Introduction
Breast cancer survival rates have seen a remarkable increase over the past few decades, largely due to advancements in early detection, targeted therapies, and chemotherapy (1,2). This progress has transformed breast cancer from a once often fatal disease to one where long-term survivorship is increasingly common (3). However, the journey to survivorship is not easy for any woman and is often filled with mental and physical challenges (4). These challenges can be due to medications taken during the diagnosis and afterward, such as chemotherapy and adjunct hormonal therapy(5–7). Chemotherapy remains a cornerstone in the treatment of breast cancer, offering potent efficacy against tumor cells (8). Medications such as Doxorubicin, Cyclophosphamide, Paclitaxel, Docetaxel, and Carboplatin are widely used for their ability to improve survival rates(9). However, these treatments are not without their challenges. The potent effects of these drugs are accompanied by a range of side effects, including neuropathy, muscle weakness, and fatigue, which can significantly increase the risk of falls—a serious and often overlooked complication among breast cancer survivors (10,11).
Moreover, health disparities can profoundly impact the long-term outcomes of breast cancer survivors and decrease their quality of life (12). Black breast cancer survivors, for instance, face disproportionate challenges compared to their White counterparts, including limited access to quality care and the financial burden of treatment (13,14). These inequities lead to varied health outcomes, making women of color more vulnerable to complications (15). The choice of chemotherapy, in particular, is sometimes influenced by a patient's socioeconomic status, depending on the availability and type of health insurance (16). Some chemotherapy drugs, known for their strong effects, such as dizziness and muscle weakness, increase the likelihood of falls and related injuries among these women (11,17).
This study aims to investigate racial disparities (Black and White) in fall-related injuries among breast cancer survivors receiving chemotherapy, focusing on the impact of chemotherapy regimens, including Doxorubicin, Cyclophosphamide, Paclitaxel, Docetaxel, and Carboplatin.
Methods
A retrospective cohort study consisted of 11,400 female breast cancer survivors (Stage 1-3), aged 18 years and older. The participants were divided into three groups for analysis: those who received chemotherapy, those who experienced fall-related injuries, and those who had both chemotherapy and fall-related injuries. The Inclusion criteria required patients between 18 to 89 years old who had undergone treatment with one or more of the following chemotherapy agents: Doxorubicin, Cyclophosphamide, Paclitaxel, Docetaxel, or Carboplatin. Additionally, patients needed to have documented fall-related injuries during the study period, which spanned from January 1, 2019, to December 31, 2023.
TriNetX is a global health research network that connects healthcare organizations and life sciences companies, utilizing real-world data to accelerate clinical research and the development of new therapies. Through its platform, TriNetX provides access to aggregated and anonymized patient data from electronic health records, enabling researchers to conduct observational studies, optimize clinical trials, and generate real-world evidence for healthcare advancements (18).
International Classification of Diseases (ICD-10)(19) codes were used to accurately identify breast cancer diagnoses and fall-related injuries. These codes allow for a standardized way of categorizing various health conditions, ensuring consistency in data collection and analysis. Personal history of malignant neoplasm of the breast are coded under ICD-10 category Z85.3, which encompasses various forms of malignant neoplasms of the breast, depending on their specific location. Fall-related injuries, which are crucial to this study, are captured under the W00-W19 range, which includes different types of falls, such as those occurring from slipping, tripping, or falling from furniture (Table1.).
Table 1.
ICD-10 Codes for Breast Cancer Diagnoses and Fall-Related Injuries Used in the Study.
Table 1.
ICD-10 Codes for Breast Cancer Diagnoses and Fall-Related Injuries Used in the Study.
| Category |
Code |
Description |
| Breast Cancer (ICD-10) |
Z85.3 |
Personal history of malignant neoplasm of the breast |
| Fall-Related Injuries (ICD-10) |
W00-W19 |
Slipping, tripping, stumbling, and falls |
| W01 |
Fall on same level from slipping, tripping, and stumbling |
| W06 |
Fall from bed |
| W07 |
Fall from chair |
| W08 |
Fall from other furniture |
| W18 |
Other fall on same level |
| W06 |
Fall from bed |
The Current Procedural Terminology (CPT) codes used to document the administration of chemotherapy drugs in the study. These codes provide a standardized way of identifying and billing for specific chemotherapy treatments administered to breast cancer survivors. Each drug listed is commonly used in the treatment of breast cancer, and its corresponding CPT code is used to track its administration in clinical settings (Table. 2).
Table 2.
CPT Codes for Chemotherapy Drugs Administered to Breast Cancer Survivors.
Table 2.
CPT Codes for Chemotherapy Drugs Administered to Breast Cancer Survivors.
| Drug |
CPT Code |
Description |
| Doxorubicin (Adriamycin) |
J9000 |
Doxorubicin hydrochloride, 10 mg |
| Cyclophosphamide (Cytoxan) |
J9070 |
Cyclophosphamide, 100 mg |
| Paclitaxel (Taxol) |
J9265 |
Paclitaxel, 30 mg |
| Docetaxel (Taxotere) |
J9171 |
Docetaxel, 1 mg |
| Carboplatin |
J9045 |
Carboplatin, 50 mg |
For missing data, the TriNetX platform aggregates real-world data from electronic health records (EHRs) across healthcare organizations, ensuring that all necessary patient data are complete and de-identified. TriNetX’s federated data network maintains rigorous data governance protocols, minimizing the occurrence of missing data. Therefore, for this analysis, there were no missing data for key variables such as diagnosis codes, demographic characteristics, or clinical outcomes.
Statistical Analysis. Our analysis was conducted on the TriNetX platform. Descriptive statistics were used to summarize demographic characteristics of the study population. Continuous variables, such as age, were expressed as means and standard deviations, while categorical variables, including sex, race, and ethnicity, were reported as frequencies and percentages.
Univariate analyses were performed using Chi-square tests for categorical variables and T-tests for continuous variables. Logistic regression analyses were conducted to assess associations between chemotherapy types, race, and the likelihood of fall-related injuries.
Software and Tools. All analyses were conducted using the TriNetX platform’s analytical tools. These tools enabled processing of large datasets and provided robust statistical evaluations and visualizations to support epidemiological analysis.
Results
Demographic Characteristics. In the study population of 11,400 breast cancer survivors, 89.2% (N = 10,170) were identified as either Black or White. The age range of these patients ranged from 18 to 89 years, with a mean age of 69 years and a standard deviation of 12 years. The majority of patients were White, accounting for 66.75% (N = 6,788), while Black or African American patients constituted 33.33% (N = 3,390). In terms of ethnicity, 64.03% (N = 6,512) had an unknown ethnicity. Non-Hispanic or Latino individuals comprised 35.78% (N = 3,639), while Hispanic or Latino individuals accounted for only 0.35% (N = 36).
Racial Distribution of Breast Cancer Survivors Who Received Chemotherapy. Among the total breast cancer survivors, 28.2% (N = 3,220) received chemotherapy. Within this group, 55.7% (N = 1,794) were White, and 44.3% (N = 1,426) were Black or African American.
Among White breast cancer survivors, Cyclophosphamide was the most common chemotherapy, given to 24.9% (N = 447) of patients, followed closely by Paclitaxel (Taxol) at 23.9% (N = 429). Docetaxel (Taxotere) was used by 19.0% (N = 341) of White patients, while both Carboplatin and Doxorubicin were each administered to 16.1% (N = 289).
For Black or African American breast cancer survivors, Cyclophosphamide was also the most commonly administered treatment, given to 25.2% (N = 359) of patients. Paclitaxel (Taxol) was used by 23.9% (N = 341), while Doxorubicin was administered to 17.8% (N = 254). Docetaxel (Taxotere) and Carboplatin were each given to 16.6% (N = 237) of Black patients.
The most commonly administered chemotherapy among the total group was Cyclophosphamide, used by 25.0% (N = 806) of patients. This was followed by Paclitaxel (Taxol), administered to 23.9% (N = 770) (Table.3).
Table 3.
Racial Distribution and Chemotherapy Utilization Among Breast Cancer Survivors.
Table 3.
Racial Distribution and Chemotherapy Utilization Among Breast Cancer Survivors.
| Chemotherapy |
White Patients (N=1,794) |
Black Patients (N=1,426) |
Total Patients (N=3,220) |
| Docetaxel (Taxotere) |
341 (19.0%) |
237 (16.6%) |
578 (17.9%) |
| Carboplatin |
289 (16.1%) |
237 (16.6%) |
526 (16.3%) |
| Doxorubicin |
289 (16.1%) |
254 (17.8%) |
543 (16.9%) |
| Cyclophosphamide |
447 (24.9%) |
359 (25.2%) |
806 (25.0%) |
| Paclitaxel (Taxol) |
429 (23.9%) |
341 (23.9%) |
770 (23.9%) |
| Total |
1,794 (55.7%) |
1,426 (44.3%) |
3,220 (100%) |
Racial Distribution of Breast Cancer Survivors Who Received Chemotherapy and Experienced Falls. In the study population of 10,170 breast cancer survivors (Black and white), 9.2% (N = 940) experienced fall-related injuries. Among these, 41.5% (N = 390) of the falls were specifically attributed to the effects of chemotherapy. The distribution of these chemotherapy-related falls varied across different chemotherapy types.
For patients treated with Doxorubicin, 50% (N = 30) of the falls were observed in White patients, and 50% (N = 30) in Black or African American patients. Cyclophosphamide was associated with falls in 44.44% (N = 40) of White patients and 55.55% (N = 50) of Black or African American patients. Paclitaxel (Taxol) resulted in an equal distribution of falls, with 50% (N = 50) occurring in White patients and 50% (N = 50) in Black or African American patients.
Docetaxel (Taxotere) was linked to falls in 42.85% (N = 30) of White patients and 57.14% (N = 40) of Black or African American patients. Similarly, Carboplatin was associated with falls in 42.85% (N = 30) of White patients and 57.14% (N = 40) of Black or African American patients (
Table 4.).
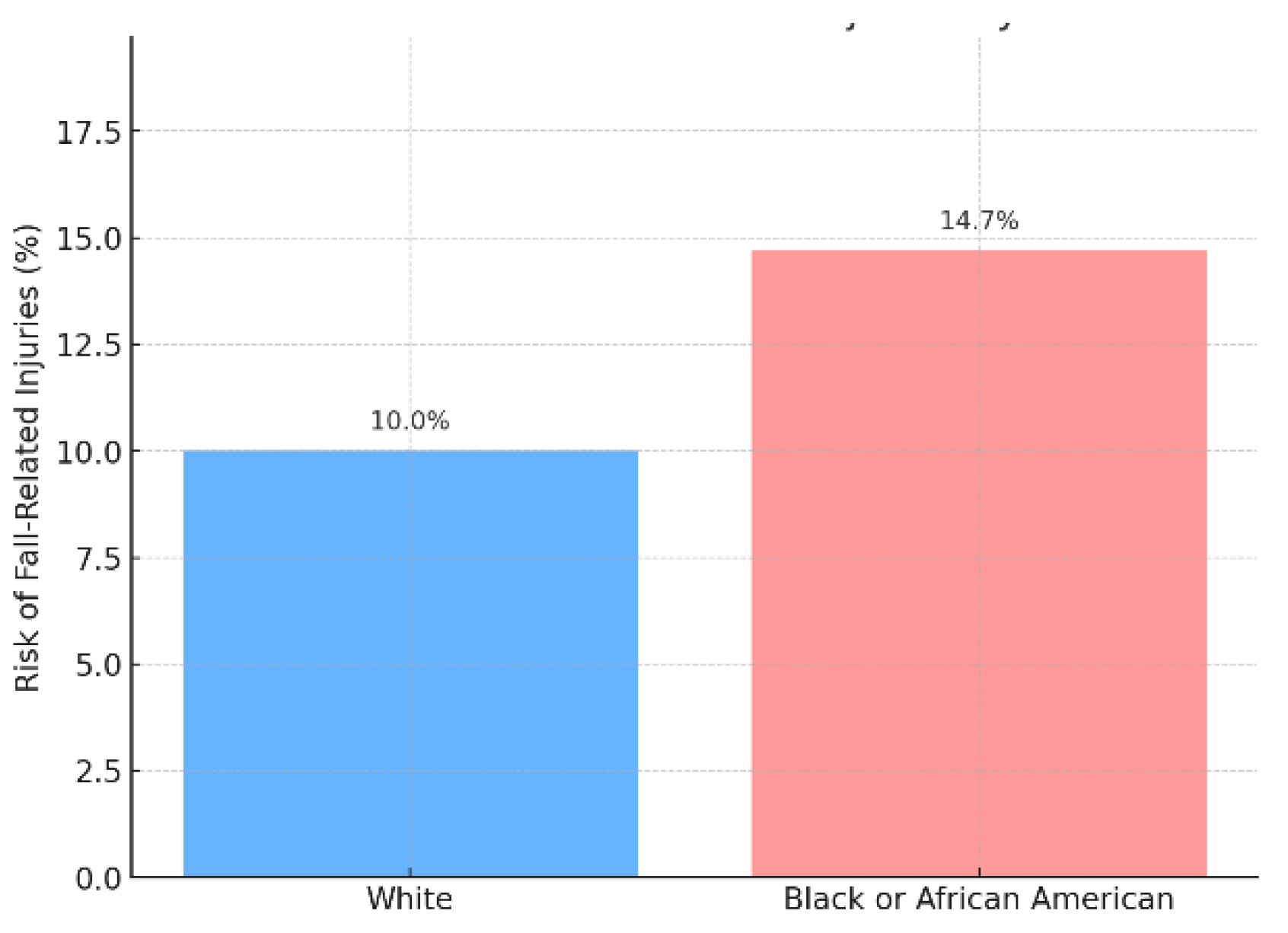
Among the 390 breast cancer survivors who received chemotherapy and experienced falls, the risk varied between White and Black or African American patients. White breast cancer survivors had a fall risk of 10.0% (N = 180), while Black or African American survivors had a higher fall risk of 14.7% (N = 210). The odds ratio (OR) for experiencing a fall-related injury was 0.65, indicating that White breast cancer survivors were 35% less likely to experience falls compared to their Black or African American counterparts. This difference in fall risk was statistically significant, with a p-value of 0.000056 (p < 0.001), suggesting a meaningful disparity between the two racial groups (Figure .1).
Figure 1.
Falls risk among breast cancer survivors who received chemotherapy.
Figure 1.
Falls risk among breast cancer survivors who received chemotherapy.
The risk of fall-related injuries among breast cancer survivors varied by chemotherapy type and race. For Doxorubicin, the risk of falls was 10.4% for White patients and 11.8% for Black patients. The odds ratio for this difference was 0.86 (p = 0.681), indicating no statistically significant difference in fall risk between the two groups for this treatment. Similarly, for Paclitaxel (Taxol), the risk of falls was 11.7% for White patients and 14.7% for Black patients, with an odds ratio of 0.77 (p = 0.236), also showing no significant difference.
In contrast, the risk of falls was significantly different for other chemotherapy types. For Cyclophosphamide, the risk was 8.9% for White patients and 13.9% for Black patients, with an odds ratio of 0.61 (p = 0.032). This suggests that White patients had a lower risk of falls when compared to Black patients, and this difference was statistically significant. Docetaxel (Taxotere) showed a similar pattern, with a fall risk of 8.8% for White patients and 16.9% for Black patients. The odds ratio was 0.48 (p = 0.004), indicating a significantly lower risk for White patients. Lastly, for Carboplatin, the fall risk was 10.4% for White patients and 16.9% for Black patients, with an odds ratio of 0.57 (p = 0.038), again pointing to a significant difference.
Discussion
This study investigates the risk of fall-related injuries among breast cancer survivors undergoing chemotherapy, with a particular emphasis on racial disparities between Black and White patients. Utilizing a retrospective cohort of 11,400 female breast cancer survivors (Stage 1-3), aged 18 and older, treated between January 1, 2019, and December 31, 2023, the study evaluates the fall risks associated with chemotherapy agents like Doxorubicin, Cyclophosphamide, Paclitaxel, Docetaxel, and Carboplatin.
Our study found that Black breast cancer survivors are disproportionately affected by falls, particularly when treated with Cyclophosphamide, Docetaxel, and Carboplatin. This aligns with prior research indicating that chemotherapy-induced peripheral neuropathy (CIPN) is a significant risk factor for falls, especially among patients undergoing aggressive chemotherapy regimens(20). Black patients receiving these chemotherapy agents were found to be at a significantly higher risk of falls compared to their White counterparts, indicating a critical disparity in treatment outcomes. This disparity may stem from various factors, including differences in drug metabolism, healthcare access, and availability of supportive care(21,22). In particular, Cyclophosphamide, one of the more affordable chemotherapy drugs(23), was associated with the highest fall risk among Black patients, in our study. This raises concerns about the influence of socioeconomic factors on treatment decisions, where less costly but potentially riskier drugs are more frequently prescribed to minority populations.
The financial burden of chemotherapy, particularly with drugs like Cyclophosphamide, Paclitaxel, Docetaxel, and Carboplatin, significantly influences treatment decisions and outcomes for breast cancer survivors(24). These drugs vary in cost, with Docetaxel and Carboplatin being more expensive than Cyclophosphamide(25). However, cost plays a crucial role in physicians’ prescribing patterns, especially when treating patients from underinsured or uninsured backgrounds(26). Cyclophosphamide, though less expensive(27), is often prescribed to Black breast cancer survivors, who are disproportionately affected by financial toxicity due to inadequate health insurance coverage(28). Moreover, limited insurance coverage can delay access to high-quality supportive care, exacerbating these side effects and increasing the likelihood of poor outcomes. The higher costs of Docetaxel and Carboplatin, coupled with limited insurance, make them less accessible to minority populations, even though these drugs could offer more favorable side effect profiles. These findings underscore the critical role that healthcare policies and insurance play in shaping equitable access to cancer treatment. Addressing these disparities requires policies that ensure all patients, regardless of financial status or race, have access to comprehensive care and the full range of chemotherapy options.
Strengths
The study boasts several strengths that enhance the robustness and relevance of its findings. First, the large sample size of 11,400 breast cancer survivors allows for sufficient statistical power to detect meaningful differences across chemotherapy regimens and racial subgroups. This extensive dataset ensures that the results are more generalizable and reflective of broader patterns in real-world clinical settings. Additionally, the use of standardized ICD-10 codes for identifying both breast cancer diagnoses and fall-related injuries improves the accuracy and consistency of the data, minimizing potential errors in diagnosis classification and treatment outcomes. The study’s focus on multiple chemotherapy agents, including Doxorubicin, Cyclophosphamide, Paclitaxel, Docetaxel, and Carboplatin, offers a comprehensive evaluation of fall risks across different treatment modalities, providing valuable insights into drug-specific side effects that could inform future clinical guidelines. Moreover, the inclusion of racial disparities as a key component of the analysis is a significant strength, as it highlights critical healthcare inequities that affect treatment outcomes for underrepresented populations, particularly Black breast cancer survivors.
Limitations
As a retrospective cohort study, the analysis is reliant on previously collected data, which may be subject to inaccuracies or incomplete entries in electronic health records (EHRs). While the TriNetX platform provides a powerful tool for analyzing large datasets, the inability to conduct follow-up assessments limits the ability to validate certain outcomes, such as the accuracy of fall-related injury reports or the precise timing of chemotherapy administration. Additionally, the study’s reliance on de-identified data restricts the potential for in-depth patient-level analysis, preventing a more personalized understanding of the factors contributing to the observed disparities. The study’s setting within a single health system may also limit the generalizability of the findings, as healthcare access, treatment practices, and socioeconomic factors can vary widely across different regions.
Conclusions
The findings reveal that Black breast cancer survivors face a disproportionately higher risk of fall-related injuries compared to their White counterparts, particularly when undergoing specific chemotherapy regimens. This disparity underscores concerns regarding healthcare access, treatment quality, and the differing physiological responses to chemotherapy between racial groups. Addressing these inequities requires a focused effort to ensure that all breast cancer survivors, regardless of race, receive equitable care, supportive treatment, and comprehensive follow-up to mitigate adverse effects such as falls. These insights point to the need for more inclusive healthcare policies that address both racial and socioeconomic barriers to effective cancer care.
Funding Statement: We extend our gratitude to the informatics team at Virginia Commonwealth University's (VCU) C. Kenneth and Dianne Wright Center for Clinical and Translational Research, especially Dr. Tamas Gal and his team members, Evan French and Patrick Shi, for their invaluable support in data extraction through TriNetX platform. This work was supported by the Wright Center under the Clinical and Translational Science Award (CTSA) Grant number UM1TR004360.
Conflicts of Interest. Each author has completed and submitted the Conflict-of-Interest Disclosure Form and has declared that:• Financial conflicts: None of the authors have financial relationships with any entities that have an interest in the information in the submitted work. There are no affiliations, memberships, funding, or financial holdings that might be perceived as affecting the objectivity of this publication.• Personal conflicts: There are no personal relationships or direct engagements that could influence or appear to influence the submitted work.
Author Contributions
A.N. Conceptualized the study, oversaw data collection and analysis, interpreted the results, and wrote the main manuscript text. R.M. and D.R. Assisted in drafting sections related to health disparities and treatment access. V.S. Provided guidance on study design, manuscript drafting and editing. N.T. Provided guidance on study design, statistical analysis, and data interpretation, and contributed to the methods and results sections. He also reviewed and critically revised the manuscript for intellectual content. All authors reviewed and approved the final manuscript.
Ethics Approval Statement: Ethical approval for this study was obtained from the Institutional Review Board (IRB) at Virginia Commonwealth University, classified as a non-human subject submission to ensure adherence to ethical guidelines and patient confidentiality. Access to the TriNetX database was secured through the observational informatics program at Virginia Commonwealth University’s, C. Kenneth and Dianne Wright Center for Clinical and Translational Research, following strict data governance protocols to protect patient privacy and comply with regulatory standards.
Data Availability Statement
The datasets generated and/or analyzed during the current study were extracted from the TriNetX database and are available upon request from the corresponding author due to privacy and ethical restrictions.
References
- Wilson, J.; Sule, A.A. Disparity in Early Detection of Breast Cancer. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2024 [cited 2024 Aug 27]. Available from: http://www.ncbi.nlm.nih. 5643. [Google Scholar]
- Guo, F. ; Kuo Y fang, Shih YCT, Giordano, S. H.; Berenson, A.B. Trends in breast cancer mortality by stage at diagnosis among US young women. Cancer. 2018, 124, 3500–3509. [Google Scholar] [CrossRef]
- Bodai, B.I.; Tuso, P. Breast Cancer Survivorship: A Comprehensive Review of Long-Term Medical Issues and Lifestyle Recommendations. Perm, J. 2015, 19, 48–79. [Google Scholar] [CrossRef] [PubMed]
- R. K, L. S, P. B, S. G, R. LP. Psychosocial experiences of breast cancer survivors: a meta-review. J Cancer Surviv. 2024, 18, 84–123. [CrossRef]
- Katta, B.; Vijayakumar, C.; Dutta, S.; Dubashi, B.; Nelamangala Ramakrishnaiah, V.P. The Incidence and Severity of Patient-Reported Side Effects of Chemotherapy in Routine Clinical Care: A Prospective Observational Study. Cureus. 15, e38301. [CrossRef]
- Hormone Therapy for Breast Cancer Fact Sheet - NCI [Internet]. 2022 [cited 2024 Aug 27]. Available from: https://www.cancer.
- Bekes, I.; Huober, J. Extended Adjuvant Endocrine Therapy in Early Breast Cancer Patients—Review and Perspectives. Cancers. 2023, 15, 4190. [Google Scholar] [CrossRef] [PubMed]
- Advances in medical treatment of breast cancer in 2022 - Zhai - 2023 - Cancer Innovation - Wiley Online Library [Internet]. [cited 2024 Aug 27]. Available from: https://onlinelibrary.wiley.com/doi/10.1002/cai2.
- Amjad, M.T.; Chidharla, A.; Kasi, A. Cancer Chemotherapy. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2024 [cited 2024 Aug 27]. Available from: http://www.ncbi.nlm.nih. 5643. [Google Scholar]
- Regan, J.N.; Mikesell, C.; Reiken, S.; Xu, H.; Marks, A.R.; Mohammad, K.S.; et al. Osteolytic Breast Cancer Causes Skeletal Muscle Weakness in an Immunocompetent Syngeneic Mouse Model. Front Endocrinol. 2017, 8, 358. [Google Scholar] [CrossRef] [PubMed]
- Tofthagen, C.; Overcash, J.; Kip, K. Falls in Persons with Chemotherapy Induced Peripheral Neuropathy. Support Care Cancer Off J Multinatl Assoc Support Care Cancer. 2012, 20, 583–589. [Google Scholar] [CrossRef] [PubMed]
- Yedjou, C.G.; Sims, J.N.; Miele, L.; Noubissi, F.; Lowe, L.; Fonseca, D.D.; et al. Health and Racial Disparity in Breast Cancer. Adv Exp Med Biol. 2019, 1152, 31–49. [Google Scholar] [CrossRef] [PubMed]
- Zeno, E.E.; Brewer, N.T.; Spees, L.P.; Des Marais, A.C.; Sanusi, B.O.; Hudgens, M.G.; et al. Racial and ethnic differences in cervical cancer screening barriers and intentions: The My Body My Test-3 HPV self-collection trial among under-screened, low-income women. PloS One. 2022, 17, e0274974. [Google Scholar] [CrossRef] [PubMed]
- Tong, M.; Hill, L.; Published, S.A. Racial Disparities in Cancer Outcomes, Screening, and Treatment [Internet]. KFF. 2022 [cited 2024 Aug 27]. Available from: https://www.kff.
- Chinn, J.J.; Martin, I.K.; Redmond, N. Health Equity Among Black Women in the United States. J Womens Health. 2021, 30, 212–219. [Google Scholar] [CrossRef] [PubMed]
- Tibi, S.; Tieu, V.; Babayigit, S.; Ling, J. Influence of Health Insurance Types on Clinical Cancer Care Accessibility and Quality Using All of Us Database. Medicina (Mex). 2024, 60, 623. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.; Wang, G.; Chen, L.; Yu, S.; Li, W. Risk factors for falls in hospitalized patients with cancer: A systematic review and meta-analysis. Asia-Pac J Oncol Nurs. 2022 Jun 29;9, 100107. [CrossRef]
- TriNetX. TriNetX [Internet]. 2013 [cited 2024 Jul 18]. Available from: https://live.trinetx. 2028.
- World Health Organization. international statistical classification of diseases and related health problems : tenth revision, 2nd ed. World Health Organization. 2004. ICD-10 : international statistical classification of diseases and related health problems : tenth revision, 2nd ed. Available from: https://iris.who. 1066.
- Yamamoto, S.; Fujikawa, N.; Asano, K.; Toki, M.; Takao, A.; Arao, H. Assessment of Fall-Related Self-Efficacy: Characteristics that Influence the Perception of Patients with Chemotherapy-Induced Peripheral Neuropathy. Asia-Pac J Oncol Nurs. 2020, 7, 190–195. [Google Scholar] [CrossRef] [PubMed]
- Esnaola, N.F.; Ford, M.E. Racial Differences and Disparities in Cancer Care and Outcomes: Where’s the Rub? Surg Oncol Clin N Am. 2012, 21, 417–viii. [Google Scholar] [CrossRef] [PubMed]
- Pathak, S.; Zajac, K.K.; Annaji, M.; Govindarajulu, M.; Nadar, R.M.; Bowen, D.; et al. Clinical outcomes of chemotherapy in cancer patients with different ethnicities. Cancer Rep. 2023, 6 (Suppl 1), e1830. [Google Scholar] [CrossRef] [PubMed]
- Younis, T.; Rayson, D.; Skedgel, C. The cost–utility of adjuvant chemotherapy using docetaxel and cyclophosphamide compared with doxorubicin and cyclophosphamide in breast cancer. Curr Oncol. 2011, 18, e288–96. [Google Scholar] [CrossRef] [PubMed]
- Smith, G.L.; Lopez-Olivo, M.A.; Advani, P.G.; Ning, M.S.; Geng, Y.; Giordano, S.H.; et al. Financial Burdens of Cancer Treatment: A Systematic Review of Risk Factors and Outcomes. J Natl Compr Cancer Netw JNCCN. 2019, 17, 1184–1192. [Google Scholar] [CrossRef] [PubMed]
- Giordano, S.H.; Niu, J.; Chavez-MacGregor, M.; Zhao, H.; Zorzi, D. ; Shih YCT, et al. Estimating regimen-specific costs of chemotherapy for breast cancer: Observational cohort study. Cancer. 2016, 122, 3447–3455. [Google Scholar] [CrossRef] [PubMed]
- Hart, J.; Salman, H.; Bergman, M.; Neuman, V.; Rudniki, C.; Gilenberg, D.; et al. Do drug costs affect physicians’ prescription decisions? J Intern Med. 1997, 241, 415–420. [Google Scholar] [CrossRef] [PubMed]
- McKinley, A.; Park, E.; Spetie, D.; Hackshaw, K.V.; Nagaraja, S.; Hebert, L.A.; et al. Oral Cyclophosphamide for Lupus Glomerulonephritis: An Underused Therapeutic Option. Clin J Am Soc Nephrol CJASN. 2009, 4, 1754–1760. [Google Scholar] [CrossRef] [PubMed]
- The influence of race on financial toxicity among cancer patients. | Journal of Clinical Oncology [Internet]. [cited 2024 Sep 20]. Available from: https://ascopubs.org/doi/10.1200/JCO.2021.39.15_suppl. 1525.
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).