Submitted:
14 October 2024
Posted:
15 October 2024
You are already at the latest version
Abstract
Migraine and stroke are neurological disorders with significant global prevalence and impact. Recent advances in migraine therapy have focused on calcitonin gene-related peptide (CGRP) pathway. This review examines the shared pathomechanisms between migraine and stroke, with emphasis on the role of CGRP. We analyze current literature on CGRP's functions in cerebrovascular regulation, edema formation, neuroinflammation, and neuroprotection. CGRP acts as a potent vasodilator and plays a crucial role in trigeminovascular activation during migraine attacks. In stroke, CGRP has demonstrated neuroprotective effects by improving collateral circulation and reducing ischemia-reperfusion injury. Concerns have been raised about the potential impact of CGRP inhibsitors on stroke risk and outcomes. Studies in animals suggest that CGRP receptor antagonists may worsen cerebral ischemia by impairing collateral flow. We discuss the implications of these findings for the use of CGRP-targeting therapies in migraine patients, especially those at increased risk of stroke. Additionally, we explore the complex interplay between CGRP, endothelial function, and platelet activity in both conditions. This review highlights the need for further research to elucidate the long-term cerebrovascular safety of CGRP pathway inhibitors and to identify potential subgroups of migraine patients who may be at higher risk of adverse cerebrovascular events with these novel therapies.
Keywords:
1.(. Patho-)Physiological Effects of Calcitonin Gene-Related Peptide (CGRP)
2. Migraine Treatment with CGRP-Pathway Targeting Therapeutics
3. The Complex Relationship Between Migraine and Stroke: Insights into Risk Factors and Vascular Mechanisms
4. The Role of Attack Medication and Cortical Spreading Depression (CSD)
5. Molecular Implications of CGRP’s Role in Stroke
6. Cardiovascular Risk Signals in Clinical Trials Using Monoclonal Antibodies and Gepants
7. Cardiovascular Risk Signals in Non-Clinical
8. CGRP Levels in Acute Stroke Patients
9. CGRP and Other Vascular Disease
10. Discussion
10.1. Concerns in Acute Vascular Events
10.2. Dual CGRP-Targeted Treatment by mAbs and Gepants
10.3. Molecular Findings Leading to Clinical Implications
11. Conclusion
Author Contributions
Funding
Conflicts of Interest
References
- Eftekhari, S.; Salvatore, C.A.; Johansson, S.; Chen, T.-B.; Zeng, Z.; Edvinsson, L. Localization of CGRP, CGRP Receptor, PACAP and Glutamate in Trigeminal Ganglion. Relation to the Blood-Brain Barrier. Brain Res 2015, 1600, 93–109. [Google Scholar] [CrossRef] [PubMed]
- Balint, L.; Nelson-Maney, N.P.; Tian, Y.; Serafin, S.D.; Caron, K.M. Clinical Potential of Adrenomedullin Signaling in the Cardiovascular System. Circ Res 2023, 132. [Google Scholar] [CrossRef] [PubMed]
- Bernstein, C.; Burstein, R. Sensitization of the Trigeminovascular Pathway: Perspective and Implications to Migraine Pathophysiology. J Clin Neurol 2012, 8, 89–99. [Google Scholar] [CrossRef] [PubMed]
- Altamura, C.; Vernieri, F. Commentary: Enhanced Hemodynamic and Clinical Response to ACGRP in Migraine Patients—A TCD Study. Front Neurol 2021, 12. [Google Scholar] [CrossRef] [PubMed]
- Okutsu, Y.; Takahashi, Y.; Nagase, M.; Shinohara, K.; Ikeda, R.; Kato, F. Potentiation of NMDA Receptor-Mediated Synaptic Transmission at the Parabrachial-Central Amygdala Synapses by CGRP in Mice. Mol Pain 2017, 13. [Google Scholar] [CrossRef]
- Carter, S.C.; Cucchiara, B.; Reehal, N.; Hamilton, K.; Kaiser, E.A.; Favilla, C.G. Effect of CGRP Inhibitors on Interictal Cerebral Hemodynamics in Individuals with Migraine. Frontiers in Neurology 2024, 15. [Google Scholar] [CrossRef]
- Zhai, L.; Sakurai, T.; Kamiyoshi, A.; Ichikawa-Shindo, Y.; Kawate, H.; Tanaka, M.; Xian, X.; Hirabayashi, K.; Dai, K.; Cui, N.; et al. Endogenous Calcitonin Gene-Related Peptide Suppresses Ischemic Brain Injuries and Progression of Cognitive Decline. J Hypertens 2018, 36, 876–891. [Google Scholar] [CrossRef]
- Guo, Z.; Liu, N.; Chen, L.; Zhao, X.; Li, M.R. Independent Roles of CGRP in Cardioprotection and Hemodynamic Regulation in Ischemic Postconditioning. Eur J Pharmacol 2018, 828. [Google Scholar] [CrossRef]
- Sorby-Adams, A.J.; Marcoionni, A.M.; Dempsey, E.R.; Woenig, J.A.; Turner, R.J. The Role of Neurogenic Inflammation in Blood-Brain Barrier Disruption and Development of Cerebral Oedema Following Acute Central Nervous System (CNS) Injury. Int J Mol Sci 2017, 18. [Google Scholar] [CrossRef]
- Zarban, A.A.; Chaudhry, H.; de Sousa Valente, J.; Argunhan, F.; Ghanim, H.; Brain, S.D. Elucidating the Ability of CGRP to Modulate Microvascular Events in Mouse Skin. Int J Mol Sci 2022, 23. [Google Scholar] [CrossRef]
- Eftekhari, S.; Salvatore, C.A.; Calamari, A.; Kane, S.A.; Tajti, J.; Edvinsson, L. Differential Distribution of Calcitonin Gene-Related Peptide and Its Receptor Components in the Human Trigeminal Ganglion. Neuroscience 2010, 169, 683–696. [Google Scholar] [CrossRef] [PubMed]
- Hage La Cour, S.; Juhler, K.; Kogelman, L.J.A.; Olesen, J.; Klærke, D.A.; Kristensen, D.M.; Jansen-Olesen, I. Characterization of Erenumab and Rimegepant on Calcitonin Gene-Related Peptide Induced Responses in Xenopus Laevis Oocytes Expressing the Calcitonin Gene-Related Peptide Receptor and the Amylin-1 Receptor. Journal of Headache and Pain 2022, 23. [Google Scholar] [CrossRef] [PubMed]
- Global Burden of 369 Diseases and Injuries in 204 Countries and Territories, 1990-2019: A Systematic Analysis for the Global Burden of Disease Study 2019. Lancet 2020, 396, 1204–1222. [CrossRef] [PubMed]
- Kamm, K.; Straube, A.; Ruscheweyh, R. Calcitonin Gene-Related Peptide Levels in Tear Fluid Are Elevated in Migraine Patients Compared to Healthy Controls. Cephalalgia 2019, 39, 1535–1543. [Google Scholar] [CrossRef]
- Cernuda-Morollon, E.; Larrosa, D.; Ramon, C.; Vega, J.; Martinez-Camblor, P.; Pascual, J.; Cernuda-Morollón, E.; Larrosa, D.; Ramón, C.; Vega, J.; et al. Interictal Increase of CGRP Levels in Peripheral Blood as a Biomarker for Chronic Migraine. Neurology 2013, 81, 1191–1196. [Google Scholar] [CrossRef]
- Hansen, J.M.; Hauge, A.W.; Olesen, J.; Ashina, M. Calcitonin Gene-Related Peptide Triggers Migraine-like Attacks in Patients with Migraine with Aura. Cephalalgia 2010, 30, 1179–1186. [Google Scholar] [CrossRef]
- Guo, S.; Vollesen, A.L.H.; Olesen, J.; Ashina, M. Premonitory and Nonheadache Symptoms Induced by CGRP and PACAP38 in Patients with Migraine. Pain 2016, 157, 2773–2781. [Google Scholar] [CrossRef]
- Messina, R.; Huessler, E.-M.; Puledda, F.; Haghdoost, F.; Lebedeva, E.R.; Diener, H.-C. Safety and Tolerability of Monoclonal Antibodies Targeting the CGRP Pathway and Gepants in Migraine Prevention: A Systematic Review and Network Meta-Analysis. Cephalalgia 2023, 43, 3331024231152169. [Google Scholar] [CrossRef]
- Bhakta, M.; Vuong, T.; Taura, T.; Wilson, D.S.; Stratton, J.R.; Mackenzie, K.D. Migraine Therapeutics Differentially Modulate the CGRP Pathway. Cephalalgia 2021, 41. [Google Scholar] [CrossRef]
- Edvinsson, L.; Nilsson, E.; Jansen-Olesen, I. Inhibitory Effect of BIBN4096BS, CGRP 8-37, a CGRP Antibody and an RNA-Spiegelmer on CGRP Induced Vasodilatation in the Perfused and Non-Perfused Rat Middle Cerebral Artery. Br J Pharmacol 2007, 150. [Google Scholar] [CrossRef]
- Lundblad, C.; Haanes, K.A.; Grände, G.; Edvinsson, L. Experimental Inflammation Following Dural Application of Complete Freund’s Adjuvant or Inflammatory Soup Does Not Alter Brain and Trigeminal Microvascular Passage. J Headache Pain 2015, 16, 91. [Google Scholar] [CrossRef] [PubMed]
- Noseda, R.; Schain, A.J.; Melo-Carrillo, A.; Tien, J.; Stratton, J.; Mai, F.; Strassman, A.M.; Burstein, R. Fluorescently-Labeled Fremanezumab Is Distributed to Sensory and Autonomic Ganglia and the Dura but Not to the Brain of Rats with Uncompromised Blood Brain Barrier. Cephalalgia 2020, 40, 229–240. [Google Scholar] [CrossRef] [PubMed]
- Eftekhari, S.; Salvatore, C.A.; Johansson, S.; Chen, T.-B.; Zeng, Z.; Edvinsson, L. Localization of CGRP, CGRP Receptor, PACAP and Glutamate in Trigeminal Ganglion. Relation to the Blood-Brain Barrier. Brain Res 2015, 1600, 93–109. [Google Scholar] [CrossRef] [PubMed]
- Salvatore, C.A.; Moore, E.L.; Calamari, A.; Cook, J.J.; Michener, M.S.; O’Malley, S.; Miller, P.J.; Sur, C.; Williams, D.L.J.; Zeng, Z.; et al. Pharmacological Properties of MK-3207, a Potent and Orally Active Calcitonin Gene-Related Peptide Receptor Antagonist. J Pharmacol Exp Ther 2010, 333, 152–160. [Google Scholar] [CrossRef] [PubMed]
- Christensen, S.L.; Ernstsen, C.; Olesen, J.; Kristensen, D.M. No Central Action of CGRP Antagonising Drugs in the GTN Mouse Model of Migraine. Cephalalgia 2020, 40, 924–934. [Google Scholar] [CrossRef]
- Dodick, D.W.; Lipton, R.B.; Ailani, J.; Lu, K.; Finnegan, M.; Trugman, J.M.; Szegedi, A. Ubrogepant for the Treatment of Migraine. N Engl J Med 2019, 381, 2230–2241. [Google Scholar] [CrossRef]
- Lipton, R.B.; Croop, R.; Stock, D.A.; Madonia, J.; Forshaw, M.; Lovegren, M.; Mosher, L.; Coric, V.; Goadsby, P.J. Safety, Tolerability, and Efficacy of Zavegepant 10 Mg Nasal Spray for the Acute Treatment of Migraine in the USA: A Phase 3, Double-Blind, Randomised, Placebo-Controlled Multicentre Trial. Lancet Neurol 2023, 22, 209–217. [Google Scholar] [CrossRef]
- Kurth, T.; Rist, P.M.; Ridker, P.M.; Kotler, G.; Bubes, V.; Buring, J.E. Association of Migraine With Aura and Other Risk Factors With Incident Cardiovascular Disease in Women. JAMA 2020, 323, 2281–2289. [Google Scholar] [CrossRef]
- West, B.H.; Noureddin, N.; Mamzhi, Y.; Low, C.G.; Coluzzi, A.C.; Shih, E.J.; Gevorgyan Fleming, R.; Saver, J.L.; Liebeskind, D.S.; Charles, A.; et al. Frequency of Patent Foramen Ovale and Migraine in Patients With Cryptogenic Stroke. Stroke 2018, 49, 1123–1128. [Google Scholar] [CrossRef]
- De Giuli, V.; Grassi, M.; Locatelli, M.; Gamba, M.; Morotti, A.; Bonacina, S.; Mazzoleni, V.; Pezzini, D.; Magoni, M.; Monastero, R.; et al. Cardiac Sources of Cerebral Embolism in People with Migraine. Eur J Neurol 2021, 28, 516–524. [Google Scholar] [CrossRef]
- De Giuli, V.; Grassi, M.; Lodigiani, C.; Patella, R.; Zedde, M.; Gandolfo, C.; Zini, A.; DeLodovici, M.L.; Paciaroni, M.; Del Sette, M.; et al. Association Between Migraine and Cervical Artery Dissection: The Italian Project on Stroke in Young Adults. JAMA Neurol 2017, 74, 512–518. [Google Scholar] [CrossRef] [PubMed]
- Kurth, T.; Rist, P.M.; Ridker, P.M.; Kotler, G.; Bubes, V.; Buring, J.E. Association of Migraine With Aura and Other Risk Factors With Incident Cardiovascular Disease in Women. JAMA 2020, 323, 2281–2289. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.-T.; Chu, K.; Jung, K.-H.; Kim, D.-H.; Kim, E.-H.; Choe, V.N.; Kim, J.-H.; Im, W.-S.; Kang, L.; Park, J.-E.; et al. Decreased Number and Function of Endothelial Progenitor Cells in Patients with Migraine. Neurology 2008, 70, 1510–1517. [Google Scholar] [CrossRef] [PubMed]
- Oterino, A.; Toriello, M.; Palacio, E.; Quintanilla, V.G.; Ruiz-Lavilla, N.; Montes, S.; Vega, M.S. de la; Martinez-Nieto, R.; Castillo, J.; Pascual, J. Analysis of Endothelial Precursor Cells in Chronic Migraine: A Case-Control Study. Cephalalgia 2013, 33, 236–244. [Google Scholar] [CrossRef]
- Liman, T.G.; Bachelier-Walenta, K.; Neeb, L.; Rosinski, J.; Reuter, U.; Böhm, M.; Endres, M. Circulating Endothelial Microparticles in Female Migraineurs with Aura. Cephalalgia 2015, 35, 88–94. [Google Scholar] [CrossRef]
- Tietjen, G.E.; Khubchandani, J.; Herial, N.; Palm-Meinders, I.H.; Koppen, H.; Terwindt, G.M.; van Buchem, M.A.; Launer, L.J.; Ferrari, M.D.; Kruit, M.C. Migraine and Vascular Disease Biomarkers: A Population-Based Case-Control Study. Cephalalgia 2018, 38, 511–518. [Google Scholar] [CrossRef]
- Fischer, M.; Gaul, C.; Shanib, H.; Holle, D.; Loacker, L.; Griesmacher, A.; Lackner, P.; Broessner, G. Markers of Endothelial Function in Migraine Patients: Results from a Bi-Center Prospective Study. Cephalalgia 2015, 35, 877–885. [Google Scholar] [CrossRef]
- Tietjen, G.E.; Al-Qasmi, M.M.; Athanas, K.; Dafer, R.M.; Khuder, S.A. Increased von Willebrand Factor in Migraine. Neurology 2001, 57, 334–336. [Google Scholar] [CrossRef]
- Tietjen, G.E.; Al-Qasmi, M.M.; Athanas, K.; Utley, C.; Herial, N.A. Altered Hemostasis in Migraineurs Studied with a Dynamic Flow System. Thromb Res 2007, 119, 217–222. [Google Scholar] [CrossRef]
- Tietjen, G.E.; Herial, N.A.; Utley, C.; White, L.; Yerga-Woolwine, S.; Joe, B. Association of von Willebrand Factor Activity with ACE I/D and MTHFR C677T Polymorphisms in Migraine. Cephalalgia 2009, 29, 960–968. [Google Scholar] [CrossRef]
- Vanmolkot, F.H.; de Hoon, J.N. Increased C-Reactive Protein in Young Adult Patients with Migraine. Cephalalgia 2007, 27, 843–846. [Google Scholar] [CrossRef] [PubMed]
- Tietjen, G.E.; Herial, N.A.; White, L.; Utley, C.; Kosmyna, J.M.; Khuder, S.A. Migraine and Biomarkers of Endothelial Activation in Young Women. Stroke 2009, 40, 2977–2982. [Google Scholar] [CrossRef] [PubMed]
- Hamed, S.A.; Hamed, E.A.; Ezz Eldin, A.M.; Mahmoud, N.M. Vascular Risk Factors, Endothelial Function, and Carotid Thickness in Patients with Migraine: Relationship to Atherosclerosis. J Stroke Cerebrovasc Dis 2010, 19, 92–103. [Google Scholar] [CrossRef] [PubMed]
- Güzel, I.; Taşdemir, N.; Celik, Y. Evaluation of Serum Transforming Growth Factor Β1 and C-Reactive Protein Levels in Migraine Patients. Neurol Neurochir Pol 2013, 47, 357–362. [Google Scholar] [CrossRef] [PubMed]
- Domínguez, C.; López, A.; Ramos-Cabrer, P.; Vieites-Prado, A.; Pérez-Mato, M.; Villalba, C.; Sobrino, T.; Rodriguez-Osorio, X.; Campos, F.; Castillo, J.; et al. Iron Deposition in Periaqueductal Gray Matter as a Potential Biomarker for Chronic Migraine. Neurology 2019, 92, e1076–e1085. [Google Scholar] [CrossRef]
- Oterino, A.; Toriello, M.; Palacio, E.; Quintanilla, V.G.; Ruiz-Lavilla, N.; Montes, S.; Vega, M.S. de la; Martinez-Nieto, R.; Castillo, J.; Pascual, J. Analysis of Endothelial Precursor Cells in Chronic Migraine: A Case-Control Study. Cephalalgia 2013, 33, 236–244. [Google Scholar] [CrossRef]
- Liman, T.G.; Bachelier-Walenta, K.; Neeb, L.; Rosinski, J.; Reuter, U.; Böhm, M.; Endres, M. Circulating Endothelial Microparticles in Female Migraineurs with Aura. Cephalalgia 2015, 35, 88–94. [Google Scholar] [CrossRef]
- Tietjen, G.E.; Khubchandani, J.; Herial, N.; Palm-Meinders, I.H.; Koppen, H.; Terwindt, G.M.; van Buchem, M.A.; Launer, L.J.; Ferrari, M.D.; Kruit, M.C. Migraine and Vascular Disease Biomarkers: A Population-Based Case-Control Study. Cephalalgia 2018, 38, 511–518. [Google Scholar] [CrossRef]
- Roberto, G.; Raschi, E.; Piccinni, C.; Conti, V.; Vignatelli, L.; D’Alessandro, R.; De Ponti, F.; Poluzzi, E. Adverse Cardiovascular Events Associated with Triptans and Ergotamines for Treatment of Migraine: Systematic Review of Observational Studies. Cephalalgia 2015, 35, 118–131. [Google Scholar] [CrossRef]
- de Vries, T.; Villalón, C.M.; MaassenVanDenBrink, A. Pharmacological Treatment of Migraine: CGRP and 5-HT beyond the Triptans. Pharmacol Ther 2020, 211. [Google Scholar] [CrossRef]
- Sueiras, M.; Thonon, V.; Santamarina, E.; Sánchez-Guerrero, Á.; Poca, M.A.; Quintana, M.; Riveiro, M.; Sahuquillo, J. Cortical Spreading Depression Phenomena Are Frequent in Ischemic and Traumatic Penumbra: A Prospective Study in Patients With Traumatic Brain Injury and Large Hemispheric Ischemic Stroke. J Clin Neurophysiol 2021, 38, 47–55. [Google Scholar] [CrossRef] [PubMed]
- Chang, C.L.; Cai, Z.; Hsu, S.Y.T. Sustained Activation of CLR/RAMP Receptors by Gel-Forming Agonists. Int J Mol Sci 2022, 23. [Google Scholar] [CrossRef] [PubMed]
- Liu, G.; Cai, H.; Xu, X.; Liu, Z.; Wang, Q.; Feng, G.; Li, Y.; Xu, C.; Li, Z. The Effects of Calcitonin Gene-Related Peptide on BFGF and AQP4 Expression after Focal Cerebral Ischemia Reperfusion in Rats. Pharmazie 2010, 65. [Google Scholar] [CrossRef]
- Tam, C.; Brain, S.D. The Assessment of Vasoactive Properties of CGRP and Adrenomedullin in the Microvasculature: A Study Using in Vivo and in Vitro Assays in the Mouse. In Proceedings of the Journal of Molecular Neuroscience; 2004; Vol. 22. [Google Scholar]
- Shin, H.K.; Hong, K.W. Importance of Calcitonin Gene-Related Peptide, Adenosine and Reactive Oxygen Species in Cerebral Autoregulation under Normal and Diseased Conditions. Clin Exp Pharmacol Physiol 2004, 31. [Google Scholar] [CrossRef]
- Leticia, F.; Anita, I. Adrenomedullin and Angiotensin II Signaling Pathways Involved in the Effects on Cerebellar Antioxidant Enzymes Activity. Brain Res Bull 2017, 128. [Google Scholar] [CrossRef]
- Rehni, A.K.; Singh, T.G.; Jaggi, A.S.; Singh, N. Pharmacological Preconditioning of the Brain: A Possible Interplay between Opioid and Calcitonin Gene Related Peptide Transduction Systems. Pharmacological Reports 2008, 60. [Google Scholar]
- Cozene, B.; Sadanandan, N.; Farooq, J.; Kingsbury, C.; Park, Y.J.; Wang, Z.J.; Moscatello, A.; Saft, M.; Cho, J.; Gonzales-Portillo, B.; et al. Mesenchymal Stem Cell-Induced Anti-Neuroinflammation Against Traumatic Brain Injury. Cell Transplant 2021, 30. [Google Scholar] [CrossRef]
- Zhu, J.; Chen, Y.H.; Ji, J.J.; Lu, C.X.; Liu, Z.F. Calcitonin Gene-Related Peptide Inhibits Neuronal Apoptosis in Heatstroke Rats via PKA/p-CREB Pathway. Chinese Journal of Traumatology - English Edition 2024, 27. [Google Scholar] [CrossRef]
- Xiong, J.; Wang, Z.; Bai, J.; Cheng, K.; Liu, Q.; Ni, J. Calcitonin Gene-Related Peptide: A Potential Protective Agent in Cerebral Ischemia–Reperfusion Injury. Front Neurosci 2023, 17. [Google Scholar] [CrossRef]
- Yang, S.; Yuan, Y.; Jiao, S.; Luo, Q.; Yu, J. Calcitonin Gene-Related Peptide Protects Rats from Cerebral Ischemia/Reperfusion Injury via a Mechanism of Action in the MAPK Pathway. Biomed Rep 2016, 4. [Google Scholar] [CrossRef]
- Kelly, P.D.; Yengo-Kahn, A.M.; Tang, A.R.; Jonathan, S. V.; Reynolds, R.A.; Ye, F.; Zhao, Z.; Froehler, M.T.; Fusco, M.R.; Morone, P.J.; et al. Conditional Vasospasm-Free Survival Following Aneurysmal Subarachnoid Hemorrhage. Neurocrit Care 2022, 37. [Google Scholar] [CrossRef] [PubMed]
- Altamura, C.; Viticchi, G.; Fallacara, A.; Costa, C.M.; Brunelli, N.; Fiori, C.; Silvestrini, M.; Vernieri, F. Erenumab Does Not Alter Cerebral Hemodynamics and Endothelial Function in Migraine without Aura. Cephalalgia 2021, 41, 90–98. [Google Scholar] [CrossRef] [PubMed]
- Lampl, C.; Kraus, V.; Lehner, K.; Loop, B.; Chehrenama, M.; Maczynska, Z.; Ritter, S.; Klatt, J.; Snellman, J. Safety and Tolerability of Erenumab in Individuals with Episodic or Chronic Migraine across Age Groups: A Pooled Analysis of Placebo-Controlled Trials. J Headache Pain 2022, 23, 104. [Google Scholar] [CrossRef] [PubMed]
- Haghdoost, F.; Puledda, F.; Garcia-Azorin, D.; Huessler, E.-M.; Messina, R.; Pozo-Rosich, P. Evaluating the Efficacy of CGRP MAbs and Gepants for the Preventive Treatment of Migraine: A Systematic Review and Network Meta-Analysis of Phase 3 Randomised Controlled Trials. Cephalalgia 2023, 43, 3331024231159366. [Google Scholar] [CrossRef] [PubMed]
- Muñoz-Vendrell, A.; Campoy, S.; Caronna, E.; Alpuente, A.; Torres-Ferrus, M.; Nieves Castellanos, C.; Olivier, M.; Campdelacreu, J.; Prat, J.; Camiña Muñiz, J.; et al. Effectiveness and Safety of Anti-CGRP Monoclonal Antibodies in Patients over 65 Years: A Real-Life Multicentre Analysis of 162 Patients. J Headache Pain 2023, 24, 63. [Google Scholar] [CrossRef] [PubMed]
- Ashina, M.; Goadsby, P.J.; Reuter, U.; Silberstein, S.; Dodick, D.W.; Xue, F.; Zhang, F.; Paiva da Silva Lima, G.; Cheng, S.; Mikol, D.D. Long-Term Efficacy and Safety of Erenumab in Migraine Prevention: Results from a 5-Year, Open-Label Treatment Phase of a Randomized Clinical Trial. Eur J Neurol 2021, 28, 1716–1725. [Google Scholar] [CrossRef]
- Haghdoost, F.; Puledda, F.; Garcia-Azorin, D.; Huessler, E.-M.; Messina, R.; Pozo-Rosich, P. Evaluating the Efficacy of CGRP MAbs and Gepants for the Preventive Treatment of Migraine: A Systematic Review and Network Meta-Analysis of Phase 3 Randomised Controlled Trials. Cephalalgia 2023, 43, 3331024231159366. [Google Scholar] [CrossRef]
- True, D.; Mullin, K.; Croop, R. Safety of Rimegepant in Adults with Migraine and Cardiovascular Risk Factors: Analysis of a Multicenter, Long-Term, Open-Label Study. Pain Ther 2024, 1–16. [Google Scholar] [CrossRef]
- Mulder, I.A.; Li, M.; de Vries, T.; Qin, T.; Yanagisawa, T.; Sugimoto, K.; van den Bogaerdt, A.; Danser, A.H.J.; Wermer, M.J.H.; van den Maagdenberg, A.M.J.M.; et al. Anti-Migraine Calcitonin Gene-Related Peptide Receptor Antagonists Worsen Cerebral Ischemic Outcome in Mice. Ann Neurol 2020, 88, 771–784. [Google Scholar] [CrossRef]
- Favoni, V.; Giani, L.; Al-Hassany, L.; Asioli, G.M.; Butera, C.; de Boer, I.; Guglielmetti, M.; Koniari, C.; Mavridis, T.; Vaikjärv, M.; et al. CGRP and Migraine from a Cardiovascular Point of View: What Do We Expect from Blocking CGRP? J Headache Pain 2019, 20, 27. [Google Scholar] [CrossRef]
- Ohlsson, L.; Haanes, K.A.; Kronvall, E.; Xu, C.; Snellman, J.; Edvinsson, L. Erenumab (AMG 334), a Monoclonal Antagonist Antibody against the Canonical CGRP Receptor, Does Not Impair Vasodilatory or Contractile Responses to Other Vasoactive Agents in Human Isolated Cranial Arteries. Cephalalgia 2019, 39, 1745–1752. [Google Scholar] [CrossRef] [PubMed]
- Bahr-Hosseini, M.; Meißner, N.; Reidler, P.; Saver, J.L.; Tiedt, S. Plasma CGRP Levels Are Not Associated With Collateral Flow and Outcome After Stroke. Stroke 2023, 54, e203–e204. [Google Scholar] [CrossRef] [PubMed]
- Sohn, I.; Sheykhzade, M.; Edvinsson, L.; Sams, A. The Effects of CGRP in Vascular Tissue - Classical Vasodilation, Shadowed Effects and Systemic Dilemmas. Eur J Pharmacol 2020, 881, 173205. [Google Scholar] [CrossRef] [PubMed]
- Smillie, S.-J.; King, R.; Kodji, X.; Outzen, E.; Pozsgai, G.; Fernandes, E.; Marshall, N.; de Winter, P.; Heads, R.J.; Dessapt-Baradez, C.; et al. An Ongoing Role of α-Calcitonin Gene-Related Peptide as Part of a Protective Network against Hypertension, Vascular Hypertrophy, and Oxidative Stress. Hypertension 2014, 63, 1056–1062. [Google Scholar] [CrossRef] [PubMed]
- Saely, S.; Croteau, D.; Jawidzik, L.; Brinker, A.; Kortepeter, C. Hypertension: A New Safety Risk for Patients Treated with Erenumab. Headache 2021, 61, 202–208. [Google Scholar] [CrossRef] [PubMed]
- de Vries Lentsch, S.; van der Arend, B.W.H.; Maassen VanDenBrink, A.; Terwindt, G.M. Blood Pressure in Patients With Migraine Treated With Monoclonal Anti-CGRP (Receptor) Antibodies: A Prospective Follow-up Study. Neurology 2022, 99, e1897–e1904. [Google Scholar] [CrossRef]
- Mair, J.; Lechleitner, P.; Längle, T.; Wiedermann, C.; Dienstl, F.; Saria, A. Plasma CGRP in Acute Myocardial Infarction. Lancet 1990, 335, 168. [Google Scholar] [CrossRef]
- Strecker, T.; Reeh, P.W.; Weyand, M.; Messlinger, K. Release of Calcitonin Gene-Related Peptide from the Isolated Mouse Heart: Methodological Validation of a New Model. Neuropeptides 2006, 40, 107–113. [Google Scholar] [CrossRef]
- Gennari, C.; Nami, R.; Agnusdei, D.; Fischer, J.A. Improved Cardiac Performance with Human Calcitonin Gene Related Peptide in Patients with Congestive Heart Failure. Cardiovasc Res 1990, 24, 239–241. [Google Scholar] [CrossRef]
- Li, D.; Peng, J.; Xin, H.-Y.; Luo, D.; Zhang, Y.-S.; Zhou, Z.; Jiang, D.-J.; Deng, H.-W.; Li, Y.-J. Calcitonin Gene-Related Peptide-Mediated Antihypertensive and Anti-Platelet Effects by Rutaecarpine in Spontaneously Hypertensive Rats. Peptides (N.Y.) 2008, 29, 1781–1788. [Google Scholar] [CrossRef]
- Kraenzlin, M.E.; Ch’ng, J.L.; Mulderry, P.K.; Ghatei, M.A.; Bloom, S.R. Infusion of a Novel Peptide, Calcitonin Gene-Related Peptide (CGRP) in Man. Pharmacokinetics and Effects on Gastric Acid Secretion and on Gastrointestinal Hormones. Regul Pept 1985, 10, 189–197. [Google Scholar] [CrossRef] [PubMed]
- Jakate, A.; Blumenfeld, A.M.; Boinpally, R.; Butler, M.; Borbridge, L.; Contreras-De Lama, J.; McGeeney, D.; Periclou, A.; Lipton, R.B. Pharmacokinetics and Safety of Ubrogepant When Coadministered with Calcitonin Gene‒related Peptide-Targeted Monoclonal Antibody Migraine Preventives in Participants with Migraine: A Randomized Phase 1b Drug–Drug Interaction Study. Headache 2021, 61. [Google Scholar] [CrossRef] [PubMed]
- Alsaadi, T.; Suliman, R.; Santos, V.; Al Qaisi, I.; Carmina, P.; Aldaher, B.; Haddad, S.; Bader, Y. Safety and Tolerability of Combining CGRP Monoclonal Antibodies with Gepants in Patients with Migraine: A Retrospective Study. Neurol Ther 2024, 13. [Google Scholar] [CrossRef] [PubMed]
- de Vries, T.; Rubio-Beltrán, E.; van den Bogaerdt, A.; Dammers, R.; Danser, A.H.J.; Snellman, J.; Bussiere, J.; MaassenVanDenBrink, A. Pharmacology of Erenumab in Human Isolated Coronary and Meningeal Arteries: Additional Effect of Gepants on Top of a Maximum Effect of Erenumab. Br J Pharmacol 2024, 181. [Google Scholar] [CrossRef]
- Cirillo, C.; Brihmat, N.; Castel-Lacanal, E.; Le Friec, A.; Barbieux-Guillot, M.; Raposo, N.; Pariente, J.; Viguier, A.; Simonetta-Moreau, M.; Albucher, J.F.; et al. Post-Stroke Remodeling Processes in Animal Models and Humans. Journal of Cerebral Blood Flow and Metabolism 2020, 40. [Google Scholar] [CrossRef]
- van der Vliet, R.; Selles, R.W.; Andrinopoulou, E.-R.; Nijland, R.; Ribbers, G.M.; Frens, M.A.; Meskers, C.; Kwakkel, G. Predicting Upper Limb Motor Impairment Recovery after Stroke: A Mixture Model. Ann Neurol 2020, 87, 383–393. [Google Scholar] [CrossRef]
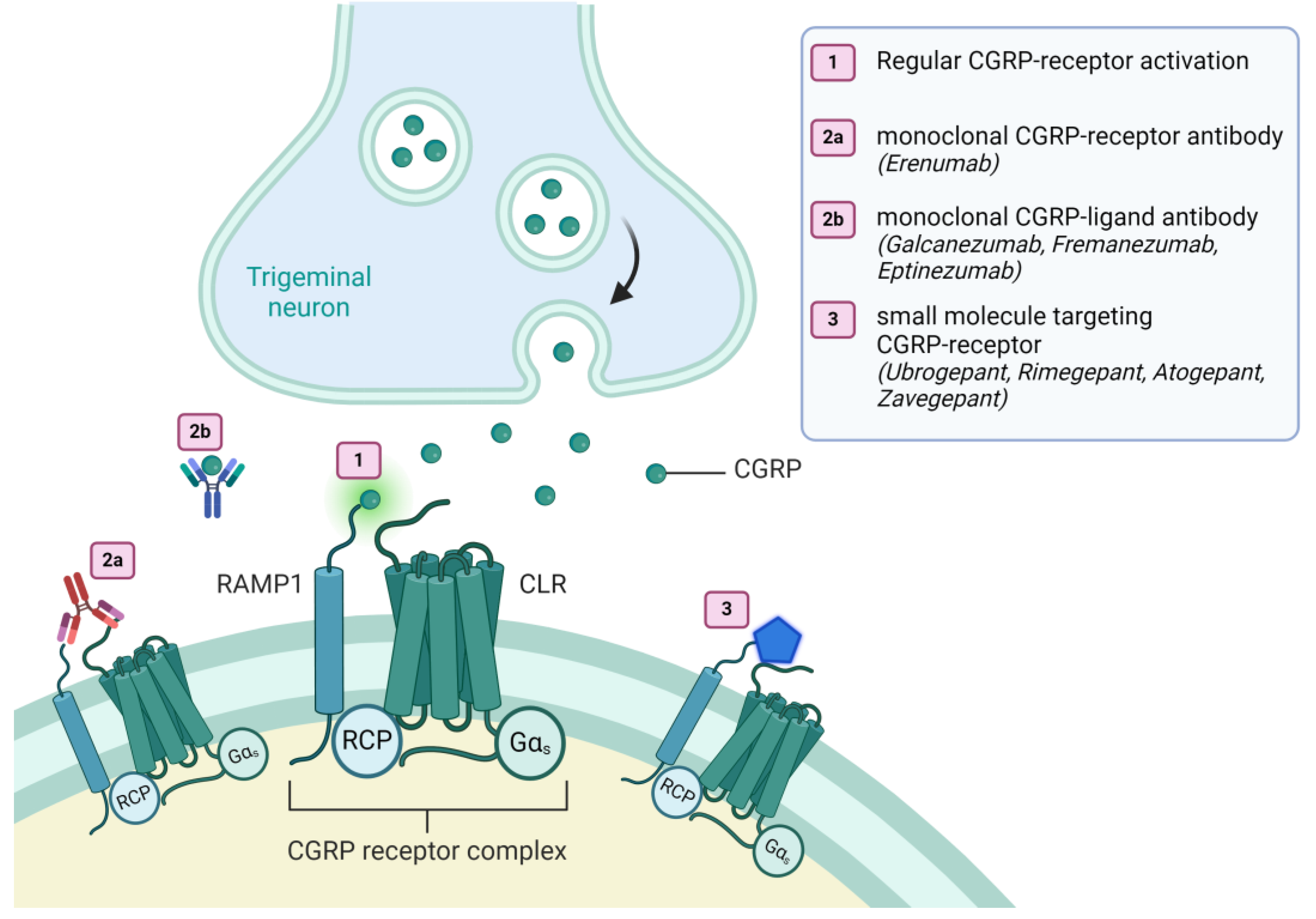
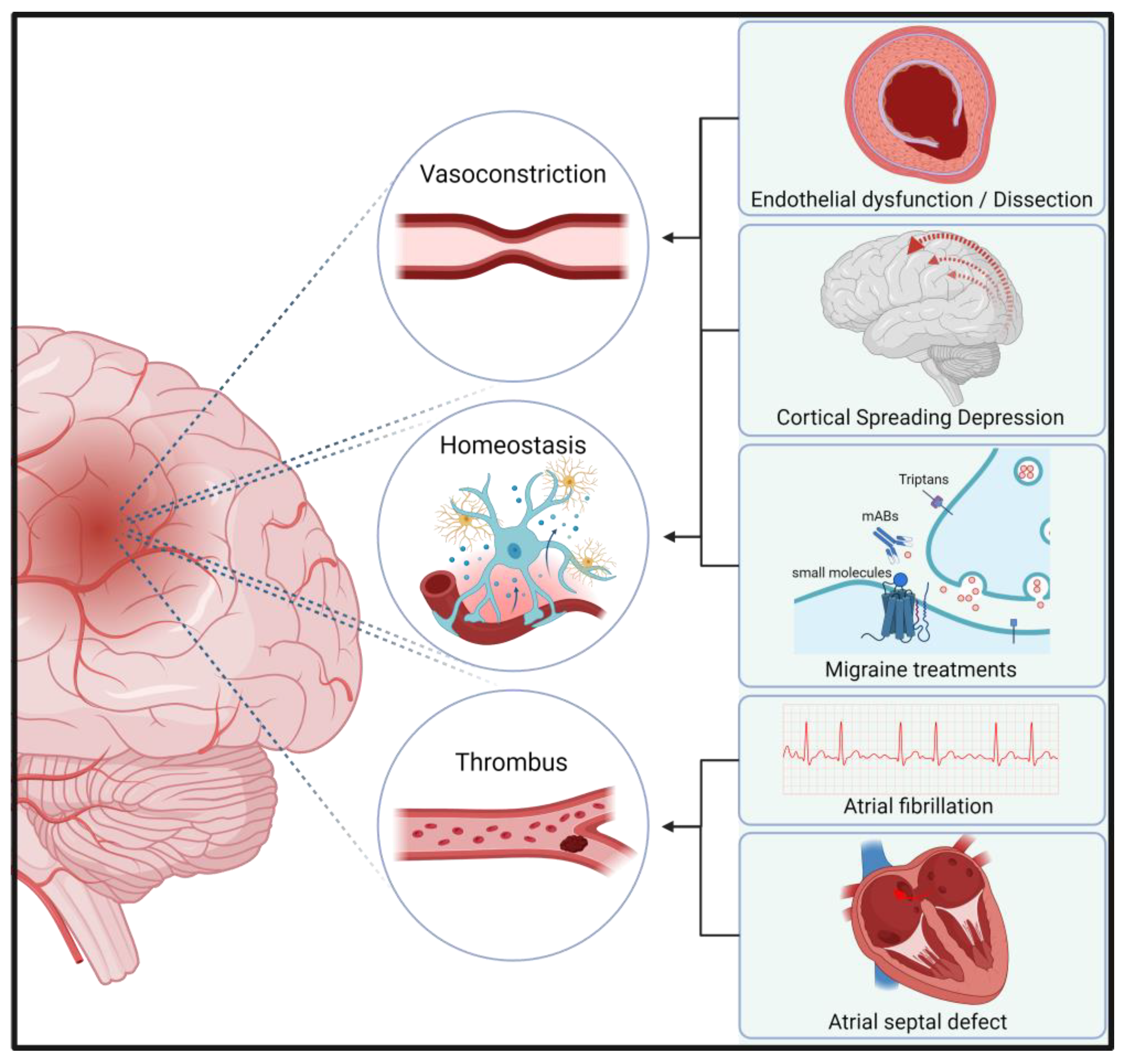
| Role of CGRP in Stroke | Molecular Mechanism | Effect in Stroke | Comments |
|---|---|---|---|
|
Blood-Brain Barrier (BBB) Influence [10,52,53] |
CGRP influences BBB permeability |
Increases permeability, which can lead to cerebral edema; conflicting findings on protective effects against BBB injury |
Increased permeability may be linked to both beneficial or detrimental outcomes depending on conditions |
|
Vasodilation [54,55] |
CGRP and adrenomedullin act as vasodilators, especially in microvasculature |
Maintains cerebral blood flow (CBF) during ischemic conditions; counteracts hypoperfusion |
Helps in regulating blood flow, particularly during systemic hypotension |
|
Antioxidative Effects [56,57] |
CGRP reduces oxidative stress during reperfusion injury |
Protects against oxidative stress induced by reintroduction of blood flow post-ischemia |
Beneficial in mitigating damage from reperfusion injury |
|
Anti-Inflammatory Effects [9,58,61,62] |
CGRP (and adrenomedullin) show anti-inflammatory properties |
Reduced inflammation |
Supports neuronal recovery by attenuating inflammatory response |
|
Neuroprotection via CREB Pathway [8,59,60] |
Activates CREB pathway leading to increased Bcl-2, reduction of caspase-3 |
Anti-apoptotic effect, protecting neurons |
Stabilizes mitochondrial membrane via Bcl-2/BAX pathway |
|
Modulation of MAPK Pathways [61] |
Increases ERK phosphorylation, reduces JNK and p38 phosphorylation |
Improves neuronal function, reduces apoptosis |
MAPK pathway modulation contributes significantly to neuroprotective role |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





