1. Introduction
1.1. Historical Overview-Definition-Classification
The diagnosis of hydrocephalus is established on a constellation of clinical and neuroradiographic characteristics, and is characterized by a pathological accumulation of CSF due to disturbances in terms of CSF absorption or circulation. In children, this condition predominantly manifests with a pathological elevation in intracranial pressure. In a limited subset of cases where this is not applicable, it is hypothesized that pressure remains within normal limits or low due to compensatory mechanism that occurs elsewhere. This may interfere either with, and at the expense of, the brain parenchyma or by a mechanism that involves expansion of the skull. In a minority of cases, both of the aforementioned mechanisms are intermingled [
1,
2]. The normal flow of CSF is from lateral ventricles through the foramen of Monroe, into the third ventricle, proceeding into the fourth ventricle, through the cerebral aqueduct, and out of the foramen of Luschka and Magendie into the spinal subarachnoid space. Communicating hydrocephalus arises when flow of CSF (within normal volumetric values) is unimpeded and is caused from an inability to absorb CSF via the normal drainage pathways or, rarely, pathological accumulation of CSF due to over-production [
3].
In accordance with its definition, obstructive hydrocephalus is established in cases where there is an obstruction to the normal CSF flow throughout its normal pathways. This can be intimately related with a wide range of underlying precipitating causes. Although the diagnostic and differentiating criteria that are commonly utilized, such as “communicating” versus “noncommunicating”, —in accordance with other classification schemes— are used, this is not always enough to delineate all subtypes of hydrocephalus. In clinical practice, we have to manage a wide spectrum of relevant conditions, among patients with hydrocephalus, which share in common a range of clinical heterogeneity. In this review we consider hydrocephalus as primary (syndromic and/or idiopathic) or secondary to a wide variety of pathologic conditions.
Primary hydrocephalus may be driven by a range of genetic factors that have an effect on fetal development. Under this term are referred several entities, although they are not restricted to them, such as neural tube defects, arachnoid cysts, Dandy Walker syndrome, and Chiari malformation [
3]. Notably, a remarkable percentage of primary hydrocephalus cases in the pediatric neurosurgical practice are collectively included under the term idiopathic. Secondary hydrocephalus may occur mainly, but not solely, due to infection, bleeding, or trauma. In developed countries, PHH represents the most common cause of secondary hydrocephalus in the pediatric population. PHH is most frequently associated with intraventricular hemorrhage of prematurity, which is universally accepted to be present at many as 40% of premature newborns younger than 37 weeks gestation. It is thought that this is caused by the rupture of small, delicate vessels along the developing germinal matrix of the brain. These hemorrhages may block or scar the outlets of the ventricular system and/or obstruct the drainage pathways along the meningeal vessels [
3,
4]. In developing countries, infections of the central nervous system such as meningitis constitute the most frequently encountered etiology of pediatric hydrocephalus resulting in PIH due to inflammation of the ependymal lining and subventricular zone cells, as well as obstruction of the CSF drainage patterns at the meninges, as well as an obliteration of CSF drainage or flow [
3].
1.2. Hydrocephalus Treatment: Evolution of Shunt Technology and the Development of Endoscopic Third Ventriculostomy
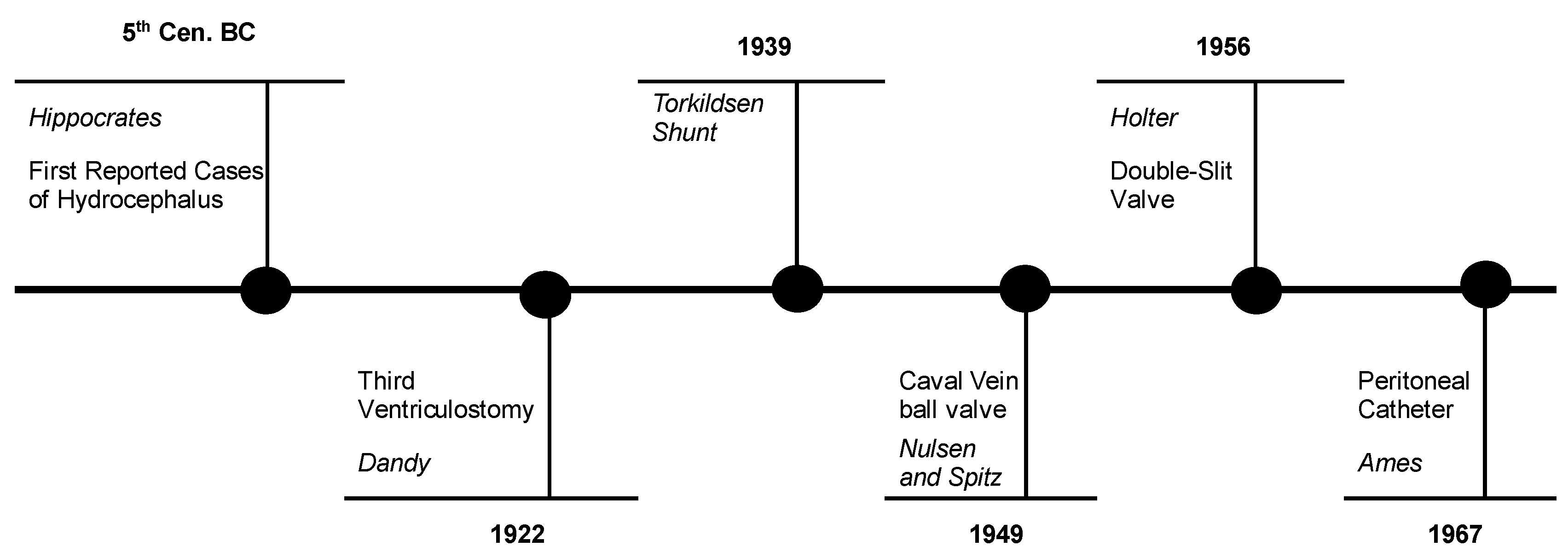
The first reported cases of hydrocephalus dates to Galen, Hippocrates, and the medieval Arabian physicians. Effective, durable treatment was unable to be registered, and the first such successfully managed cases were recorded with Torkildsen in 1939 using an intracranial bypass system from the third ventricle to the cisterna magna to overcome an obstruction of the aqueduct of Sylvius [
5,
6]. The invention of a one-way valve drainage system did not occur until the 1950s [
7,
8]. Recently published studies have examined the evolution of shunt technology in the meantime [
9,
10]. During the 1950s and early 1960s, the value of insertion of a drainage catheter within the ventricular system of patients suffering from hydrocephalus into the vascular system was verified [
7,
11]. Nulsen and Spitz placed the first valve, constituted by a spring and ball system. Later this was replaced by a double-slit valve developed by Holter [
11]. By 1969, at Children’s Hospital of Philadelphia, 90% of shunt placements involved VA shunts with a Spitz-Holter valve. The major advantage of the VA shunt was that over-drainage was not a major concern in those patients with a closed fontanelle. Over-drainage with the VA shunt was rarely a concern since the atrial pressure was higher than the negative ICP in the erect position minus the gravitational column of CSF in the shunt tubing. The most serious disadvantages that were associated with the VA shunts are summarized as following:
1. As the child growths, the peripheral catheter is gradually getting shorter and eventually needs to be elongated. Sometimes this is quite technically demanding due to clot formation around the tube in the lumen of the jugular vein.
2. If bacterial infection complicates our case and this involves the shunt system, the risk of development of septicemia is significantly increased.
Nevertheless, the invention of the VA shunt should be considered as a major advancement in the treatment of hydrocephalus, being a live-saving procedure for many children. Apart from that, due to the potential side effects that were associated with its use, it became obvious that it could not be considered as the therapeutic option of choice.
VPS were rarely involved in the treatment of hydrocephalus in the 1960s, due to the high prevalence of obstruction of the distal catheters. In 1967, Ames [
12] introduced a valve system whose peripheral catheter was ending at the peritoneal cavity. This shunt system demonstrated a favorable safety profile, with minimal side effects and over the following few years VP shunting replaced VA shunts, being considered as the first option drainage system [
13].
Although valve technology has progressed, there are several issues that need to be resolved. It is widely accepted that the most prevalent cause of shunt malfunction is obliteration of the central catheter [
14,
15,
16]. The offending mechanism may be due to mechanical obstruction of the proximal catheter with choroid plexus or ventricular debris. Nevertheless, another contributory mechanism, potentially tied to overdrainage, is suspected. This predisposes to ventricular catheter obliteration against the ventricular wall. Apart from the subgroup of children which present with non-functioning shunts and elevated ICP, there is another subgroup of children who present with symptoms attributable to intracranial hypotension, along with a working shunt. This is in accordance with the concept of overdrainage due to inadequacy of the valvular system to overcome this complication. [
17,
18,
19]. The widespread availability of imaging of the dimensions of the ventricular system in the shunted children enhanced our knowledge about what is included under the term “SVS” or shunt inappropriate function, which is not coupled with ventricular enlargement [
20]. Apart from that, a lot of referrals exist about children who were harboring well-functioning shunts, in association with small ventricular dimensions. A common complaint of children that are harboring a shunt refers to the frequent occurrence of headache· this clinical observation, in conjunction with a relevant imaging study showing a ventricular system that is smaller than normal, altogether was taken as symptomatic overdrainage by the inserted system. The estimated incidence of either symptomatic low pressure or SVS varied from as low as 8–10% to as high as 40–50% [
21]. The current hypothesis is that siphoning is believed to occur when children transition from a supine to an upright position· this is capable of resulting in excess CSF drainage. In order to counteract the siphoning effect of currently available valvular mechanisms, antisiphon devices were invented to be inserted in line with the valves [
22]. The beneficial results reported were not verified by all of the initially published series. More precisely, some publishments demonstrated a significant diminishment in the rate of proximal catheter obliteration, whereas others were unable to support any evidence of benefit [
23]. When standard differential pressure valves were utilized, the rates of shunt failure remained largely unchanged and relatively high, with approximately 40% of shunts associated with malfunction within the first year of insertion [
24]. Newer technical innovations have been introduced, referring to both the shunt and the ventricular catheter, in order to overcome side effects associated with either to overdrainage, or to the issue of shunt malfunction due to obliteration. These technical advancements involve the utilization of programmable valves with externally adjustable opening pressures, valves with antisiphon devices, gravity-assist devices, and flow-regulating valves [
25]. Current valve technology includes the following:
Fixed differential pressure (DP) valves
Overdrainage control devices (OCD)
Adjustable DP valves
Fixed DP valves with OCD
Adjustable DP valves with OCD.
1.3. Lumboperitoneal Shunts
LP shunts offer the advantage of mitigating complications that are inherently associated with any kind of brain operation (hemorrhage, venous infarct, etc.). Moreover, by adopting a drainage system that drains downstream from the ventricular system, the potential complications that are associated with the drainage of an individual ventricle (based on a single central catheter), can be overcome. LPs have been utilized in the management of a wide spectrum of pathologies that are included under the term communicating hydrocephalus. Nevertheless, the most widely accepted indication is for the treatment of idiopathic intracranial hypertension as the lumbar subarachnoid space allows access for CSF diversion even in the presence of a ventricular system that radiographically seems to be slit. These types of shunts have also been implicated with a lower infection rate, as well as with a lower prevalence of obliteration of the central catheter [
26,
27]. The initially introduced types of LP shunts mostly used slit valves, which are characterized by a simplified hardware design, which reduces the likelihood of failure. Nevertheless, a significant limitation of these valve types is that they are able to offer limited control regarding CSF flow, thus they could be implicated for shunt overdrainage [
28]. In a retrospective review of 143 pediatric patients harboring LP shunts, 70% of patients showed radiographic evidence of tonsillar on follow-up imaging. This should to be attributed to the resultant overdrainage of CSF. Nevertheless, only 4% of this subpopulation of patients ultimately necessitated any intervention [
29]. These shunt systems share in common another inherent drawback, that is they do not offer the opportunity to clinically assess their functional status· this limitation stems from the absence of an access reservoir, which enables us for aspiration and measurement of the opening pressure. LP valves have been introduced to our therapeutic armamentarium in order to control more effectively CSF flow and a reservoir can be connected with them. The Integra horizontal-vertical valve incorporates two different valve mechanisms, a spring-actuated valve that controls a lower pressure range of drainage while the patient is horizontal and a gravity-actuated valve controlling a higher-pressure drainage when the patient adopts an erect position [
21]. When literature data that are relevant with the cumulative rate of complication associated with LP shunts are analyzed, it seems that they are not enlightening. More precisely, there are several reports that mention an increased failure rate of shunt revision [
30,
31]. However, a recently published retrospective study demonstrated that the rates of shunt failure, number of failures, and overall complication rates were comparable to those associated with VP shunts [
32].
1.4. Endoscopic Third Ventriculostomy
At present, endoscopic third ventriculostomy is the sole surgical alternative option to shunt placement for the management of hydrocephalus. Since 1910, multiple efforts have been undertaken to refine an endoscopic approach to the ventricular system. Hoffman [
33] reported a cohort of children with noncommunicating hydrocephalus treated effectively with ETV, and Cinalli [
34] published his results based on the encouraging results of third ventriculostomy in patients necessitating shunt revision. He demonstrated that 76.6% of patients became shunt-independent with mean follow-up of 7 years. Better lighting and visualization, that was made possible from technological improvement, by the late 1990’s, attracted increased interest by neurosurgeons. These technical innovations facilitated us to attempt to treat with third ventriculostomy, not only pathologies related with noncommunicating hydrocephalus, but also patients suffering from communicating hydrocephalus [
35,
36]. The outcomes were highly promising [
37,
38], the only exception been infants and children under 1 year, especially those under 6 months [
39]. In all groups, the failure rates associated with ETV were higher than their counterparts, associated with shunts, when the evaluation period was restricted in the first few months. Nevertheless, when the investigation period was extended over the next few years, ETVs were associated with less revisions and, accordingly, demonstrated a lower incidence of failure [
40,
41,
42]. After a 8 years follow-up period, the revision rate for ETV (which included a revision of ETV or the insertion of a shunt) was 49% with failures occurring late, in the time range of 5–8 years [
43]. A scale was developed in order to be able to predict which patients would be most likely to benefit in the long-term from ETV [
44] called the ETVSS. The ETVSS score seems to be a valuable predictor of successful outcome [
45,
46] and may even underestimate the success rate of ETV [
42]. A modification of this score has been advocated [
47]. Nowadays, 50% or more of children with hydrocephalus should be considered for ETV as the optimum treatment option. A recently introduced technique incorporates the performance, in addition to ETV, of choroid plexus cauterization in infants. However, the relative benefits of this are still under investigation [
48,
49,
50]. The rationale for their use primarily hinges on the observation that the pathological substrate of the hydrocephalus, e.g., post infectious, may be an important contributing factor to the potential benefit from choroid plexus coagulation [
51].
Figure 1.
Schematic diagram, representing the main historical evolutions in the diagnosis and management of hydrocephalus.
Figure 1.
Schematic diagram, representing the main historical evolutions in the diagnosis and management of hydrocephalus.
2. Materials and Methods
The vast majority of recently published review articles centered on hydrocephalus have concentrated on particular parameters of its pathophysiology. In accordance with that, we would like to mention to our readers the following topics that most review papers are dealt with: CSF and ventricular system [
52,
53], hemorrhagic hydrocephalus [
4,
54], congenital [
4,
55,
56,
57], and idiopathic hydrocephalus [
58,
59,
60]. The purpose of this review article is to briefly highlight the complexity of the pathophysiology of hydrocephalus as an entity, to review the current treatment protocols and compare the available treatment modalities, in terms of relative indications according to the underlying pathology, safety and long- term efficacy, as well as future potential considerations.
Articles were collected utilizing PubMed, Google Scholar, BioRxiv, and MedRxiv search terms: hydrocephalus, CSF, ventricles, ventricular system, and intracranial pressure. There were no exclusion criteria, thus the searches gathered articles of all styles (reviews, primary literature, clinical trials, etc.) and from all publication years. These articles were reviewed via title and abstract to ensure that their focus was related to hydrocephalus, to control ICP, along with ventricle size. The basic tenet of the authors of this review article is to provide historical and background and updated information about the treatment armamentarium for hydrocephalus, detailed analysis of current categorization of hydrocephalus based on the pathophysiology of this entity, followed by a presentation of the currently available established options, focused to the treatment of hydrocephalus.
3. Discussion
3.1. Treatment Paradigms-Standards of Surgical Intervention
When we are dealing with patients who are suffering from hydrocephalus, there are several options in our therapeutic armamentarium regarding the intervention procedure. Our final treatment decision depends on a wide spectrum of variables, including patient weight, severity of symptoms, and clinical recordings. These are fairly well analyzed by a systematic bibliographic review and literature-based guidelines, which are included in recently published manuscripts [
61,
62]. In general, they are subdivided into transient surgical interventions, as well as permanent surgical treatments.
Temporary surgical measures for hydrocephalus include the insertion of an EVD or the development of a VSGS. The choice of incorporating NEL to our treatment plan, aiming to suction or dilute the intraventricular debris, resulting from infection and hemorrhage, is being currently under investigation using the international multicenter “TROPHY” registry [
63].
Permanent surgical options for hydrocephalus include two basic subcategories. One of them attempts to overcome an obstruction-obstacle, that was due to an anatomical dome, to the flow of CSF (i.e., tumor of the third ventricle) using neuroendoscopy (e.g., ETV). The other main subcategory of therapeutic interventions incorporates the insertion of a shunt in order to facilitate CSF diversion from the ventricular system to a body cavity which can effectively absorb the excess of fluid. The most common options include the peritoneum, atrium, or pleural cavity. However, it is well known among pediatric neurosurgeons that permanent CSF diversion procedures are generally prone to a high rate of failure and often re-implantation of the shunt system is required.
Currently, the most commonly used shunt type is the VPS, which is thought to be our first-choice approach for the greater percentage of patients. Nevertheless, while the peritoneal cavity is the most commonly selected location for drainage of CSF, VA and VPS are currently considered as acceptable alternative distal targets, in cases where the abdomen is deemed to be inappropriate.
Neuroendoscopy is a surgical technique that can be used as an alternative option to shunting therapy in a subcategory of patients who share in common a certain number of specific indications. More precisely, they involve a subpopulation of patients who present with obstructive hydrocephalus, regardless of its underlying pathophysiology and etiology. It involves placement of an endoscope into the ventricular system in order to address any underlying primary pathology or to provide an alternative pathway for the bulk flow of CSF. Walter Dandy is assigned as the one who has first attempted to utilize the neuroendoscope in order to address the excess of flow of CSF and popularized ETV in the early 1900s [
64]. This technique was effective in resolving obstructive hydrocephalus at the level of the third ventricle by the development of a septostomy, thus creating a communication between the floor of the third ventricle (after perforation of the premammillary membrane) to the subarachnoid space (that is chiasmatic, interpeduncular and prepontine cisterns). Initially, ETV-related morbidity and mortality was unacceptably high. This should be attributed to severe inherent limitations of the visualization capacity, with early patient series demonstrating a mortality in the range of 75% [
64].
ETV underwent a resurgence in popularity from the beginning of the 21st century, mainly due to the work of Dr. Benjamin Warf, which was based on patients from sub-Saharan Africa· the fact that they shared in common was the restricted access to shunt devices [
65,
66]. His patient’s cohort, based on the utilization of ETV, has shown satisfactory management of hydrocephalus which fluctuated in the range of approximately 80% of his patients. ETV is sometimes followed with CPC. The theoretical basis under this manipulation was to decrease the amount of CSF produced by the choroid plexus of the lateral ventricles. There is a number of risk factors that are associated with an increased likelihood of failure of the ETV. These include younger age, hydrocephalus of non-obstructive origin, and pre-existence of a drainage system. The combined interaction of all these factors constitutes the main reason to form the ETVSS, that is utilized as a tool in order to predict whether it is reasonable to attempt an ETV in patients harboring specific characteristics [
44].
It is common concept that several valuable improvements are established in our technical and theoretical perspective in the context of CSF diversion as a management option for hydrocephalus. Nevertheless, infection and failure of therapy due to shunt obstruction, complicate the treatment strategy of these at-risk patients. Especially in children, the estimated prevalence of shunt failure is very common, with a frequency of up to 50% in the first two years [
67,
68,
69]. The most frequently recorded causes of shunt failure are categorized under the general term ‘mechanical reasons’ (catheter breakage, or disconnection), malposition (central end of the catheter not in the ventricular cavity, distal end not in the peritoneal cavity), or obstruction (choroid plexus ingrowth or debris, epithelialization). By definition, each case of shunt failure necessitates at least one re-operation, with any further attempt at shunt repair being associated with an increased risk of morbidity and mortality [
70]. ETV with or without CPC seems to be able to alter the clinical course of these patients, as they could impair the long-term management of hydrocephalus, depending on its underlying etiology. Based on that, cases associated with long-term failures that necessitate revision are common. The Kaplan–Meier curves for patients with a 60–70% ETVSS cross at 6 months following the index procedure.
3.2. Comparison of Endoscopic Third Ventriculostomy and Shunt Placement in the Pediatric Population
Hydrocephalus in the pediatric population constitutes one of the most frequently encountered diagnoses, registered at admission in the pediatric neurosurgical units [
71,
72]. Currently, VPS is considered the standard of treatment, although a lot of advancements have been achieved and several new CSF diversion approaches have been introduced in the management of hydrocephalus. A very promising approach that has been included in our range of therapeutic options for the management of this entity includes the ETV [
73,
74].
Nevertheless, VPS is implicated with a wide spectrum of adverse effects, including shunt malfunction, infections, and inconsistent long-term motor and cognitive outcomes [
75]. A lot of clinical trials have attempted to compare and evaluate the current shunt success and relevant failure rates, in comparison with those of the previous decades; however, the existing data in the literature are inconsistent and thus unable to extract a definite conclusion [
76,
77].
ETV has been established as an alternative CSF diversion procedure, most commonly preferred in cases of hydrocephalus that are non-communicating in terms of their underlying cause [
78,
79] · apart from that, it has been proposed that ETV might also be useful for a specific subgroup of pediatric patients who are suffering from communicating hydrocephalus [
34]. A recent trend includes the addition of CPC during ETV· preliminary results have been published, supporting that the efficacy of the endoscopic approach is enhanced [
66,
80]. This new therapeutic trend is scientifically supported by the growing number of studies that attempt to evaluate and compare the relative effectiveness of VPS and ETV [
81,
82].
One of the objectives of our narrative review is to systematically evaluate the literature and analyze the comparative safety and efficacy of valve-based drainage systems and ETV in pediatric hydrocephalus cases. Most of the involved studies share in common several intrinsic limitations that weaken the validity of their results. Some of these include the finding that in the majority of cases the etiology of hydrocephalus is mixed, as well as the fact that geographical location, along with technical differentiations in the performance of ETV, influence our patient’s ultimate outcome. However, the aforementioned studies have tried to investigate the relevant effect of all of these parameters by conducting subgroup analyses.
The extracted conclusions verified that patients who underwent ETV shared in common a statistically significant decreased incidence of any procedural-related infection. This was reaffirmed in the analysis of the subgroup of cases that included only patients suffering from obstructive hydrocephalus. No differences in terms of repeat operations, mortality, and CSF leak were identified in the original pooled analyses that was based on the comparison of these two groups [
71].
Despite the fact that VPS has been performed as operation for several decades, a lot of complications continue to be intimately related with it, including procedure-related infections, CSF leaks, a high failure, and re-operation rate and even mortality [
72]. When ETV was introduced as a promising alternate of shunting, the initial indication for its use was strictly restricted to cases of hydrocephalus associated with aqueductal stenosis. Nevertheless, the spectrum of indications was gradually expanding. This fact was also evident when we were recording the indications for performance of an ETV. All our data were included in the studies that were incorporated as part of a relevant meta-analysis, based on obstructive and non-obstructive hydrocephalus cases [
74,
83]. ETV and shunting can be alternatively used for cases with pathologies that do not rule out any one of them. Nevertheless, there is always a number of variables, based on individual patient characteristics (i.e., previous CNS infection, number of previous attempts at shunt revision, patient age), which could predict procedural success and influence ultimate patient selection.
Infections are generally considered to be one of the most common and devastating complications in CSF diversion procedures [
81]. Although several attempts have been performed from organizations such as Hydrocephalus Clinical Research Network, shunt infection constitutes a major issue, adding significantly to the overall morbidity and mortality [
84]. The previously reported meta-analysis seemed to verify that ETV is inherently related with a statistically significant reduced prevalence of procedure-related infection compared to shunt insertion, during the follow-up period. Nevertheless, there was not any individual study, focused on this comparison, which was capable of supporting by its own these statistically significant differences between ETV and shunt. It could be supposed that these studies were inherently unable to individually prove this difference; nevertheless, the utilization of meta-analysis was able to substantially enhance the statistical power, and therefore, statistical significance was detected.
When we encounter patient cases where the primary CSF diversion procedure fails, a reoperation is commonly needed· this could involve a repeat of the first procedure, or the application of another technique [
74]. According to the studies that are included in the reported analysis [
71], the relevant details that would refer to the technical aspect of the repeat operation were lucking. This inconsistently present information is a fact that constitutes an inherent limitation. The attempted pooled analysis was not capable of verifying any significant differences between ETV vs. drainage procedures, when re-operation was the term that the comparison was based upon.
The aforementioned study suggested that, when all cases of mortality of any cause and rates of CSF leak were compared between the two main treatment modalities, the results from the ETV and shunt groups did not show any significant difference. These results are in alignment with the sole RCT by Kulkarni et al. [
41], who was not able to document any statistically significant differences between the study groups in terms mortality, regardless of the underlying cause. CSF leak was not reported by this RCT but was in accordance with all relevant studies that were included and contributed to this outcome. Future RCTs are warranted to validate the results of these published series. In particular, future studies would be able to yield more valuable conclusions if they could be based upon standardized outcomes definitions for success and failure of ETV or shunt, uniform age and hydrocephalus etiology subgroups, and a predetermined long-term follow-up.
3.3. Comparison of Endoscopic Third Ventriculostomy and Ventriculoperitoneal Shunt Placement in Infants and Children in Terms of Safety and Efficacy
We reviewed studies comparing ETV and shunts in pediatric population, with the goal of offering consultation in the selection of the optimum treatment strategy [
85]. According to our cumulative data, we should suggest that there are no statistically significant verified differences in terms of success and failure rates within 1 year after surgical intervention between ETV and shunting. Therefore, based on that evidence, at present, neither technique is justified to be considered as significantly superior. Cheng et al., in a recent meta-analysis reported that ETV and VPS can be used as alternating therapeutic options, without any compromise of the final patient outcome, for the treatment of non-communicating hydrocephalus in heterogenous population of adults and children. Nevertheless, ETV is associated with lower surgery time, postoperative complication and reoperation rates. On the other hand, there are several authors who suggested that ETV is significantly more efficient in pediatric patients who are older than 2 years of age [
86,
87]. On the contrary, others present evidence that the etiology of the pathology is a more important determinant factor than is age [
88]. Rasul et al. stated that while both ETV and shunting procedures are associated with a high failure rate, there is some evidence that ETV may be inherently related with improved long-term success rates [
87,
89]. No significant difference was established, when ETV and shunting were compared, in terms of both success and failure rates at one-year follow-up. Koch et al., in a relatively recently published review, showed that the ETV failure rate was high, up to 68.8%, within a median of 38 days [
90]. More recently published series, however, report a failure rate as low as 26.5% [
91]. In a recent systematic review, Bouras et al., mentioned that complication rate of ETV procedure reached 8.5 % and mortality risk was 0.28% [
92], and that improvement was based mainly on advanced technology, and better surgical skills as well. On the contrary, shunt procedure, while offering low mortality risk (0.1 %) [
76], is constantly associated with high failure rates (31.3% for the 1st year and 4.5% per year thereafter), with no significant advancements over the past several years. Except from efficacy and failure rates of any individual treatment, a constellation of other parameters should be revealed during the selection of the best treatment modality. Such factors include, but are not restricted to, individual patient anatomy and baseline clinical characteristics, patients’ age, etiology of hydrocephalus, surgeon-related factors, and equipment availability.
3.4. Shunt Independence and the Role of Endoscopic Third Ventriculostomy
There is only restricted bulk of evidence dedicated to the issue of shunt independence, although this is a permanent and of paramount significance concern for patients to whom an internal drainage system is implanted. Moreover, treating neurosurgeons play a pivotal role, as they are dealing with the potential shunt-related complications [
34,
93,
94,
95,
96,
97,
98,
99,
100]. Another issue that further complicates this discussion and necessitates further determination is centered on the determination of a generally accepted definition of shunt independency, the most effective method to attain it and what is the real prevalence of that side effect. Based on the fact that there is lack of relevant prospective studies and also on the consideration that there is only a minority of patients who present with spontaneous independence [
97], shunt removal is frequently elective. Moreover, this maneuver is frequently related with our efforts to calculate the estimated success rate of secondary ETV [
101] and is usually performed in patients with a medical history of frequent shunt dysfunctions and revision surgeries [
34]. The estimated rate of accomplishment of shunt independence is currently in the range of 3 to 9% of paediatric patients, suffering from hydrocephalus [
98,
99].
Shunt independence has been the ultimate tenet for neurosurgeons since shunt surgery became a common clinical practice. According to several anecdotal reports, it has been intimately related with their efforts to manage shunt-related complications. These are more frequently associated with shunt overdrainage and their efforts to prevent or even treat this adverse effect of shunt devices. In this context, several technical innovations have been adopted in order to facilitate independence (intermittent cranial compression with ICP monitoring, “on-off” type of shunts, subtemporal craniectomy) [
98]. Several meetings discussed that issue at the late 1980’s and early 1990’s (Shunts and Problems in Shunts Symposium, Marseille, June 1980; Consensus Conference: Hydrocephalus ‘92, Assisi, Italy). Several proposals have been submitted in order to better discriminate the term shunt independence and compensated or arrested hydrocephalus. Based on that, initial efforts to define the concept of shunt dependency were recorded, albeit with equivocal conclusions. The prevailing view among the scientists at that time period was that no individual test is reliable on its own and the safety of shunt removal remains uncertain. According to the policy that was adopted recently to a large pediatric department [
93], secondary ETV is more often proposed for individuals suffering from with obstructive hydrocephalus, of primary or acquired origin [
102]. These patients usually share in common a medical history that is complicated with recurrent episodes of shunt malfunction or a medical history of complex surgical procedures, attempting to handle the issue of shunt malfunction. The whole intervention is usually accomplished as part of a treatment algorithm, usually with prior emergent externalization of the shunt or EVD surgery.
3.5. Endoscopic Third Ventriculostomy and Infant Patient Population
There are ample, albeit contradictory, referrals from pediatric patients centered on which individuals are most likely to benefit from ETV [
103]. These controversial results constitute the basis for a debate which mainly centers on the success rates of ETV, the significance of age as an isolated factor, aetiology of hydrocephalus or both. Some neurosurgeons have advocated ETV as the management modality of choice for the obstructive subtype of hydrocephalus, which is attributed to primary aqueductal stenosis and other closely related pathological situations [
104]. More precisely, according to the previously mentioned study, ETV was selected as the most appropriate surgical option in 96.5% of individuals that were incorporated in that study. Nevertheless, this study was unable to verify if patients that are less than 1 year of age share in common a higher risk of ETV failure, when a comparison was made with older participants. Authors mention that when all included studies were taken into consideration, the success rate was 51.6%. When individual published reports were taken into consideration, the reported success rates were ranging from 0% [
105] to as high as 83% [
106]. There were published studies which recorded lower success rates when they were dealing with younger patients [
107,
108,
109] ·however, the aforementioned results were not always supported by a level of statistical significance [
79,
106,
110]. One report concluded that the underlying pathology and not the patient’s age was the main determinant factor that was determining the outcomes of ETV. More precisely, patients with an underlying diagnosis of congenital aqueduct stenosis were inherently associated with a better long-term outcome, compared with their counterparts associated with other offending pathologies [
111]. Moreover, an outcome that was dependent simultaneously with patient’s age and aetiology of hydrocephalus was reported by four studies [
112,
113,
114]. One study stated that patients with aqueductal stenosis that belong to a younger age group as well as those who are suffering from Chiari malformation had statistically significant worse outcomes [
112]. The remaining studies concluded that older infants with stenosis of the aqueduct were intimately related with improved outcomes [
113,
114].
Recently, a meta-analysis was published, centered on the role of ETV in patients manifested with shunt malfunction and they belong to the pediatric population [
115]. In conclusion, it manifests that when secondary ETV is selected as the treatment modality in this cohort of patients, it should be regarded as an acceptable option, accompanied with relatively good success rates, along with low complication rates. Based on those remarks, it could be justified as worth considering for cases that are complicated with shunt malfunction.
3.6. The Role of Neuroendoscopy in the Management of Post-Infection Hydrocephalus
Management of multiloculated hydrocephalus remains an intractable problem, mainly due to our inability to simultaneously and effectively drain compartments that are in isolation from the remaining ventricular system [
116]. The ultimate target lies to the creation of a single, freely-communicating cavity which could be effectively drained by one ventricular catheter, by establishing a communication between the different entrapped compartments [
117,
118,
119,
120,
121,
122,
123]. The more traditional treatment algorithm includes the insertion of multiple, separate ventricular catheters, which could be able to effectively drain non-communicating compartments. The main drawback of this approach is that, by increasingly the overall complexity of the shunt system, is far from desirable as it multiplicate the risk of complications, mainly central catheter obstruction. [
117,
121,
123,
124,
125]. Wide fenestration of membranes separating the individual ventricular compartments appears to be the procedure that is more advantageous and more akin to normal CSF circulation. The sole means to achieve this is through an open microsurgery or endoscopic surgery [
117,
118]. Endoscopic fenestration has inherently been associated with reduced blood loss, decreased operative time, as well as with lower morbidity and reduced time period of hospital recovery [
117,
121]. An alternative, as well as complementary option includes the placement of proximal catheters via the aid of endoscopic guidance· this may also be associated with a decreased risk of shunt malposition [
126]. Therefore, endoscopic fenestration nowadays consists of the optimum management option for multiloculated hydrocephalus, and this is more evident in infants. Apart from that, open fenestration is currently been our ultimate solution, reserved for severe or refractory cases [
117,
119,
121,
123].
3.7. Posthemorrhagic Hydrocephalus in Premature Infants and Available Treatment Modalities
We have performed bibliographic research in an attempt to determine the current, if any, recommendation guidelines, concerning the optimum time frame of shunt insertion in premature infants. To the best of our knowledge, there is inadequate evidence, that is they are unable to recommend a specific weight or a peculiar CSF value, capable to determine the optimum time point of shunt insertion in premature infants with PHH [
127]. On the contrary, clinical judgment is always required in such cases. Based on the guidelines that were extracted from an extended literature review, the current strength of Recommendation: Level III, that is not absolute clinical certainty. Another relevant issue is centered on the current recommendation concerning the utilization of ETV. According to the previously reported reference, there is not plenty of evidence able to justify the utilization of ETV in premature infants suffering from posthemorrhagic hydrocephalus. The strength of recommendation is Level III, that meaning not verified clinical certainty.
3.8. The Entity of Isolated Fourth Ventricle and Available Treatment Options: Relative Advantages and Disadvantages
TFV is a relatively uncommon, as well as critical and difficult to manage pathological entity, which usually refers to patients who have suffered from an intraventricular hemorrhage, inflammation or infection, tumor resection that was involving the fourth ventricle, and ventricular shunt placement. It is usually coming to clinical attention by delayed onset of its clinical signs, following a period of relative neurological amelioration [
127,
128]. The pathophysiological mechanism that is implicated in cases of TFV is intimately related with the arachnoidal blockage of the inlets and outlets of the fourth ventricle, the aqueduct of Sylvius, and the foramina of Magendie and Luschka, respectively [
129]. The available management strategies consist of placement of a separate drainage system (central catheter within the fourth ventricle), endoscopic and microsurgical fenestration. Nowadays, in the vast majority of cases, the selected treatment modality is the insertion of a shunt with a central catheter directed into the fourth ventricle [
130]. Nevertheless, this option is intimately related with several complications, including infection, malposition, malfunction, and brain stem injury [
131]. Nowadays, taking into consideration the widespread evolution of endoscopic techniques, a lot of published reports have presented individual cases of patients treated by endoscopic aqueductoplasty, either with or without stent placement [
132]. The ultimate tenet of these techniques is to reestablish the communication between the TFV, and the third ventricle or the subarachnoid space, thus circumventing the dependence from a separate fourth ventricular drainage system.
Endoscopy should be regarded as the most appropriate management strategy, and this should be attributed to its lower revision rate and its tendency to be associated with a considerably higher overall clinical improvement, in comparison with shunt placement. Another viable and effective treatment option remains the use of an open microsurgical fenestration. This is further supported by the fact that is accompanied with a similar clinical outcome and its revision rate is comparable to that of endoscopy. However, we should underline the fact that it is inextricably linked to a more invasive surgical approach, and thus should not be considered as a first-line treatment option. Shunt insertion could be managed as a rescue type procedure in the treatment of TFV, as it is frequently accompanied with serious complications and associated with a n increased rate of failure, and subsequent revision, compared to endoscopy. Endoscopy could even be considered as the most appropriate management option in the infantile population (< 1 year old). This can be attributed to the fact that it is a minimally invasive procedure and there is no definitive evidence that can justify its inferiority when compared with other treatment options.
4. Conclusions
ETV is generally related with a statistically significantly decreased risk of operation-related infection, in comparison with shunt placement. Apart from that, overall mortality rates, CSF leak, and re-operation rates seem to be within the same range when the two groups of patients are compared.
As far as we were able to know, based on current literature data, ETV and shunts are associated with similar 1 year success, as well as failure rates. Based on that, there is no justified data which could be able to recommend one procedure over the other. Definitively, there is need for the execution of more randomized studies with age and etiology subgroup analysis are needed in order to identify the most effective option regarding the treatment modality that should be followed for all of the divergent groups of hydrocephalus patients.
Shunt independence following a subsequent ETV in cases of obstructive hydrocephalus, performed at the time of shunt malfunction has significantly enhanced the rate of success and should be discussed in a selected, yet appropriate, subpopulation of patients. Intended removal of the shunt in cases of symptomatic shunt overdrainage constitutes a procedure that is accompanied with significant risks and should only be discussed in a particular subset of patients that fulfill specific and strict criteria. These criteria include close monitoring and the ability to obtain a long-term follow-up. There is compelling evidence which supports the concept that secondary ETV in pediatric patients is an alternative option, with encouraging success rates and low complication rates, and meriting consideration in individuals suffering from shunt malfunction.
ETV has been proved to be a valuable tool in our therapeutic armamentarium in order to avoid shunt placement in selected cases of post-infective hydrocephalus. Moreover, there is cumulative evidence to support that this could be accompanied with a high success rate in selected cases of shunt malfunction, associated with infection or previous post-infective hydrocephalus.
ETV can be considered as an effective and safe alternative for children experiencing shunt dysfunction, being able to offer shunt independence in cases that complete several indications and prerequisites: primary obstructive hydrocephalus with aqueductal stenosis, and post-inflammatory hydrocephalus in children ≥ 36 months.
Hydrocephalus has been recognized as a pathological entity from ancient history, and the first recorded placement of a shunt in 1950 was a historical achievement, adding a major innovation in the management of this condition. Unfortunately, despite the attempts of the scientific community, since that time there remains a significant number of issues to be elucidated and, more importantly, treat the underlying cause. Because of that, more research should be scheduled, aiming to determine the etiology of hydrocephalus. However, we should mention that, the current therapeutic armamentarium, although it necessitates several improvements, is able to provide lifesaving interventions and cognitive enhancements for many children. Even if it is below the expectations of the scientific community, it should be widely available to all the world’s children!
Abbreviations
| ETV |
endoscopic third ventriculostomy |
| CSF |
cerebrospinal fluid |
| PHH |
post-hemorrhagic hydrocephalus |
| CPC |
choroid plexus cauterization |
| RCT |
randomized controlled trials |
| PHH |
post-hemorrhagic hydrocephalus |
| IVH |
intraventricular hemorrhage |
| PIH |
post-infectious hydrocephalus |
| ICP |
intra-cranial pressure |
| TFV |
Trapped or isolated fourth ventricle |
| VA shunt |
ventriculo-atrial shunt |
| VPlS |
ventriculo-pleural shunt |
| VSGS |
ventriculo-subgaleal shunt |
| NEL |
neuroendoscopic lavage |
| LPs |
Lumboperitoneal shunts |
| SVS |
slit ventricle syndrome |
| VPS |
Ventriculoperitoneal shunts |
| EVD |
external ventricular drain |
| ETVSS |
ETV success score |
References
- Hochstetler, A.; Raskin, J.; Blazer-Yost, B.L. Hydrocephalus: historical analysis and considerations for treatment. Eur. J. Med Res. 2022, 27, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Mokri, B. The Monro–Kellie hypothesis: applications in CSF volume depletion. Neurology 2001, 56, 1746–8. [Google Scholar] [CrossRef] [PubMed]
- Fact Sheet: Hydrocephalus Fact Sheet [Internet]. Bethesda (MD): National Library of Medicine (US). Available online: https://www.ninds.nih.gov/Disorders/Patient-Caregiver-Education/Fact-Sheets/Hydrocephalus-Fact-Sheet (accessed on 7 January 2022).
- Robinson, S. Neonatal posthemorrhagic hydrocephalus from prematurity: pathophysiology and current treatment concepts. J. Neurosurgery: Pediatr. 2012, 9, 242–258. [Google Scholar] [CrossRef]
- Torkildsen, A. A new palliative procedure in cases of inoperable occlusion of the Sylvian duct. Acta Chir Scand 1939, 82, 177–185. [Google Scholar]
- Fleming, C.H.; Ritter, A.M.; Bruce, D.A. Development of shunt valves used for treating hydrocephalus: comparison with endoscopy treatment. Child's Nerv. Syst. 2023, 39, 2709–2717. [Google Scholar] [CrossRef]
- Nulsen, F.E.S.E. Treatment of hydrocephalus by a direct shunt from ventricle to jugular vein. Surgical Forum 1952, 399–402. [Google Scholar]
- Pudenz, R.H.; Russell, F.E.; Hurd, A.H.; Shelden, C.H. Ventriculo-auriculostomy; a technique for shunting cerebrospinal fluid into the right auricle; preliminary report. J Neurosurg 1957, 14, 171–179. [Google Scholar] [CrossRef]
- Cornejo, V.J.F.E.S. Shunt technology for infants and lifetime. Child’s Nervous System 2021, 37, 3475–3484. [Google Scholar] [CrossRef]
- Tomei, K.L. The Evolution of Cerebrospinal Fluid Shunts: Advances in Technology and Technique. Pediatr. Neurosurg. 2017, 52, 369–380. [Google Scholar] [CrossRef]
- Boockvar, J.A.; Loudon, W.; Sutton, L.N. Development of the Spitz—Holter valve in Philadelphia. J. Neurosurg. 2001, 95, 145–147. [Google Scholar] [CrossRef]
- Ames, R.H. Ventriculo-Peritoneal Shunts in the Management of Hydrocephalus. J. Neurosurg. 1967, 27, 525–529. [Google Scholar] [CrossRef] [PubMed]
- Olsen, L.; Frykberg, T. Complications in the treatment of hydrocephalus in children. A comparison of ventriculoatrial and ventriculoperitoneal shunts in a 20-year material. Acta Paediatr Scand 1983, 72, 385–390. [Google Scholar] [CrossRef] [PubMed]
- Kast, J.; Duong, D.; Nowzari, F.; Chadduck, W.M.; Schiff, S.J. Time-related patterns of ventricular shunt failure. Child's Nerv. Syst. 1994, 10, 524–528. [Google Scholar] [CrossRef] [PubMed]
- Mazza, C.; Pasqualin, A.; Da Pian, R. Results of treatment with ventriculoatrial and ventriculoperitoneal shunt in infantile nontumoral hydrocephalus. Childs Brain 1980, 7, 1–14. [Google Scholar] [PubMed]
- Sainte-Rose, C. Shunt obstruction: a preventable complication? Pediatr Neurosurg 1993, 19, 156–164. [Google Scholar] [CrossRef]
- Foltz, E.L.; Blanks, J.P. Symptomatic low intracranial pressure in shunted huydrocephalus. J Neurosurg 1988, 68, 401–408. [Google Scholar] [CrossRef]
- Pedersen, S.H.; Prein, T.H.; Ammar, A.; Grotenhuis, A.; Hamilton, M.G.; Hansen, T.S.; Kehler, U.; Rekate, H.; Thomale, U.-W.; Juhler, M. How to define CSF overdrainage: a systematic literature review. Acta Neurochir. 2023, 165, 429–441. [Google Scholar] [CrossRef]
- Pudenz, R.H.; Foltz, E.L. Hydrocephalus: Overdrainage by ventricular shunts. A review and recommendations. Surg. Neurol. 1991, 35, 200–212. [Google Scholar] [CrossRef]
- Rekate, H.L. Classification of Slit-Ventricle Syndromes Using Intracranial Pressure Monitoring. Pediatr. Neurosurg. 1993, 19, 15–20. [Google Scholar] [CrossRef]
- Alghamdi, K.T.; Alghamdi, M.D.; Neazy, S.; Algamdi, M.M.; Alzahrani, A.; A Khan, M.; Algahtani, A. Incidental and Clinical Significance of Slit Ventricles in Fixed Pressure Valves. Cureus 2022, 14, e30902. [Google Scholar] [CrossRef]
- Pudenz, R.H.; Constantini, S. Pudenz antisiphon device tear as a cause of shunt malfunction. Child's Nerv. Syst. 1990, 6, 117–117. [Google Scholar] [CrossRef] [PubMed]
- Koueik, J.; Kraemer, M.R.; Hsu, D.; Rizk, E.; Zea, R.; Haldeman, C.; Iskandar, B.J. A 12-year single-center retrospective analysis of antisiphon devices to prevent proximal ventricular shunt obstruction for hydrocephalus. J. Neurosurgery: Pediatr. 2019, 24, 642–651. [Google Scholar] [CrossRef] [PubMed]
- Bierbrauer, K.S.; Storrs, B.B.; McLone, D.G.; Tomita, T.; Dauser, R. A Prospective, Randomized Study of Shunt Function and Infections as a Function of Shunt Placement. Pediatr. Neurosurg. 1990, 16, 287–291. [Google Scholar] [CrossRef] [PubMed]
- Drake, J.M.; Kestle, J.R.W.; Tuli, S. CSF shunts 50 years on –past, present and future. Child’s Nervous System 2000, 16, 800–804. [Google Scholar] [CrossRef] [PubMed]
- Aoki, N. Lumboperitoneal shunt: clinical applications, complications, and comparison with ventriculoperitoneal shunt. Neurosurgery 1990, 26, 998–1003; discussion 1003–1004. [Google Scholar] [CrossRef]
- Wang, V.Y.; Barbaro, N.M.; Lawton, M.T.; Pitts, L.; Kunwar, S.; Parsa, A.T.; Gupta, N.; McDermott, M.W. Complications of lumboperitoneal shunts. Neurosurgery 2007, 60, 1045–1048; discussion 1049. [Google Scholar] [CrossRef]
- Mirzayan, M.J.; Klinge, P.M.; Samii, M.; Goetz, F.; Krauss, J.K. MRI safety of a programmable shunt assistant at 3 and 7 Tesla. Br. J. Neurosurg. 2012, 26, 397–400. [Google Scholar] [CrossRef]
- Chumas, P.D.; Armstrong, D.C.; Drake, J.M.; Kulkarni, A.V.; Hoffman, H.J.; Humphreys, R.P.; Rutka, J.T.; Hendrick, E.B. Tonsillar herniation: the rule rather than the exception after lumboperitoneal shunting in the pediatric population. J. Neurosurg. 1993, 78, 568–573. [Google Scholar] [CrossRef]
- Mcgirt, M.J.; Woodworth, G.; Thomas, G.; Miller, N.; Williams, M.; Rigamonti, D. Cerebrospinal fluid shunt placement for pseudotumor cerebri—associated intractable headache: predictors of treatment response and an analysis of long-term outcomes. J. Neurosurg. 2004, 101, 627–632. [Google Scholar] [CrossRef]
- Menger, R.P.; Connor, D.E.; Thakur, J.D.; Sonig, A.; Smith, E.; Guthikonda, B.; Nanda, A. A comparison of lumboperitoneal and ventriculoperitoneal shunting for idiopathic intracranial hypertension: an analysis of economic impact and complications using the Nationwide Inpatient Sample. Neurosurg. Focus 2014, 37, E4–E4. [Google Scholar] [CrossRef]
- Azad, T.D.; Zhang, Y.; Varshneya, K.; Veeravagu, A.; Ratliff, J.K.; Li, G. Lumboperitoneal and ventriculoperitoneal shunting for idiopathic intracranial hypertension demonstrate comparable failure and complication rates. Neurosurgery 2020, 86, 272–280. [Google Scholar] [CrossRef] [PubMed]
- Hoffman, H.; Harwood-Nash, D.; Gilday, D. Percutaneous third ventriculostomy in the management of noncommunicating hydrocephalus. Neurosurgery 1980, 7, 313–321. [Google Scholar] [CrossRef] [PubMed]
- Cinalli, G.; Salazar, C.; Mallucci, C.; Yada, J.Z.; Zerah, M.; Sainte-Rose, C. The role of endoscopic third ventriculostomy in the management of shunt malfunction. Neurosurgery 1998, 43, 1323–1327; discussion 1327–1329. [Google Scholar]
- Warf, B.C. Comparison of 1-year outcomes for the Chhabra and Codman-Hakim Micro Precision shunt systems in Uganda: a prospective study in 195 children. J. Neurosurgery: Pediatr. 2005, 102, 358–362. [Google Scholar] [CrossRef]
- Kulkarni, A.V.; Warf, B.C.; Drake, J.M.; Mallucci, C.L.; Sgouros, S.; Constantini, S.; the Canadian Pediatric Neurosurgery Study Group. Surgery for hydrocephalus in sub-Saharan Africa versus developed nations: a risk-adjusted comparison of outcome. Child's Nerv. Syst. 2010, 26, 1711–1717. [Google Scholar] [CrossRef]
- de Ribaupierre, S.; Rilliet, B.; Vernet, O.; Regli, L.; Villemure, J.-G. Third ventriculostomy vs ventriculoperitoneal shunt in pediatric obstructive hydrocephalus: results from a Swiss series and literature review. Child's Nerv. Syst. 2007, 23, 527–533. [Google Scholar] [CrossRef]
- Roth, J.; Bo, X.; Beni-Adani, L.; Elran, H.; Constantini, S. [Endoscopic third ventriculostomy--a physiological alternative to shunts as treatment for obstructive hydrocephalus in children]. Harefuah 2007, 146, 660–5, 735. [Google Scholar]
- Lam, S.; Harris, D.; Rocque, B.G.; Ham, S.A. Pediatric endoscopic third ventriculostomy: a population-based study. J. Neurosurgery: Pediatr. 2014, 14, 455–464. [Google Scholar] [CrossRef]
- Dewan, M.C.; Lim, J.; Shannon, C.N.; Wellons, J.C. The durability of endoscopic third ventriculostomy and ventriculoperitoneal shunts in children with hydrocephalus following posterior fossa tumor resection: a systematic review and time-to-failure analysis. J. Neurosurgery: Pediatr. 2017, 19, 578–584. [Google Scholar] [CrossRef]
- Kulkarni, A.V.; Drake, J.M.; Kestle, J.R.; Mallucci, C.L.; Sgouros, S.; Constantini, S. Endoscopic third ventriculostomy vs cerebrospinal fluid shunt in the treatment of hydrocephalus in children: a propensity score-adjusted analysis. Neurosurgery 2010, 67, 588–593. [Google Scholar] [CrossRef]
- Kulkarni, A.V.; Riva-Cambrin, J.; Holubkov, R.; Browd, S.R.; Cochrane, D.D.; Drake, J.M.; Limbrick, D.D.; Rozzelle, C.J.; Simon, T.D.; Tamber, M.S.; et al. Endoscopic third ventriculostomy in children: prospective, multicenter results from the Hydrocephalus Clinical Research Network. J. Neurosurgery: Pediatr. 2016, 18, 423–429. [Google Scholar] [CrossRef] [PubMed]
- Stovell, M.G.; Zakaria, R.; Ellenbogen, J.R.; Gallagher, M.J.; Jenkinson, M.D.; Hayhurst, C.; Mallucci, C.L. Long-term follow-up of endoscopic third ventriculostomy performed in the pediatric population. J. Neurosurgery: Pediatr. 2016, 17, 734–738. [Google Scholar] [CrossRef] [PubMed]
- Kulkarni, A.V.; Drake, J.M.; Kestle, J.R.W.; Mallucci, C.L.; Sgouros, S.; Constantini, S. Predicting who will benefit from endoscopic third ventriculostomy compared with shunt insertion in childhood hydrocephalus using the ETV Success Score. J. Neurosurgery: Pediatr. 2010, 6, 310–315. [Google Scholar] [CrossRef]
- Furtado, L.M.F.; Filho, J.A.d.C.V.; Júnior, E.C.d.S. External validation of the ETV success score in 313 pediatric patients: a Brazilian single-center study. Neurosurg. Rev. 2021, 44, 2727–2734. [Google Scholar] [CrossRef]
- Kulkarni, A.V.; Riva-Cambrin, J.; Browd, S.R. Use of the ETV Success Score to explain the variation in reported endoscopic third ventriculostomy success rates among published case series of childhood hydrocephalus. J. Neurosurgery: Pediatr. 2011, 7, 143–146. [Google Scholar] [CrossRef]
- Yordanov, S.; Garnett, M.R.; Santarius, T.; Holland, K.; Jalloh, I.; Naushahi, M.J. An audit of endoscopic third ventriculostomy (ETV) in a regional paediatric neurosurgical centre assessing the accuracy and feasibility of the ETV success score. Acta Neurochir. 2022, 164, 1453–1458. [Google Scholar] [CrossRef]
- Ben-Israel, D.; Mann, J.A.; Yang, M.M.H.; Isaacs, A.M.; Cadieux, M.; Sader, N.; Muram, S.; Albakr, A.; Manoranjan, B.; Yu, R.W.; et al. Clinical outcomes in pediatric hydrocephalus patients treated with endoscopic third ventriculostomy and choroid plexus cauterization: a systematic review and meta-analysis. J. Neurosurgery: Pediatr. 2022, 30, 18–30. [Google Scholar] [CrossRef]
- Ellenbogen, Y.; Brar, K.; Yang, K.; Lee, Y.; Ajani, O. Comparison of endoscopic third ventriculostomy with or without choroid plexus cauterization in pediatric hydrocephalus: a systematic review and meta-analysis. J. Neurosurgery: Pediatr. 2020, 26, 371–378. [Google Scholar] [CrossRef]
- Stone, S.; Warf, B.C. Combined endoscopic third ventriculostomy and choroid plexus cauterization as primary treatment for infantshydrocephalus: a prospective North American series. J Neurosurg Pediatrics 2014, 14, 439–446. [Google Scholar] [CrossRef]
- Warf, B.C. The Impact of Combined Endoscopic Third Ventriculostomy and Choroid Plexus Cauterization on the Management of Pediatric Hydrocephalus in Developing Countries. World Neurosurg. 2011, 79, S23.e13–S23.e15. [Google Scholar] [CrossRef]
- Fame, R.M.; Cortés-Campos, C.; Sive, H.L. Brain ventricular system and cerebrospinal fluid development and function: light at the end of the tube: a primer with latest insights. BioEssays. 2020, 42, e1900186. [Google Scholar] [CrossRef] [PubMed]
- Fame, R.M.; Lehtinen, M.K. Emergence and Developmental Roles of the Cerebrospinal Fluid System. Dev. Cell 2020, 52, 261–275. [Google Scholar] [CrossRef] [PubMed]
- Karimy, J.K.; Reeves, B.C.; Damisah, E.; Duy, P.Q.; Antwi, P.; David, W.; Wang, K.; Schiff, S.J.; Limbrick, D.D.; Alper, S.L.; et al. Inflammation in acquired hydrocephalus: pathogenic mechanisms and therapeutic targets. Nat. Rev. Neurol. 2020, 16, 285–296. [Google Scholar] [CrossRef]
- McAllister, J.P. Pathophysiology of congenital and neonatal hydrocephalus. Semin. Fetal Neonatal Med. 2012, 17, 285–294. [Google Scholar] [CrossRef]
- Kahle, K.T.; Kulkarni, A.V.; Limbrick, D.D.; Warf, B.C. Hydrocephalus in children. Lancet 2016, 387, 788–99. [Google Scholar] [CrossRef]
- Li, J.; Zhang, X.; Guo, J.; Yu, C.; Yang, J. Molecular Mechanisms and Risk Factors for the Pathogenesis of Hydrocephalus. Front. Genet. 2022, 12, 777926. [Google Scholar] [CrossRef]
- Kelly, E.J.; Yamada, S. Cerebrospinal Fluid Flow Studies and Recent Advancements. Semin. Ultrasound, CT MRI 2016, 37, 92–99. [Google Scholar] [CrossRef]
- Reeves, B.C.; Karimy, J.K.; Kundishora, A.J.; Mestre, H.; Cerci, H.M.; Matouk, C.; Alper, S.L.; Lundgaard, I.; Nedergaard, M.; Kahle, K.T. G-lymphatic System impairment in Alzheimer’s disease and idiopathic normal pressure hydrocephalus. Trends Mol Med. 2020, 26, 285–95. [Google Scholar] [CrossRef]
- Williams, M.A.; Malm, J. Diagnosis and Treatment of Idiopathic Normal Pressure Hydrocephalus. Contin. Lifelong Learn. Neurol. 2016, 22, 579–599. [Google Scholar] [CrossRef]
- Wang, Z.; Zhang, Y.; Hu, F.; Ding, J.; Wang, X. Pathogenesis and pathophysiology of idiopathic normal pressure hydrocephalus. CNS Neurosci. Ther. 2020, 26, 1230–1240. [Google Scholar] [CrossRef]
- Jin, S.C.; Dong, W.; Kundishora, A.J.; Panchagnula, S.; Moreno-De-Luca, A.; Furey, C.G.; Allocco, A.A.; Walker, R.L.; Nelson-Williams, C.; Smith, H.; et al. Exome sequencing implicates genetic disruption of prenatal neuro-gliogenesis in sporadic congenital hydrocephalus. Nat. Med. 2020, 26, 1754–1765. [Google Scholar] [CrossRef] [PubMed]
- Thomale, U.W.; Ginalli, G.; Kulkarni, A.V.; Al-Hakin, S.; Roth, J.; Schaumann, A.; Buhrer, C.; Cavalheiro, S.; Sgouros, S.; Constanini, S.; Bock, H.C. TROPHY registry study design: a prospective, international multicenter study for the surgical treatment of post-hemorrhagic hydrocephalus in neonates. Child Nerv Sys. 2019, 35, 613–9. [Google Scholar] [CrossRef] [PubMed]
- Blitz, A.M.; Ahmed, A.K.; Rigamonti, D. Founder of modern hydrocephalus diagnosis and therapy: Walter Dandy at the Johns Hopkins Hospital. J Neurosurg. 2018. [Google Scholar] [CrossRef] [PubMed]
- Stagno, V.; Navarrete, E.A.; Mirone, G.; Esposito, F. Management of Hydrocephalus Around the World. World Neurosurg. 2013, 79, S23.e17–S23.e20. [Google Scholar] [CrossRef] [PubMed]
- Warf, B.C. Comparison of endoscopic third ventriculostomy alone and combined with choroid plexus cauterization in infants younger than 1 year of age: a prospective study in 550 African children. J. Neurosurgery: Pediatr. 2005, 103, 475–481. [Google Scholar] [CrossRef]
- Drake, J.M.; Kestle, J. Rational and methodology of the multicenter pediatric cerebrospinal fluid shunt design trial. Pediatric hydrocephalus treatment evaluation group. Childs Nerv Sys. 1996, 12, 434–47. [Google Scholar] [CrossRef]
- Kestle, J.; Milner, R.; Drake, J. The Shunt Design Trial: Variation in Surgical Experience Did Not Influence Shunt Survival. Pediatr. Neurosurg. 1999, 30, 283–287. [Google Scholar] [CrossRef]
- Kestle, J.; Drake, J.; Milner, R.; Sainte-Rose, C.; Cinalli, G.; Boop, F.; Piatt, J.; Haines, S.; Schiff, S.; Cochrane, D.; et al. Long-Term Follow-Up Data from the Shunt Design Trial. Pediatr. Neurosurg. 2000, 33, 230–236. [Google Scholar] [CrossRef]
- Shannon, C.N.; Carr, K.R.; Tomycz, L.; Wellons, J.C.; Tulipan, N. Time to First Shunt Failure in Pediatric Patients over 1 Year Old: A 10-Year Retrospective Study. Pediatr. Neurosurg. 2013, 49, 353–359. [Google Scholar] [CrossRef]
- Texakalidis, P.; Tora, M.S.; Wetzel, J.S.; Chern, J.J. Endoscopic third ventriculostomy versus shunt for pediatric hydrocephalus: a systematic literature review and meta-analysis. Child's Nerv. Syst. 2019, 35, 1283–1293. [Google Scholar] [CrossRef]
- Beuriat, P.-A.; Puget, S.; Cinalli, G.; Blauwblomme, T.; Beccaria, K.; Zerah, M.; Sainte-Rose, C. Hydrocephalus treatment in children: long-term outcome in 975 consecutive patients. J. Neurosurgery: Pediatr. 2017, 20, 10–18. [Google Scholar] [CrossRef] [PubMed]
- Pople, I.K. Hydrocephalus and shunts: what the neurologist should know. J Neurol Neurosurg Psychiatry 2002, 73 (Suppl 1), i17–i22. [Google Scholar] [PubMed]
- Jernigan, S.C.; Berry, J.G.; Graham, D.A.; Goumnerova, L. The comparative effectiveness of ventricular shunt placement versus endoscopic third ventriculostomy for initial treatment of hydrocephalus in infants. J. Neurosurgery: Pediatr. 2014, 13, 295–300. [Google Scholar] [CrossRef] [PubMed]
- Uche, E.; Onyia, E.; Mezue, U.; Okorie, E.; Ozor, I.; Chikani, M. Determinants and Outcomes of Ventriculoperitoneal Shunt Infections in Enugu, Nigeria. Pediatr. Neurosurg. 2013, 49, 75–80. [Google Scholar] [CrossRef] [PubMed]
- Stein, S.C.; Guo, W. Have we made progress in preventing shunt failure? A critical analysis. J. Neurosurgery: Pediatr. 2008, 1, 40–47. [Google Scholar] [CrossRef]
- Kulkarni, A.V.; Riva-Cambrin, J.; Butler, J.; Browd, S.R.; Drake, J.M.; Holubkov, R.; Kestle, J.R.W.; Limbrick, D.D.; Simon, T.D.; Tamber, M.S.; et al. Outcomes of CSF shunting in children: comparison of Hydrocephalus Clinical Research Network cohort with historical controls. J. Neurosurgery: Pediatr. 2013, 12, 334–338. [Google Scholar] [CrossRef]
- Greitz, D. Paradigm shift in hydrocephalus research in legacy of Dandy’s pioneering work: rationale for third ventriculostomy in communicating hydrocephalus. Child's Nerv. Syst. 2007, 23, 487–489. [Google Scholar] [CrossRef]
- Koch-Wiewrodt, D.; Wagner, W. Success and failure of endoscopic third ventriculostomy in young infants: are there different age distributions? Child's Nerv. Syst. 2006, 22, 1537–1541. [Google Scholar] [CrossRef]
- Warf, B.C.; Tracy, S.; Mugamba, J. Long-term outcome for endoscopic third ventriculostomy alone or in combination with choroid plexus cauterization for congenital aqueductal stenosis in African infants. J Neurosurg Pediatr 2012, 10, 108–111. [Google Scholar] [CrossRef]
- Pan, I.-W.; A Harris, D.; Luerssen, T.G.; Lam, S.K. Comparative Effectiveness of Surgical Treatments for Pediatric Hydrocephalus. Neurosurgery 2017, 83, 480–487. [Google Scholar] [CrossRef]
- Kulkarni, A.V.; Schiff, S.J.; Mbabazi-Kabachelor, E.; Mugamba, J.; Ssenyonga, P.; Donnelly, R.; Levenbach, J.; Monga, V.; Peterson, M.; MacDonald, M.; et al. Endoscopic Treatment versus Shunting for Infant Hydrocephalus in Uganda. New Engl. J. Med. 2017, 377, 2456–2464. [Google Scholar] [CrossRef] [PubMed]
- Uche, E.O.; Okorie, C.; Iloabachie, I.; Amuta, D.S.; Uche, N.J. Endoscopic third ventriculostomy (ETV) and ventriculoperitoneal shunt (VPS) in non-communicating hydrocephalus (NCH): comparison of outcome profiles in Nigerian children. Child's Nerv. Syst. 2018, 34, 1683–1689. [Google Scholar] [CrossRef] [PubMed]
- Kestle, J.R.W.; Holubkov, R.; Cochrane, D.D.; Kulkarni, A.V.; Limbrick, D.D., Jr.; Luerssen, T.G.; Oakes, W.J.; Riva-Cambrin, J.; Rozzelle, C.; Simon, T.D.; et al. A new Hydrocephalus Clinical Research Network protocol to reduce cerebrospinal fluid shunt infection. J. Neurosurgery: Pediatr. 2016, 17, 391–396. [Google Scholar] [CrossRef]
- Li, C.; Gui, S.; Zhang, Y. Compare the safety and efficacy of endoscopic third ventriculostomy and ventriculoperitoneal shunt placement in infants and children with hydrocephalus: a systematic review and meta-analysis. Int. J. Neurosci. 2023, 134, 1–10. [Google Scholar] [CrossRef]
- Jones, R.F.C.; Kwok, B.C.T.; Stening, W.A.; Vonau, M. The Current Status of Endoscopic Third Ventriculostomy in the Management of Non-Communicating Hydrocephalus. min - Minim. Invasive Neurosurg. 1994, 37, 28–36. [Google Scholar] [CrossRef]
- Rasul, F.T.; Marcus, H.J.; Toma, A.K.; Thorne, L.; Watkins, L.D. Is endoscopic third ventriculostomy superior to shunts in patients with non-communicating hydrocephalus? A systematic review and meta-analysis of the evidence. Acta Neurochir. 2013, 155, 883–889. [Google Scholar] [CrossRef]
- Ojo, O.; Bankole, O.; Kanu, O.; Okubadejo, N. Efficacy of endoscopic third ventriculostomy in the management of hydrocephalus in children under 2 years of age: Experience from a tertiary institution in Nigeria. Niger. J. Clin. Pr. 2015, 18, 318–22. [Google Scholar] [CrossRef]
- Bouras, T.; Sgouros, S. Complications of endoscopic third ventriculostomy. World Neurosurg. 2013, 79, e9–e12. [Google Scholar] [CrossRef]
- Warf, B.C. Hydrocephalus in Uganda: the predominance of infectious origin and primary management with endoscopic third ventriculostomy. J. Neurosurgery: Pediatr. 2005, 102, 1–15. [Google Scholar] [CrossRef]
- Ogiwara, H.; Dipatri, A.J.; Alden, T.D.; Bowman, R.M.; Tomita, T. Endoscopic third ventriculostomy for obstructive hydrocephalus in children younger than 6 months of age. Child's Nerv. Syst. 2009, 26, 343–347. [Google Scholar] [CrossRef]
- Di Rocco, C.; Marchese, E.; Velardi, F.A. Survey of the first complication of newly implanted CSF shunt devices for the treatment of nontumoral hydrocephalus Cooperative survey of the 1991-1992 Education Committee of the, I.S.P.N. Childs Nerv Syst. 1994, 10, 321–327. [Google Scholar] [CrossRef] [PubMed]
- Iglesias, S.; Ros, B.; Ibáñez, G.; Delgado, A.; Ros, A.; Arráez, M.A. Shunt independence in paediatric hydrocephalus: our 16-year experience and review. Child's Nerv. Syst. 2019, 35, 1547–1555. [Google Scholar] [CrossRef]
- Baskin, J.J.; Manwaring, K.H.; Rekate, H.L. Ventricular shunt removal: the ultimate treatment of the slit ventricle syndrome. J. Neurosurg. 1998, 88, 478–484. [Google Scholar] [CrossRef]
- Chernov, M.F.; Kamikawa, S.; Yamane, F.; Ishihara, S.; Hori, T. Neurofiberscope-guided management of slit-ventricle syndrome due to shunt placement. J. Neurosurgery: Pediatr. 2005, 102, 260–267. [Google Scholar] [CrossRef]
- Epstein, F.J.; Hochwald, G.M.; Wald, A.; Ransohoff, J. Avoidance of Shunt Dependency in Hydrocephalus. Dev. Med. Child Neurol. 1975, 17, 71–77. [Google Scholar] [CrossRef]
- Iannelli, A.; Rea, G.; Di Rocco, C. CSF shunt removal in children with hydrocephalus. Acta Neurochir. 2005, 147, 503–507. [Google Scholar] [CrossRef]
- Iglesias, S.; Ros, B.; Martín, A.; Carrasco, A.; Segura, M.; Delgado, A.; Rius, F.; Arráez, M.A. Surgical outcome of the shunt: 15-year experience in a single institution. Child's Nerv. Syst. 2016, 32, 2377–2385. [Google Scholar] [CrossRef]
- Vinchon, M.; Rekate, H.; Kulkarni, A.V. Pediatric hydrocephalus outcomes: a review. Fluids Barriers CNS 2012, 9, 18–18. [Google Scholar] [CrossRef]
- Waqar, M.; Ellenbogen, J.R.; Mallucci, C. Endoscopic third ventriculostomy for shunt malfunction in children: A review. J. Clin. Neurosci. 2018, 51, 6–11. [Google Scholar] [CrossRef]
- Kulkarni, A.V.; Drake, J.M.; Mallucci, C.L.; Sgouros, S.; Roth, J.; Constantini, S. Endoscopic Third Ventriculostomy in the Treatment of Childhood Hydrocephalus. J. Pediatr. 2009, 155, 254–259.e1. [Google Scholar] [CrossRef]
- Scavarda, D.; Bednarek, N.; Litre, F.; Koch, C.; Lena, G.; Morville, P.; Rousseaux, P. Acquired aqueductal stenosis in preterm infants: an indication for neuroendoscopic third ventriculostomy. Child's Nerv. Syst. 2003, 19, 756–759. [Google Scholar] [CrossRef] [PubMed]
- Zaben, M.; Manivannan, S.; Sharouf, F.; Hammad, A.; Patel, C.; Bhatti, I.; Leach, P. The efficacy of endoscopic third ventriculostomy in children 1 year of age or younger: A systematic review and meta-analysis. Eur. J. Paediatr. Neurol. 2020, 26, 7–14. [Google Scholar] [CrossRef] [PubMed]
- Kadrian, D.; Van Gelder, J.; Florida, D.; Jones, R.; Vonau, M.; Teo, C.; Stening, W.; Kwok, B. Long-term reliability of endoscopic third ventriculostomy. Neurosurgery 2005, 56, 1271–1278, discussion 1278. [Google Scholar] [CrossRef] [PubMed]
- El Beltagy, M.A.; Kamal, H.M.; Taha, H.; Awad, M.; El Khateeb, N. Endoscopic third ventriculostomy before tumor surgery in children with posterior fossa tumors, CCHE experience. Child's Nerv. Syst. 2010, 26, 1699–1704. [Google Scholar] [CrossRef] [PubMed]
- Yadav, Y.R.; Jaiswal, S.; Adam, N.; Basoor, A.; Jain, G. Endoscopic third ventriculostomy in infants. Neurol. India 2006, 54, 161–3. [Google Scholar]
- Kim, S.K.; Wang, K.C.; Cho, B.K. Surgical outcome of pediatric hydrocephalus treated by endoscopic III ventriculostomy: prognostic factors and interpretation of postoperative neuroimaging. Child's Nerv. Syst. 2000, 16, 161–168. [Google Scholar] [CrossRef]
- Gallo, P.; Szathmari, A.; De Biasi, S.; Mottolese, C. Endoscopic Third Ventriculostomy in Obstructive Infantile Hydrocephalus: Remarks about the So-Called ‘Unsuccessful Cases’. Pediatr. Neurosurg. 2010, 46, 435–441. [Google Scholar] [CrossRef]
- Tewuerbati, S.; Maimaitili, M.; Zhu, G.; Du, G.; Liu, B.; Sailike, D.; et al. Timing of endoscopic third ventriculostomy in pediatric patients with congenital obstructive hydrocephalus: assessment of neurodevelopmental outcome short-term operative success rate. J. Clin. Neurosci 2015, 22, 1292–1297. [Google Scholar] [CrossRef]
- Koch, D.; Wagner, W. Endoscopic third ventriculostomy in infants of less than1 year of age: which factors influence the outcome? Child’s Nerv. Syst. 2004, 20, 405–411. [Google Scholar] [CrossRef]
- Javadpour, M.; Mallucci, C.; Brodbelt, A.; Golash, A.; May, P. The Impact of Endoscopic Third Ventriculostomy on the Management of Newly Diagnosed Hydrocephalus in Infants. Pediatr. Neurosurg. 2001, 35, 131–135. [Google Scholar] [CrossRef]
- Gorayeb, R.P.; Cavalheiro, S.; Zymberg, S.T. Endoscopic third ventriculostomy in children younger than 1 year of age. J. Neurosurgery: Pediatr. 2004, 100, 427–429. [Google Scholar] [CrossRef] [PubMed]
- Baldauf, J.; Oertel, J.; Gaab, M.R.; Schroeder, H.W.S. Endoscopic third ventriculostomy in children younger than 2 years of age. Child's Nerv. Syst. 2007, 23, 623–626. [Google Scholar] [CrossRef] [PubMed]
- Ogiwara, H.; Dipatri Jr, A.J.; Alden, T.D.; Bowman, R.M.; Tomita, T. Endoscopic third ventriculostomy for obstructive hydrocephalus in children younger than 6 months of age. Child’s Nerv. Syst 2010, 26, 343–347. [Google Scholar] [CrossRef]
- Lee, K.S.; Chari, A.; Gillespie, C.S.; Ekert, J.O.; Saffari, S.E.; James, G.; Aquilina, K. Endoscopic third ventriculostomy for shunt malfunction in the pediatric population: a systematic review, meta-analysis, and meta-regression analysis. J. Neurosurgery: Pediatr. 2023, 31, 1–10. [Google Scholar] [CrossRef]
- Deopujari, C.E.; Padayachy, L.; Azmi, A.; Figaji, A.; Samantray, S.K. Neuroendoscopy for post-infective hydrocephalus in children. Child's Nerv. Syst. 2018, 34, 1905–1914. [Google Scholar] [CrossRef]
- Akbari, S.H.A.; Holekamp, T.F.; Murphy, T.M.; Mercer, D.; Leonard, J.R.; Smyth, M.D.; Park, T.S.; Limbrick, D.D. Surgical management of complex multiloculated hydrocephalus in infants and children. Child's Nerv. Syst. 2014, 31, 243–249. [Google Scholar] [CrossRef]
- Andresen, M.; Juhler, M. Multiloculated hydrocephalus: a review of current problems in classification and treatment. Child's Nerv. Syst. 2012, 28, 357–362. [Google Scholar] [CrossRef]
- Fritsch, M.J.; Mehdorn, M. Endoscopic Intraventricular Surgery for Treatment of Hydrocephalus and Loculated CSF Space in Children Less than One Year of Age. Pediatr. Neurosurg. 2002, 36, 183–188. [Google Scholar] [CrossRef]
- Gandhoke, G.S.; Frassanito, P.; Chandra, N.; Ojha, B.K.; Singh, A. Role of magnetic resonance ventriculography in multiloculated hydrocephalus. J. Neurosurgery: Pediatr. 2013, 11, 697–703. [Google Scholar] [CrossRef]
- Lewis, A.I.; Keiper, G.L.; Crone, K.R. Endoscopic treatment of loculated hydrocephalus. J. Neurosurg. 1995, 82, 780–785. [Google Scholar] [CrossRef]
- Spennato, P.; Cinalli, G.; Ruggiero, C.; Aliberti, F.; Trischitta, V.; Cianciulli, E.; Maggi, G. Neuroendoscopic treatment of multiloculated hydrocephalus in children. J. Neurosurgery: Pediatr. 2007, 106, 29–35. [Google Scholar] [CrossRef] [PubMed]
- Zuccaro, G.; Ramos, J.G. Multiloculated hydrocephalus. Childs Nerv Syst 2011, 27, 1609–1619. [Google Scholar] [CrossRef] [PubMed]
- Nowoslawska, E.; Polis, L.; Kaniewska, D.; Mikolajczyk, W.; Krawczyk, J.; Szymanski, W.; Zakrzewski, K.; Podciechowska, J. Effectiveness of neuroendoscopic procedures in the treatment of complex compartmentalized hydrocephalus in children. Child's Nerv. Syst. 2003, 19, 659–665. [Google Scholar] [CrossRef]
- Sandberg, D.I.; McComb, J.G.; Krieger, M.D. Craniotomy for Fenestration of Multiloculated Hydrocephalus in Pediatric Patients. Neurosurg. 2005, 57, 100–106. [Google Scholar] [CrossRef]
- Schulz, M.; Bohner, G.; Knaus, H.; Haberl, H.; Thomale, U.-W. Navigated endoscopic surgery for multiloculated hydrocephalus in children. J. Neurosurgery: Pediatr. 2010, 5, 434–442. [Google Scholar] [CrossRef]
- Mazzola, C.A.; Choudhri, A.F.; Auguste, K.I.; Limbrick Jr, D.D.; Rogido, M.; Mitchell, L. Pediatric hydrocephalus: systematic literature review and evidence-based guidelines. Part 2: Management of posthemorrhagic hydrocephalus in premature infants. J Neurosurg Pediatr 2014, 14 (Suppl 1), 8–23. [Google Scholar] [CrossRef]
- Sadigh, Y.; van Surksum, C.; Schröder, P.H.D.; Cozar, A.; Khandour, D.; Talbi, L.; Spoor, J.K.H.; Rooda, O.H.J.E.; Volovici, V.; van Veelen, M.-L.C. Trapped fourth ventricle: to stent, shunt, or fenestrate—a systematic review and individual patient data meta-analysis. Neurosurg. Rev. 2023, 46, 1–10. [Google Scholar] [CrossRef]
- Hawkins, J.C.; Hoffman, H.J.; Humphreys, R.P. Isolated fourth ventricle as a complication of ventricular shunting. J. Neurosurg. 1978, 49, 910–913. [Google Scholar] [CrossRef]
- Foltz, E.L.; Shurtleff, D.B. Conversion of Communicating Hydrocephalus to Stenosis or Occlusion of the Aqueduct during Ventricular Shunt. J. Neurosurg. 1966, 24, 520–529. [Google Scholar] [CrossRef]
- Harter, D.H. Management strategies for treatment of the trapped fourth ventricle. Child's Nerv. Syst. 2004, 20, 710–716. [Google Scholar] [CrossRef]
- Lee, M.; Leahu, D.; Weiner, H.L.; Abbott, R.; Wisoff, J.H.; Epstein, F.J. Complications of Fourth-Ventricular Shunts. Pediatr. Neurosurg. 1995, 22, 309–314. [Google Scholar] [CrossRef] [PubMed]
- Fritsch, M.J.; Kienke, S.; Manwaring, K.H.; Mehdorn, H.M. Endoscopic aqueductoplasty and interventriculostomy for the treatment of isolated fourth ventricle in children. Neurosurgery 2004, 55, 372–377; discussion 7-9. [Google Scholar] [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).