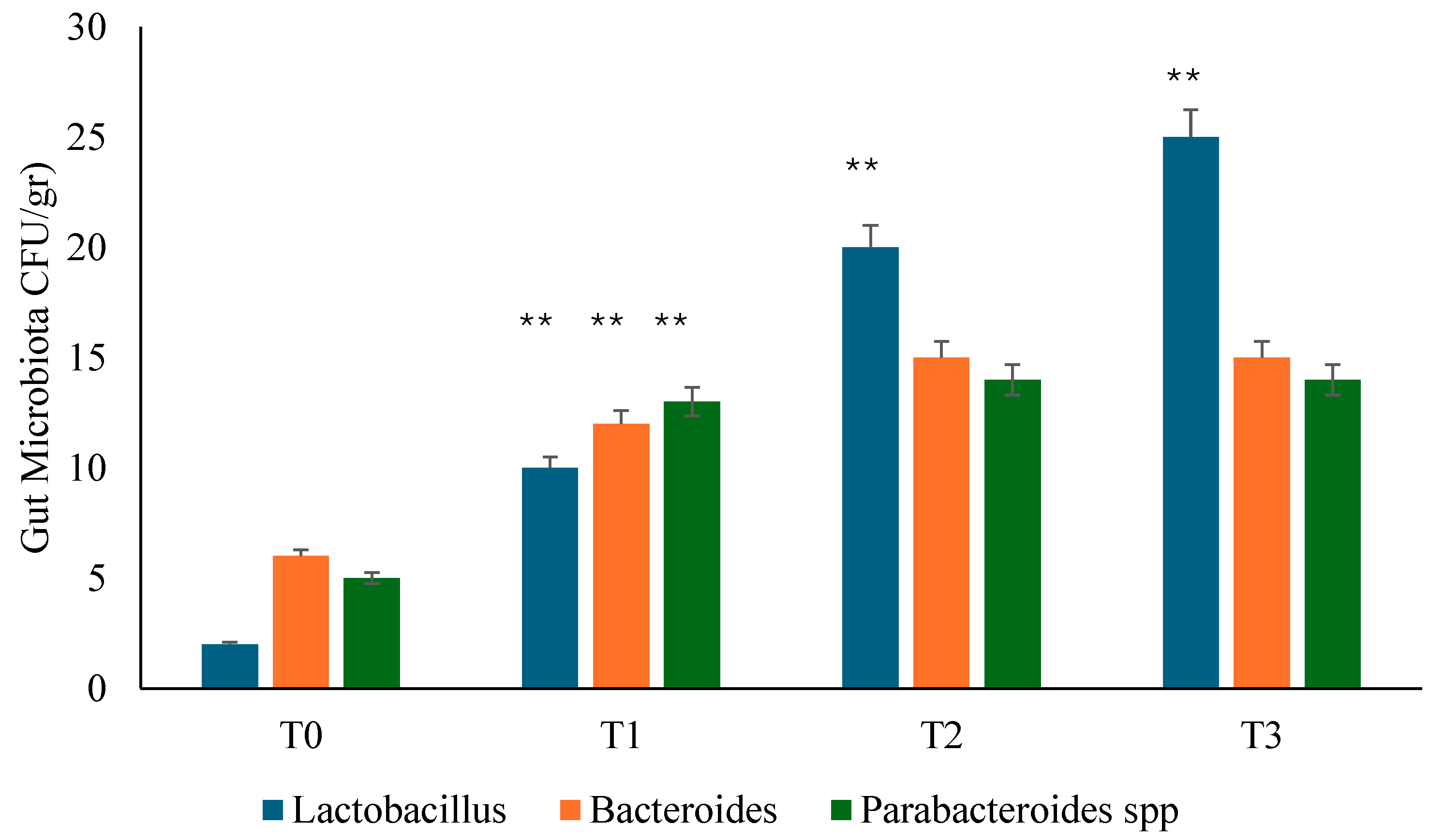
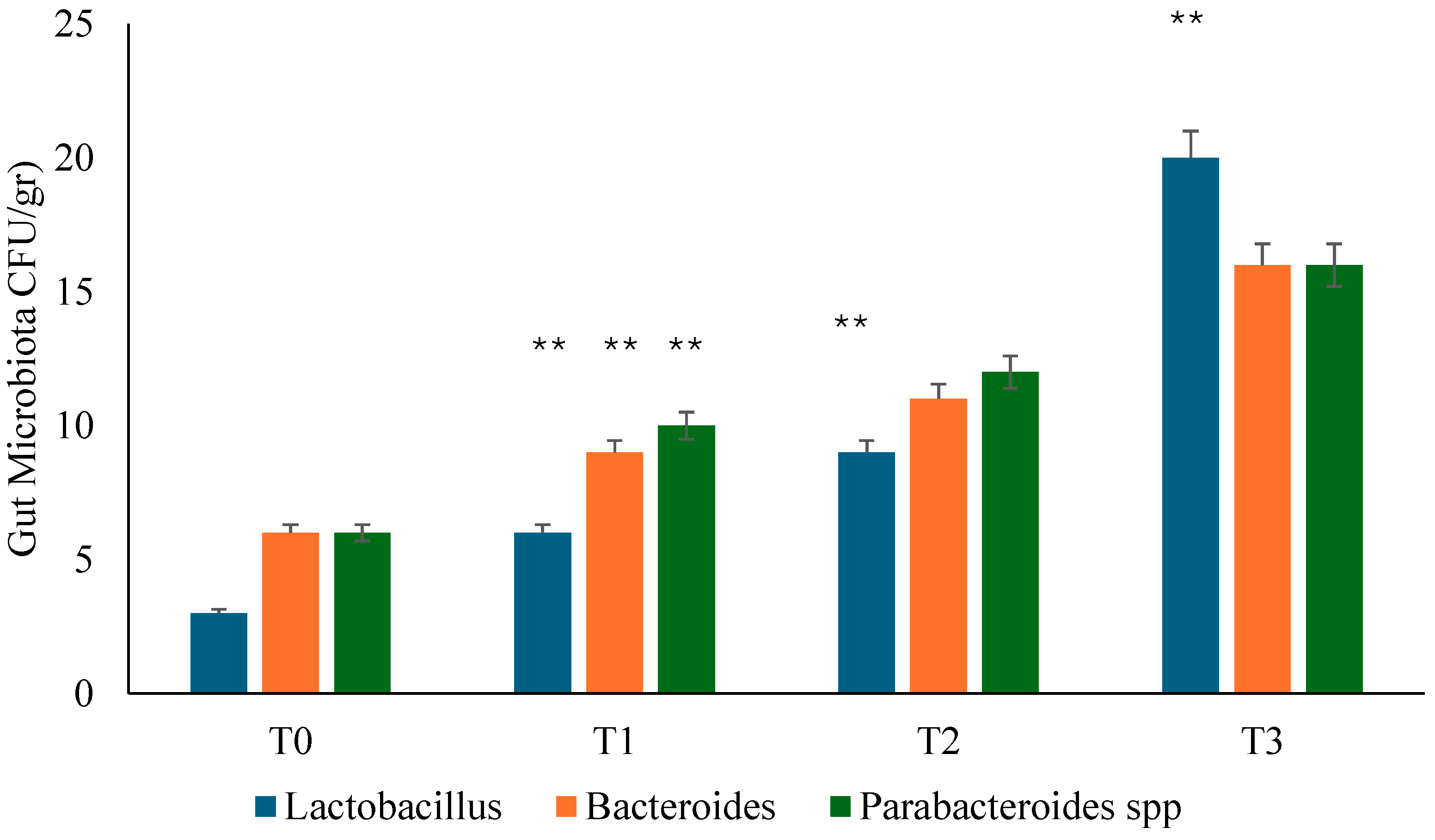
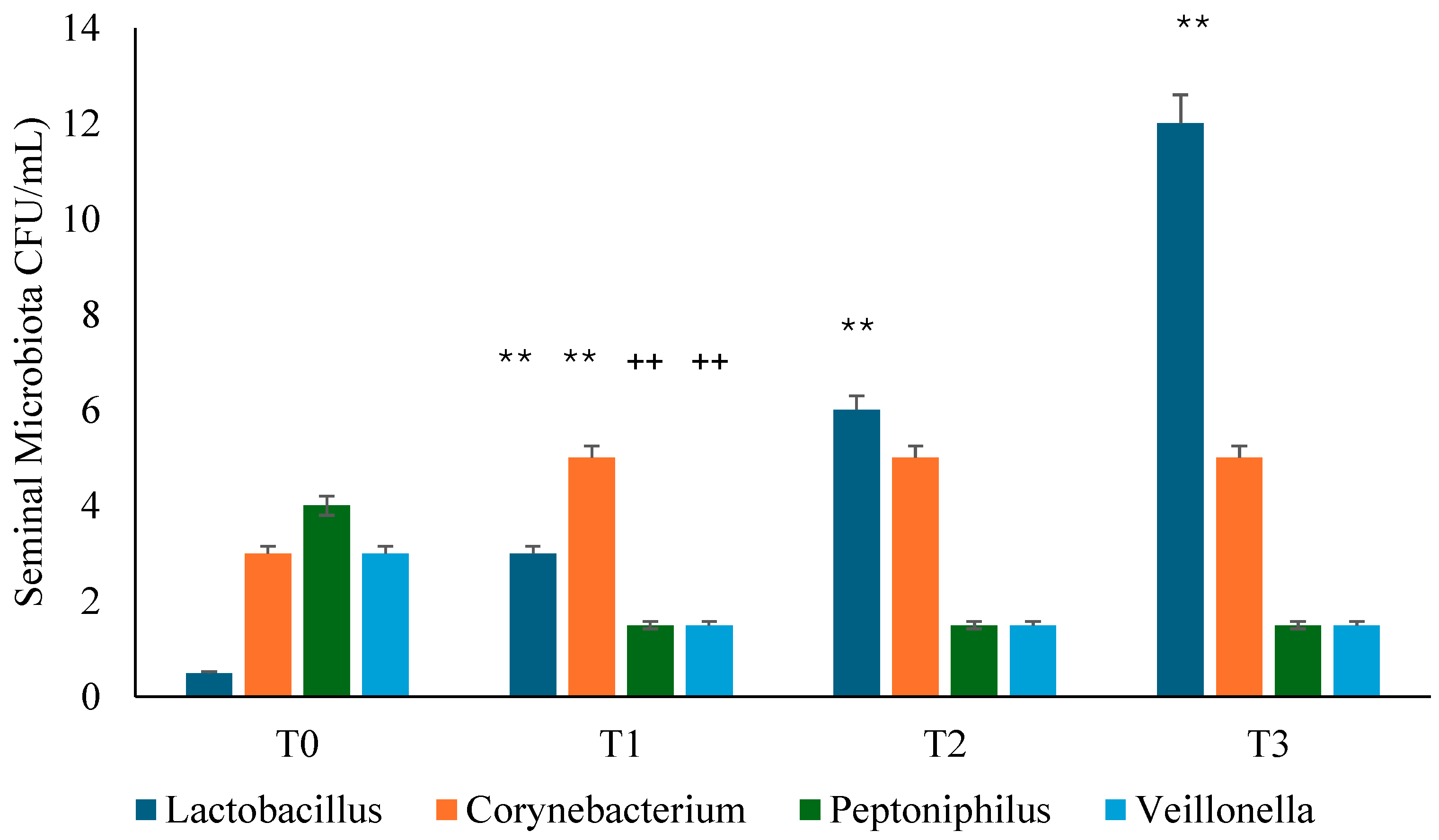
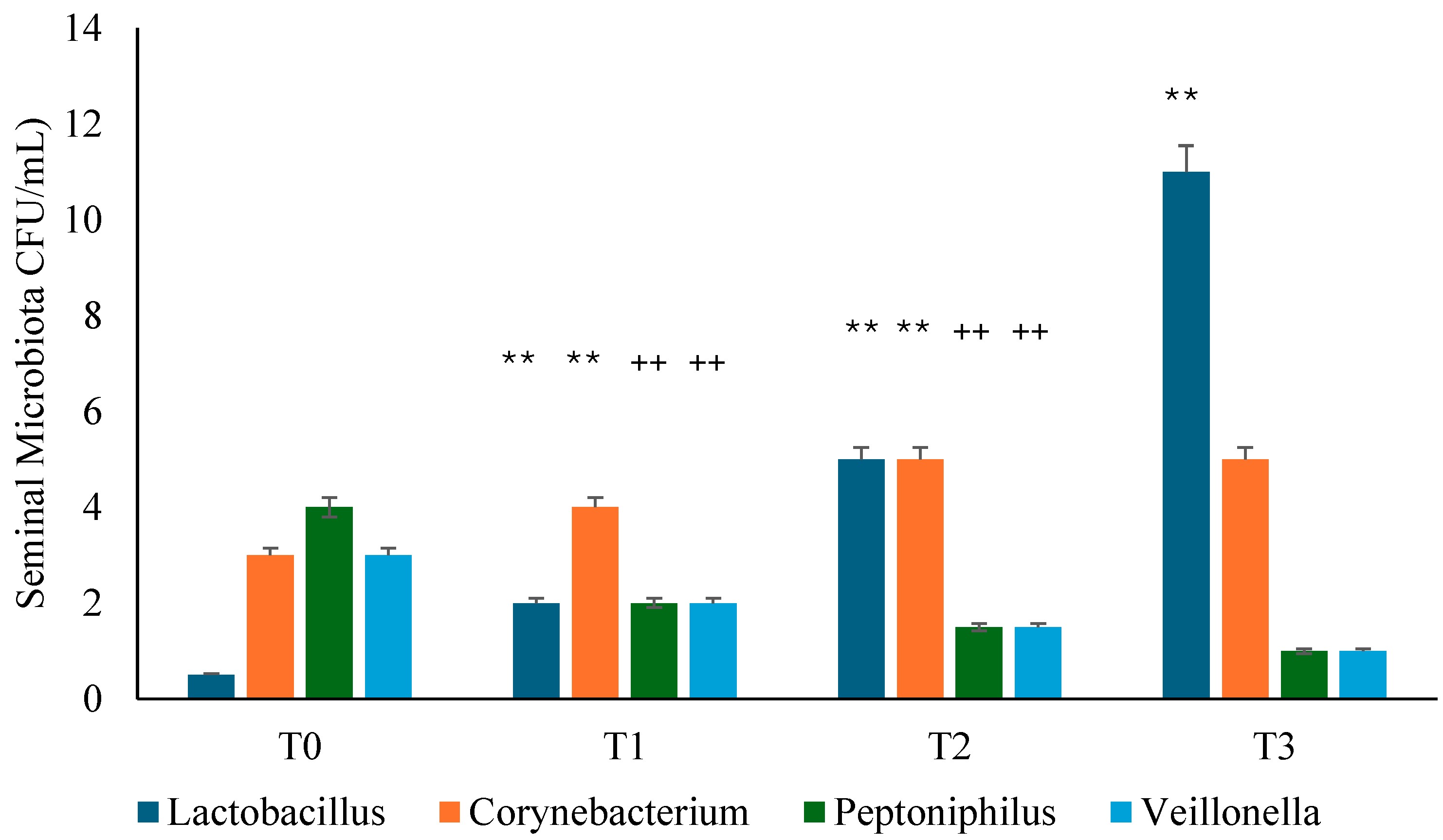
Background: Several studies hypothesized a therapeutic role of probiotics in the management of chronic bacterial prostatitis (CBP) patients. Here, we assessed the effect of probiotics as an add-on treatment in patients with clinical recurrences of CBP, through gut microbiota modification analysis. Methods: This study has been planned as a randomized, double-blind, placebo-controlled, multicenter clinical trial examining the efficacy and safety of consumption of probiotics containing human Lactobacillus casei DG® or placebo following 1 month-treatment with ciprofloxacin. Twenty-four patients with CBP were recruited and treated for 3 months with placebo (n. 12) or with Lactobacillus Casei DG® (n. 12). During the enrollment and follow-ups, IPSS, NIH-CPSI and SF-36 were used. Urological examinations and microbiological tests were performed to analyze the symptomatology, recurrences frequency, and gut and seminal microbiota. Results: The treatment with Lactobacillus Casei DG® induced a significantly (p<0.01) faster recovery of symptoms (2 days vs. 8 days) than placebo and an increased time free from symptoms (86 days vs. 42 days) without the occurrence of adverse events. In the treatment group, the appearance of Lactobacilli after 30 days (T1) was higher in the probiotic group, and a significant reduction of Corynebacterium, Peptoniphilus, Pseudomonas, Veillonella, Staphylococcus and Streptococcus has been observed. Conclusion: These data suggest that in patients with CBP, the use of Lactobacillus casei DG after an antimicrobial treatment is safe and effective in improving the days free of symptoms and the quality of life.
Keywords:
1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Participants
2.3. Drugs
2.4. Experimental Protocol
2.5. Questionnaires
2.6. Meares-Stamey Test
2.7. Fecal Samples
2.8. Microbiological Identification Tests
2.9. Admission (T0)
2.10. Follow-Up Visits (T1, T2, T3)
2.11. Outcomes
2.12. Statistical Analysis
3. Results
3.1. Population
3.2. Gut Microbiota Analysis
3.3. Seminal Microbiota Analysis
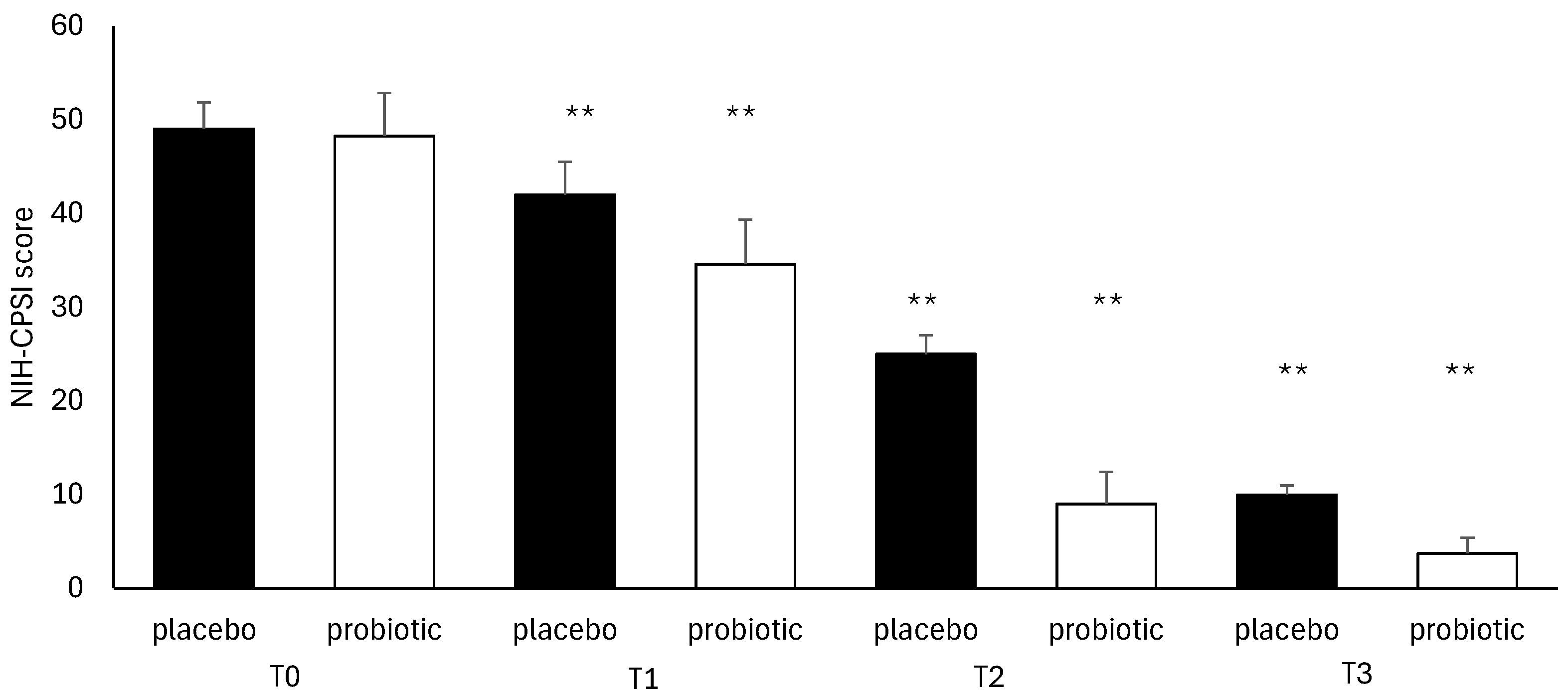
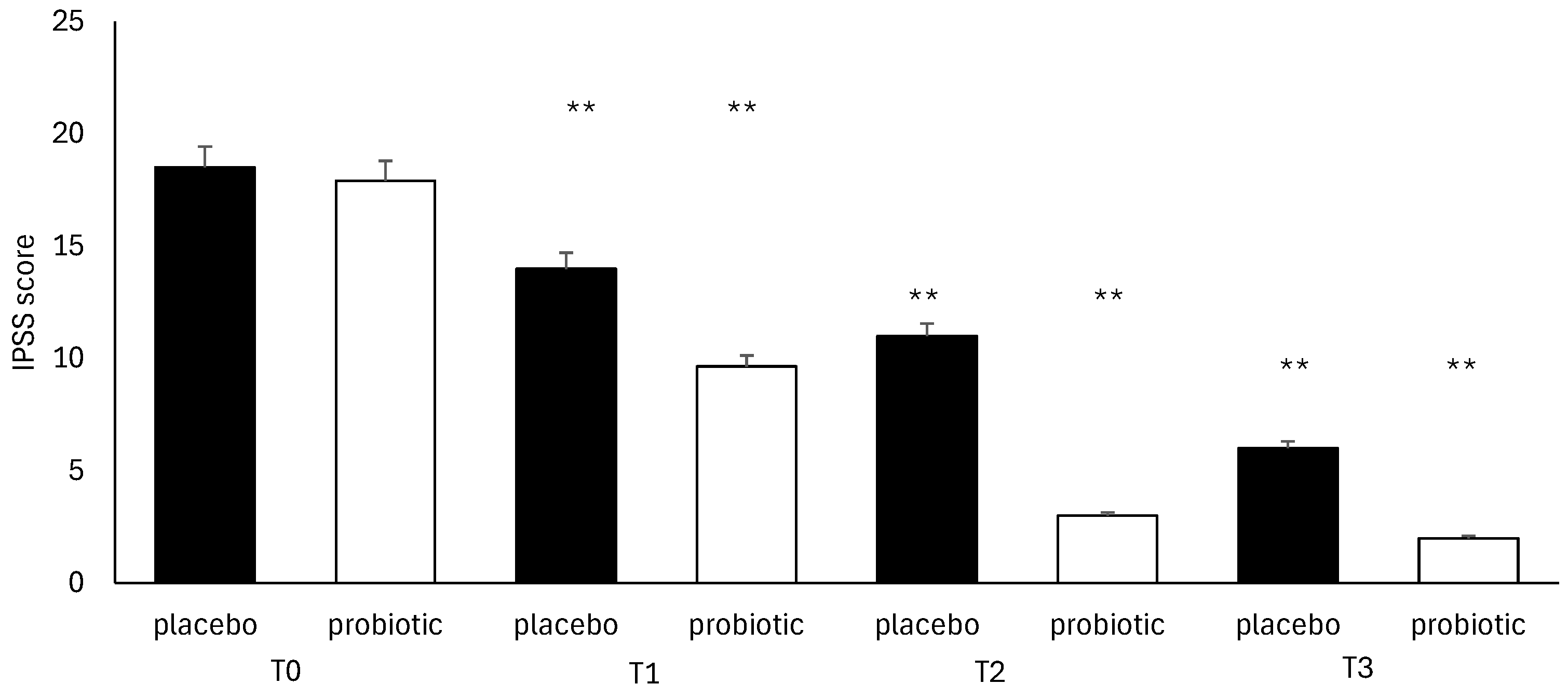
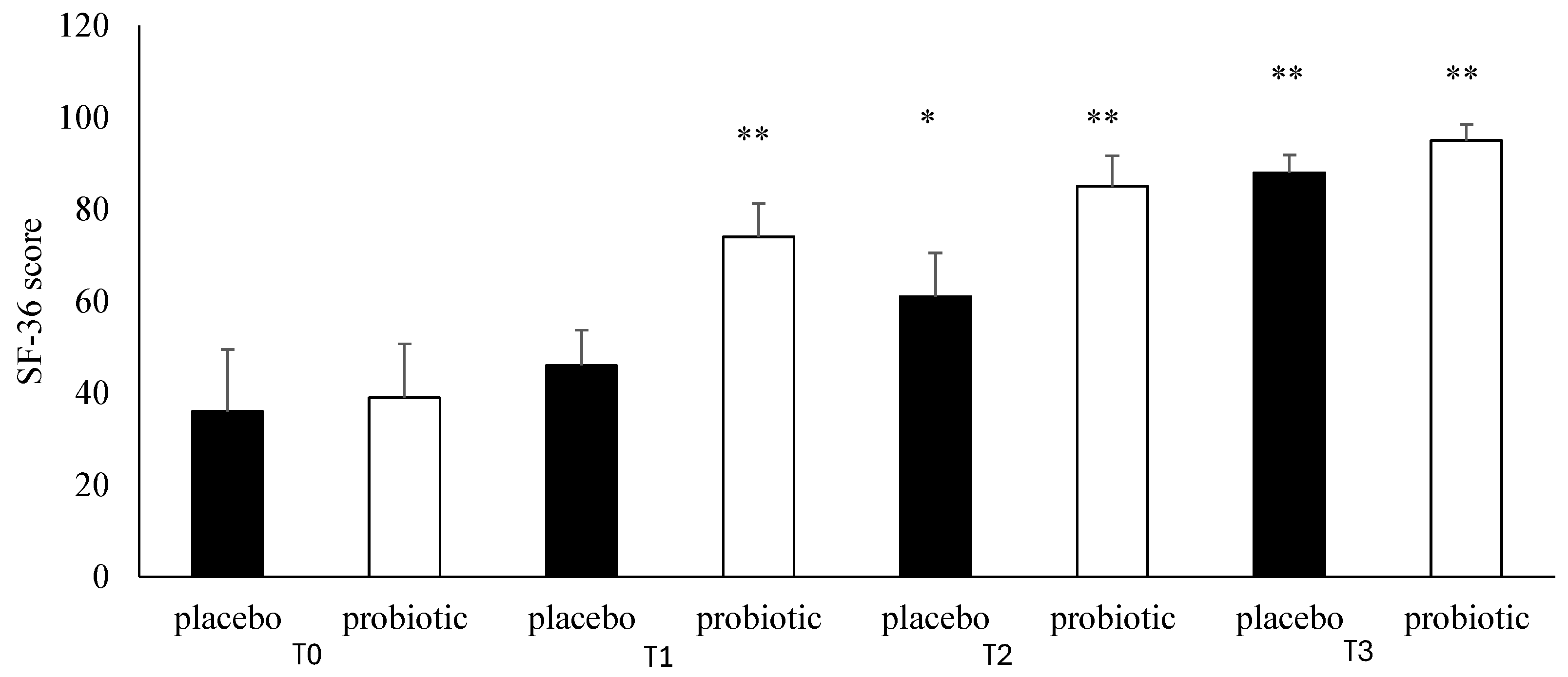
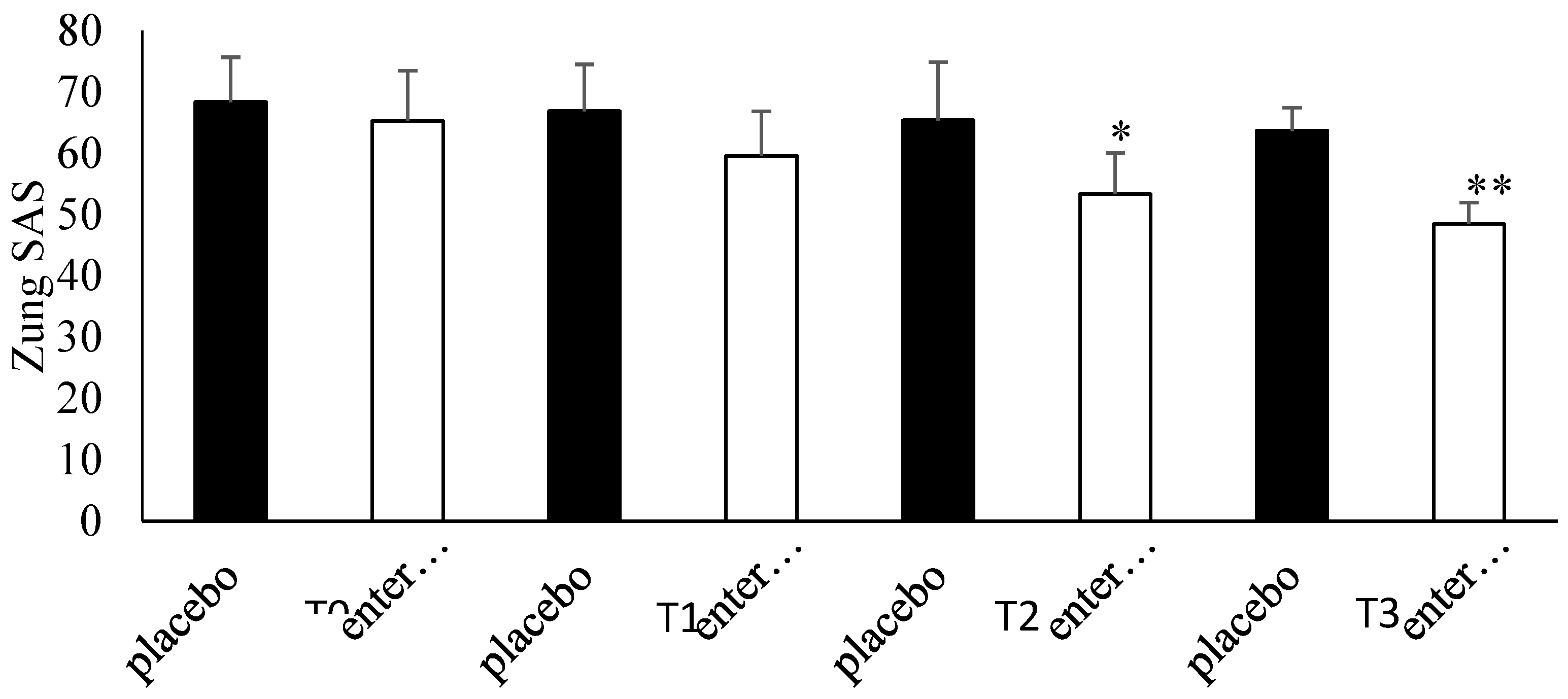
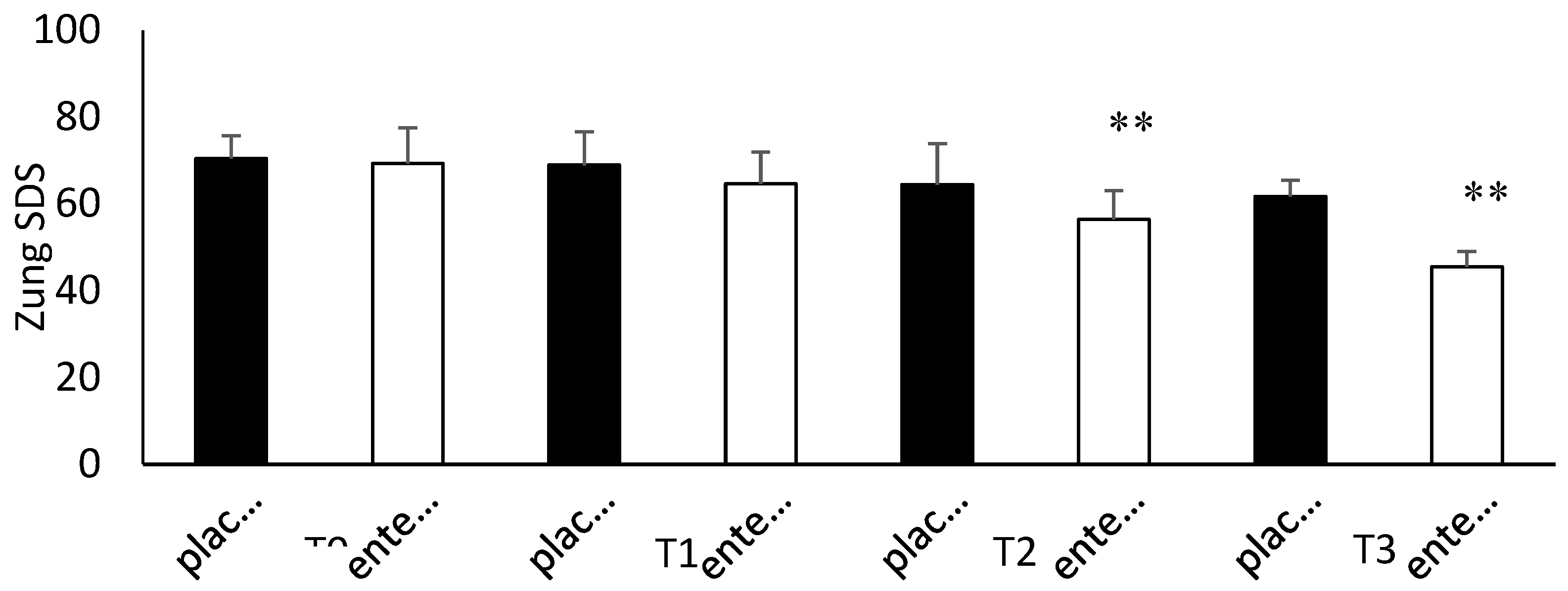
3.4. Questionnaire Analysis
3.5. Clinical Evaluation
4. Discussion
5. Conclusions
6. Limitations, Strengths and Future Directions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Rees, J.; Abrahams, M.; Doble, A.; Cooper, A. Prostatitis Expert Reference Group (PERG). Diagnosis and treatment of chronic bacterial prostatitis and chronic prostatitis/chronic pelvic pain syndrome: a consensus guideline. BJU Int 2015, 116(4):509-25. [CrossRef] [PubMed] [PubMed Central]
- Cai, T.; Alidjanov, J.; Palagin, I.; Medina-Polo, J.; Nickel, J.C.; Wagenlehner, F.M.E. Chronic prostatitis/chronic pelvic pain syndrome (CP/CPPS): look to the future. Prostate Cancer Prostatic Dis 2024, 27(2):239-241. [CrossRef] [PubMed]
- Krieger, J.N.; Lee, S.W.; Jeon, J.; Cheah, P.Y.; Liong, M.L.; Riley, D.E. Epidemiology of prostatitis. Int J Antimicrob Agents 2008, 31 Suppl 1(Suppl 1):S85-90. [CrossRef] [PubMed] [PubMed Central]
- Bartoletti, R.; Cai, T.; Nesi, G.; Albanese, S.; Meacci, F.; Mazzoli, S.; Naber, K. The impact of biofilmproducing bacteria on chronic bacterial prostatitis treatment: results from a longitudinal cohort study. World J Urol 2014, 32(3):737-42.
- Magri, V.; Boltri, M.; Cai, T.; Colombo, R.; Cuzzocrea, S.; De Visschere, P.; Giuberti, R.; Granatieri, C.M.,;Latino, M.A.; Larganà, G.; Leli, C.; Maierna, G.; Marchese, V.; Massa, E.; Matteelli, A.; Montanari, E.; Morgia, G.; Naber, K.G.; Papadouli, V.; Perletti, G.; Rekleiti, N.; Russo, G.I.; Sensini, A.; Stamatiou, K.; Trinchieri, A.; Wagenlehner, F.M.E. MultidisciplinaryApproach to Prostatitis. Arch Ital Urol Androl 2019;90(4):227-248.
- He, H.; Luo, H.; Xu, H.; Qian, B.; Zou, X.; Zhang, G.; Zeng, F.; Zou, J. Preclinical models and evaluation criteria of prostatitis. Front Immunol 2023, 14:1183895. [CrossRef] [PubMed] [PubMed Central]
- Cai, T.; Verze, P.; La Rocca, R.; Palmieri, A.; Tiscione, D.; Luciani, L.G.; Mazzoli, S.; Mirone, V.; Malossini, G. The Clinical Efficacy of Pollen Extract and Vitamins on Chronic Prostatitis/Chronic Pelvic Pain Syndrome Is Linked to a Decrease in the Pro-Inflammatory Cytokine Interleukin-8. World J Mens Health 2017, 35(2):120-128.
- Romano, L.; Napolitano, L.; Crocetto, F.; Sciorio, C.; Priadko, K.; Fonticelli, M.; Federico, A.; Romano, M.; Gravina, A.G. The potential therapeutic role of Hericium erinaceus extract in pathologic conditions involving the urogenital-gut axis: insights into the involved mechanisms and mediators. J Physiol Pharmacol 2024, 75(1). Epub 2024 Apr 3. [CrossRef] [PubMed]
- Gravina, A.G.; Pellegrino, R.; Palladino, G.; Coppola, A.; Brandimarte, G.; Tuccillo, C.; Ciardiello, F.; Romano, M.; Federico, A. Hericium erinaceus, in combination with natural flavonoid/alkaloid and B3/B8 vitamins, can improve inflammatory burden in Inflammatory bowel diseases tissue: an ex vivo study. Front Immunol 2023, 14:1215329. [CrossRef] [PubMed] [PubMed Central]
- Cai, T.; Gallelli, L.; Cione, E.; Perletti, G.; Ciarleglio, F.; Malossini, G.; De Pretis, G.; Palmieri, A.; Mirone, V.; Bartoletti, R.; Johansen, T.E.B. The use of Lactobacillus casei DG® prevents symptomatic episodes and reduces the antibiotic use in patients affected by chronic bacterial prostatitis: results from a phase IV study. World J Urol 2021, 39(9):3433-3440. [CrossRef] [PubMed] [PubMed Central]
- Food and Agricultural Organization of the United Nations and World Health Organization. Health and nutritional properties of probiotics in food including powder milk with live lactic acid bacteria. World Health Organization [online]. https://www.who.int/foodsafety/publications/fs_management/en/probiotics.pdf (2001).
- Kranz, J.; Bartoletti, R.; Bruyère, F.; Cai, T.; Geerlings, S., Köves, B.; Schubert, S.; Pilatz, A.; Veeratterapillay, R.; Wagenlehner, F.M.E.; Bausch, K.; Devlies, W.; Horváth, J.; Leitner, L.; Mantica, G.; Mezei, T.; Smith, E.J.; Bonkat, G. European Association of Urology Guidelines on Urological Infections: Summary of the 2024 Guidelines. Eur Urol 2024, 86(1):27-41. [CrossRef] [PubMed]
- Trinchieri, A. Role of levofloxacin in the treatment of urinary tract infections. Arch Ital Urol Androl 2001, 73(2):105-13. [PubMed]
- Nickel, J.C.; Downey, J.; Hunter, D.; Clark, J. Prevalence of prostatitis-like symptoms population based study using the National Institutes of Health chronic prostates symptoms index. J Urol 2001, 165:843–845.
- Badia, X.; Garcia-Losa, M.; Dal-Re, R. Ten-language translation and harmonization of the International Prostate Symptom Score: developing a methodology for multinational clinical trials. Eur Urol 1997, 31:129–40.
- Giubilei, G.; Mondaini, N.; Crisci, A.; Raugei, A.; Lombardi, G.; Travaglini, F.; Del Popolo, G.; Bartoletti, R. The Italian version of the National Institutes of Health Chronic Prostatitis Symptom Index. Eur Urol 2005, 47:805–11.
- Cappelleri, J.C.; Rosen, R.C.; Smith, M.D.; Mishra, A.; Osterloh, I.H. Diagnostic evaluation of the erectile function domain of the International Index of Erectile Function. Urology 1999, 54(2):346-51. [CrossRef] [PubMed]
- Zhang, Y.; Qu, B.; Lun, S.S.; Guo, Y.; Liu, J. The 36-item short form health survey: reliability and validity in Chinese medical students. Int J Med Sci 2012, 9(7):521-6. [CrossRef] [PubMed] [PubMed Central]
- Laucis, N.C.; Hays, R.D.; Bhattacharyya, T. Scoring the SF-36 in Orthopaedics: A Brief Guide. J Bone Joint Surg Am 2015, 97(19):1628-34. [CrossRef] [PubMed] [PubMed Central]
- Dunstan, D.A.; Scott, N. Norms for Zung's Self-rating Anxiety Scale. BMC Psychiatry 2020, 20(1):90. [CrossRef] [PubMed] [PubMed Central]
- Dunstan, D.A.; Scott, N. Clarification of the cut-off score for Zung's self-rating depression scale. BMC Psychiatry 2019, 19(1):177. [CrossRef] [PubMed] [PubMed Central]
- Cai, T.; Tamanini, I.; Odorizzi, K.; Gallelli, L.; Lanzafame, M.; Mazzoli, S.; Lanzafame, P.; Massidda, O.; Palmieri, A.; Wagenlehner, F.M.E.; Bjerklund Johansen, T.E.; De Nunzio, C. The diagnostic yield of the Meares & Stamey test can be significantly improved by symptom-based patient selection and the experience of the test performer. Prostate Cancer Prostatic Dis 2024, 27(2):300-304. [CrossRef] [PubMed]
- Cossart, Y. Bergey’s Manual of Systematic Bacteriology Volume 2. Pathology 1987, 19, 324; Holt, J.G.; Krieg, N.R.; Sneath, P.H.A. Bergey’s Manual of Determinative Bacterology; The Williams and Wilkins Co.: Baltimore, MD, USA, 1994.
- Rognes, T.; Flouri, T.; Nichols, B.; Quince, C.; Mahé, F. VSEARCH: a versatile open source tool for metagenomics. PeerJ 2016, 4:e2584. eCollection 2016. [CrossRef]
- Magri, V.; Perletti, G.; Cai, T.; Stamatiou, K.; Trinchieri, A.; Montanari, E. Levofloxacin for NIH Category II Chronic Bacterial Prostatitis: A Real-Life Study. Chemotherapy 2019, 64(1):8-16. [CrossRef] [PubMed]
- O'Hara, A.M.; Shanahan, F. The gut flora as a forgotten organ. EMBO Rep 2006, 7(7):688-93. [CrossRef] [PubMed] [PubMed Central]
- Isolauri, E.; Sütas, Y.; Kankaanpää, P.; Arvilommi, H.; Salminen, S. Probiotics: effects on immunity. Am J Clin Nutr 2001, 73(2 Suppl):444S-450S. [CrossRef] [PubMed]
- Mohamadzadeh, M.; Olson, S.; Kalina, W.V.; Ruthel, G.; Demmin, G.L.; Warfield, K.L.; Bavari, S.; Klaenhammer, T.R. Lactobacilli activate human dendritic cells that skew T cells toward T helper 1 polarization. Proc Natl Acad Sci U S A 2005, 102(8):2880-5. [CrossRef] [PubMed] [PubMed Central]
- Compare, D.; Rocco, A.; Coccoli, P.; Angrisani, D.; Sgamato, C.; Iovine, B.; Salvatore, U.; Nardone, G. Lactobacillus casei DG and its postbiotic reduce the inflammatory mucosal response: an ex-vivo organ culture model of post-infectious irritable bowel syndrome. BMC Gastroenterol 2017, 17(1):53. [CrossRef] [PubMed] [PubMed Central]
- D'Incà, R.; Barollo, M.; Scarpa, M.; Grillo, A.R.; Brun, P.; Vettorato, M.G.; Castagliuolo, I.; Sturniolo, G.C. Rectal administration of Lactobacillus casei DG modifies flora composition and Toll-like receptor expression in colonic mucosa of patients with mild ulcerative colitis. Dig Dis Sci 2011, 56(4):1178-87. Epub 2010 Aug 25. [CrossRef] [PubMed]
- Vicari, E.; La Vignera, S.; Castiglione, R.; Condorelli, R.A.; Vicari, L.O.; Calogero, A.E. Chronic Bacterial Prostatitis and Irritable Bowel Syndrome: Effectiveness of Treatment With Rifaximin Followed by the Probiotic VSL#3. Asian J Androl 2014, 16(5):735-9.
- Hamamah, S.; Aghazarian, A.; Nazaryan, A.; Hajnal, A.; Covasa, M. Role of Microbiota-Gut-Brain Axis in Regulating Dopaminergic Signaling. Biomedicines 2022, 10(2):436. [CrossRef] [PubMed] [PubMed Central]
| Characteristics | Data | Probiotic Group | Placebo Group |
| Age | 50 ± 3.1 | 50.4 ± 2.5 | 49.7 ± 3.4 |
| Body Mass Index (Kg/m2) | 27.7 ± 2.7 | 28.0 ± 2.5 | 27.4 ± 2.7 |
| Occupation Status | |||
| Sedentary | 11 | 4 | 7 |
| Manual | 13 | 8 | 5 |
| Comorbidity | |||
| Copd | 3 | 2 | 1 |
| Diabetes Mellitus Type 2 | 6 | 3 | 3 |
| Hypertension | 8 | 1 | 7 |
| Dyslipidemia | 4 | 4 | 0 |
| Metabolic Syndrome | 3 | 2 | 1 |
| Bacteria | T0 | T1 | T2 | T3 |
| Escherichia coli | 36 | 34 | 38 | 36 |
| C. difficile | 51 | 49 | 52 | 54 |
| Campylobacter spp. (C. jejuni, C. upsaliensis, and C. coli) | 59 | 53 | 54 | 56 |
| Clostridioides difficile tcdA/tcdB | 51 | 52 | 51 | 54 |
| Enteroaggregative E. coli | 56 | 58 | 59 | 59 |
| Enteropathogenic E. coli | 57 | 58 | 59 | 58 |
| Enterotoxigenic E. coli eltA/estA | 61 | 62 | 59 | 62 |
| Shiga toxin–producing E. coli stx1/stx2 | 59 | 62 | 61 | 58 |
| Shiga toxin–producing E. coli stx1/stx2 O157 | 56 | 58 | 59 | 61 |
| Enteroinvasive E. coli /Shigella (S. sonnei, S. fexneri, S. boydii, and S. dysenteriae) | 61 | 62 | 64 | 62 |
| Plesiomonas shigelloides | 59 | 60 | 58 | 59 |
| Salmonella spp. | 63 | 64 | 61 | 62 |
| Symptoms | ||||
|---|---|---|---|---|
| IIEF-5 (range: 0–25 points) | Groups at T0 | Groups at T3 | ||
| Probiotic | Placebo | Probiotic | Placebo | |
| Normal (IIEF-5 22–25 points) | 1 | 2 | 4 | 2 |
| Mild (IIEF-5 17–21 points) | 5 | 5 | 7 | 6 |
| Mild to moderate (IIEF 12–16 points) | 3 | 1 | 1 | 2 |
| Moderate to severe (IIEF 8–11 points) | 1 | 2 | 0 | 1 |
| Severe (IIEF-5 0–7 points) | 2 | 2 | 0 | 1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





