Submitted:
30 September 2024
Posted:
30 September 2024
You are already at the latest version
Abstract
Keywords:
1. Introduction
1.1. WFS1: Patterns of Expression in Brain Development
1.2. WFS1: Key Regulator of ER Stress and Calcium Homeostasis
1.3. WFS1 Dysfunction: A Link between WS1, AD, and Sleep Disorders
1.4. ER Stress and the Unfolded Protein Response Alterations
1.5. Calcium Homeostasis and Synaptic Dysfunction
1.6. Mitochondrial Dysfunction and Dysregulated ER-Mitochondria Communication
1.7. Neuroinflammation and Oxidative Stress
1.8. Dysregulation of Autophagy and Protein Clearance
1.9. Altered Aβ Production and WFS1
1.10.WFS1, Tau Phosphorylation, and Neurofibrillary Tangles
1.11. Altered Synaptic Plasticity and Cognitive Decline
1.12. The Role of WFS1 in Sleep Regulation: Mechanisms and Implications for Neurodegenerative and Psychiatric Disorders
Dopaminergic Neurons and Sleep Regulation
1.13. Calcium Homeostasis and ER Stress in the Sleep Regulation
1.13.1. WFS1 and Sleep Apnea
1.13.2. WFS1 in Neuropsychiatric Sleep Disorders
2. Conclusions
Author Contributions
Funding
Informed Consent Statement
Conflicts of Interest
References
- Inoue, H.; Tanizawa, Y.; Wasson, J.; Behn, P.; Kalidas, K.; Bernal-Mizrachi, E.; Mueckler, M.; Marshall, H.; Donis-Keller, H.; Crock, P.; et al. A gene encoding a transmembrane protein is mutated in patients with diabetes mellitus and optic atrophy (Wolfram syndrome). Nat. Genet. 1998, 20, 143–148. [Google Scholar] [CrossRef] [PubMed]
- Strom, T.M.; Hörtnagel, K.; Hofmann, S.; Gekeler, F.; Scharfe, C.; Rabl, W.; Gerbitz, K.D.; Meitinger, T. Diabetes insipidus, diabetes mellitus, optic atrophy and deafness (DIDMOAD) caused by mutations in a novel gene (wolframin) coding for a predicted transmembrane protein. Hum. Mol. Genet. 1998, 7, 2021–2028. [Google Scholar] [CrossRef] [PubMed]
- Delpech, J.C.; Pathak, D.; Varghese, M.; et al. Wolframin-1-expressing neurons in the entorhinal cortex propagate tau to CA1 neurons and impair hippocampal memory in mice. Sci. Transl. Med. 2021, 13, eabe8455. [Google Scholar] [CrossRef] [PubMed]
- Hao, H.; Song, L.; Zhang, L. Wolfram syndrome 1 regulates sleep in dopamine receptor neurons by modulating calcium homeostasis. PLoS Genet. 2023, 19, e1010827. [Google Scholar] [CrossRef] [PubMed]
- Callens, M.; Loncke, J.; Bultynck, G. Dysregulated Ca2+ homeostasis as a central theme in neurodegeneration: Lessons from Alzheimer's disease and Wolfram syndrome. Cells 2022, 11, 1963. [Google Scholar] [CrossRef]
- Caruso, V.; Raia, A.; Rigoli, L. Wolfram Syndrome 1: A neuropsychiatric perspective on a rare disease. Genes 2024, 15, 984. [Google Scholar] [CrossRef]
- Rigoli, L.; Caruso, V.; Salzano, G.; Lombardo, F. Wolfram syndrome 1: From genetics to therapy. Int. J. Environ. Res. Public Health 2022, 19, 3225. [Google Scholar] [CrossRef]
- Barrett, T.G.; Poulton, K.; Bundey, S. DIDMOAD syndrome; further studies and muscle biochemistry. J. Inherit. Metab. Dis. 1995, 18, 218–220. [Google Scholar] [CrossRef]
- Rohayem, J.; Ehlers, C.; Wiedemann, B.; et al. Diabetes and neurodegeneration in Wolfram syndrome: A multicenter study of phenotype and genotype. Diabetes Care 2011, 34, 1503–1510. [Google Scholar] [CrossRef]
- Chaussenot, A.; Bannwarth, S.; Rouzier, C.; et al. Neurologic features and genotype-phenotype correlation in Wolfram syndrome. Ann. Neurol. 2011, 69, 501–508. [Google Scholar] [CrossRef]
- Scolding, N.J.; Kellar-Wood, H.F.; Shaw, C.; Shneerson, J.M.; Antoun, N. Wolfram syndrome: hereditary diabetes mellitus with brainstem and optic atrophy. Ann. Neurol. 1996, 39, 352–360. [Google Scholar] [CrossRef] [PubMed]
- Licis, A.; Davis, G.; Eisenstein, S.A.; Lugar, H.M.; Hershey, T. Sleep disturbances in Wolfram syndrome. Orphanet J. Rare Dis. 2019, 14, 188. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Venkataraman, L.; Chen, S.; Fu, H. Function of WFS1 and WFS2 in the Central Nervous System: Implications for Wolfram Syndrome and Alzheimer's disease. Neurosci. Biobehav. Rev. 2020, 118, 775–783. [Google Scholar] [CrossRef] [PubMed]
- Munshani, S.; Ibrahim, E.Y.; Domenicano, I.; Ehrlich, B.E. The Impact of Mutations in Wolframin on Psychiatric Disorders. Front. Pediatr. 2021, 9, 718132. [Google Scholar] [CrossRef] [PubMed]
- Blackman, J.; Swirski, M.; Clynes, J.; Harding, S.; Leng, Y.; Coulthard, E. Pharmacological and non-pharmacological interventions to enhance sleep in mild cognitive impairment and mild Alzheimer's disease: A systematic review. J. Sleep Res. 2021, 30, e13229. [Google Scholar] [CrossRef]
- Tombini, M.; Boscarino, M.; Di Lazzaro, V. Tackling seizures in patients with Alzheimer's disease. Expert Rev. Neurother. 2023, 23, 1131–1145. [Google Scholar] [CrossRef]
- Cacabelos, R.; Carril, J.C.; Corzo, L.; et al. Pharmacogenetics of anxiety and depression in Alzheimer's disease. Pharmacogenomics 2023, 24, 27–57. [Google Scholar] [CrossRef]
- Huang, Y.Y.; Gan, Y.H.; Yang, L.; Cheng, W.; Yu, J.T. Depression in Alzheimer's Disease: Epidemiology, Mechanisms, and Treatment. Biol. Psychiatry 2024, 95, 992–1005. [Google Scholar] [CrossRef]
- Prodhan, A.H.M.S.U.; Cavestro, C.; Kamal, M.A.; Islam, M.A. Melatonin and Sleep Disturbances in Alzheimer's Disease. CNS Neurol. Disord. Drug Targets 2021, 20, 736–754. [Google Scholar] [CrossRef]
- Swift, M.; Swift, R.G. Psychiatric disorders and mutations at the Wolfram syndrome locus. Biol. Psychiatry 2000, 47, 787–793. [Google Scholar] [CrossRef]
- Mühlbauer, V.; Möhler, R.; Dichter, M.N.; Zuidema, S.U.; Köpke, S.; Luijendijk, H.J. Antipsychotics for agitation and psychosis in people with Alzheimer's disease and vascular dementia. Cochrane Database Syst. Rev. 2021, 12, CD013304. [Google Scholar] [CrossRef] [PubMed]
- Abbott, S.M.; Videnovic, A. Chronic sleep disturbance and neural injury: links to neurodegenerative disease. Nat. Sci. Sleep 2016, 8, 55–61. [Google Scholar] [CrossRef] [PubMed]
- Hofmann, S.; Philbrook, C.; Gerbitz, K.D.; Bauer, M.F. Wolfram syndrome: structural and functional analyses of mutant and wild-type wolframin, the WFS1 gene product. Hum. Mol. Genet. 2003, 12, 2003–2012. [Google Scholar] [CrossRef] [PubMed]
- Luuk, H.; Koks, S.; Plaas, M.; Hannibal, J.; Rehfeld, J.F.; Vasar, E. Distribution of Wfs1 protein in the central nervous system of the mouse and its relation to clinical symptoms of the Wolfram syndrome. J. Comp. Neurol. 2008, 509, 642–660. [Google Scholar] [CrossRef]
- Swift, R.G.; Polymeropoulos, M.H.; Torres, R.; Swift, M. Predisposition of Wolfram syndrome heterozygotes to psychiatric illness. Mol. Psychiatry 1998, 3, 86–91. [Google Scholar] [CrossRef]
- Fonseca, S.G.; Ishigaki, S.; Oslowski, C.M.; Lu, S.; Lipson, K.L.; Ghosh, R.; Hayashi, E.; Ishihara, H.; Oka, Y.; Permutt, M.A.; Urano, F. Wolfram syndrome 1 gene negatively regulates ER stress signaling in rodent and human cells. J. Clin. Invest. 2010, 120, 744–755. [Google Scholar] [CrossRef]
- De Falco, M.; Manente, L.; Lucariello, A.; Baldi, G.; Fiore, P.; Laforgia, V.; Baldi, A.; Iannaccone, A.; De Luca, A. Localization and distribution of wolframin in human tissues. Front. Biosci. 2012, 4, 1986–1998. [Google Scholar] [CrossRef]
- Tekko, T.; Lilleväli, K.; Luuk, H.; Sütt, S.; Truu, L.; Örd, T.; Möls, M.; Vasar, E. Initiation and developmental dynamics of Wfs1 expression in the context of neural differentiation and ER stress in mouse forebrain. Int. J. Dev. Neurosci. 2014, 35, 80–88. [Google Scholar] [CrossRef]
- Abramov, A.Y. The brain—from neurodevelopment to neurodegeneration. FEBS J. 2022, 289, 2010–2012. [Google Scholar] [CrossRef]
- Kawano, J.; Fujinaga, R.; Yamamoto-Hanada, K.; Oka, Y.; Tanizawa, Y.; Shinoda, K. Wolfram syndrome 1 (Wfs1) mRNA expression in the normal mouse brain during postnatal development. Neurosci. Res. 2009, 64, 213–230. [Google Scholar] [CrossRef]
- Sakakibara, Y.; Sekiya, M.; Fujisaki, N.; Quan, X.; Iijima, K.M. Knockdown of wfs1, a fly homolog of Wolfram syndrome 1, in the nervous system increases susceptibility to age- and stress-induced neuronal dysfunction and degeneration in Drosophila. PLoS Genet. 2018, 14, e1007196. [Google Scholar] [CrossRef] [PubMed]
- Yamada, T.; Ishihara, H.; Tamura, A.; Takahashi, R.; Yamaguchi, S.; Takei, D.; Tokita, A.; Satake, C.; Tashiro, F.; Katagiri, H.; Aburatani, H.; Miyazaki, J.; Oka, Y. WFS1-deficiency increases endoplasmic reticulum stress, impairs cell cycle progression and triggers the apoptotic pathway specifically in pancreatic beta-cells. Hum. Mol. Genet. 2006, 15, 1600–1609. [Google Scholar] [CrossRef] [PubMed]
- Rigoli, L.; Lombardo, F.; Di Bella, C. Wolfram syndrome and WFS1 gene. Clin. Genet. 2011, 79, 103–117. [Google Scholar] [CrossRef] [PubMed]
- Lipson, K.L.; Ghosh, R.; Urano, F. The role of IRE1alpha in the degradation of insulin mRNA in pancreatic beta-cells. PLoS One 2008, 3, e1648. [Google Scholar] [CrossRef] [PubMed]
- So, J.S. Roles of endoplasmic reticulum stress in immune responses. Mol. Cells 2018, 41, 705–716. [Google Scholar] [CrossRef]
- Gardner, B.M.; Walter, P. Unfolded proteins are Ire1-activating ligands that directly induce the unfolded protein response. Science 2011, 333, 1891–1894. [Google Scholar] [CrossRef]
- Eratne, D.; Loi, S.M.; Farrand, S.; Kelso, W.; Velakoulis, D.; Looi, J.C. Alzheimer's disease: clinical update on epidemiology, pathophysiology and diagnosis. Australas. Psychiatry 2018, 26, 347–357. [Google Scholar] [CrossRef]
- Ogbodo, J.O.; Agbo, C.P.; Njoku, U.O.; Ogugofor, M.O.; Egba, S.I.; Ihim, S.A.; Echezona, A.C.; Brendan, K.C.; Upaganlawar, A.B.; Upasani, C.D. Alzheimer's disease: Pathogenesis and therapeutic interventions. Curr. Aging Sci. 2022, 15, 2–25. [Google Scholar] [CrossRef]
- Murphy, M.P.; LeVine, H. III. Alzheimer's disease and the amyloid-beta peptide. J. Alzheimers Dis. 2010, 19, 311–323. [Google Scholar] [CrossRef]
- Uddin, M.S.; Tewari, D.; Sharma, G.; et al. Molecular mechanisms of ER stress and UPR in the pathogenesis of Alzheimer's disease. Mol. Neurobiol. 2020, 57, 2902–2919. [Google Scholar] [CrossRef]
- Ekundayo, B.E.; Obafemi, T.O.; Adewale, O.B.; Obafemi, B.A.; Oyinloye, B.E.; Ekundayo, S.K. Oxidative stress, endoplasmic reticulum stress and apoptosis in the pathology of Alzheimer's disease. Cell Biochem. Biophys. 2024, 82, 457–477. [Google Scholar] [CrossRef] [PubMed]
- Ho, Y.S.; Yang, X.; Lau, J.C.; et al. Endoplasmic reticulum stress induces tau pathology and forms a vicious cycle: implication in Alzheimer's disease pathogenesis. J. Alzheimers Dis. 2012, 28, 839–854. [Google Scholar] [CrossRef] [PubMed]
- Princen, K.; Van Dooren, T.; van Gorsel, M.; et al. Pharmacological modulation of septins restores calcium homeostasis and is neuroprotective in models of Alzheimer's disease. Science 2024, 384, eadd6260. [Google Scholar] [CrossRef]
- Tönnies, E.; Trushina, E. Oxidative stress, synaptic dysfunction, and Alzheimer's disease. J. Alzheimers Dis. 2017, 57, 1105–1121. [Google Scholar] [CrossRef]
- Ryan, K.C.; Ashkavand, Z.; Norman, K.R. The role of mitochondrial calcium homeostasis in Alzheimer's and related diseases. Int. J. Mol. Sci. 2020, 21, 9153. [Google Scholar] [CrossRef]
- Esteras, N.; Abramov, A.Y. Mitochondrial calcium deregulation in the mechanism of beta-amyloid and tau pathology. Cells 2020, 9, 2135. [Google Scholar] [CrossRef]
- Britti, E.; Ros, J.; Esteras, N.; Abramov, A.Y. Tau inhibits mitochondrial calcium efflux and makes neurons vulnerable to calcium-induced cell death. Cell Calcium 2020, 86, 102150. [Google Scholar] [CrossRef]
- Chen, S.; Acosta, D.; Li, L.; et al. Wolframin is a novel regulator of tau pathology and neurodegeneration. Acta Neuropathol. 2022, 143, 547–569. [Google Scholar] [CrossRef]
- LaFerla, F.M. Calcium dyshomeostasis and intracellular signalling in Alzheimer's disease. Nat. Rev. Neurosci. 2002, 3, 862–872. [Google Scholar] [CrossRef]
- Liiv, M.; Vaarmann, A.; Safiulina, D.; et al. ER calcium depletion as a key driver for impaired ER-to-mitochondria calcium transfer and mitochondrial dysfunction in Wolfram syndrome. Nat. Commun. 2024, 15, 6143. [Google Scholar] [CrossRef]
- Ajoolabady, A.; Lindholm, D.; Ren, J.; Pratico, D. ER stress and UPR in Alzheimer's disease: mechanisms, pathogenesis, treatments. Cell Death Dis. 2022, 13, 706. [Google Scholar] [CrossRef] [PubMed]
- Crouzier, L.; Danese, A.; Yasui, Y.; et al. Activation of the sigma-1 receptor chaperone alleviates symptoms of Wolfram syndrome in preclinical models. Sci. Transl. Med. 2022, 14, eabh3763. [Google Scholar] [CrossRef] [PubMed]
- Kaarniranta, K.; Ojala, J. ER stress in Alzheimer's disease: a novel neuronal trigger for inflammation and Alzheimer's pathology. J. Neuroinflammation 2009, 6, 41. [Google Scholar] [CrossRef]
- Thakur, S.; Dhapola, R.; Sarma, P.; Medhi, B.; Reddy, D.H. Neuroinflammation in Alzheimer's disease: Current progress in molecular signaling and therapeutics. Inflammation 2023, 46, 1–17. [Google Scholar] [CrossRef]
- Dela Cruz, C.S.; Kang, M.J. Mitochondrial dysfunction and damage associated molecular patterns (DAMPs) in chronic inflammatory diseases. Mitochondrion 2018, 41, 37–44. [Google Scholar] [CrossRef]
- Zhang, Z.; Yang, X.; Song, Y.Q.; Tu, J. Autophagy in Alzheimer's disease pathogenesis: Therapeutic potential and future perspectives. Ageing Res. Rev. 2021, 72, 101464. [Google Scholar] [CrossRef]
- Reddy, P.H.; Oliver, D.M. Amyloid beta and phosphorylated tau-induced defective autophagy and mitophagy in Alzheimer's disease. Cells 2019, 8, 488. [Google Scholar] [CrossRef]
- Kim, Y.C.; Guan, K.L. mTOR: a pharmacologic target for autophagy regulation. J. Clin. Invest. 2015, 125, 25–32. [Google Scholar] [CrossRef]
- Cao, W.; Li, J.; Yang, K.; Cao, D. An overview of autophagy: Mechanism, regulation and research progress. Bull. Cancer 2021, 108, 304–322. [Google Scholar] [CrossRef]
- Hampel, H.; Vassar, R.; De Strooper, B.; et al. The β-secretase BACE1 in Alzheimer's disease. Biol. Psychiatry 2021, 89, 745–756. [Google Scholar] [CrossRef]
- Cho, Y.; Bae, H.G.; Okun, E.; Arumugam, T.V.; Jo, D.G. Physiology and pharmacology of amyloid precursor protein. Pharmacol. Ther. 2022, 235, 108122. [Google Scholar] [CrossRef] [PubMed]
- Jiang, H.; Jayadev, S.; Lardelli, M.; Newman, M. A review of the familial Alzheimer's disease locus PRESENILIN 2 and its relationship to PRESENILIN 1. J. Alzheimers Dis. 2018, 66, 1323–1339. [Google Scholar] [CrossRef] [PubMed]
- Han, J.; Park, H.; Maharana, C.; et al. Alzheimer's disease-causing presenilin-1 mutations have deleterious effects on mitochondrial function. Theranostics 2021, 11, 8855–8873. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Islam, S.; Michikawa, M.; Zou, K. Presenilin: A multi-functional molecule in the pathogenesis of Alzheimer's disease and other neurodegenerative diseases. Int. J. Mol. Sci. 2024, 25, 1757. [Google Scholar] [CrossRef]
- Sasaguri, H.; Hashimoto, S.; Watamura, N.; et al. Recent advances in the modeling of Alzheimer's disease. Front. Neurosci. 2022, 16, 807473. [Google Scholar] [CrossRef] [PubMed]
- Busche, M.A.; Hyman, B.T. Synergy between amyloid-β and tau in Alzheimer's disease. Nat. Neurosci. 2020, 23, 1183–1193. [Google Scholar] [CrossRef]
- Muyllaert, D.; Terwel, D.; Borghgraef, P.; Devijver, H.; Dewachter, I.; Van Leuven, F. Transgenic mouse models for Alzheimer's disease: the role of GSK-3B in combined amyloid and tau-pathology. Rev. Neurol. (Paris) 2006, 162, 903–907. [Google Scholar] [CrossRef]
- Sayehmiri, F.; Motamedi, F.; Batool, Z.; et al. Mitochondrial plasticity and synaptic plasticity crosstalk; in health and Alzheimer's disease. CNS Neurosci. Ther. 2024, 30, e14897. [Google Scholar] [CrossRef]
- Hayashi, Y. Molecular mechanism of hippocampal long-term potentiation: Towards multiscale understanding of learning and memory. Neurosci. Res. 2022, 175, 3–15. [Google Scholar] [CrossRef]
- Castell, L.; Le Gall, V.; Cutando, L.; et al. Dopamine D2 receptors in WFS1-neurons regulate food-seeking and avoidance behaviors. Prog. Neuropsychopharmacol. Biol. Psychiatry 2024, 129, 110883. [Google Scholar] [CrossRef]
- Tekko, T.; Lakspere, T.; Allikalt, A.; et al. Wfs1 is expressed in dopaminoceptive regions of the amniote brain and modulates levels of D1-like receptors. PLoS One 2017, 12, e0172825. [Google Scholar] [CrossRef] [PubMed]
- Goto, A. Synaptic plasticity during systems memory consolidation. Neurosci. Res. 2022, 183, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Yamada, M.; Yasuda, T.; Niwa, F.; Inoue, Y.; Takano, H. WFS1 protein modulates synaptic plasticity through the regulation of endoplasmic reticulum stress signaling. Mol. Brain Res. 2006, 144, 123–133. [Google Scholar] [CrossRef]
- Vyazovskiy, V.V.; Harris, K.D. Sleep and the single neuron: the role of global slow oscillations in individual cell rest. Nat. Rev. Neurosci. 2013, 14, 443–451. [Google Scholar] [CrossRef]
- Phillips, B.; Clark, J.; Martineau, É.; Rungta, R.L. Orai, RyR, and IP3R channels cooperatively regulate calcium signaling in brain mid-capillary pericytes. Commun. Biol. 2023, 6, 493. [Google Scholar] [CrossRef]
- Luuk, H.; Fahrenkrug, J.; Hannibal, J. Circadian rhythms and food anticipatory behavior in Wfs1-deficient mice. Biochem. Biophys. Res. Commun. 2012, 424, 717–723. [Google Scholar] [CrossRef]
- Harris, J.C.; Kenkare, J.D.; Schramm, C.M. An adolescent with Wolfram syndrome and central sleep apnea. J. Clin. Sleep Med. 2024, 20, 1205–1208. [Google Scholar] [CrossRef]
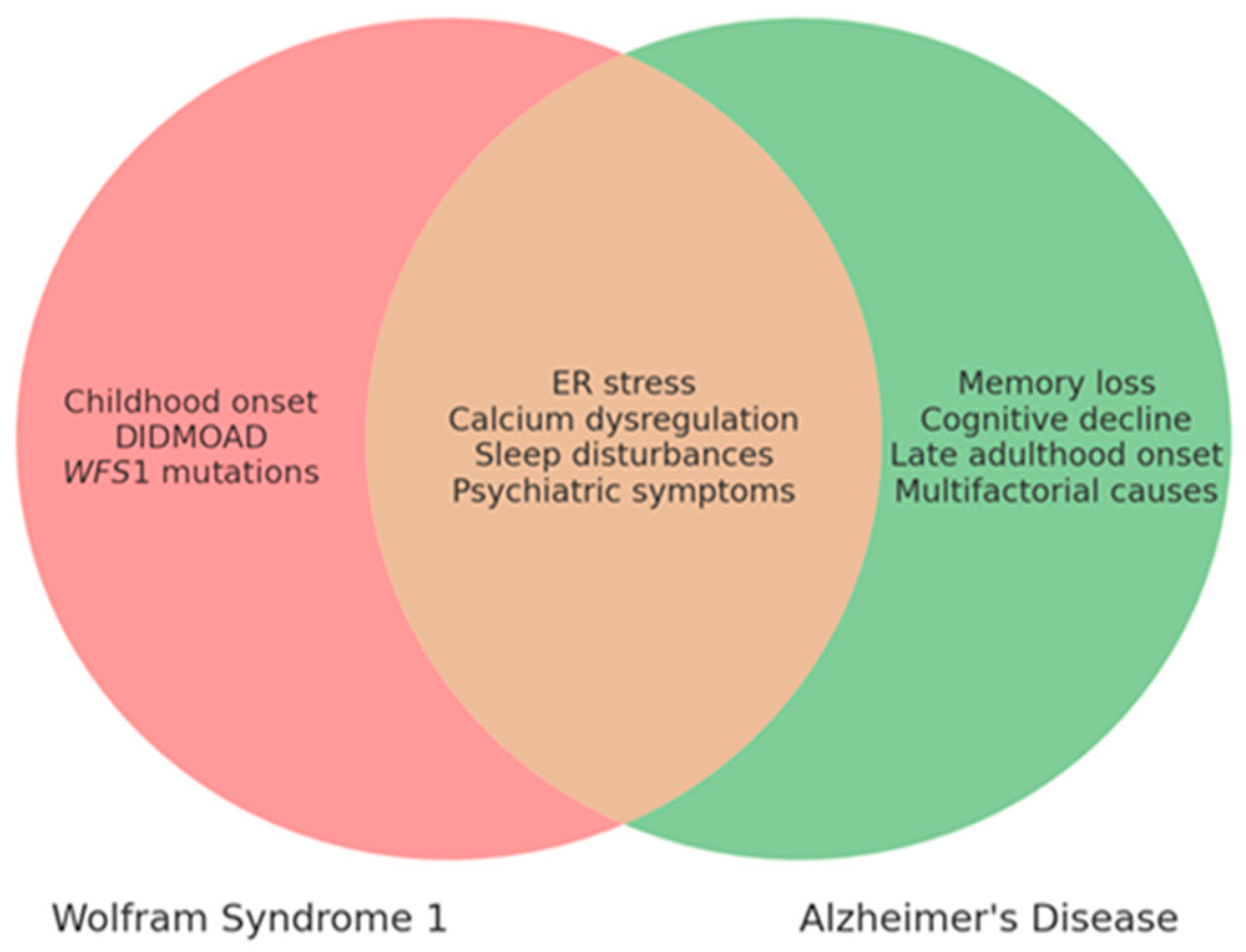
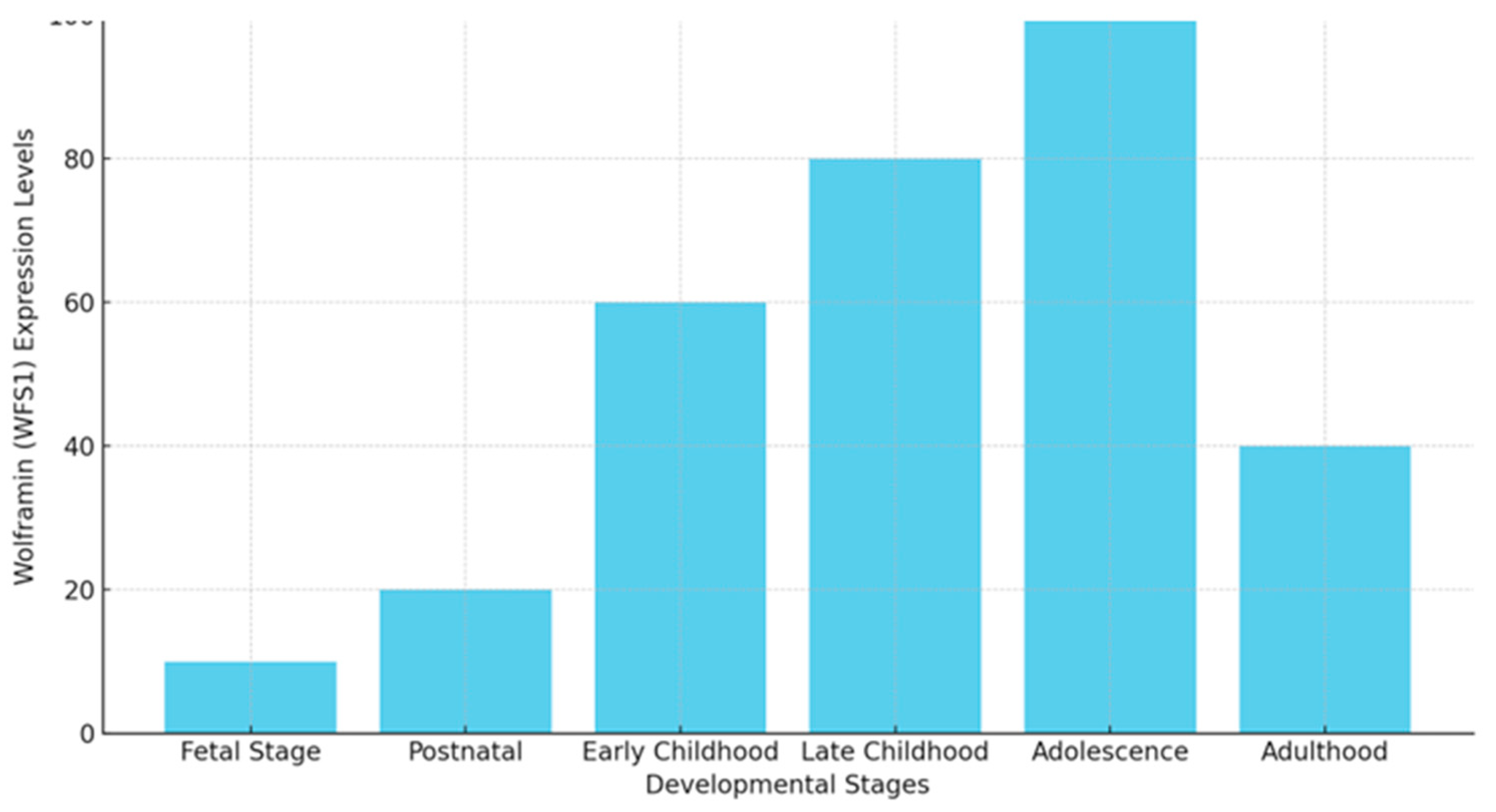
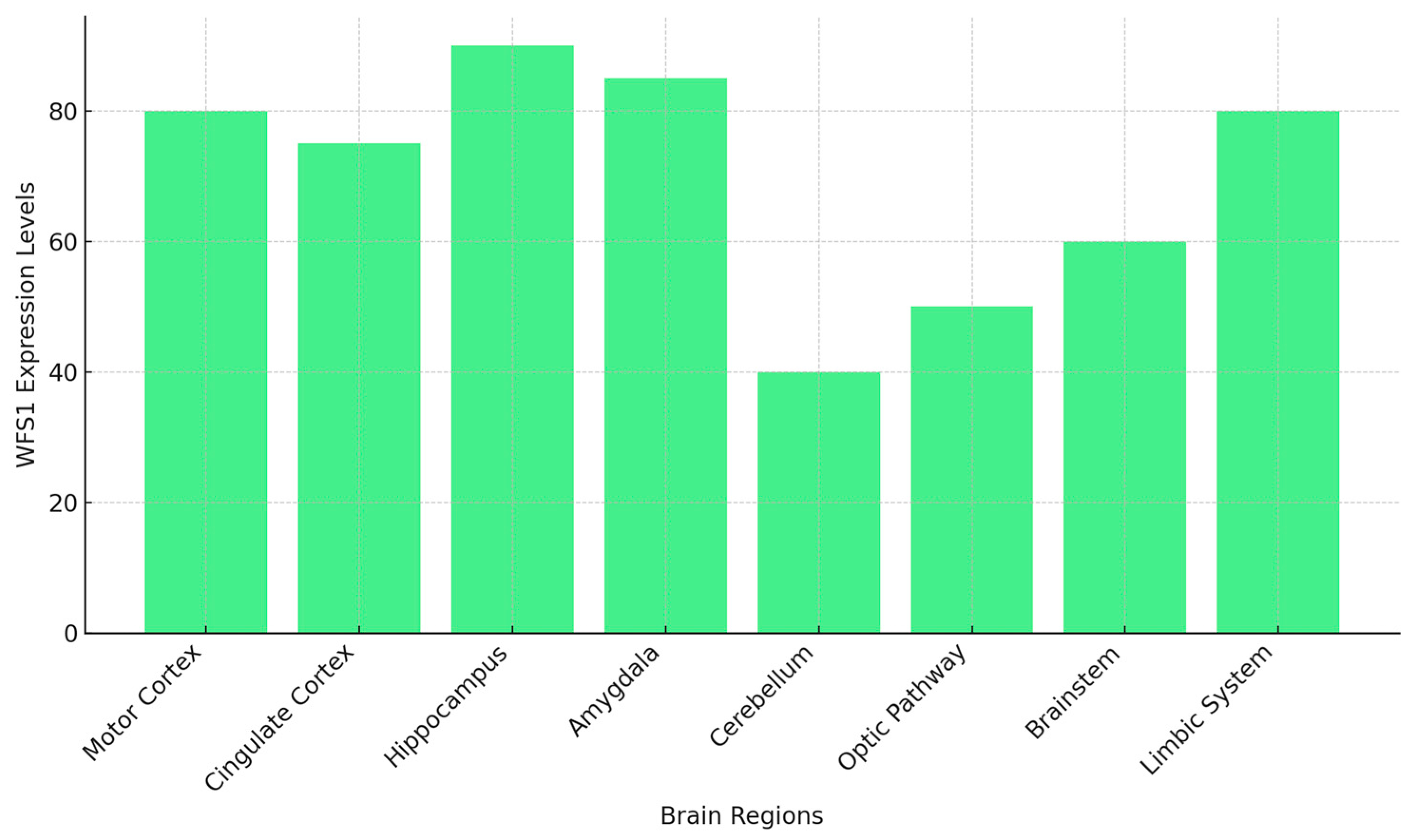
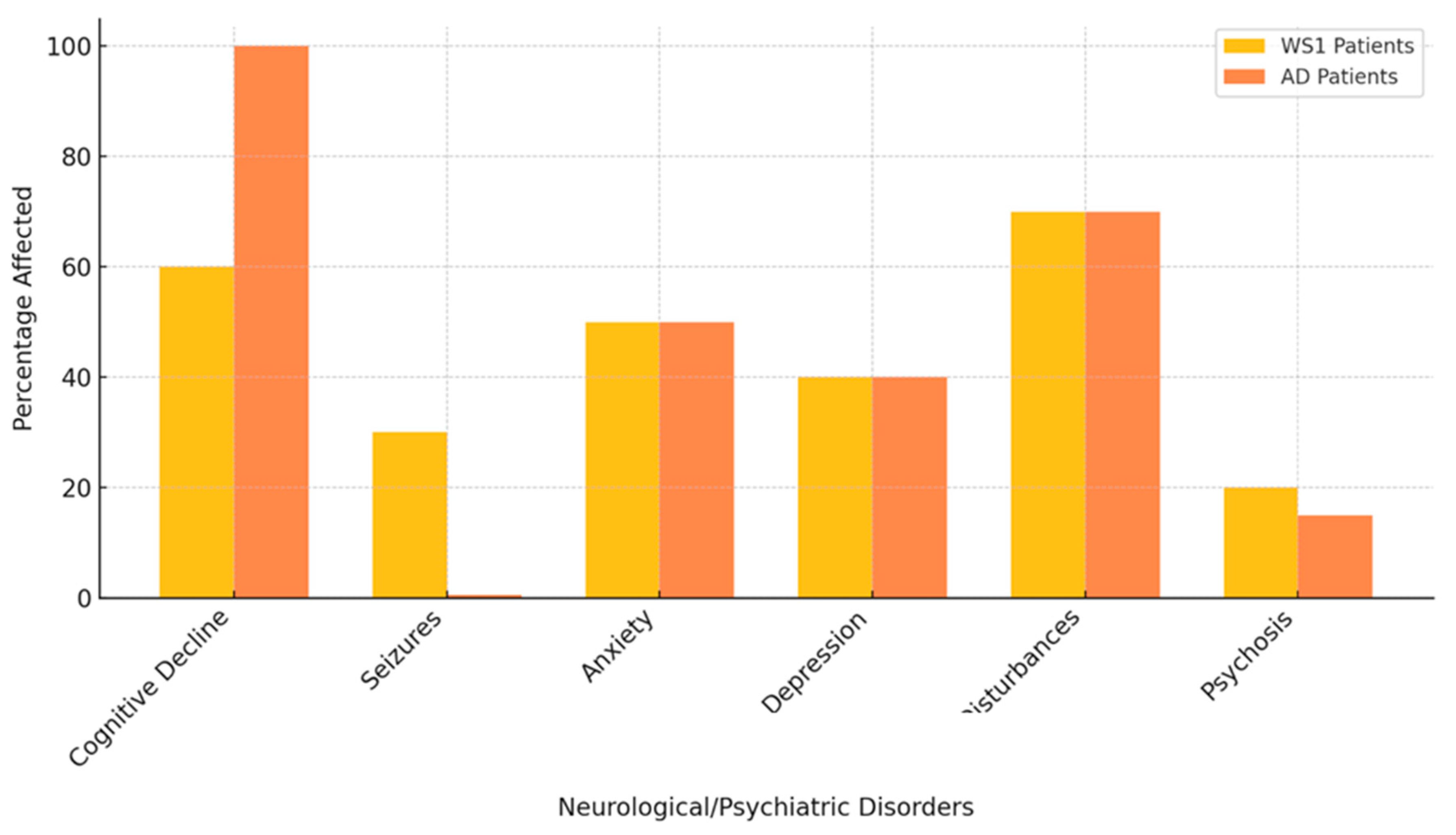
| Mechanism | WS1 | AD |
|---|---|---|
| ER stress | Chronic ER stress, apoptosis | Excessive UPR, neuronal death |
| Calcium dysregulation | Dysregulated calcium signaling | Calcium influx, excitotoxicity |
| Neurodegeneration | Neuronal death in brainstem, cerebellum | Synaptic dysfunction, brain atrophy |
| Aβ and Tau accumulation | Absent | Present (Aβ plaques, tau tangles) |
| Clinical Feature | WS1 | AD |
|---|---|---|
| Age of Onset | Childhood to adolescence | Late adulthood (65+) |
| Main Symptoms | DIDMOAD, neuropsychiatric disorders, urinary/endocrine issues | Memory loss, cognitive decline, behavioral changes |
| Neurodegeneration | Optic pathway, cerebellum, brainstem alterations | Hippocampus, cortex |
| ER Stress Involvement | Yes | Yes |
| Calcium Dysregulation | Yes | Yes |
| Sleep Disturbances | Common (up to 70%) | Common |
| Psychiatric Symptoms | Anxiety, depression, seizures | Depression, anxiety, agitation |
| Genetic Cause | WFS1 gene mutation | Multifactorial (genetic/environmental) |
| Neurological/Psychiatric Disorders | Percentage of WS1 Patients Affected | References (WS1) | Percentage of AD Patients Affected | References (AD) |
|---|---|---|---|---|
| Cognitive Decline | 60% | Barrett et al [8]; Rohayem et al 2011 [9] | 100% | Huang et al [17] |
| Seizures | 30% | Chaussenot et al. [10] | Rare | Prodhan AHMSU et al [18] |
| Anxiety | 50% | Barrett et al [8] | 40-60% | Cacabelos et al [16] |
| Depression | 40% | Scolding et al [12] | 40-50% | Huang et al [17] |
| Sleep Disturbances | 70% | Munshani et al [13] | 60-70% | Prodhan AHMSU [18] |
| Psychosis | 20% | Swift et al [19] | 10-15% | Muhlbauer et al [20] |
| Brain Region | Level of WFS1 Expression |
|---|---|
| Hippocampus | High |
| Amygdala | High |
| Brainstem | Moderate |
| Thalamus | High |
| Cerebellum | Low |
| Prefrontal cortex | Moderate |
| Function of WFS1 | Effect of Dysfunction |
|---|---|
| Regulates calcium in ER | Calcium imbalance, hyperexcitability, impaired protein folding |
| Coordinates ER-mitochondria calcium signaling | Disrupted ATP production, mitochondrial damage, oxidative stress |
| Modulates calcium influx from extracellular space | Altered calcium signaling, impaired synaptic transmission |
| Maintains synaptic plasticity | Synaptic failure, cognitive decline, neurodegeneration |
| Regulates stress response pathways (e.g., UPR) | Chronic ER stress, increased apoptosis, neuronal dysfunction |
| Supports autophagy and protein clearance | Disrupted autophagy, protein aggregation, neurodegenerative diseases |
| Molecular Pathway | Normal Role of WFS1 | Effect of WFS1 Dysfunction |
|---|---|---|
| Unfolded Protein Response | Maintains ER homeostasis, regulates UPR sensors (ATF6, IRE1, PERK) | Chronic ER stress, prolonged UPR, increased apoptosis |
| Calcium Homeostasis | Controls ER calcium levels and signaling between ER and mitochondria | Calcium dysregulation, excitability, mitochondrial dysfunction |
| Mitochondrial Function | Supports ER-mitochondria communication for ATP production | Impaired ATP production, oxidative stress, ROS increase |
| Autophagy & Protein Clearance | Prevents protein aggregation via proper folding and degradation | Misfolded proteins, amyloid-β plaques, tau tangles |
| Dopaminergic Signaling | Modulates neurotransmitter release and neuronal excitability | Altered release, excitability, sleep disturbances |
| Neuroinflammation | Mitigates stress-induced inflammation | Activation of inflammatory pathways, cytokine release |
| Sleep Disorder | WFS1 Dysfunction Mechanism |
|---|---|
| Sleep apnea | Disrupted brainstem regulation of breathing |
| Insomnia | Hyperexcitability due to calcium dysregulation |
| Circadian rhythm disorders | Altered dopaminergic signaling |
| REM sleep behavior disorder | Impaired neuronal excitability |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





