Submitted:
22 September 2024
Posted:
23 September 2024
You are already at the latest version
Abstract
Keywords:
Combined Hypertension and Tachycardia in Acute Lymphoblastic Leukemia: An Overlooked Problem in Literature
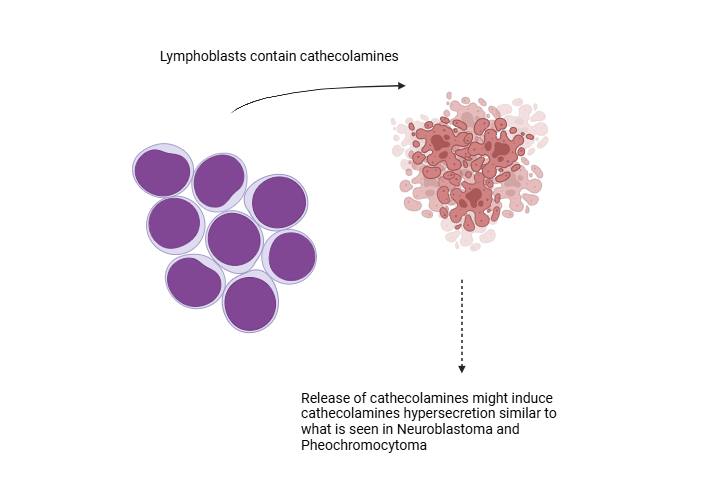
Catecholamine Levels and Paracrine Regulation in ALL
Choice of Antihypertensives in Cancer-Related Hypertension and Implications of This Hypothesis
Author Contributions
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
References
- Bhojwani D, Yang JJ, Pui C-H (2015) Biology of Childhood Acute Lymphoblastic Leukemia. Pediatr Clin North Am 62:47–60. [CrossRef]
- Murphy L, Maloney K, Gore L, Blanchette E (2022) Hypertension in Pediatric Acute Lymphoblastic Leukemia Patients: Prevalence, Impact, and Management Strategies. Integr Blood Press Control 15:1–10. [CrossRef]
- Khandelwal K, Madathala RR, Chennaiahgari N, Yousuffuddin M (2021) Steroid-Induced Sinus Bradycardia. Cureus. [CrossRef]
- Bertrand É, Caru M, Harvey A, Dodin P, Jacquemet V, Curnier D (2023) Cardiac electrical abnormalities in childhood acute lymphoblastic leukemia survivors: a systematic review. Cardio-Oncology 9:1–23. [CrossRef]
- Lipshultz SE, Colan SD, Gelber RD, Perez-Atayde AR, Sallan SE, Sanders SP (1991) Late Cardiac Effects of Doxorubicin Therapy for Acute Lymphoblastic Leukemia in Childhood. N Engl J Med 324:808–815. [CrossRef]
- Steinherz LJ, Steinherz PG, Tan C (1995) Cardiac Failure and Dysrhythmias 6–19 Years After Anthracycline Therapy: A Series of 15 Patients. Med Pediatr Oncol 24:352–361. [CrossRef]
- Rajda C, Bencsik K, Vécsei L L, Bergquist J (2002) Catecholamine levels in peripheral blood lymphocytes from multiple sclerosis patients. J Neuroimmunol 124:93–100. [CrossRef]
- Flierl MA, Rittirsch D, Huber-Lang M, Vidya Sarma J, Award P (2008) Catecholamines - Crafty weapons in the inflammatory arsenal of immune/inflammatory cells or opening Pandora’s box§? Mol Med 14:195–204. [CrossRef]
- Bergquist J, Tarkowski A, Ekman R, Ewing A (1994) Discovery of endogenous catecholamines in lymphocytes and evidence for catecholamine regulation of lymphocyte function via an autocrine loop. Proc Natl Acad Sci U S A 91:12912–12916. [CrossRef]
- Eisenhofer G, Peitzsch M, Bechmann N, Huebner A (2022) Biochemical Diagnosis of Catecholamine-Producing Tumors of Childhood: Neuroblastoma, Pheochromocytoma and Paraganglioma. Front Endocrinol (Lausanne) 13:1–15. [CrossRef]
- Hanns P, Paczulla AM, Medinger M, Konantz M, Lengerke C (2019) Stress and catecholamines modulate the bone marrow microenvironment to promote tumorigenesis. Cell Stress 3:221–235. [CrossRef]
- Paczulla AM, Hanns P, Konantz M, Lundberg P, Dirnhofer S, Lengerke C (2018) Catecholamine Exposure Accelerates In Vivo Leukemogenesis in Acute Myeloid Leukemia Patient Derived Xenografts. Blood 132:1475–1475. [CrossRef]
- Askarinejad A, Alizadehasl A, Jolfayi AG, Adimi S (2023) Hypertension in Cardio-Oncology Clinic: an update on etiology, assessment, and management. Cardio-Oncology 9:1–13. [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).




