Submitted:
04 October 2024
Posted:
04 October 2024
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Materials and Methods
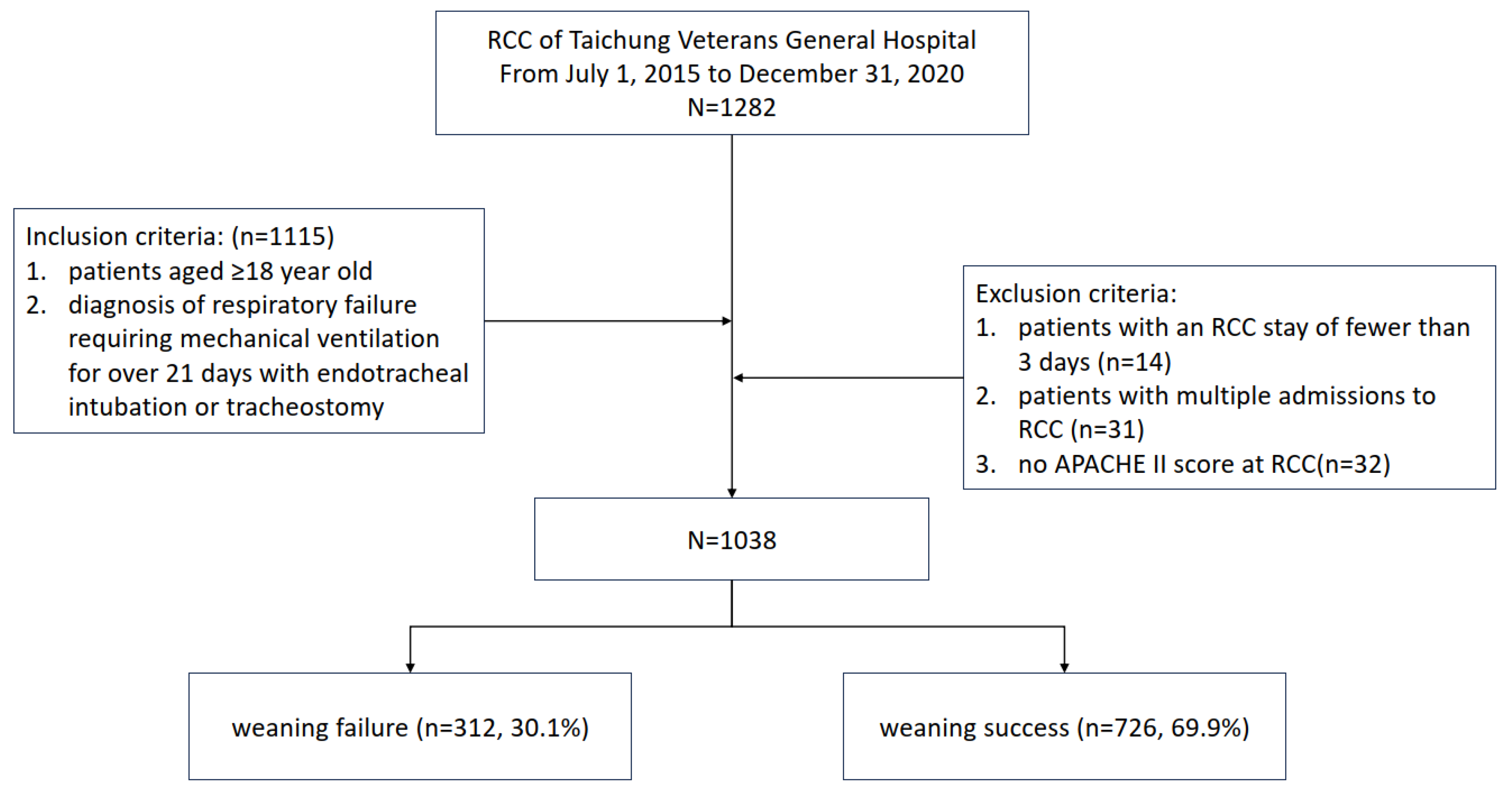
2.1. Study Design and Patient Enrollment
2.2. Data Collection and Outcome Measures
2.3. Statistical Analysis
3. Results
3.1. Clinicodemographic Characteristics of the Participants
3.2. Intergroup Differences between the Weaning-Failure and Weaning-Success Sub-Cohorts
3.3. Daily Calorie Intake in PMV Patients Stratified by Successful Weaning
3.4. Analysis of Factors Associated with Successful Weaning
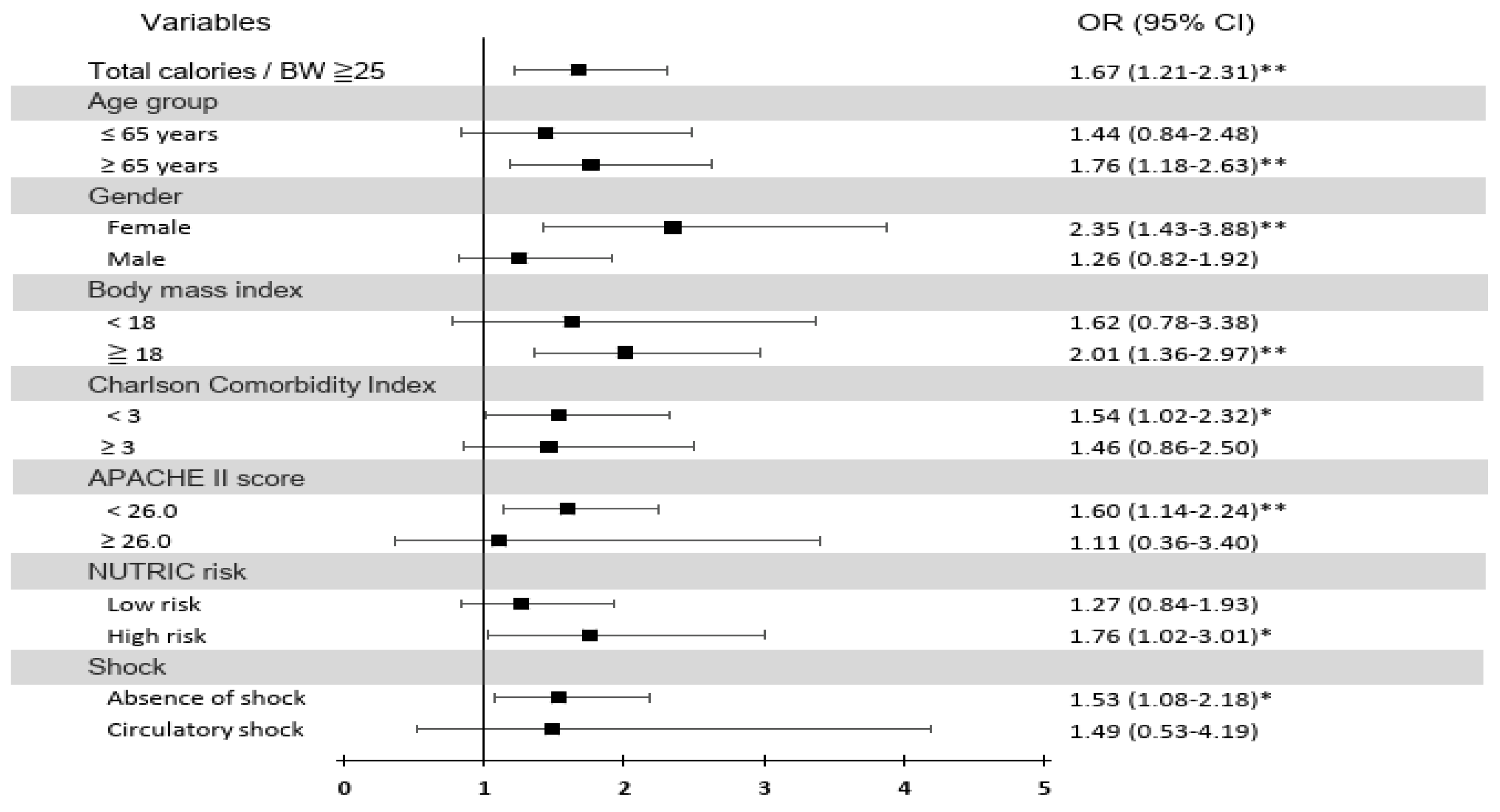
3.5. Stratified Analysis of the Association of the Initial 7-day Average Daily Total Calorie Intake/BW ≥25 kcal/kg/day with Successful Weaning
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Cox, C.E.; Carson, S.S.; Govert, J.A.; Chelluri, L.; Sanders, G.D. An economic evaluation of prolonged mechanical ventilation. Crit Care Med 2007, 35, 1918–1927. [Google Scholar] [CrossRef]
- Cox, C.E.; Carson, S.S.; Lindquist, J.H.; Olsen, M.K.; Govert, J.A.; Chelluri, L. Differences in one-year health outcomes and resource utilization by definition of prolonged mechanical ventilation: a prospective cohort study. Crit Care 2007, 11, R9. [Google Scholar] [CrossRef]
- Damuth, E.; Mitchell, J.A.; Bartock, J.L.; Roberts, B.W.; Trzeciak, S. Long-term survival of critically ill patients treated with prolonged mechanical ventilation: a systematic review and meta-analysis. Lancet Respir Med 2015, 3, 544–553. [Google Scholar] [CrossRef]
- Dettmer, M.R.; Damuth, E.; Zarbiv, S.; Mitchell, J.A.; Bartock, J.L.; Trzeciak, S. Prognostic Factors for Long-Term Mortality in Critically Ill Patients Treated With Prolonged Mechanical Ventilation: A Systematic Review. Crit Care Med 2017, 45, 69–74. [Google Scholar] [CrossRef]
- Ghiani, A.; Paderewska, J.; Sainis, A.; Crispin, A.; Walcher, S.; Neurohr, C. Variables predicting weaning outcome in prolonged mechanically ventilated tracheotomized patients: a retrospective study. J Intensive Care 2020, 8, 19. [Google Scholar] [CrossRef]
- Leonov, Y.; Kisil, I.; Perlov, A.; Stoichev, V.; Ginzburg, Y.; Nazarenko, A.; Gimelfarb, Y. Predictors of successful weaning in patients requiring extremely prolonged mechanical ventilation. Adv Respir Med 2020, 88, 477–484. [Google Scholar] [CrossRef]
- Arora, N.S.; Rochester, D.F. Respiratory muscle strength and maximal voluntary ventilation in undernourished patients. Am Rev Respir Dis 1982, 126, 5–8. [Google Scholar] [CrossRef]
- McWhirter, J.P.; Pennington, C.R. Incidence and recognition of malnutrition in hospital. Bmj 1994, 308, 945–948. [Google Scholar] [CrossRef]
- Huang, Y.C.; Yen, C.E.; Cheng, C.H.; Jih, K.S.; Kan, M.N. Nutritional status of mechanically ventilated critically ill patients: comparison of different types of nutritional support. Clin Nutr 2000, 19, 101–107. [Google Scholar] [CrossRef]
- Kan, M.N.; Chang, H.H.; Sheu, W.F.; Cheng, C.H.; Lee, B.J.; Huang, Y.C. Estimation of energy requirements for mechanically ventilated, critically ill patients using nutritional status. Crit Care 2003, 7, R108–R115. [Google Scholar] [CrossRef]
- Barr, J.; Hecht, M.; Flavin, K.E.; Khorana, A.; Gould, M.K. Outcomes in critically ill patients before and after the implementation of an evidence-based nutritional management protocol. Chest 2004, 125, 1446–1457. [Google Scholar] [CrossRef]
- Singer, P.; Blaser, A.R.; Berger, M.M.; Alhazzani, W.; Calder, P.C.; Casaer, M.P.; Hiesmayr, M.; Mayer, K.; Montejo, J.C.; Pichard, C.; et al. ESPEN guideline on clinical nutrition in the intensive care unit. Clin Nutr 2019, 38, 48–79. [Google Scholar] [CrossRef]
- Weijs, P.J.; Looijaard, W.G.; Beishuizen, A.; Girbes, A.R.; Oudemans-van Straaten, H.M. Early high protein intake is associated with low mortality and energy overfeeding with high mortality in non-septic mechanically ventilated critically ill patients. Crit Care 2014, 18, 701. [Google Scholar] [CrossRef]
- McClave, S.A.; Taylor, B.E.; Martindale, R.G.; Warren, M.M.; Johnson, D.R.; Braunschweig, C.; McCarthy, M.S.; Davanos, E.; Rice, T.W.; Cresci, G.A.; et al. Guidelines for the Provision and Assessment of Nutrition Support Therapy in the Adult Critically Ill Patient: Society of Critical Care Medicine (SCCM) and American Society for Parenteral and Enteral Nutrition (A.S.P.E.N.). JPEN J Parenter Enteral Nutr 2016, 40, 159–211. [Google Scholar] [CrossRef]
- Azoulay, E.; Vincent, J.L.; Angus, D.C.; Arabi, Y.M.; Brochard, L.; Brett, S.J.; Citerio, G.; Cook, D.J.; Curtis, J.R.; Dos Santos, C.C.; et al. Recovery after critical illness: putting the puzzle together-a consensus of 29. Crit Care 2017, 21, 296. [Google Scholar] [CrossRef]
- Herridge, M.S.; Chu, L.M.; Matte, A.; Tomlinson, G.; Chan, L.; Thomas, C.; Friedrich, J.O.; Mehta, S.; Lamontagne, F.; Levasseur, M.; et al. The RECOVER Program: Disability Risk Groups and 1-Year Outcome after 7 or More Days of Mechanical Ventilation. Am J Respir Crit Care Med 2016, 194, 831–844. [Google Scholar] [CrossRef]
- Reignier, J.; Boisrame-Helms, J.; Brisard, L.; Lascarrou, J.B.; Ait Hssain, A.; Anguel, N.; Argaud, L.; Asehnoune, K.; Asfar, P.; Bellec, F.; et al. Enteral versus parenteral early nutrition in ventilated adults with shock: a randomised, controlled, multicentre, open-label, parallel-group study (NUTRIREA-2). Lancet 2018, 391, 133–143. [Google Scholar] [CrossRef]
- Koekkoek, W.; van Setten, C.H.C.; Olthof, L.E.; Kars, J.; van Zanten, A.R.H. Timing of PROTein INtake and clinical outcomes of adult critically ill patients on prolonged mechanical VENTilation: The PROTINVENT retrospective study. Clin Nutr 2019, 38, 883–890. [Google Scholar] [CrossRef]
- Rahman, A.; Hasan, R.M.; Agarwala, R.; Martin, C.; Day, A.G.; Heyland, D.K. Identifying critically-ill patients who will benefit most from nutritional therapy: Further validation of the “modified NUTRIC” nutritional risk assessment tool. Clin Nutr 2016, 35, 158–162. [Google Scholar] [CrossRef]
- Knaus, W.A.; Draper, E.A.; Wagner, D.P.; Zimmerman, J.E. APACHE II: a severity of disease classification system. Crit Care Med 1985, 13, 818–829. [Google Scholar] [CrossRef]
- Vincent, J.L.; Moreno, R.; Takala, J.; Willatts, S.; De Mendonça, A.; Bruining, H.; Reinhart, C.K.; Suter, P.M.; Thijs, L.G. The SOFA (Sepsis-related Organ Failure Assessment) score to describe organ dysfunction/failure. On behalf of the Working Group on Sepsis-Related Problems of the European Society of Intensive Care Medicine. Intensive Care Med 1996, 22, 707–710. [Google Scholar] [CrossRef]
- Boles, J.M.; Bion, J.; Connors, A.; Herridge, M.; Marsh, B.; Melot, C.; Pearl, R.; Silverman, H.; Stanchina, M.; Vieillard-Baron, A.; et al. Weaning from mechanical ventilation. Eur Respir J 2007, 29, 1033–1056. [Google Scholar] [CrossRef]
- Mirtallo, J.M. Assessing the nutritional needs of the critically ill patient. DICP 1990, 24, S20–S23. [Google Scholar]
- Chao, D.C.; Scheinhorn, D.J.; Stearn-Hassenpflug, M. Impact of renal dysfunction on weaning from prolonged mechanical ventilation. Crit Care 1997, 1, 101–104. [Google Scholar] [CrossRef]
- Wu, Y.K.; Kao, K.C.; Hsu, K.H.; Hsieh, M.J.; Tsai, Y.H. Predictors of successful weaning from prolonged mechanical ventilation in Taiwan. Respir Med 2009, 103, 1189–1195. [Google Scholar] [CrossRef]
- Lo, S.C.; Ma, K.S.; Li, Y.R.; Li, Z.Y.; Lin, C.H.; Lin, H.C.; Yang, S.F. Nutritional support for successful weaning in patients undergoing prolonged mechanical ventilation. Sci Rep 2022, 12, 12044. [Google Scholar] [CrossRef]
- Sapijaszko, M.J.; Brant, R.; Sandham, D.; Berthiaume, Y. Nonrespiratory predictor of mechanical ventilation dependency in intensive care unit patients. Crit Care Med 1996, 24, 601–607. [Google Scholar] [CrossRef]
- Huang, S.W.; Lin, H.C.; Chou, Y.F.; Lin, T.Y.; Lo, C.Y.; Huang, H.Y.; Fang, Y.F.; Hsieh, M.H.; Lin, S.M.; Lo, Y.L.; et al. The Impact of Higher Protein Intake in Patients with Prolonged Mechanical Ventilation. Nutrients 2022, 14. [Google Scholar] [CrossRef]
- El Hadidy, S.; Saad, M.; El Hossany, R.; El Gohary, T.; El Ghobashy, M. Coinciding Changes in B Lines Patterns, Haemoglobin and Hematocrit Values Can Predict Outcomes of Weaning from Mechanical Ventilation. Open Access Maced J Med Sci 2019, 7, 4010–4014. [Google Scholar] [CrossRef]
- Chen-Roetling, J.; Ma, S.K.; Cao, Y.; Shah, A.; Regan, R.F. Hemopexin increases the neurotoxicity of hemoglobin when haptoglobin is absent. J Neurochem 2018, 145, 464–473. [Google Scholar] [CrossRef]
- Lai, Y.C.; Ruan, S.Y.; Huang, C.T.; Kuo, P.H.; Yu, C.J. Hemoglobin levels and weaning outcome of mechanical ventilation in difficult-to-wean patients: a retrospective cohort study. PLoS One 2013, 8, e73743. [Google Scholar] [CrossRef]
- Upadya, A.; Tilluckdharry, L.; Muralidharan, V.; Amoateng-Adjepong, Y.; Manthous, C.A. Fluid balance and weaning outcomes. Intensive Care Med 2005, 31, 1643–1647. [Google Scholar] [CrossRef]
- Frutos-Vivar, F.; Ferguson, N.D.; Esteban, A.; Epstein, S.K.; Arabi, Y.; Apezteguia, C.; Gonzalez, M.; Hill, N.S.; Nava, S.; D’Empaire, G.; et al. Risk factors for extubation failure in patients following a successful spontaneous breathing trial. Chest 2006, 130, 1664–1671. [Google Scholar] [CrossRef]
- Maezawa, S.; Kudo, D.; Miyagawa, N.; Yamanouchi, S.; Kushimoto, S. Association of Body Weight Change and Fluid Balance With Extubation Failure in Intensive Care Unit Patients: A Single-Center Observational Study. J Intensive Care Med 2021, 36, 175–181. [Google Scholar] [CrossRef]
- Messmer, A.S.; Zingg, C.; Muller, M.; Gerber, J.L.; Schefold, J.C.; Pfortmueller, C.A. Fluid Overload and Mortality in Adult Critical Care Patients-A Systematic Review and Meta-Analysis of Observational Studies. Crit Care Med 2020, 48, 1862–1870. [Google Scholar] [CrossRef]
- Wang, T.J.; Pai, K.C.; Huang, C.T.; Wong, L.T.; Wang, M.S.; Lai, C.M.; Chen, C.H.; Wu, C.L.; Chao, W.C. A Positive Fluid Balance in the First Week Was Associated With Increased Long-Term Mortality in Critically Ill Patients: A Retrospective Cohort Study. Front Med (Lausanne) 2022, 9, 727103. [Google Scholar] [CrossRef]
- Gungabissoon, U.; Hacquoil, K.; Bains, C.; Irizarry, M.; Dukes, G.; Williamson, R.; Deane, A.M.; Heyland, D.K. Prevalence, risk factors, clinical consequences, and treatment of enteral feed intolerance during critical illness. JPEN J Parenter Enteral Nutr 2015, 39, 441–448. [Google Scholar] [CrossRef]
- Chiang, C.Y.; Lan, C.C.; Yang, C.H.; Hou, Y.C. Investigating the differences in nutritional status between successfully weaned and unsuccessfully weaned respirator patients. Sci Rep 2023, 13, 7144. [Google Scholar] [CrossRef]
- Compher, C.; Bingham, A.L.; McCall, M.; Patel, J.; Rice, T.W.; Braunschweig, C.; McKeever, L. Guidelines for the provision of nutrition support therapy in the adult critically ill patient: The American Society for Parenteral and Enteral Nutrition. JPEN J Parenter Enteral Nutr 2022, 46, 12–41. [Google Scholar] [CrossRef]
- van Zanten, A.R.H.; De Waele, E.; Wischmeyer, P.E. Nutrition therapy and critical illness: practical guidance for the ICU, post-ICU, and long-term convalescence phases. Crit Care 2019, 23, 368. [Google Scholar] [CrossRef]
- Doley, J.; Mallampalli, A.; Sandberg, M. Nutrition management for the patient requiring prolonged mechanical ventilation. Nutr Clin Pract 2011, 26, 232–241. [Google Scholar] [CrossRef]
- Supinski, G.S.; Morris, P.E.; Dhar, S.; Callahan, L.A. Diaphragm Dysfunction in Critical Illness. Chest 2018, 153, 1040–1051. [Google Scholar] [CrossRef]
| All (n=1038) | successful weaning for 48 hrs | p-value | ||
|---|---|---|---|---|
| Weaning failure (n=312) | Weaning success (n=726) | |||
| Mean±SD | Mean±SD | Mean±SD | ||
| Basic characteristics | ||||
| Age, years | 68.51±16.01 | 71.04±15.06 | 67.42±16.30 | 0.001** |
| Sex-Male, n (%) | 595 (57.32%) | 187 (59.94%) | 408 (56.20%) | 0.264 |
| Charlson Comorbidity Index | 2.30±1.56 | 2.71±1.59 | 2.12±1.51 | <0.001** |
| Nutrition relevant variables | ||||
| Body mass index | 22.59±4.44 | 22.55±4.50 | 22.61±4.42 | 0.951 |
| mNUTRIC score(ICU) | 4.10±1.87 | 4.74±1.87 | 3.82±1.80 | <0.001** |
| Albumin (g/dL) | 3.03±0.47 | 2.89±0.46 | 3.09±0.46 | <0.001** |
| Hemoglobin (g/dL) | 9.86±1.45 | 9.37±1.41 | 10.07±1.42 | <0.001** |
| Disease severityand managements | ||||
| APACHE II score (in the RCC) | 18.05±5.51 | 19.85±5.86 | 17.27±5.16 | <0.001** |
| SOFA score on Day 1 | 4.85±2.54 | 5.37±2.84 | 4.63±2.37 | <0.001** |
| Presence of shock, n (%) | ||||
| Absence of shock, n (%) | 923 (88.92%) | 231 (74.04%) | 692 (95.32%) | |
| Norepinephrine-alone, n (%) | 82 (7.90%) | 55 (17.63%) | 27 (3.72%) | |
| Norepinephrine and other vasopressors, n (%) | 33 (3.18%) | 26 (8.33%) | 7 (0.96%) | |
| Renal replacement therapy (RRT), n (%) | 52 (5.01%) | 22 (7.05%) | 30 (4.13%) | 0.048* |
| Fluid balance, day 1-7 | 1167.79±2995.60 | 1768.93±3344.02 | 909.45±2795.74 | <0.001** |
| Calorie intake, days 1–7 (kcal/day) | 1151.35±324.33 | 1054.76±338.10 | 1192.85±309.33 | <0.001** |
| Total calories/BW, days 1–7 (kcal/kg/day) | 20.68±7.25 | 19.17±7.77 | 21.33±6.92 | <0.001** |
| Outcomes | ||||
| Duration of hospital stay, days | 57.87±47.21 | 61.61±68.16 | 56.27±34.44 | 0.096 |
| Ventilator-use duration, days | 41.16±21.15 | 52.78±25.80 | 36.17±16.47 | <0.001** |
| All (n=1038) | RCC successful weaning for 48 h | p-value | |||||
|---|---|---|---|---|---|---|---|
| Weaning failure (n=312) | Weaning success (n=726) | ||||||
| Mean±SD | Mean±SD | Mean±SD | |||||
| Total calories (kcal/day) | |||||||
| Day 1 | 1178.95±361.14 | 1117.01±369.86 | 1205.57±354.26 | <0.001** | |||
| Day 2 | 1174.71±362.98 | 1100.20±374.50 | 1206.73±353.38 | <0.001** | |||
| Day 3 | 1172.30±356.20 | 1084.94±371.50 | 1209.85±342.89 | <0.001** | |||
| Day 4 | 1156.08±374.54 | 1055.76±395.00 | 1199.20±357.11 | <0.001** | |||
| Day 5 | 1142.30±390.71 | 1036.26±425.54 | 1187.87±365.72 | <0.001** | |||
| Day 6 | 1135.44±414.54 | 994.85±454.96 | 1195.86±380.59 | <0.001** | |||
| Day 7 | 1099.64±440.15 | 994.33±473.09 | 1144.90±417.45 | <0.001** | |||
| Days 1–7 | 1151.35±324.33 | 1054.76±338.10 | 1192.85±309.33 | <0.001** | |||
| Enteral Nutrition | |||||||
| Day 1 | 1137.95±387.02 | 1059.90±406.42 | 1171.50±373.68 | <0.001** | |||
| Day 2 | 1135.42±389.69 | 1043.87±419.09 | 1174.77±369.74 | <0.001** | |||
| Day 3 | 1135.49±382.30 | 1032.00±409.63 | 1179.96±361.22 | <0.001** | |||
| Day 4 | 1119.67±397.82 | 1001.29±425.34 | 1170.54±374.36 | <0.001** | |||
| Day 5 | 1106.70±413.83 | 982.21±454.49 | 1160.20±383.17 | <0.001** | |||
| Day 6 | 1097.95±433.70 | 936.23±478.97 | 1167.46±393.11 | <0.001** | |||
| Day 7 | 1066.22±455.45 | 941.69±494.17 | 1119.74±427.08 | <0.001** | |||
| Days 1–7 | 1114.20±350.87 | 999.60±378.17 | 1163.45±326.59 | <0.001** | |||
| Parenteral Nutrition | |||||||
| Day 1 | 41.00±116.78 | 57.11±166.05 | 34.07±86.71 | 0.108 | |||
| Day 2 | 39.29±112.43 | 56.33±154.86 | 31.96±87.26 | 0.205 | |||
| Day 3 | 36.82±105.34 | 52.93±137.09 | 29.89±87.46 | 0.004** | |||
| Day 4 | 36.41±114.02 | 54.47±154.27 | 28.65±90.48 | 0.001** | |||
| Day 5 | 35.60±110.16 | 54.05±146.67 | 27.67±89.00 | 0.002** | |||
| Day 6 | 37.49±134.49 | 58.63±175.66 | 28.40±111.17 | 0.001** | |||
| Day 7 | 33.42±117.31 | 52.64±167.44 | 25.16±86.19 | 0.003** | |||
| Days 1–7 | 37.15±95.15 | 55.17±123.72 | 29.40±78.63 | <0.001** | |||
| Characteristics | Univariate | Multivariate | ||||
|---|---|---|---|---|---|---|
| OR (95% CI) | p-value | OR (95% CI) | p-value | |||
| Age, per year increment | 0.99 (0.98–0.99) | 0.001** | 0.99 (0.98–1.01) | 0.341 | ||
| Sex (male) | 0.86 (0.65–1.12) | 0.264 | ||||
| CCI, per 1 score increment | 0.79 (0.72–0.86) | <0.001** | 0.91 (0.82–1.00) | 0.060 | ||
| BMI, per 1 kg/m2 increment | 1.00 (0.97–1.03) | 0.833 | ||||
| NUTRIC score, high risk (≥5) | 0.43 (0.33–0.57) | <0.001** | 0.76 (0.49–1.17) | 0.210 | ||
| Albumin, per 1 g/dL increment | 2.63 (1.94–3.56) | <0.001** | 1.93 (1.35–2.75) | <0.001** | ||
| Hemoglobin, per 1 g/dL increment | 1.44 (1.30–1.60) | <0.001** | 1.20 (1.07–1.36) | 0.003** | ||
| APACHE II score, per 1 score increment | 0.92 (0.90–0.94) | <0.001** | 0.99 (0.95–1.03) | 0.596 | ||
| Absence of shock | 1.00 | |||||
| Norepinephrine-alone | 0.16 (0.10–0.27) | <0.001** | 0.27 (0.16–0.46) | <0.001** | ||
| Norepinephrine and other vasopressors | 0.09 (0.04–0.21) | <0.001** | 0.15 (0.06–0.37) | <0.001** | ||
| RRT during RCC | 0.57 (0.32–1.00) | 0.051 | ||||
| Fluid balance, days 1–7, per liter | 0.91 (0.86–0.95) | <0.001** | 0.87 (0.83–0.92) | <0.001** | ||
| Calories intake, days 1–7, per kcal | 1.21 (1.14–1.29) | <0.001** | ||||
| Total calories/BW ≥25 kcal/kg/day (days 1–7) | 1.67 (1.21–2.31) | 0.002** | 1.50 (1.05–2.15) | 0.026* | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).




