Submitted:
05 August 2024
Posted:
06 August 2024
You are already at the latest version
Abstract
Keywords:
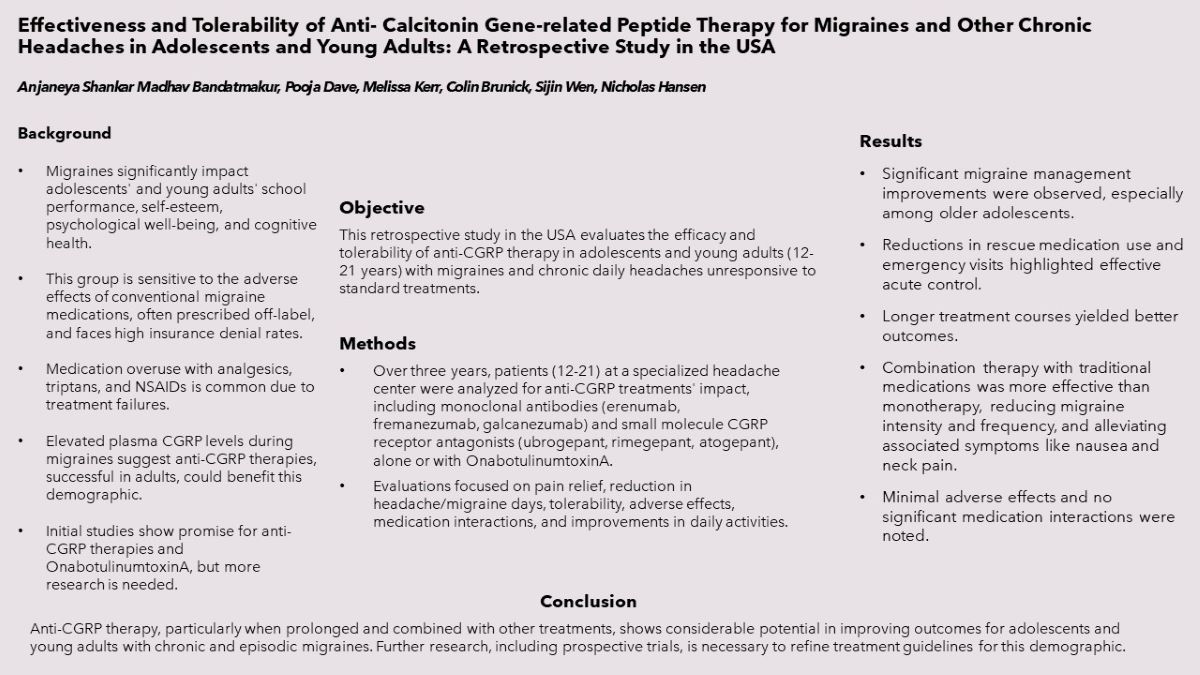
Main Manuscript
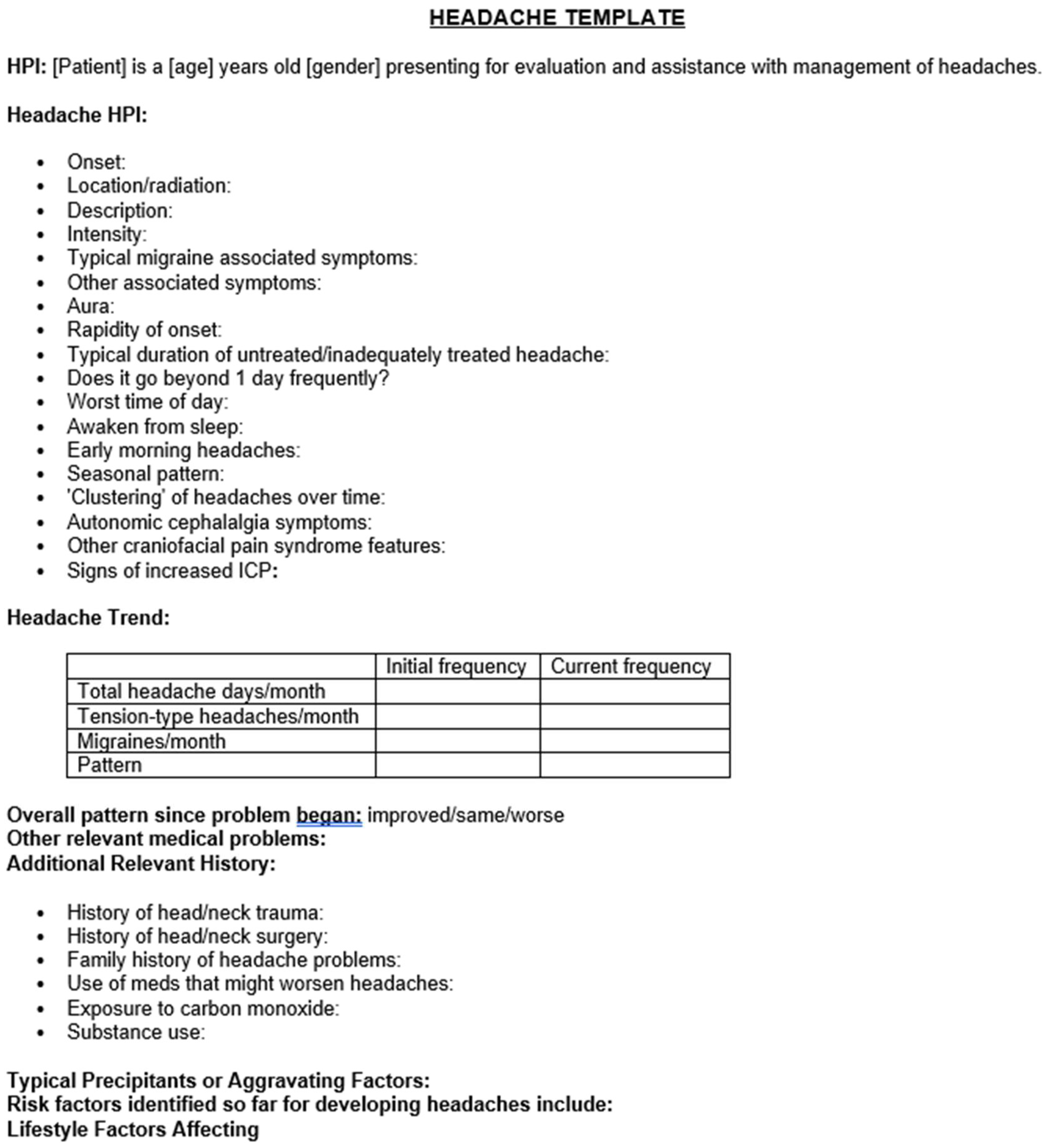
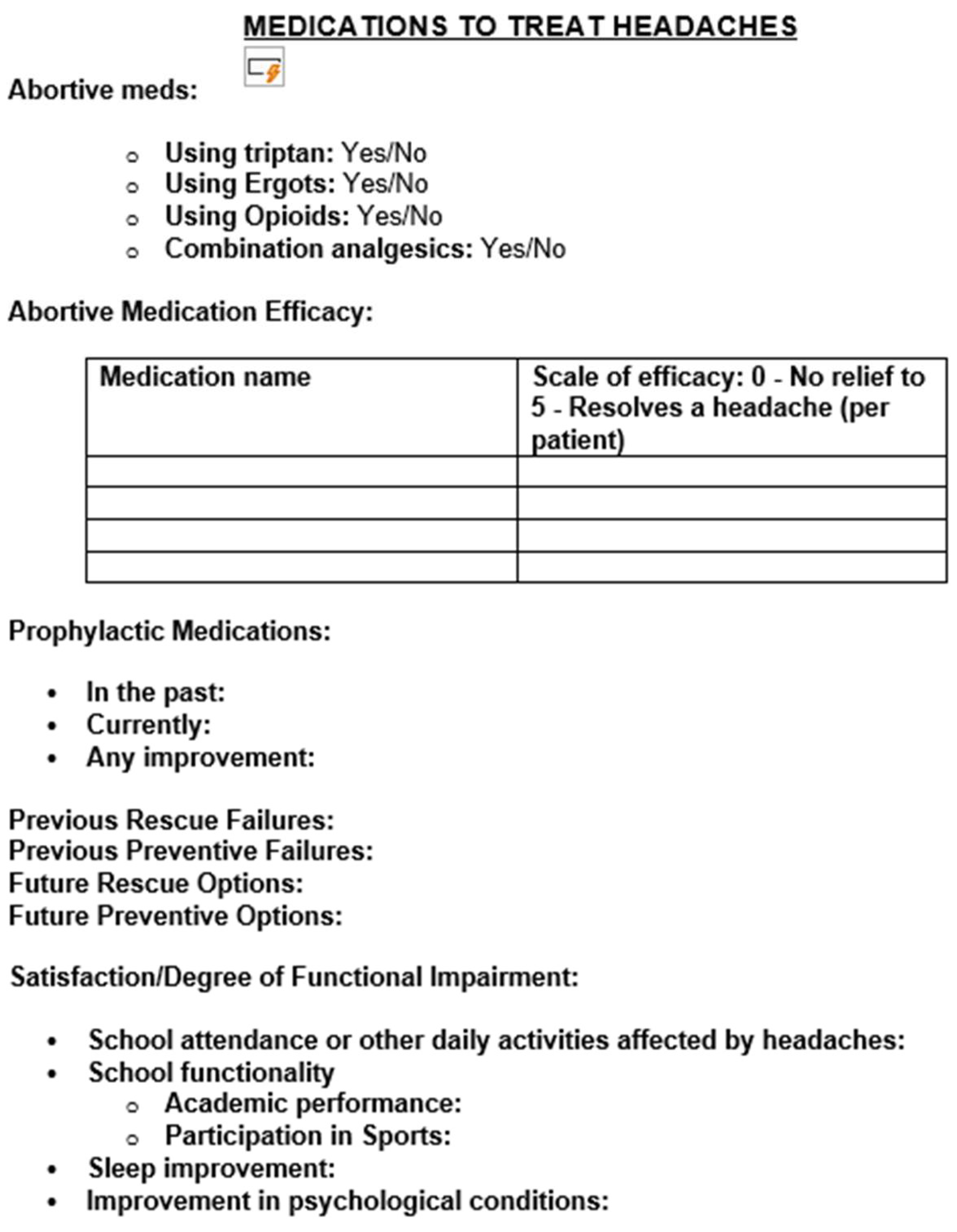
Methodology
Results
Discussion
Conclusions
References
- Fuh JL, Wang SJ, Lu SR, Liao YC, Chen SP, Yang CY. Headache disability among adolescents: a student population-based study. Headache. 2010;50(2):210-218.
- Rocha-Filho PA, Santos PV. Headaches, quality of life, and academic performance in schoolchildren and adolescents. Headache. 2014;54(7):1194-1202.
- El-Chammas K, Keyes J, Thompson N, Vijayakumar J, Becher D, Jackson JL. Pharmacologic treatment of pediatric headaches: a meta-analysis. JAMA Pediatr. 2013;167(3):250-258.
- Goadsby PJ, Reuter U, Hallstrom Y, et al. A controlled trial of erenumab for episodic migraine. N Engl J Med. 2017;377(22):2123-2132.
- Tepper S, Ashina M, Reuter U, et al. Safety and efficacy of erenumab for preventive treatment of chronic migraine: a randomized, double-blind, placebo-controlled phase 2 trial. Lancet Neurol. 2017;16(6):425-434.
- Silberstein SD, Dodick DW, Bigal ME, et al. Fremanezumab for the preventive treatment of chronic migraine. N Engl J Med. 2017;377(22):2113-2122.
- Stauffer VL, Dodick DW, Zhang Q, Carter JN, Ailani J, Conley RR. Evaluation of galcanezumab for the prevention of episodic migraine: The EVOLVE-1 randomized clinical trial. JAMA Neurol. 2018.
- Skljarevski V, Matharu M, Millen BA, Ossipov MH, Kim BK, Yang JY. Efficacy and safety of galcanezumab for the prevention of episodic migraine: Results of the EVOLVE-2 Phase 3 randomized controlled clinical trial. Cephalalgia. 2018;38(6):1026-1037.
- Martinez JM, Goadsby PJ, Dodick DW, et al. A placebo-controlled study of galcanezumab in patients with episodic cluster headache: from the 8-week double-blind treatment phase. Headache. 2018;58:1247-1258.
- Dodick DW, Ashina M, Brandes JL, et al. ARISE: A Phase 3 randomized trial of erenumab for episodic migraine. Cephalalgia. 2018;38(6):1026-1037.
- Dodick DW, Silberstein SD, Bigal ME, et al. Effect of fremanezumab compared with placebo for prevention of episodic migraine: a randomized clinical trial. JAMA. 2018;319(19):1999-2008.
- Bigal ME, Edvinsson L, Rapoport AM, et al. Safety, tolerability, and efficacy of TEV-48125 for preventive treatment of chronic migraine: a multicenter, randomized, double-blind, placebo-controlled, phase 2b study. Lancet Neurol. 2015;14(11):1091-1100.
- Deen M, Correnti E, Kamm K, et al. Blocking CGRP in migraine patients - a review of pros and cons. J Headache Pain. 2017;18(1):96.
- Goadsby PJ, Lipton RB, Ferrari MD. Migraine: current understanding and treatment. N Engl J Med. 2002;346:257-270. [CrossRef]
- Gallai V, Sarchielli P, Floridi A, et al. Vasoactive peptide levels in the plasma of young migraine patients with and without aura assessed both interictally and ictally. Cephalalgia. 1995;15(5):384-390. [CrossRef]
- Fan PC, Kuo PH, Chang SH, Lee WT, Wu RM, Chiou LC. Plasma calcitonin gene-related peptide in diagnosing and predicting pediatric migraine. Cephalalgia. 2009;29(8):883-890. [CrossRef]
- Juhasz G, Zsombok T, Modos EA, et al. NO-induced migraine attack: strong increase in plasma calcitonin gene-related peptide (CGRP) concentration and negative correlation with platelet serotonin release. Pain. 2003;106(3):461-470. [CrossRef]
- Fan PC, Kuo PH, Lee MT, et al. Plasma calcitonin gene-related peptide: a potential biomarker for diagnosis and therapeutic responses in pediatric migraine. Front Neurol. 2019;10:10. [CrossRef]
- Berger A, Bloudek LM, Varon SF, Oster G. Adherence with migraine prophylaxis in clinical practice. Pain Pract. 2012;12(7):541-549.
- Szperka CL, VanderPluym J, Orr SL, et al. Recommendations on the use of anti-CGRP monoclonal antibodies in children and adolescents. Headache. 2018;58(10):1658-1669. [CrossRef]
- Greene KA, Gentile CP, Szperka CL, et al. Calcitonin gene-related peptide monoclonal antibody use for the preventive treatment of refractory headache disorders in adolescents. Pediatr Neurol. 2021;114:62-67. [CrossRef]
- Headache Classification Committee of the International Headache Society (IHS). The International Classification of Headache Disorders, 3rd edition. Cephalalgia. 2018;38:1-211.
- Grengs LR, Mack KJ. New daily persistent headache is most likely to begin at the start of school. J Child Neurol. 2016;31(7):864-868.
- Kacperski J. Pharmacotherapy for persistent posttraumatic headaches in children and adolescents: a brief review of the literature. Paediatr Drugs. 2018.
- Nierenburg H, Newman LC. Update on new daily persistent headache. Curr Treat Options Neurol. 2016;18(6):25.
- Joshi SG, Mathew PG, Markley HG. New daily persistent headache and potential new therapeutic agents. Curr Neurol Neurosci Rep. 2014;14(2):425.
- Dodick DW, Goadsby PJ, Silberstein SD, et al. Safety and efficacy of ALD403, an antibody to calcitonin gene-related peptide, for the prevention of frequent episodic migraine: a randomized, double-blind, placebo-controlled, exploratory phase 2 trial. Lancet Neurol. 2014;13(11):1100-1107.
- Tyburski AL, Cheng L, Assari S, Darvish K, Elliott MB. Frequent mild head injury promotes trigeminal sensitivity concomitant with microglial proliferation, astrocytosis, and increased neuropeptide levels in the trigeminal pain system. J Headache Pain. 2017;18(1):16.
- Al-Hassany L, Goadsby PJ, Danser AHJ, MaassenVanDenBrink A. Calcitonin gene-related peptide-targeting drugs for migraine: how pharmacology might inform treatment decisions. Lancet Neurol. 2022;21(3):284-294. [CrossRef]
- Charles A, Pozo-Rosich P. Targeting calcitonin gene-related peptide: a new era in migraine therapy. Lancet. 2019;394(10210):1765-1774. [CrossRef]
- Mechtler L, Saikali N, McVige J, et al. Real-world evidence for the safety and efficacy of CGRP monoclonal antibody therapy added to onabotulinumtoxinA treatment for migraine prevention in adult patients with chronic migraine. Front Neurol. 2022;12:788159. [CrossRef]
- Blumenfeld AM, Frishberg BM, Schim JD, et al. Real-world evidence for control of chronic migraine patients receiving CGRP monoclonal antibody therapy added to onabotulinumtoxinA: a retrospective chart review. Pain Ther. 2021;10(2):809-826. [CrossRef]
| Table 1 | |
|---|---|
| Column (Variables) | Summary |
| Age Group | n - out of 23 (%) |
| 12-15Y | 9 (39.1) |
| 16-18Y | 12 (52.2) |
| >18 Y | 2 (8.7) |
| Indication | |
| Episodic Migraine | 3 (13) |
| Chronic migraine | 18 (78.3) |
| NDPH | 1 (4.3) |
| Post traumatic (Chronic migraine, NDPH) | 1 (4.3) |
| 1st line preventive failures | |
| 1-2 meds | 13 (56.5) |
| 3-4 meds | 7 (30.4) |
| >4 meds | 3 (13) |
| History of concussion | |
| Yes | 8 (34.8) |
| No | 15 (65.2) |
| Psychiatric conditions | |
| Yes | 21 (91.3) |
| No | 2 (8.7) |
| Sleep disorder | |
| Yes | 16 (69.6) |
| No | 7 (30.4) |
| Other neurological problems | |
| Yes | 5 (21.7) |
| No | 18 (78.3) |
| Table 2 | |
|---|---|
| Column (Variables) | Summary |
| Treatment | n - out of 23 (%) |
| Anti -CGRP + Traditional | 9 (39.1) |
| Anti -CGRP + OnabotulinumtoxinA | 3 (13) |
| Anti -CGRP + OnabotulinumtoxinA + traditional | 6 (26.1) |
| Anti -CGRP alone | 5 (21.8) |
| Months of use | |
| <3 months | 3 (13) |
| 4-6 months | 2 (8.7) |
| >6 months | 18 (78.3) |
| Anti-CGRP therapy for rescue or prevention or both | |
| Rescue | |
| Prevention | 14 (60.9) |
| Both | 9 (39.1) |
| Table 3 | |
|---|---|
| Column (Variables) | Summary |
| Response to rescue meds | n - out of 23 (%) |
| improved | 17 (73.9) |
| no change | 6 (26.1) |
| Reduction in rescue med use in a week | |
| Yes (<50%) | 18 (78.3) |
| No (>50%) | 5 (21.7) |
| ER visits reduction | |
| Yes | 13 (56.5) |
| No | 10 (43.5) |
| Duration of migraines reduced | |
| A lot | 7 (30.4) |
| Some | 14 (60.9) |
| None | 2 (8.7) |
| Intensity of migraines reduced | |
| A lot | 9 (39.1) |
| Some | 12 (52.2) |
| None | 2 (8.7) |
| Other bothersome symptoms improvement | |
| A lot | 9 (39.1) |
| Some | 10 (43.5) |
| None | 4 (17.4) |
| Number of headache days reduction after 1 month | |
| >50% | 9 (39.1) |
| <50% | 10 (43.5) |
| same | 4 (17.4) |
| Number of headache days reduction after 3 months | |
| >50% | 9 (39.1) |
| <50% | 11 (47.8) |
| Same | 3 (13) |
| Adverse effects | |
| Few | 1 (4.3) |
| None | 22 (95.7) |
| Discontinued for any reason | |
| Yes | 7 (30.4) |
| No | 16 (69.6) |
| Patient’s satisfaction | |
| Strongly | 16 (69.6) |
| Doesn’t matter | 5 (21.7) |
| No | 2 (8.7) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).





