1. Introduction
Uric acid (UA) is the end product of the purine degradation pathway in humans. In this pathway, xanthine oxidase (XO) is the enzyme that transforms hypoxanthine and xanthine into UA. Hyperuricemia, which is the excess accumulation of UA in the blood, can be caused by the metabolic overproduction or reduced renal excretion of UA [
1]. Two-thirds of the total UA elimination primarily occurs via excretion by the kidney. Thus, as kidney function declines, uric acid is retained to a certain degree because other compensatory excretion pathways are activated. Consequently, hyperuricemia can result from impaired kidney function; however, in the clinical setting, hyperuricemia commonly precedes the development of chronic kidney disease. In this way, hyperuricemia causes kidney injury through the deposition of monosodium urate crystals in renal structures and surrounding vascular capillaries, as well as through the intracellular activation of mechanisms related to impaired renal flow, oxidative stress induction, and ischemia mediated by the soluble UA fraction [
2].
The two main effective strategies used to prevent or treat hyperuricemia in patients are the use of XO inhibitors (e.g., allopurinol, AL), which leads to decreased UA production, and the use of uricosuric agents (e.g., benzbromarone), which are capable of increasing UA excretion. Nevertheless, both classes of drugs may cause serious side effects. For example, with regard to AL, there have been reports of reactions involving eosinophilia and systemic symptoms, toxic epidermal necrolysis, and gastrointestinal toxicity, among others, and, with regard to benzbromarone, there have been reports of hepatotoxicity in patients. Therefore, there is a need for effective therapies without serious or fewer side effects to regulate serum UA levels in patients. In this way, some extracts from natural sources are useful against hyperuricemia [
1,
3,
4].
Dactylopius opuntiae, known as wild cochineal, is an insect considered an invasive pest of Opuntia species, and the female adult insects are a source of carminic acid (CA) [
5]. CA has been described as a potential therapeutic strategy for fructose-induced kidney injury because it restricts inflammation and reactive oxygen species production during the injury and reduces the progression to chronic kidney damage [
6]. Additional biological actions have been described for CA, such as antioxidant abilities, the attenuation of fatty liver disease progression, and suppressive activity against tumors. Meanwhile, a CA-rich extract obtained from
D. opuntiae female insects has been reported to have properties of interest to the food industry, such as antioxidant activity and biochemical actions against the discoloration and oxidation of proteins and lipids in refrigerated meat [
5].
To the best of our knowledge, D. opuntiae extracts have not been evaluated against a hyperuricemic state related to kidney injury. Therefore, the present study aimed to evaluate the effects of a D. opuntiae aqueous extract on XO inhibition, serum purine levels, and renal antioxidant markers in mice with hyperuricemia related to kidney dysfunction.
3. Discussion
The CA contents in the D2 and D1 extracts were superior to those reported in a
D. opuntiae extract sample obtained using a procedure similar to that presented here (4497.1 ± 113.8 µg of CA/mL of M3 extract) and those reported in
Dactylopius coccus extract samples obtained via solid–liquid extractions with different solvents and temperatures (concentrations below 450 µg of CA/mL of extract) [
5,
7]. The present and previous extracts differed because of the amount of cochineal powder, type of cochineal, and volume of solvent used during the extraction procedures. Our data show that the extraction technique performed without citric acid was superior to that performed with citric acid (
Table 1) despite previous data indicating that citric acid is an enhancer of CA extraction from a water matrix [
7]. An explanation for this situation is that our procedure in which citric acid was used may have lost CA during sample processing due to it having more preparative steps than the technique without citric acid. However, it is important to note that, although there are other methods that can be used to obtain CA-rich extracts from insect powder, they involve sophisticated equipment and high costs, such as those used for supercritical fluid extraction [
7]. In contrast, solid–liquid extractions are easy and accessible procedures, as observed in the present methodology.
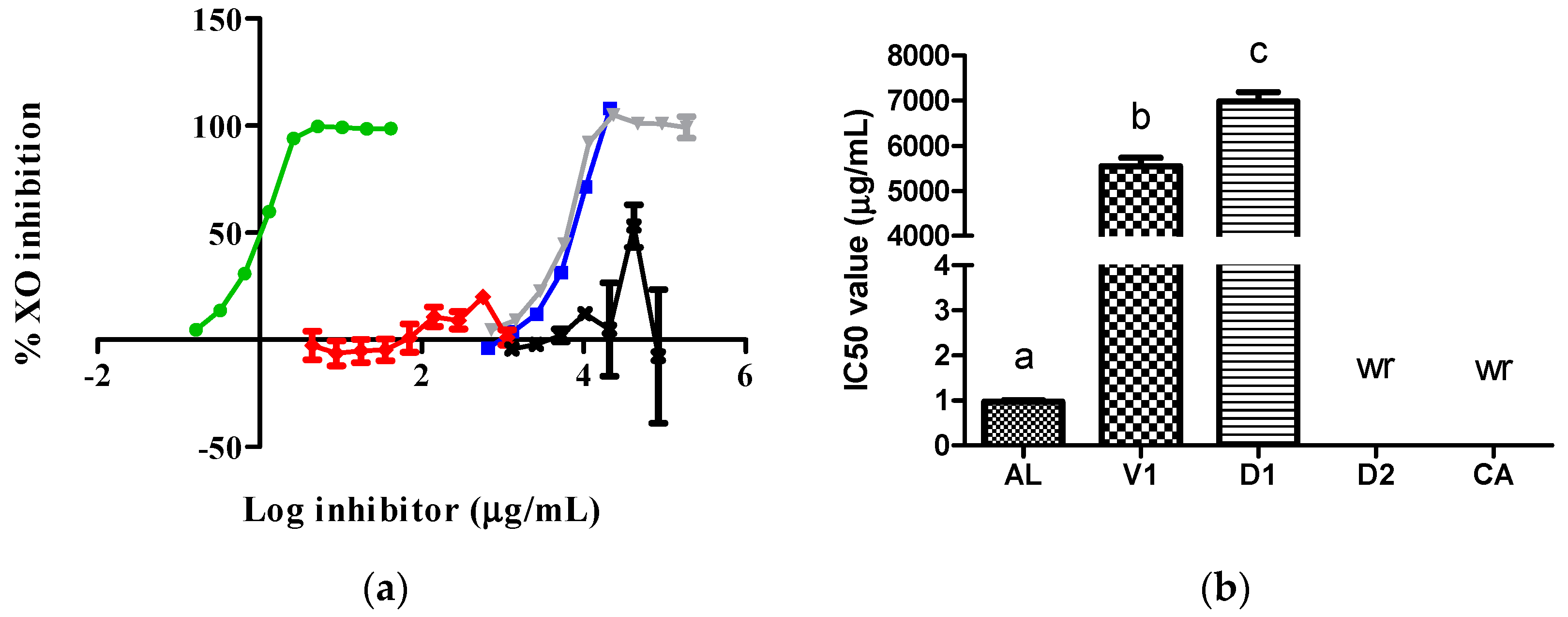
To the best of our knowledge, this is the first time that an evaluation against the XO of extracts obtained from insects belonging to the Dactylopius genus has been performed. Our data show that the
D. opuntiae aqueous extract had a very limited inhibitory effect on XO due to its key component CA showing an effect close to inactivity against XO in com-parison with AL (
Figure 1). The inhibitory effect of the D1 extract on XO was due to its citric acid content, which was confirmed by the results obtained from the V1 samples. However, the D2 extract exhibited important action against the enzyme only at one concentration (
Figure 1). The action of the D2 extract on XO was not a common behavior of the XO inhibitors. Possible reasons for this are the type of assay used, as it was a discontinuous assay, and the reaction time, which may have been insufficient to achieve an appropriate interaction between the inhibitor and the enzyme [
8,
9]. It is important to note that our finding that the V1 sample functioned as an XO inhibitor has not yet been reported. Only one prior study mentioned that citrate preparations, which are conjugate bases of citric acid, alongside AL improve serum UA levels through an enhanced renal function in patients with hyperuricemia [
10].
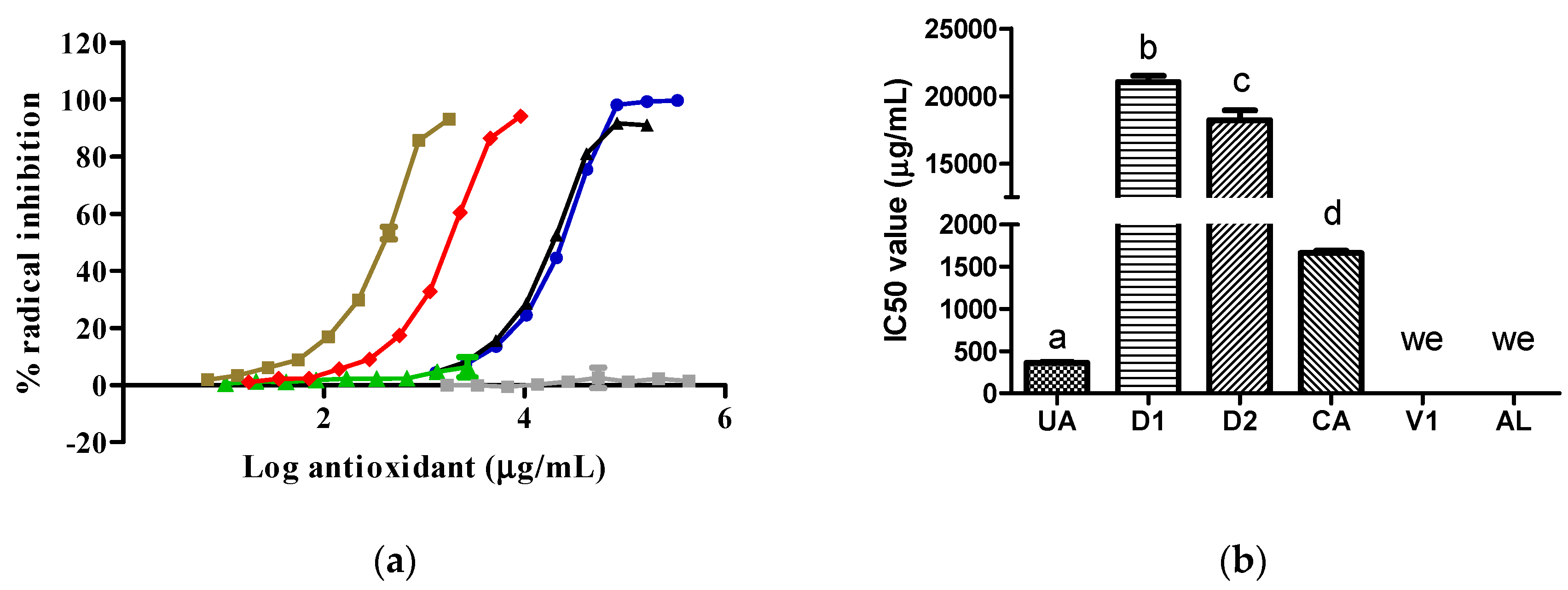
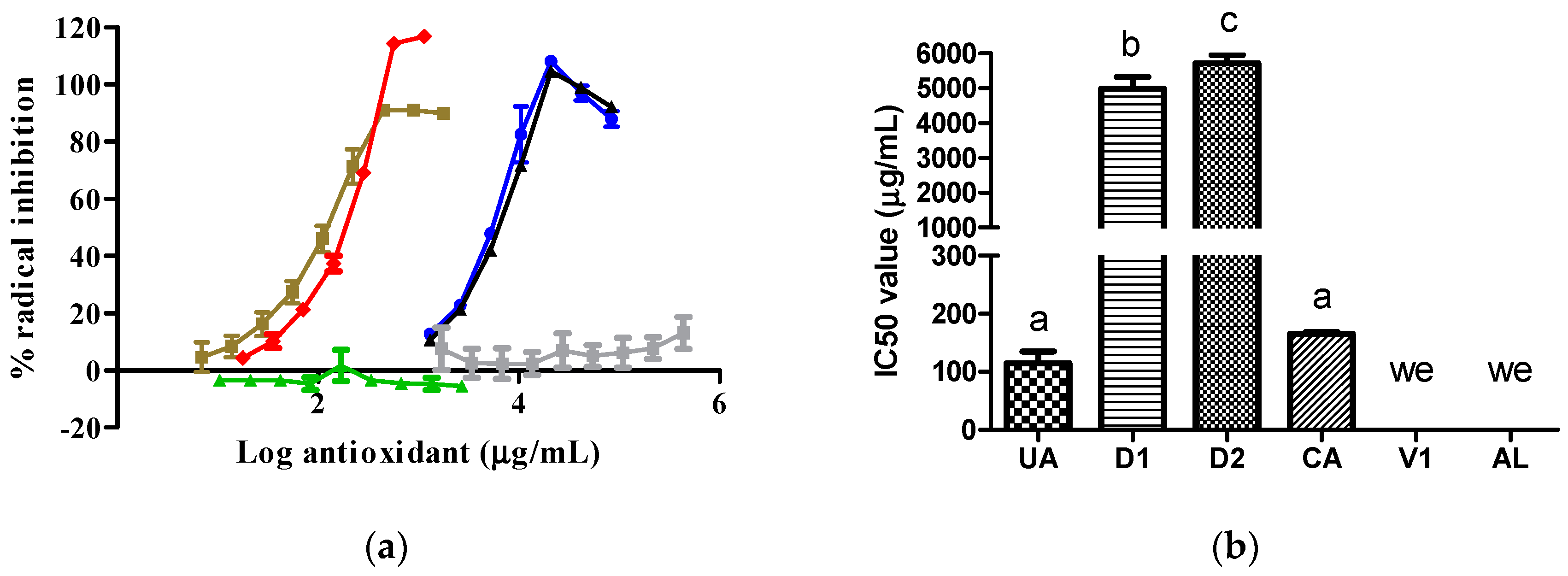
The antioxidant abilities of the D2 and D1 extracts (
Figure 2 and
Figure 3) were due to the content of CA, as this compound possesses free radical scavenging properties [
6]. The antioxidant abilities found in D2 and D1 varied according to the CA content, type of radical, and CA solubility in the reaction medium. We report for the first time that the AL molecule did not possess antioxidant abilities by itself. In line with our result, previous data show that AL can inhibit free radical formation through its specific action against XO [
11]. The V1 sample did not inhibit the two synthetic radicals tested because its major component, citric acid, did not possess antioxidant abilities [
12]. Under the present experimental conditions, UA exhibited only antioxidant abilities without prooxidant actions [
13].
Despite the confirmed XO inhibitory activity of the V1 samples, it did not prove to be useful in the preclinical setting, as its administration killed the mice in the hyperuricemia model. The V1 administration involved a dose of citric acid/citrate at 3.98 g/kg bw, which was inferior to the No Observed Adverse Effect Level (NOAEL) reported for citric acid in mice (7.5 g/kg bw/day) and to a dose of citric acid used in albino mice (4 g/kg bw) in a lipopolysaccharide-induced endotoxemia model, where animal deaths were not reported [
14,
15]. A possible explanation for this is that the V1 sample caused a fatal cytotoxic and prooxidant effect in the mice in the hyperuricemia model [
15,
16]. As the D1 extract contained the same amount of citric acid/citrate as the V1 samples, D1 administration also killed the mice in the present model.
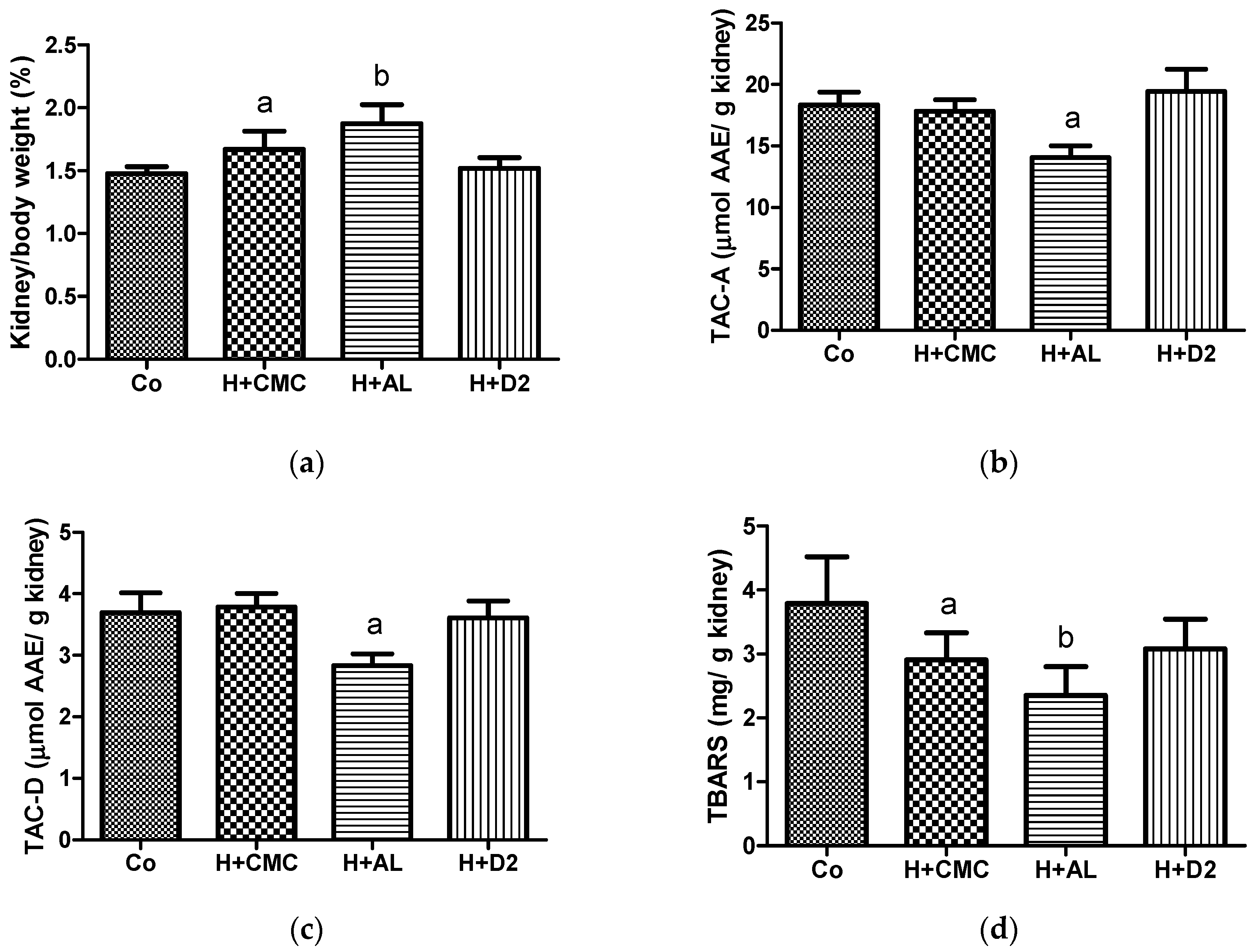
For the first time, our preclinical data demonstrate that the D2 insect extract can be used as a therapy for hyperuricemia associated with renal dysfunction. The administration of the D2 extract resulted in a low serum UA level due to high renal clearance, as indicated by the H+D2 group having a low level of serum creatinine in comparison with the group with hyperuricemia only (
Table 2). The enhanced renal clearance by the D2 extract was caused by improved kidney function, as kidney function is related to an appropriate renal antioxidant capacity [
17]. In our case, the D2 extract protected the kidney weight and antioxidant renal status in the mice subjected to hyperuricemia induction and AL administration (
Figure 4). Our data show that AL had a deleterious effect on the kidneys during hyperuricemia induction in the mice (
Figure 4), which is consistent with previously reported data [
4]. Our study shows that damage to the kidney caused by hyperuricemia induction activated the antioxidant system, reflected by low TBARS values together with unchanged antioxidant levels. Moreover, this insult was exacerbated by AL administration, as a very low TBARS value was accompanied by a reduced antioxidant capacity in the kidneys (
Figure 4). In some cases, inconsistent results between the TBARS and antioxidant levels were found. One explanation for these conflicting results is that antioxidant defense can be activated in response to an insult, which, in turn, prevents oxidative damage to lipids, and, thus, the generation of TBARSs is reduced while antioxidant levels remain unchanged [
18,
19]. Taking into account this explanation and the H+AL data, we suggest that the insult caused by AL was superior to the velocity of the response of the antioxidant renal system.
The H+CMC data confirmed that hyperuricemia was successfully induced under the present animal protocol. In this case, hyperuricemia was induced via the administration of gentamicin, potassium oxonate, and hypoxanthine, as the application of potassium oxonate and hypoxanthine did not produce hyperuricemia in this mouse strain by day 3 [
1]. Previous reports have confirmed that gentamicin causes nephrotoxicity, where the generation of free radicals and other pathways are involved in causing kidney damage with a renal inflammation response, alongside an increase in creatinine and UA concentrations in plasma [
20,
21,
22]. The use of gentamicin in our hyperuricemia mouse model increased kidney weight and serum UA levels. However, there was no statistically significant increase in the serum creatinine level following gentamicin administration because the duration of aminoglycoside exposure was short in comparison with that in previously published studies [
20,
21,
22]. Additionally, the H+AL data supported the in vivo inhibitory action of AL against the XO enzyme, while the H+D2 data did not show statistically significant changes in the xanthine or hypoxanthine levels (
Table 2). This latter finding was in line with the poor activity of the D2 extract against the XO enzyme in vitro. It is known that the measurement of xanthine, hypoxanthine, and UA, which are involved in the purine catabolism pathway, is desirable to effectively interpret XO inhibition in vivo [
1].
As this work involved in vitro and preclinical experiments, further evaluations such as clinical studies are necessary to contribute to the evidence. Nevertheless, our study is the beginning of the use of a cactus pest insect as a therapy for a common disorder, such as hyperuricemia linked to kidney dysfunction.
4. Materials and Methods
4.1. Insect Breeding
D. opuntiae [Cockerell] specimens and cladodes of
Opuntia ficus-indica cv. Rojo pelón were obtained from an orchard belonging to the Colegio de Postgraduados, Campus San Luis, Salinas de Hidalgo, S.L.P., Mexico. Since 2011, Dactylopius specimens have been identified using microscopic features, and their reproduction has been maintained until now [
23]. Cleaned and healed cladodes were placed in a container containing wet soil, and then they were exposed to
D. opuntiae nymphs for 48 h. The infested cladodes were placed in a container covered with a fine mesh to produce adult female insects under controlled humidity (40%) and temperature (23.5 ⁰C) conditions [
24].
4.2. Cochineal Pigment Extraction
Adult female insects, alongside their wax, were collected, dried at 40 ⁰C, and powdered with a blender. Subsequently, the insect powder was stored in polypropylene opaque containers at room temperature. Two conventional solid–liquid extraction methods were performed to obtain two cochineal extracts.
For the first method [
5], a 433 mg/mL citric acid solution was prepared using drinking water, and then 3.34 g of the powdered insect sample was mixed with 10 mL of the citric acid solution. This mixture was incubated at 50 ⁰C for 30 min, cooled at 4 ⁰C for 14 min, and centrifugated at 1500 rpm for 10 min at room temperature. After centrifugation, three phases were identified, and the intermediate layer was collected. Then, 1.14 g of sodium carbonate was gradually added to the collected layer and maintained at room temperature until the formed foam disappeared completely. This mixture was centrifugated again, and two layers were produced. The lower layer was collected, weighed, and stored at -20 ⁰C until use. In addition, the same procedure was applied for a citric acid solution without insect powder to obtain the V1 sample.
For the second method, 20 mL of drinking water was heated at 50⁰C, and 6.68 g of the powered insect sample was mixed with the water for 5 min. This mixture was centrifugated at 1500 rpm for 10 min at room temperature. After centrifugation, four phases were identified, and the second layer from the top was collected, weighed, and stored at -20 ⁰C until use.
4.3. CA Quantification
A CA quantification analysis was performed as described earlier [
25] but with modifications. Calibrators containing CA (0.73-145 µg/mL) and fortified with AL (81.3 µg/mL) as an internal standard were prepared in deionized water. Subsequently, these calibrators were mixed with methanol at a ratio of 1:1. Then, these mixtures were centrifugated (at 12,100 rpm for 10 min at 4 ⁰C), and their supernatants were placed in vial inserts inside amber glass tubes for a chromatographic analysis.
A 1100 series Agilent liquid chromatography system consisting of a quaternary pump with a degasser, autosampler, thermostated column compartment, and diode-array detector, alongside Agilent ChemStation software for LC 3D systems, was used for measurements and data analyses (Agilent Technologies, Palo Alto, CA, USA). For chromatographic separation, a 250 mm long Syncronis C18 column was used, with a 5 µm particle size and a 4.6 mm internal diameter (Thermo Fisher Scientific, CA, USA). The column compartment was maintained at 30 ⁰C during isocratic separation, and a mixture of acetic acid solution (3.49 g/L) and methanol at a ratio of 1:1 v/v was used as the mobile phase.
The preparation of extract samples included a dilution of 1:400 v/v with deionized water alongside fortification with AL. Each chromatographic peak in the extract samples was identified by its retention time and spectrum record, which were compared with those obtained from the calibrators. Monitoring was carried out at 274 and 254 nm for CA and AL alongside a drawing of the spectra from 190 to 400 nm. A flow of 1 mL/min for an 8 min run time and an injection volume of 10 µL for each sample were programmed in the chromatographic system for the separation. The CA concentration in each extracted sample was obtained by interpolating its response (ratio of the CA and AL peak areas) into the curve obtained with the calibrators.
4.4. XO Inhibition by Experimental Samples
This assay was performed as described previously [
1]. In brief, working standards for the AL, CA, V1, D1, and D2 samples were prepared using drinking water (six to nine points per sample, n = 5). The assay was initiated with a mixture containing 100 µL of the test sample, 70 µL of 70 mM phosphate-buffered saline (PBS) solution (pH 7.5), and 60 µL of 0.37 U/mL XO, which was incubated at 37 ⁰C for 30 min. After this incubation period, 120 µL of a 1 mM xanthine solution was added and mixed with the sample, and this subsequent mixture was incubated again at 37 ⁰C for 30 min. After this second incubation period, the reaction was stopped by adding 50 µL of 1N HCl solution to the sample. Then, 200 µL of each sample was placed into a well of a 96-well plate, and the absorbance was read at 290 nm using a Cytation™ 3 microplate reader controlled using Gen5™ software version 2.06 (Biotek Instruments Inc., Winooski, VT, USA).
Using the data obtained from blank samples (samples processed as mentioned above but with the HCl solution added before XO), an interference-free absorbance was calculated for each tested sample. Each experimental result is expressed as the percentage of XO inhibition, which was calculated using the interference-free absorbance of the assay mixture, both with and without the test material.
4.5. Radical Inhibition by Test Samples
To evaluate the antioxidant capacity of the experimental samples, the DPPH and ABTS radicals were employed. These assays were carried out as described previously [
26,
27]. For both assays, working standards for the UA, AL, CA, V1, D1, and D2 samples were prepared using drinking water (seven to nine points per sample, n = 5). In this case, UA was used as a positive control, as it is a powerful antioxidant found in blood and renal tissues [
13].
For the DPPH radical scavenging assay, a mixture of each sample (50 µL) and 450 µmol/L DPPH solution (200 µL) was incubated at 30 ⁰C for 20 min. After the incubation period, the mixture was centrifugated at 1500 rpm for 5 min at 15 ⁰C, and the resultant supernatant (150 µL) was placed into a well of a 96-well plate to monitor the absorbance at 517 nm. Before the ABTS radical scavenging assay was started, the ABTS radical was produced by a reaction between ABTS diammonium salt and potassium persulfate in an aqueous medium. Subsequently, it was diluted with deionized water to obtain a 95.7 µmol/L ABTS radical solution. Then, a mixture of each sample (3 µL) and the diluted ABTS radical solution (300 µL) was incubated at 30 ⁰C for 4 min. Finally, each mixture (150 µL) was placed into a well of a 96-well plate to monitor the absorbance at 730 nm.
For both radical scavenging assays, the microplate reader and software described above were used to measure the absorbance units. The percentage of inhibition against the DPPH or ABTS radical was calculated using the absorbance values of the blank (drinking water) and experimental samples.
4.6. Mouse Model and Sampling
The procedure for the mouse model was carried out according to a prior study [
1] but with modifications. Male BALB/c mice weighing between 15 and 20 g were kept at room temperature (21 ± 1 °C) and housed in acrylic cages with free access to water and a standard diet under a 12 h dark and light cycle. The mice were housed under these laboratory conditions for one week before the experiment.
Hypoxanthine and AL were suspended in a 0.3% CMC aqueous solution, potassium oxonate was suspended in a 0.5 % CMC aqueous solution, and gentamicin (160 mg/ 2 mL, Antibióticos de México, Coyoacán, Ciudad de México, México) was diluted at a ratio of 1:1 v/v with a physiological saline solution. The mice were randomly divided into the following six groups (n = 6 for each group): Co, H+CMC, H+AL, H+D1, H+D2, and H+V1 groups.
After 2 h of fasting, gentamicin (96 mg/kg bw, i.p.) alongside 0.3% CMC (9.2 mL/kg, p.o.), AL (2.5 mg/kg bw, p.o.), D1 (9.2 mL/kg bw, p.o.), D2 (9.2 mL/kg bw, p.o.), or V1 (9.2 mL/kg bw, p.o.) was administered once daily to the animals for three consecutive days.
On the third day, potassium oxonate (280 mg/kg bw, i.p.) was mixed with gentamicin for administration to the mice in the same injection. After one hour of the potassium oxonate/gentamicin injection and experimental oral therapy, a hypoxanthine suspension (268.0 mg/kg bw, p.o.) was administered to the mice. For the Co group, a physiological saline solution or a physiological saline solution plus 0.5% CMC was administered via an intraperitoneal injection, while 0.3% CMC was administered via the oral route for the same periods of time as the other groups.
One hour after hypoxanthine administration, the mice were anesthetized with a combination of ketamine and xylazine (100 and 20 mg/kg bw, respectively). Then, blood and kidneys were collected from the mice. The kidneys were placed in liquid nitrogen, while the blood was kept at 4 °C for 1 h. Subsequently, the blood samples were centrifuged at 5600 rpm for 10 min at 4 °C. The resultant serum samples were separated and frozen at -20 °C for further analysis. To continue the processing of the kidneys, they were thawed at 4 °C, weighed, and homogenized in 2 mL of a cold 70 mM PBS solution (pH 7.5). Subsequently, each homogenate was centrifugated at 11,200 rpm for 10 min at 4 °C. Finally, each supernatant was frozen at -20 °C for further analysis.
4.7. Serum UA, Xanthine, Hypoxanthine, and Creatinine Levels
A liquid chromatography method was used for the quantification of purines and creatinine, as described in a prior study [
1]. In brief, the chromatographic conditions used were as follows: a column compartment maintained at 32 °C, a mobile phase consisting of a PBS solution at pH 6.0 in an acetonitrile gradient, an injection sample volume of 20 µL, a flow rate of 1 mL/min, and multiple monitoring wavelengths (236, 248, 263, and 292 nm for creatinine, hypoxanthine, xanthine, and UA, respectively) together with spectrum drawing (range from 200 to 400 nm). The same apparatus, stationary phase, and software as described above were used for this chromatographic analysis. The compounds were identified by retention times and spectrum records. The compound concentration in the serum samples was calculated from the calibration curves obtained with the pure compounds. The results are expressed as μmol of compound/L of serum sample.
4.8. Antioxidant and Lipid Peroxidation Levels in Kidney Homogenate Supernatant
The ABTS and DPPH radical scavenging assays described above were employed. In the ABTS radical inhibition assay, the kidney supernatants were undiluted, and the blank sample was the 70 mM PBS solution (pH 7.5). For the DPPH radical inhibition assay, the kidney supernatants were diluted 1:1 with the 70 mM PBS solution (pH 7.5) before the reaction with the radical, and the blank sample was the PBS solution. The absorbance value obtained from each tissue supernatant was interpolated in a calibration curve prepared with ascorbic acid. Using the kidney weight and interpolated ascorbic acid concentration, the antioxidant ability of each sample is expressed in µmol of ascorbic acid equivalents (AAE)/g of the kidney.
To measure the lipid peroxidation in the samples, the TBARS assay was used. This assay was adapted from prior studies [
28,
29]. A sample of the kidney supernatant (200 µL), 20 µL of a 493 mg/mL trichloroacetic acid solution, and 2.5 µL of a 1 mg/mL butylated hydroxytoluene solution were mixed for 1 min. This mixture was centrifuged at 4400 rpm for 10 min at 18 °C. A volume of the supernatant or malondialdehyde calibrator (100 µL) was mixed with 60 µL of a 0.8% thiobarbituric acid solution. Then, this mixture was incubated at 75 °C for 30 min and kept at 4 °C for 3 min. A volume of the mixture (120 µL) was placed in 1 well of a 96-well plate to read the absorbance at 532 nm using the same microplate reader and software mentioned above. The TBARS value for each sample was calculated using the calibration curves of malondialdehyde. The malondialdehyde stock solution was obtained as described previously [
28]. Considering the TBARS concentration, volume of the sample, and kidney weight, the results are expressed as mg TBARS/g of the kidney.
4.9. Data Analysis
Data are presented as the mean and standard deviation. Student’s t-test with Welch's correction was used for the analysis of the CA content. A one-way ANOVA with Tukey's post-test was used for the analyses of the data obtained from the XO inhibition experiments, antioxidant abilities in vitro, kidney weighing, and kidney homogenates. A p-value of <0.05 was considered statistically significant. The calculation of IC50 values and statistical analyses were performed using GraphPad Prism 5 software (San Diego, CA, USA).
Author Contributions
Conceptualization, O.H.A.-M., M.M.G.-C., and S.d.J.M.-G.; methodology, O.H.A.-M., M.M.G.-C., O.G.G.-C., S.d.J.M.-G., and F.M.-M.; formal analysis, O.H.A.-M., M.M.G.-C., and S.d.J.M.-G.; data curation, O.H.A.-M., M.M.G.-C., O.G.G.-C., F.M.-M., and M.A.I.-E.; writing—original draft preparation, O.H.A.-M. and M.A.I.-E.; writing—review and editing, M.M.G.-C., M.A.I.-E., O.G.G.-C., O.H.A.-M., and F.M.-M.; supervision, M.M.G.-C., O.H.A.-M., and O.G.G.-C.; funding acquisition, M.M.G.-C. All authors have read and agreed to the published version of the manuscript.