Submitted:
20 September 2024
Posted:
23 September 2024
You are already at the latest version
Abstract
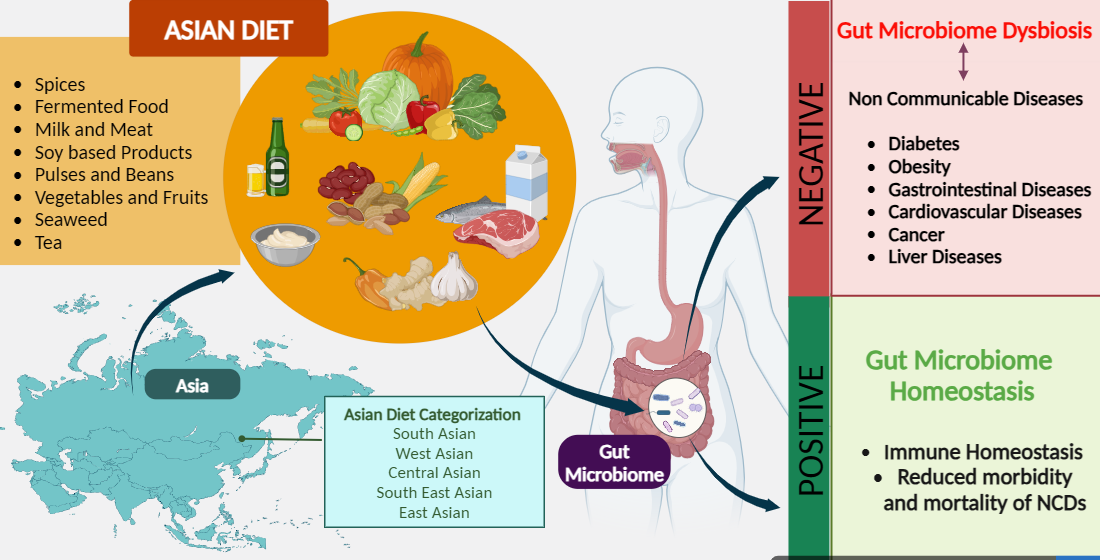
Keywords:
1. Introduction
2. Human Gut Microbiome
3. Asian Gut Microbiome
4. Functions of Gut Microbiome In Human
5. Factors Affecting on Gut Microbiome
5.1. Effect of Diet on the Gut Microbiome
6. Asian Diet
7. East Asian and Southeast Asian diet
7.1. Fermented Food in the Diet
7.2. Seaweed in the Diet
7.3. Consumption of Soybeans
7.3.1. Soy Isoflavones in the Diet.
7.3.2. Soy Oligosaccharides in the Diet.
7.3.3. Fermented Soy Products in the Diet
7.4. Tea, a Widely Consumed Beverage
7.4.1. Effect of Black Tea on Gut Microbiome.
7.4.2. Effect of Green Tea on Gut Microbiome.
8. South Asian Diet
8.1. Spices
8.2. Legumes in the Diet
8.3. Vegetarianism and Gut Microbiota
9. Diet in Central Asia
9.1. Red meat in the Central Asian Diet
9.2. Mare’s milk in Central Asian Diet
10. Western Asia
10.1. Fasting as a Dietary Regimen
11. Recommendations and Way Forward
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Ren, Y.; Wu, J.; Wang, Y.; Zhang, L.; Ren, J.; Zhang, Z.; Chen, B.; Zhang, K.; Zhu, B.; Liu, W.; et al. Lifestyle Patterns Influence the Composition of the Gut Microbiome in a Healthy Chinese Population. Sci Rep 2023, 13, 1–16. [Google Scholar] [CrossRef]
- Nakayama, J.; Watanabe, K.; Jiang, J.; Matsuda, K.; Chao, S.H.; Haryono, P.; La-Ongkham, O.; Sarwoko, M.A.; Sujaya, I.N.; Zhao, L.; et al. Diversity in Gut Bacterial Community of School-Age Children in Asia. Sci Rep 2015, 5, 1–11. [Google Scholar] [CrossRef]
- Das, B.; Ghosh, T.S.; Kedia, S.; Rampal, R.; Saxena, S.; Bag, S.; Mitra, R.; Dayal, M.; Mehta, O.; Surendranath, A.; et al. Analysis of the Gut Microbiome of Rural and Urban Healthy Indians Living in Sea Level and High Altitude Areas. Sci Rep 2018, 8, 1–15. [Google Scholar] [CrossRef]
- Ang, Q.Y.; Alba, D.L.; Upadhyay, V.; Bisanz, J.E.; Cai, J.; Lee, H.L.; Barajas, E.; Wei, G.; Noecker, C.; Patterson, A.D.; et al. The East Asian Gut Microbiome Is Distinct from Colocalized White Subjects and Connected to Metabolic Health. Elife 2021, 10, 1–28. [Google Scholar] [CrossRef]
- Nakayama, J.; Zhang, H.; Lee, Y.-K. Asian Gut Microbiome. Sci Bull (Beijing) 2017, 62, 816–817. [Google Scholar] [CrossRef]
- Lim, M.Y.; Hong, S.; Bang, S.-J.; Chung, W.-H.; Shin, J.-H.; Kim, J.-H.; Nam, Y.-D. Gut Microbiome Structure and Association with Host Factors in a Korean Population. mSystems 2021, 6. [Google Scholar] [CrossRef]
- Shinoda, A.; Shirchin, D.; Jamiyan, D.; Lkhagvajav, T.; Purevdorj, C.; Sonomtseren, S.; Chimiddorj, B.; Namdag, B.; Therdtatha, P.; Nakayama, J. Comparative Study of Gut Microbiota Mongolian and Asian People. Mongolian Journal of Agricultural Sciences 2021, 33, 1–7. [Google Scholar] [CrossRef]
- Syromyatnikov, M.; Nesterova, E.; Gladkikh, M.; Smirnova, Y.; Gryaznova, M.; Popov, V. Characteristics of the Gut Bacterial Composition in People of Different Nationalities and Religions. Microorganisms 2022, 10. [Google Scholar] [CrossRef]
- Yang, B.; Yan, S.; Chen, Y.; Ross, R.P.; Stanton, C.; Zhao, J.; Zhang, H.; Chen, W. Diversity of Gut Microbiota and Bifidobacterial Community of Chinese Subjects of Different Ages and from Different Regions. Microorganisms 2020, 8, 1108. [Google Scholar] [CrossRef]
- United Nations Classification and Definition of Regions.
- Holcombe, C. A History of East Asia. A History of East Asia 2017, 907. [Google Scholar] [CrossRef]
- Phan, U.T.X. Meals and Snacks in Southeast and East Asia. Handbook of Eating and Drinking: Interdisciplinary Perspectives. [CrossRef]
- Cornish, M.L.; Critchley, A.T.; Mouritsen, O.G. Consumption of Seaweeds and the Human Brain. J Appl Phycol 2017, 29, 2377–2398. [Google Scholar] [CrossRef]
- Mouritsen, O.G.; Rhatigan, P.; Pérez-Lloréns, J.L. World Cuisine of Seaweeds: Science Meets Gastronomy. Int J Gastron Food Sci 2018, 14, 55–65. [Google Scholar] [CrossRef]
- Cai, J. Global Status of Seaweed Production, Trade and Utilization; 2021.
- Fleurence, J. Seaweeds as Food; Elsevier Inc., 2016; ISBN 9780128027936.
- Rogel-Castillo, C.; Latorre-Castañeda, M.; Muñoz-Muñoz, C.; Agurto-Muñoz, C. Seaweeds in Food: Current Trends. Plants 2023, 12. [Google Scholar] [CrossRef]
- Pati, M.P.; Das Sharma, S.; Nayak, L.; Panda, C.R. Uses of Seaweed and Its Application to Human Welfare: A Review. Int J Pharm Pharm Sci 2016, 8, 12–20. [Google Scholar]
- Buschmann, A.H.; Camus, C.; Infante, J.; Neori, A.; Israel, Á.; Hernández-González, M.C.; Pereda, S.V.; Gomez-Pinchetti, J.L.; Golberg, A.; Tadmor-Shalev, N.; et al. Seaweed Production: Overview of the Global State of Exploitation, Farming and Emerging Research Activity. Eur J Phycol 2017, 52, 391–406. [Google Scholar] [CrossRef]
- Ficheux, A.-S.; Pierre, O.; Le Garrec, R.; Roudot, A.-C. Seaweed Consumption in France: Key Data for Exposure and Risk Assessment. Food and Chemical Toxicology 2022, 159, 112757. [Google Scholar] [CrossRef] [PubMed]
- Mouritsen, O.G.; Rhatigan, P.; Pérez-Lloréns, J.L. World Cuisine of Seaweeds: Science Meets Gastronomy. Int J Gastron Food Sci 2018, 14, 55–65. [Google Scholar] [CrossRef]
- Hwang, E.K.; Park, C.S. Seaweed Cultivation and Utilization of Korea. Algae 2020, 35, 107–121. [Google Scholar] [CrossRef]
- Rioux, L.E.; Beaulieu, L.; Turgeon, S.L. Seaweeds: A Traditional Ingredients for New Gastronomic Sensation. Food Hydrocoll 2017, 68, 255–265. [Google Scholar]
- Olsson, J.; Toth, G.B.; Albers, E. Biochemical Composition of Red, Green and Brown Seaweeds on the Swedish West Coast. J Appl Phycol 2020, 32, 3305–3317. [Google Scholar] [CrossRef]
- Thiviya, P.; Gamage, A.; Gama-Arachchige, N.S.; Merah, O.; Madhujith, T. Seaweeds as a Source of Functional Proteins. Phycology 2022, 2, 216–243. [Google Scholar] [CrossRef]
- Lopez-Santamarina, A.; Miranda, J.M.; Mondragon, A.D.C.; Lamas, A.; Cardelle-Cobas, A.; Franco, C.M.; Cepeda, A. Potential Use of Marine Seaweeds as Prebiotics: A Review. Molecules 2020, 25. [Google Scholar] [CrossRef] [PubMed]
- Rocha, C.P.; Pacheco, D.; Cotas, J.; Marques, J.C.; Pereira, L.; Gonçalves, A.M.M. Seaweeds as Valuable Sources of Essential Fatty Acids for Human Nutrition. Int J Environ Res Public Health 2021, 18, 4968. [Google Scholar] [CrossRef]
- Siddik, M.A.B.; Francis, P.; Rohani, M.F.; Azam, M.S.; Mock, T.S.; Francis, D.S. Seaweed and Seaweed-Based Functional Metabolites as Potential Modulators of Growth, Immune and Antioxidant Responses, and Gut Microbiota in Fish. Antioxidants 2023, 12, 2066. [Google Scholar] [CrossRef]
- Dawczynski, C.; Schubert, R.; Jahreis, G. Amino Acids, Fatty Acids, and Dietary Fibre in Edible Seaweed Products. Food Chem 2007, 103, 891–899. [Google Scholar] [CrossRef]
- Zheng, L.X.; Chen, X.Q.; Cheong, K.L. Current Trends in Marine Algae Polysaccharides: The Digestive Tract, Microbial Catabolism, and Prebiotic Potential. Int J Biol Macromol 2020, 151, 344–354. [Google Scholar] [CrossRef] [PubMed]
- Flores-Contreras, E.A.; Araújo, R.G.; Rodríguez-Aguayo, A.A.; Guzmán-Román, M.; García-Venegas, J.C.; Nájera-Martínez, E.F.; Sosa-Hernández, J.E.; Iqbal, H.M.N.; Melchor-Martínez, E.M.; Parra-Saldivar, R. Polysaccharides from the Sargassum and Brown Algae Genus: Extraction, Purification, and Their Potential Therapeutic Applications. Plants 2023, 12, 2445. [Google Scholar] [CrossRef]
- Kraan, S. Algal Polysaccharides, Novel Applications and Outlook. In; Chang, C.-F., Ed.; IntechOpen: Rijeka, 2012; p. Ch. 22.
- Yatsunenko, T.; Rey, F.E.; Manary, M.J.; Trehan, I.; Dominguez-Bello, M.G.; Contreras, M.; Magris, M.; Hidalgo, G.; Baldassano, R.N.; Anokhin, A.P.; et al. Human Gut Microbiome Viewed across Age and Geography. Nature 2012, 486, 222–227. [Google Scholar] [CrossRef]
- Zang, L.; Baharlooeian, M.; Terasawa, M.; Shimada, Y.; Nishimura, N. Beneficial Effects of Seaweed-Derived Components on Metabolic Syndrome via Gut Microbiota Modulation. Front Nutr 2023, 10. [Google Scholar] [CrossRef]
- Shannon, E.; Conlon, M.; Hayes, M. Seaweed Components as Potential Modulators of the Gut Microbiota. Mar Drugs 2021, 19, 1–50. [Google Scholar] [CrossRef]
- Jiang, P.; Zheng, W.; Sun, X.; Jiang, G.; Wu, S.; Xu, Y.; Song, S.; Ai, C. Sulfated Polysaccharides from Undaria Pinnatifida Improved High Fat Diet-Induced Metabolic Syndrome, Gut Microbiota Dysbiosis and Inflammation in BALB/c Mice. Int J Biol Macromol 2020, 167. [Google Scholar] [CrossRef]
- Zhang, Y.; Zuo, J.; Yan, L.; Cheng, Y.; Li, Q.; Wu, S.; Chen, L.; Thring, R.W.; Yang, Y.; Gao, Y.; et al. Sargassum Fusiforme Fucoidan Alleviates High-Fat Diet-Induced Obesity and Insulin Resistance Associated with the Improvement of Hepatic Oxidative Stress and Gut Microbiota Profile. J Agric Food Chem 2020, 68, 10626–10638. [Google Scholar] [CrossRef]
- Zheng, W.; Duan, M.; Jia, J.; Song, S.; Ai, C.; Li, S.; Wang, L.; Liu, B.; He, N.; Wang, Y.; et al. Sulfated Polysaccharides from Undaria Pinnatifida Improved High Fat Diet-Induced Metabolic Syndrome, Gut Microbiota Dysbiosis and Inflammation in BALB/c Mice. Int J Biol Macromol 2020, 167, 4773–4784. [Google Scholar] [CrossRef]
- Li, S.; Wang, L.; Liu, B.; He, N. Unsaturated Alginate Oligosaccharides Attenuated Obesity-Related Metabolic Abnormalities by Modulating Gut Microbiota in High-Fat-Diet Mice. Food Funct 2020, 11, 4773–4784. [Google Scholar] [CrossRef]
- Wu, Q.; Wu, S.; Cheng, Y.; Zhang, Z.; Mao, G.; Li, S.; Yang, Y.; Zhang, X.; Wu, M.; Tong, H. Sargassum Fusiforme Fucoidan Modifies Gut Microbiota and Intestinal Metabolites during Alleviation of Hyperglycemia in Type 2 Diabetic Mice. Food Funct 2021, 12, 3572–3585. [Google Scholar] [CrossRef]
- Cheng, Y.; Sibusiso, L.; Hou, L.; Jiang, H.; Chen, P.; Zhang, X.; Wu, M.; Tong, H. Sargassum Fusiforme Fucoidan Modifies the Gut Microbiota during Alleviation of Streptozotocin-Induced Hyperglycemia in Mice. Int J Biol Macromol 2019, 131, 1162–1170. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Liu, J.; Mao, G.; Zuo, J.; Li, S.; Yang, Y.; Thring, R.W.; Wu, M.; Tong, H. Sargassum Fusiforme Fucoidan Alleviates Diet-Induced Insulin Resistance by Inhibiting Colon-Derived Ceramide Biosynthesis. Food Funct 2021, 12, 8440–8453. [Google Scholar] [CrossRef] [PubMed]
- Ding, L.; Yang, L.; Wang, Z.; Huang, W. Bile Acid Nuclear Receptor FXR and Digestive System Diseases. Acta Pharm Sin B 2015, 5, 135–144. [Google Scholar] [CrossRef]
- Mandal, N.; Grambergs, R.; Mondal, K.; Basu, S.K.; Tahia, F.; Dagogo-Jack, S. Role of Ceramides in the Pathogenesis of Diabetes Mellitus and Its Complications. J Diabetes Complications 2021, 35, 107734. [Google Scholar] [CrossRef]
- Lee, M.-K.; Kim, I.-H.; Choi, Y.-H.; Nam, T.-J. A Peptide from Porphyra Yezoensis Stimulates the Proliferation of IEC-6 Cells by Activating the Insulin-like Growth Factor I Receptor Signaling Pathway. Int J Mol Med 2015, 35, 533–538. [Google Scholar] [CrossRef]
- Freile-Pelegrín, Y.; Robledo, D. Bioactive Phenolic Compounds from Algae. Bioactive Compounds from Marine Foods: Plant and Animal Sources. [CrossRef]
- Cotas, J.; Leandro, A.; Monteiro, P.; Pacheco, D.; Figueirinha, A.; Gonçalves, A.M.M.; da Silva, G.J.; Pereira, L. Seaweed Phenolics: From Extraction to Applications. Mar Drugs 2020, 18. [Google Scholar] [CrossRef]
- Yuan, Y.; Zheng, Y.; Zhou, J.; Geng, Y.; Zou, P.; Li, Y.; Zhang, C. Polyphenol-Rich Extracts from Brown Macroalgae Lessonia Trabeculate Attenuate Hyperglycemia and Modulate Gut Microbiota in High-Fat Diet and Streptozotocin-Induced Diabetic Rats. J Agric Food Chem 2019, 67, 12472–12480. [Google Scholar] [CrossRef] [PubMed]
- Yan, X.; Yang, C.; Lin, G.; Chen, Y.; Miao, S.; Liu, B.; Zhao, C. Antidiabetic Potential of Green Seaweed Enteromorpha Prolifera Flavonoids Regulating Insulin Signaling Pathway and Gut Microbiota in Type 2 Diabetic Mice. J Food Sci 2019, 84, 165–173. [Google Scholar] [CrossRef] [PubMed]
- Juturu, V.; Wu, A.H.; Ameer, K.; Messina, M.; Jr, E.J.; Duncan, A.; Messina, V.; Lynch, H.; Kiel, J.; Erdman Jr, J.W. The Health Effects of Soy: A Reference Guide for Health Professionals. Front Nutr 2022, 1–33. [Google Scholar]
- do Prado, F.G.; Pagnoncelli, M.G.B.; de Melo Pereira, G.V.; Karp, S.G.; Soccol, C.R. Fermented Soy Products and Their Potential Health Benefits: A Review. Microorganisms 2022, 10, 1–24. [Google Scholar] [CrossRef]
- Belobrajdic, D.P.; James-Martin, G.; Jones, D.; Tran, C.D. Soy and Gastrointestinal Health: A Review. Nutrients 2023, 15, 1959. [Google Scholar] [CrossRef]
- Clemente, T.E.; Cahoon, E.B. Soybean Oil: Genetic Approaches for Modification of Functionality and Total Content. Plant Physiol 2009, 151, 1030–1040. [Google Scholar] [CrossRef] [PubMed]
- Gu, C.; Pan, H.; Sun, Z.; Qin, G. Effect of Soybean Variety on Anti-Nutritional Factors Content, and Growth Performance and Nutrients Metabolism in Rat. Int J Mol Sci 2010, 11, 1048–1056. [Google Scholar] [CrossRef]
- Barac, M.; Stanojevic, S.; Pesic, M. Biologically Active Components of Soybeans and Soy Protein Products: A Review. Acta Periodica Technologica 2005, 266, 155–168. [Google Scholar] [CrossRef]
- Li, K.J.; Burton-Pimentel, K.J.; Vergères, G.; Feskens, E.J.M.; Brouwer-Brolsma, E.M. Fermented Foods and Cardiometabolic Health: Definitions, Current Evidence, and Future Perspectives. Front Nutr 2022, 9. [Google Scholar] [CrossRef]
- Kumari, M.; Kokkiligadda, A.; Dasriya, V.; Naithani, H. Functional Relevance and Health Benefits of Soymilk Fermented by Lactic Acid Bacteria. J Appl Microbiol 2022, 133, 104–119. [Google Scholar] [CrossRef] [PubMed]
- Elhalis, H.; Chin, X.H.; Chow, Y. Soybean Fermentation : Microbial Ecology and Starter Culture Technology. Crit Rev Food Sci Nutr 2023, 0, 1–23. [Google Scholar] [CrossRef] [PubMed]
- Bahuguna, A.; Kumar, V.; Bodkhe, G.; Ramalingam, S.; Lim, S.M.; Joe, A.R.; Lee, J.S.; Kim, S.Y.; Kim, M. Safety Analysis of Korean Cottage Industries’ Doenjang, a Traditional Fermented Soybean Product: A Special Reference to Biogenic Amines. Foods 2023, 12. [Google Scholar] [CrossRef] [PubMed]
- Yi, S.H.; Hong, S.P. Characteristics of Bacterial Strains with Desirable Flavor Compounds from Korean Traditional Fermented Soybean Paste (Doenjang). Molecules 2021, 26. [Google Scholar] [CrossRef]
- Zhang, X.; Zhang, C.; Xiao, L.; Wang, S.; Wang, X.; Ma, K.; Ji, F.; Azarpazhooh, E.; Ajami, M.; Rui, X.; et al. Effects of Lactiplantibacillus Plantarum with Different Phenotypic Features on the Nutrition, Flavor, Gel Properties, and Digestion of Fermented Soymilk. Food Biosci 2023, 55, 103026. [Google Scholar] [CrossRef]
- Nguyen, N.-N.; Do, D.; Van, T.; Nguyen, V.; Tran, M.; Nguyen, Q.-D. Development of Dairy-free Soybean-based Yoghurt by Active Dry Starter Culture from Kombucha: An Investigation into Microencapsulation, Curd Formation, Protein and Texture Profiles during Storage. Int J Food Sci Technol 2024, 59. [Google Scholar] [CrossRef]
- Leonard, L.M.; Choi, M.S.; Cross, T.W.L. Maximizing the Estrogenic Potential of Soy Isoflavones Through the Gut Microbiome: Implication for Cardiometabolic Health in Postmenopausal Women. Nutrients 2022, 14. [Google Scholar] [CrossRef]
- do Prado, F.G.; Pagnoncelli, M.G.B.; de Melo Pereira, G.V.; Karp, S.G.; Soccol, C.R. Fermented Soy Products and Their Potential Health Benefits: A Review. Microorganisms 2022, 10. [Google Scholar] [CrossRef]
- Huang, L.; Zheng, T.; Hui, H.; Xie, G. Soybean Isoflavones Modulate Gut Microbiota to Benefit the Health Weight and Metabolism. Front Cell Infect Microbiol 2022, 12, 1–11. [Google Scholar] [CrossRef]
- Liu, B.; Qin, L.; Liu, A.; Uchiyama, S.; Ueno, T.; Li, X.; Wang, P. Prevalence of the Equol-Producer Phenotype and Its Relationship with Dietary Isoflavone and Serum Lipids in Healthy Chinese Adults. J Epidemiol 2010, 20, 377–384. [Google Scholar] [CrossRef]
- Paul, B.; Royston, K.J.; Li, Y.; Stoll, M.L.; Skibola, C.F.; Wilson, L.S.; Barnes, S.; Morrow, C.D.; Tollefsbol, T.O. Impact of Genistein on the Gut Microbiome of Humanized Mice and Its Role in Breast Tumor Inhibition. PLoS One 2017, 12, e0189756. [Google Scholar] [CrossRef] [PubMed]
- Hagan, M.; Fungwe, T. Determining the Effect of Seaweed Intake on the Microbiota: A Systematic Review. Functional Food Science 2023, 3. [Google Scholar] [CrossRef]
- Andres, S.; Abraham, K.; Appel, K.E.; Lampen, A. Risks and Benefits of Dietary Isoflavones for Cancer. Crit Rev Toxicol 2011, 41, 463–506. [Google Scholar]
- do Prado, F.G.; Pagnoncelli, M.G.B.; de Melo Pereira, G.V.; Karp, S.G.; Soccol, C.R. Fermented Soy Products and Their Potential Health Benefits: A Review. Microorganisms 2022, 10. [Google Scholar] [CrossRef]
- Teoh, S.Q.; Chin, N.L.; Chong, C.W.; Ripen, A.M.; How, S.; Lim, J.J.L. A Review on Health Benefits and Processing of Tempeh with Outlines on Its Functional Microbes. Future Foods 2024, 9, 100330. [Google Scholar] [CrossRef]
- Guetterman, H.M.; Huey, S.L.; Knight, R.; Fox, A.M.; Mehta, S.; Finkelstein, J.L. Vitamin B-12 and the Gastrointestinal Microbiome: A Systematic Review. Adv Nutr 2022, 13, 530–558. [Google Scholar] [CrossRef]
- Huang, H.; Krishnan, H.B.; Pham, Q.; Yu, L.L.; Wang, T.T.Y. Soy and Gut Microbiota: Interaction and Implication for Human Health. J Agric Food Chem 2016, 64, 8695–8709. [Google Scholar] [CrossRef] [PubMed]
- Mitsuoka, T. Prebiotics and Intestinal Flora. Biosci Microflora 2002, 21, 3–12. [Google Scholar] [CrossRef]
- Ma, Y.; Wu, X.; Giovanni, V.; Meng, X. Effects of Soybean Oligosaccharides on Intestinal Microbial Communities and Immune Modulation in Mice. Saudi J Biol Sci 2017, 24, 114–121. [Google Scholar] [CrossRef]
- Qiang, X.; YongLie, C.; QianBing, W. Health Benefit Application of Functional Oligosaccharides. Carbohydr Polym 2009, 77, 435–441. [Google Scholar] [CrossRef]
- Basson, A.R.; Ahmed, S.; Almutairi, R.; Seo, B.; Cominelli, F. Regulation of Intestinal Inflammation by Soybean and Soy-Derived Compounds. Foods 2021, 10, 1–30. [Google Scholar] [CrossRef] [PubMed]
- Kono, K.; Murakami, Y.; Ebara, A.; Okuma, K.; Tokuno, H.; Odachi, A.; Ogasawara, K.; Hidaka, E.; Mori, T.; Satoh, K.; et al. Fluctuations in Intestinal Microbiota Following Ingestion of Natto Powder Containing Bacillus Subtilis Var. Natto SONOMONO Spores: Considerations Using a Large-Scale Intestinal Microflora Database. Nutrients 2022, 14, 3839. [Google Scholar] [CrossRef]
- Minamiyama, Y.; Okada, S. Miso: Production, Properties, And. Handbook of fermented functional foods 2003, 27.
- Jang, C.H.; Oh, J.; Lim, J.S.; Kim, H.J.; Kim, J.S. Fermented Soy Products: Beneficial Potential in Neurodegenerative Diseases. Foods 2021, 10. [Google Scholar] [CrossRef] [PubMed]
- Wambulwa, M.C.; Meegahakumbura, M.K.; Kamunya, S.; Wachira, F.N. From the Wild to the Cup: Tracking Footprints of the Tea Species in Time and Space. Front Nutr 2021, 8, 706770. [Google Scholar] [CrossRef]
- Stagg, G.V.; Millin, D.J. The Nutritional and Therapeutic Value of Tea—a Review. J Sci Food Agric 1975, 26, 1439–1459. [Google Scholar] [CrossRef]
- Bond, T.; Derbyshire, E. Tea Compounds and the Gut Microbiome: Findings from Trials and Mechanistic Studies. Nutrients 2019, 11, 1–13. [Google Scholar] [CrossRef]
- FAO International Tea Market : Market Situation, Prospects and Emerging Issues. food and Agriculture Organization of the United Nations 2022, 1–11.
- Gross, G.; Jacobs, D.M.; Peters, S.; Possemiers, S.; Van Duynhoven, J.; Vaughan, E.E.; Van De Wiele, T. In Vitro Bioconversion of Polyphenols from Black Tea and Red Wine/Grape Juice by Human Intestinal Microbiota Displays Strong Interindividual Variability. J Agric Food Chem 2010, 58, 10236–10246. [Google Scholar] [CrossRef]
- Chen, H.; Sang, S. Biotransformation of Tea Polyphenols by Gut Microbiota. J Funct Foods 2014, 7, 26–42. [Google Scholar] [CrossRef]
- Mandal, S.; DebMandal, M.; Pal, N.K.; Saha, K. Inhibitory and Killing Activities of Black Tea (Camellia Sinensis) Extract against Salmonella Enterica Serovar Typhi and Vibrio Cholerae O1 Biotype El Tor Serotype Ogawa Isolates. Jundishapur J Microbiol 2011, 4, 115–121. [Google Scholar]
- TOMIOKA, R.; TANAKA, Y.; SUZUKI, M.; EBIHARA, S. The Effects of Black Tea Consumption on Intestinal Microflora—A Randomized Single-Blind Parallel-Group, Placebo-Controlled Study. J Nutr Sci Vitaminol (Tokyo) 2023, 69, 326–339. [Google Scholar] [CrossRef] [PubMed]
- Gao, Y.; Xu, Y.; Yin, J. Black Tea Benefits Short-chain Fatty Acid Producers but Inhibits Genus Lactobacillus in the Gut of Healthy Sprague–Dawley Rats. J Sci Food Agric 2020, 100, 5466–5475. [Google Scholar] [CrossRef] [PubMed]
- Sun, H.; Chen, Y.; Cheng, M.; Zhang, X.; Zheng, X.; Zhang, Z. The Modulatory Effect of Polyphenols from Green Tea, Oolong Tea and Black Tea on Human Intestinal Microbiota in Vitro. J Food Sci Technol 2018, 55, 399–407. [Google Scholar] [CrossRef]
- Sun, L.; Su, Y.; Hu, K.; Li, D.; Guo, H.; Xie, Z. Microbial-Transferred Metabolites of Black Tea Theaflavins by Human Gut Microbiota and Their Impact on Antioxidant Capacity. Molecules 2023, 28. [Google Scholar] [CrossRef] [PubMed]
- Szliszka, E.; Czuba, Z.P.; Domino, M.; Mazur, B.; Zydowicz, G.; Krol, W. Green Tea and Its Relation to Human Gut Microbiome. Molecules 2009, 14, 738–754. [Google Scholar]
- Liu, Y.C.; Li, X.Y.; Shen, L. Modulation Effect of Tea Consumption on Gut Microbiota. Appl Microbiol Biotechnol 2020, 104, 981–987. [Google Scholar] [CrossRef]
- Jin, J.-S.; Touyama, M.; Hisada, T.; Benno, Y. Effects of Green Tea Consumption on Human Fecal Microbiota with Special Reference to Bifidobacterium Species. Microbiol Immunol 2012, 56, 729–739. [Google Scholar] [CrossRef]
- Wang, L.; Zeng, B.; Liu, Z.; Liao, Z.; Zhong, Q.; Gu, L.; Wei, H.; Fang, X. Green Tea Polyphenols Modulate Colonic Microbiota Diversity and Lipid Metabolism in High-Fat Diet Treated HFA Mice. J Food Sci 2018, 83, 864–873. [Google Scholar] [CrossRef]
- United Nations, Department of Economic and Social Affairs, P.D. World Population Prospects: 2022 Revision. Available online: https://population.un.org/wpp/.
- Garduño-Diaz, S.D.; Khokhar, S. South Asian Dietary Patterns and Their Association with Risk Factors for the Metabolic Syndrome. Journal of Human Nutrition and Dietetics 2013, 26, 145–155. [Google Scholar] [CrossRef]
- Bishwajit, G.; Sarker, S.; Kpoghomou, M.-A.; Gao, H.; Jun, L.; Yin, D.; Ghosh, S. Self-Sufficiency in Rice and Food Security: A South Asian Perspective. Agric Food Secur 2013, 2, 10. [Google Scholar] [CrossRef]
- Pingali, P. Westernization of Asian Diets and the Transformation of Food Systems: Implications for Research and Policy. Food Policy 2007, 32, 281–298. [Google Scholar] [CrossRef]
- Bishwajit, G. Nutrition Transition in South Asia: The Emergence of Non-Communicable Chronic Diseases. F1000Res 2015, 4. [Google Scholar] [CrossRef]
- Misra, A.; Khurana, L.; Isharwal, S.; Bhardwaj, S. South Asian Diets and Insulin Resistance. British Journal of Nutrition 2009, 101, 465–473. [Google Scholar] [PubMed]
- Bhathal, S.K.; Kaur, H.; Bains, K.; Mahal, A.K. Assessing Intake and Consumption Level of Spices among Urban and Rural Households of Ludhiana District of Punjab, India. Nutr J 2020, 19, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Kumar, V. RETRACTED ARTICLE: Seven Spices of India—from Kitchen to Clinic. Journal of Ethnic Foods 2020, 7, 23. [Google Scholar] [CrossRef]
- Pandey, I.R.; Pandey, P.R. Challenges and Opportunities in Value Chain of Spices in South Asia (SAARC Agricultural Centre (SAC)); 2017; Vol. 73; ISBN 9789843441713.
- Shahidi, F.; Hossain, A. Bioactives in Spices, and Spice Oleoresins: Phytochemicals and Their Beneficial Effects in Food Preservation and Health Promotion. 2018, 8–75. [CrossRef]
- Dacrema, M.; Ali, A.; Ullah, H.; Khan, A.; Di Minno, A.; Xiao, J.; Martins, A.M.C.; Daglia, M. Spice-Derived Bioactive Compounds Confer Colorectal Cancer Prevention via Modulation of Gut Microbiota. Cancers (Basel) 2022, 14. [Google Scholar]
- Gidwani, B.; Bhattacharya, R.; Shukla, S.S.; Pandey, R.K. Indian Spices: Past, Present and Future Challenges as the Engine for Bio-Enhancement of Drugs: Impact of COVID-19. J Sci Food Agric 2022, 102, 3065–3077. [Google Scholar] [CrossRef]
- Duda-Chodak, A. The Inhibitory Effect of Polyphenols on Human Gut Microbiota. Journal of Physiology and Pharmacology 2012, 63, 497–503. [Google Scholar]
- Hervert-Hernández, D.; Goñi, I. Dietary Polyphenols and Human Gut Microbiota: A Review. Food Reviews International 2011, 27, 154–169. [Google Scholar] [CrossRef]
- Lu, Q.Y.; Summanen, P.H.; Lee, R.P.; Huang, J.; Henning, S.M.; Heber, D.; Finegold, S.M.; Li, Z. Prebiotic Potential and Chemical Composition of Seven Culinary Spice Extracts. J Food Sci 2017, 82, 1807–1813. [Google Scholar] [CrossRef]
- Scazzocchio, B.; Minghetti, L.; D’archivio, M. Interaction between Gut Microbiota and Curcumin: A New Key of Understanding for the Health Effects of Curcumin. Nutrients 2020, 12, 1–18. [Google Scholar] [CrossRef] [PubMed]
- Sun, Z.-Z.; Li, X.-Y.; Wang, S.; Shen, L.; Ji, H.-F. Bidirectional Interactions between Curcumin and Gut Microbiota in Transgenic Mice with Alzheimer’s Disease. Appl Microbiol Biotechnol 2020, 104, 3507–3515. [Google Scholar] [CrossRef] [PubMed]
- Dacrema, M.; Ali, A.; Ullah, H.; Khan, A.; Di Minno, A.; Xiao, J.; Martins, A.M.C.; Daglia, M. Spice-Derived Bioactive Compounds Confer Colorectal Cancer Prevention via Modulation of Gut Microbiota. Cancers (Basel) 2022, 14. [Google Scholar] [CrossRef]
- Peterson, C.T.; Vaughn, A.R.; Sharma, V.; Chopra, D.; Mills, P.J.; Peterson, S.N.; Sivamani, R.K. Effects of Turmeric and Curcumin Dietary Supplementation on Human Gut Microbiota: A Double-Blind, Randomized, Placebo-Controlled Pilot Study. J Evid Based Integr Med 2018, 23. [Google Scholar] [CrossRef] [PubMed]
- Di Cerbo, A.; Palmieri, B.; Aponte, M.; Morales-Medina, J.C.; Iannitti, T. Mechanisms and Therapeutic Effectiveness of Lactobacilli. J Clin Pathol 2015. [Google Scholar] [CrossRef]
- Bereswill, S.; Muñoz, M.; Fischer, A.; Plickert, R.; Haag, L.-M.; Otto, B.; Kühl, A.A.; Loddenkemper, C.; Göbel, U.B.; Heimesaat, M.M. Anti-Inflammatory Effects of Resveratrol, Curcumin and Simvastatin in Acute Small Intestinal Inflammation. PLoS One 2010, 5, e15099. [Google Scholar] [CrossRef] [PubMed]
- Fattori, V.; Hohmann, M.S.N.; Rossaneis, A.C.; Pinho-Ribeiro, F.A.; Verri, W.A. Capsaicin: Current Understanding of Its Mechanisms and Therapy of Pain and Other Pre-Clinical and Clinical Uses. Molecules 2016, 21. [Google Scholar] [CrossRef]
- Peng, Z.; Zhang, W.; Zhang, X.; Mao, J.; Zhang, Q.; Zhao, W.; Zhang, S.; Xie, J. Recent Advances in Analysis of Capsaicin and Its Effects on Metabolic Pathways by Mass Spectrometry. Front Nutr 2023, 10, 1–8. [Google Scholar] [CrossRef]
- Xiang, Q.; Tang, X.; Cui, S.; Zhang, Q.; Liu, X.; Zhao, J.; Zhang, H.; Mao, B.; Chen, W. Capsaicin, the Spicy Ingredient of Chili Peppers: Effects on Gastrointestinal Tract and Composition of Gut Microbiota at Various Dosages. Foods 2022, 11, 686. [Google Scholar] [CrossRef]
- Wang, F.; Huang, X.; Chen, Y.; Zhang, D.; Chen, D.; Chen, L.; Lin, J. Study on the Effect of Capsaicin on the Intestinal Flora through High-Throughput Sequencing. ACS Omega 2020, 5, 1246–1253. [Google Scholar] [CrossRef]
- Hui, S.; Liu, Y.; Chen, M.; Wang, X.; Lang, H.; Zhou, M.; Yi, L.; Mi, M. Capsaicin Improves Glucose Tolerance and Insulin Sensitivity through Modulation of the Gut Microbiota-bile Acid-FXR Axis in Type 2 Diabetic Db/Db Mice. Mol Nutr Food Res 2019, 63, 1900608. [Google Scholar] [CrossRef] [PubMed]
- Guo, S.; Geng, W.; Chen, S.; Wang, L.; Rong, X.; Wang, S.; Wang, T.; Xiong, L.; Huang, J.; Pang, X. Ginger Alleviates DSS-Induced Ulcerative Colitis Severity by Improving the Diversity and Function of Gut Microbiota. Front Pharmacol 2021, 12, 632569. [Google Scholar] [CrossRef]
- Zhou, X.; Liu, X.; He, Q.; Wang, M.; Lu, H.; You, Y.; Chen, L.; Cheng, J.; Li, F.; Fu, X. Ginger Extract Decreases Susceptibility to Dextran Sulfate Sodium-Induced Colitis in Mice Following Early Antibiotic Exposure. Front Med (Lausanne) 2022, 8, 755969. [Google Scholar] [CrossRef]
- Van Hul, M.; Geurts, L.; Plovier, H.; Druart, C.; Everard, A.; Ståhlman, M.; Rhimi, M.; Chira, K.; Teissedre, P.-L.; Delzenne, N.M.; et al. Reduced Obesity, Diabetes, and Steatosis upon Cinnamon and Grape Pomace Are Associated with Changes in Gut Microbiota and Markers of Gut Barrier. American Journal of Physiology-Endocrinology and Metabolism 2018, 314, E334–E352. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.I.; Lee, J.H.; Song, Y.; Kim, Y.T.; Lee, Y.H.; Kang, H. Oral Consumption of Cinnamon Enhances the Expression of Immunity and Lipid Absorption Genes in the Small Intestinal Epithelium and Alters the Gut Microbiota in Normal Mice. J Funct Foods 2018, 49, 96–104. [Google Scholar] [CrossRef]
- Li, A.L.; Ni, W.W.; Zhang, Q.M.; Li, Y.; Zhang, X.; Wu, H.Y.; Du, P.; Hou, J.C.; Zhang, Y. Effect of Cinnamon Essential Oil on Gut Microbiota in the Mouse Model of Dextran Sodium Sulfate-Induced Colitis. Microbiol Immunol 2020, 64, 23–32. [Google Scholar] [CrossRef]
- Lu, Q.-Y.; Rasmussen, A.M.; Yang, J.; Lee, R.-P.; Huang, J.; Shao, P.; Carpenter, C.L.; Gilbuena, I.; Thames, G.; Henning, S.M.; et al. Mixed Spices at Culinary Doses Have Prebiotic Effects in Healthy Adults: A Pilot Study. Nutrients 2019, 11. [Google Scholar] [CrossRef]
- Khine, W.W.T.; Haldar, S.; De Loi, S.; Lee, Y.-K. A Single Serving of Mixed Spices Alters Gut Microflora Composition: A Dose–Response Randomised Trial. Sci Rep 2021, 11, 11264. [Google Scholar] [CrossRef] [PubMed]
- Hughes, J.; Pearson, E.; Grafenauer, S. Legumes-A Comprehensive Exploration of Global Food-Based Dietary Guidelines and Consumption. Nutrients 2022, 14. [Google Scholar] [CrossRef]
- Joshi, P.K.; Rao, P.P. Global and Regional Pulse Economies: Current Trends and Outlook. 2016, 26–57.
- Didinger, C.; Thompson, H.J. Defining Nutritional and Functional Niches of Legumes: A Call for Clarity to Distinguish a Future Role for Pulses in the Dietary Guidelines for Americans. Nutrients 2021, 13. [Google Scholar] [CrossRef]
- Sánchez, X.; Jimenez Martinez, C.; Dávila Ortiz, G.; Álvarez-González, I.; Madrigal-Bujaidar, E. Nutrient and Nonnutrient Components of Legumes, and Its Chemopreventive Activity: A Review. Nutr Cancer 2015, 67, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Didinger, C.; Thompson, H.J. Defining Nutritional and Functional Niches of Legumes: A Call for Clarity to Distinguish a Future Role for Pulses in the Dietary Guidelines for Americans. Nutrients 2021, 13. [Google Scholar] [CrossRef] [PubMed]
- Afriana, riza devi Role of Probiotic α-Galactosidases in the Reduction of Flatulence Causing Raffinose Oligosaccharides (RFOs. Angewandte Chemie International Edition, 6(11), 951–952. 2017, 6, 5–24.
- Khan, A.R.; Alam, S.; Ali, S.; Bibi, S.; Khalil, I.A. Dietary Fiber Profile of Food Legumes. Sarhad J. Agric 2007, 23, 763–766. [Google Scholar]
- Tosh, S.M.; Yada, S. Dietary Fibres in Pulse Seeds and Fractions: Characterization, Functional Attributes, and Applications. Food research international 2010, 43, 450–460. [Google Scholar]
- Wu, D.; Wan, J.; Li, W.; Li, J.; Guo, W.; Zheng, X.; Gan, R.Y.; Hu, Y.; Zou, L. Comparison of Soluble Dietary Fibers Extracted from Ten Traditional Legumes: Physicochemical Properties and Biological Functions. Foods 2023, 12. [Google Scholar] [CrossRef] [PubMed]
- Krishna, H.; Suthari, S. Application of Legume Seed Galactomannan Polysaccharides. In; 2020; pp. 97–113 ISBN 978-3-030-53016-7.
- Wu, G.-J.; Liu, D.; Wan, Y.-J.; Huang, X.-J.; Nie, S.-P. Comparison of Hypoglycemic Effects of Polysaccharides from Four Legume Species. Food Hydrocoll 2019, 90, 299–304. [Google Scholar] [CrossRef]
- Cichońska, P.; Ziarno, M. Legumes and Legume-Based Beverages Fermented with Lactic Acid Bacteria as a Potential Carrier of Probiotics and Prebiotics. Microorganisms 2022, 10. [Google Scholar] [CrossRef]
- Chen, Y.; Huang, C. Effects of Chickpea ( Cicer Arietinum ) on Metabolic Dysfunction by Modulation of Gut Microbiota in Diet- Induced Obese Mice. 2020.
- Monk, J.M.; Lepp, D.; Wu, W.; Graf, D.; McGillis, L.H.; Hussain, A.; Carey, C.; Robinson, L.E.; Liu, R.; Tsao, R.; et al. Chickpea-Supplemented Diet Alters the Gut Microbiome and Enhances Gut Barrier Integrity in C57Bl/6 Male Mice. J Funct Foods 2017, 38, 663–674. [Google Scholar] [CrossRef]
- Fernando, W.M.U.; Hill, J.E.; Zello, G.A.; Tyler, R.T.; Dahl, W.J.; Van Kessel, A.G. Diets Supplemented with Chickpea or Its Main Oligosaccharide Component Raffinose Modify Faecal Microbial Composition in Healthy Adults. Benef Microbes 2010, 1, 197–207. [Google Scholar] [CrossRef]
- Kang, S.; Xu, Y.; Zhang, Y.; Gao, P.; Guan, Y.; Ku, S.; Xu, J.; Zhu, X.; Li, H. Modulation of Gut Microbiota by Chickpea-Derived Proteins and Peptides with Antioxidant Capabilities. LWT 2023, 187, 115341. [Google Scholar] [CrossRef]
- Johnson, C.R.; Thavarajah, D.; Combs, G.F.; Thavarajah, P. Lentil (Lens Culinaris L.): A Prebiotic-Rich Whole Food Legume. Food Research International 2013, 51, 107–113. [Google Scholar] [CrossRef]
- Siva, N.; Johnson, C.R.; Richard, V.; Jesch, E.D.; Whiteside, W.; Abood, A.A.; Thavarajah, P.; Duckett, S.; Thavarajah, D. Lentil (Lens Culinaris Medikus) Diet Affects the Gut Microbiome and Obesity Markers in Rat. J Agric Food Chem 2018, 66, 8805–8813. [Google Scholar] [CrossRef]
- Graf, D.; Monk, J.M.; Lepp, D.; Wu, W.; McGillis, L.; Roberton, K.; Brummer, Y.; Tosh, S.M.; Power, K.A. Cooked Red Lentils Dose-Dependently Modulate the Colonic Microenvironment in Healthy C57Bl,/6 Male Mice. Nutrients 2019, 11, 1–21. [Google Scholar] [CrossRef]
- Hargreaves, S.M.; Raposo, A.; Saraiva, A.; Zandonadi, R.P. Vegetarian Diet: An Overview through the Perspective of Quality of Life Domains. Int J Environ Res Public Health 2021, 18. [Google Scholar] [CrossRef]
- Natrajan, B.; Jacob, S. “Provincialising” Vegetarianism: Putting Indian Food Habits in Their Place. Econ Polit Wkly 2018, 53, 54–64. [Google Scholar]
- Xiao, W.; Zhang, Q.; Yu, L.; Tian, F.; Chen, W.; Zhai, Q. Effects of Vegetarian Diet-Associated Nutrients on Gut Microbiota and Intestinal Physiology. Food Science and Human Wellness 2022, 11, 208–217. [Google Scholar] [CrossRef]
- Melina, V.; Craig, W.; Levin, S. Position of the Academy of Nutrition and Dietetics: Vegetarian Diets. J Acad Nutr Diet 2016, 116, 1970–1980. [Google Scholar] [CrossRef] [PubMed]
- Rizzo, N.S.; Jaceldo-Siegl, K.; Sabate, J.; Fraser, G.E. Nutrient Profiles of Vegetarian and Nonvegetarian Dietary Patterns. J Acad Nutr Diet 2013, 113, 1610–1619. [Google Scholar] [CrossRef]
- Johnston, C.S. 28 - Vegetarian Diet and Possible Mechanisms for Impact on Mood. In; Mariotti, F.B.T.-V. and P.-B.D. in H. and D.P., Ed.; Academic Press, 2017; pp. 493–509 ISBN 978-0-12-803968-7.
- Iikura, M. 27 - Plant-Based Diets and Asthma. In; Mariotti, F.B.T.-V. and P.-B.D. in H. and D.P., Ed.; Academic Press, 2017; pp. 483–491 ISBN 978-0-12-803968-7.
- Kahleova, H.; Pelikanova, T. 21 - Vegetarian Diets in People With Type 2 Diabetes. In; Mariotti, F.B.T.-V. and P.-B.D. in H. and D.P., Ed.; Academic Press, 2017; pp. 369–393 ISBN 978-0-12-803968-7.
- Mann, J. 23 - Ischemic Heart Disease in Vegetarians and Those Consuming a Predominantly Plant-Based Diet. In; Mariotti, F.B.T.-V. and P.-B.D. in H. and D.P., Ed.; Academic Press, 2017; pp. 415–427 ISBN 978-0-12-803968-7.
- Tonstad, S.; Clifton, P. 20 - Vegetarian Diets and the Risk of Type 2 Diabetes. In; Mariotti, F.B.T.-V. and P.-B.D. in H. and D.P., Ed.; Academic Press, 2017; pp. 355–367 ISBN 978-0-12-803968-7.
- Yokoyama, Y.; Nishimura, K.; Barnard, N.D.; Miyamoto, Y. 22 - Blood Pressure and Vegetarian Diets. In; Mariotti, F.B.T.-V. and P.-B.D. in H. and D.P., Ed.; Academic Press, 2017; pp. 395–413 ISBN 978-0-12-803968-7.
- Yeh, M.-C.; Glick-Bauer, M.; Katz, D.L. 18 - Weight Maintenance and Weight Loss: The Adoption of Diets Based on Predominantly Plants. In; Mariotti, F.B.T.-V. and P.-B.D. in H. and D.P., Ed.; Academic Press, 2017; pp. 333–344 ISBN 978-0-12-803968-7.
- Orlich, M.J.; Siapco, G.; Jung, S. 24 - Vegetarian Diets and the Microbiome. In; Mariotti, F.B.T.-V. and P.-B.D. in H. and D.P., Ed.; Academic Press, 2017; pp. 429–461 ISBN 978-0-12-803968-7.
- Mangano, K.M.; Tucker, K.L. 17 - Bone Health and Vegan Diets. In; Mariotti, F.B.T.-V. and P.-B.D. in H. and D.P., Ed.; Academic Press, 2017; pp. 315–331 ISBN 978-0-12-803968-7.
- Nath, P.; Singh, S.P. 26 - Defecation and Stools in Vegetarians: Implications in Health and Disease. In; Mariotti, F.B.T.-V. and P.-B.D. in H. and D.P., Ed.; Academic Press, 2017; pp. 473–481 ISBN 978-0-12-803968-7.
- Key, T.J. 19 - Cancer Risk and Vegetarian Diets. In; Mariotti, F.B.T.-V. and P.-B.D. in H. and D.P., Ed.; Academic Press, 2017; pp. 345–354 ISBN 978-0-12-803968-7.
- Jung, J.G.; Kang, H.W. 25 - Vegetarianism and the Risk of Gastroesophageal Reflux Disease. In; Mariotti, F.B.T.-V. and P.-B.D. in H. and D.P., Ed.; Academic Press, 2017; pp. 463–472 ISBN 978-0-12-803968-7.
- Pem, D.; Jeewon, R. Fruit and Vegetable Intake: Benefits and Progress of Nutrition Education Interventions-Narrative Review Article. Iran J Public Health 2015, 44, 1309–1321. [Google Scholar]
- Walsh, S.; Hebbelinck, M.; Deriemaeker, P.; Clarys, P. 11 - Dietary Patterns in Plant-Based, Vegetarian, and Omnivorous Diets. In; Mariotti, F.B.T.-V. and P.-B.D. in H. and D.P., Ed.; Academic Press, 2017; pp. 175–196 ISBN 978-0-12-803968-7.
- van Berleere, M.; Dauchet, L. 13 - Fruits, Vegetables, and Health: Evidence From Meta-Analyses of Prospective Epidemiological Studies. In; Mariotti, F.B.T.-V. and P.-B.D. in H. and D.P., Ed.; Academic Press, 2017; pp. 215–248 ISBN 978-0-12-803968-7.
- Boutron-Ruault, M.-C.; Mesrine, S.; Pierre, F. 12 - Meat Consumption and Health Outcomes. In; Mariotti, F.B.T.-V. and P.-B.D. in H. and D.P., Ed.; Academic Press, 2017; pp. 197–214 ISBN 978-0-12-803968-7.
- Sun, C.; Li, A.; Xu, C.; Ma, J.; Wang, H.; Jiang, Z.; Hou, J. Comparative Analysis of Fecal Microbiota in Vegetarians and Omnivores. Nutrients 2023, 15. [Google Scholar] [CrossRef]
- de Jonge, N.; Carlsen, B.; Christensen, M.H.; Pertoldi, C.; Nielsen, J.L. The Gut Microbiome of 54 Mammalian Species. Front Microbiol 2022, 13, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Tomova, A.; Bukovsky, I.; Rembert, E.; Yonas, W.; Alwarith, J.; Barnard, N.D.; Kahleova, H. The Effects of Vegetarian and Vegan Diets on Gut Microbiota. Front Nutr 2019, 6. [Google Scholar] [CrossRef] [PubMed]
- Buell, P.D.; Anderson, E.N.; de Pablo Moya, M.; Oskenbay, M. Crossroads of Cuisine. In Crossroads of Cuisine; BRILL, 2020 ISBN 9789004432109.
- Xiao, W.; Zhang, Q.; Yu, L.; Tian, F.; Chen, W.; Zhai, Q. Effects of Vegetarian Diet-Associated Nutrients on Gut Microbiota and Intestinal Physiology. Food Science and Human Wellness 2022, 11, 208–217. [Google Scholar] [CrossRef]
- Sidhu, S.R.; Kok, C.W.; Kunasegaran, T.; Ramadas, A. Effect of Plant-Based Diets on Gut Microbiota: A Systematic Review of Interventional Studies. Nutrients 2023, 15. [Google Scholar] [CrossRef]
- Zhang, C.; Björkman, A.; Cai, K.; Liu, G.; Wang, C.; Li, Y.; Xia, H.; Sun, L.; Kristiansen, K.; Wang, J.; et al. Impact of a 3-Months Vegetarian Diet on the Gut Microbiota and Immune Repertoire. Front Immunol 2018, 9, 1–13. [Google Scholar] [CrossRef]
- Kim, M.; Hwang, S.; Park, E.; Bae, J. Strict Vegetarian Diet Improves the Risk Factors Associated with Metabolic Diseases by Modulating Gut Microbiota and Reducing Intestinal Inflammation. Environ Microbiol Rep 2013, 5, 765–775. [Google Scholar] [CrossRef]
- Buell, P.D.; Anderson, E.N.; de Pablo Moya, M.; Oskenbay, M. Crossroads of Cuisine; 2020; ISBN 9789004432109.
- Muratalieva, E.; Nendaz, M.; Beran, D. Strategies to Address Non-Communicable Diseases in the Commonwealth of Independent States Countries: A Scoping Review. Prim Health Care Res Dev 2022, 23. [Google Scholar] [CrossRef]
- Karabay, A.; Bolatov, A.; Varol, H.A.; Chan, M.-Y. A Central Asian Food Dataset for Personalized Dietary Interventions. Nutrients 2023, 15, 1728. [Google Scholar] [CrossRef]
- Auyeskhan, U.; Azhbagambetov, A.; Sadykov, T.; Dairabayeva, D.; Talamona, D.; Chan, M.Y. Reducing Meat Consumption in Central Asia through 3D Printing of Plant-Based Protein—Enhanced Alternatives—a Mini Review. Front Nutr 2023, 10. [Google Scholar] [CrossRef]
- Otunchieva, A.; Borbodoev, J.; Ploeger, A. The Transformation of Food Culture on the Case of Kyrgyz Nomads—A Historical Overview. Sustainability (Switzerland) 2021, 13. [Google Scholar] [CrossRef]
- Belaunzaran, X.; Bessa, R.J.B.; Lavín, P.; Mantecón, A.R.; Kramer, J.K.G.; Aldai, N. Horse-Meat for Human Consumption - Current Research and Future Opportunities. Meat Sci 2015, 108, 74–81. [Google Scholar] [CrossRef] [PubMed]
- Shi, W.; Huang, X.; Schooling, C.M.; Zhao, J.V. Red Meat Consumption, Cardiovascular Diseases, and Diabetes: A Systematic Review and Meta-Analysis. Eur Heart J 2023, 44, 2626–2635. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Guo, Z.; Lim, A.A.Q.; Zheng, Y.; Koh, E.Y.; Ho, D.; Qiao, J.; Huo, D.; Hou, Q.; Huang, W.; et al. Mongolians Core Gut Microbiota and Its Correlation with Seasonal Dietary Changes. Sci Rep 2014, 4, 5001. [Google Scholar] [CrossRef] [PubMed]
- Kilmer, P.D. Review Article: Review Article. Journalism 2010, 11, 369–373. [Google Scholar] [CrossRef]
- Kondybayev, A.; Loiseau, G.; Achir, N.; Mestres, C.; Konuspayeva, G. Fermented Mare Milk Product (Qymyz, Koumiss). Int Dairy J 2021, 119, 105065. [Google Scholar] [CrossRef]
- Kushugulova, A.; Löber, U.; Akpanova, S.; Rysbekov, K.; Kozhakhmetov, S.; Khassenbekova, Z.; Essex, M.; Nurgozhina, A.; Nurgaziyev, M.; Babenko, D.; et al. Dynamic Changes in Microbiome Composition Following Mare’s Milk Intake for Prevention of Collateral Antibiotic Effect. Front Cell Infect Microbiol 2021, 11, 1–11. [Google Scholar] [CrossRef]
- Holmes, A.D.; Spelman, A.F.; Tyson Smith, C.; Kuzmeski, J.W. Composition of Mares’ Milk as Compared with That of Other Species. J Dairy Sci 1947, 30, 385–395. [Google Scholar] [CrossRef]
- Nurgaziyev, M.; Aitenov, Y.; Khassenbekova, Z.; Akpanova, S.; Rysbekov, K.; Kozhakhmetov, S.; Nurgozhina, A.; Sergazy, S.; Chulenbayeva, L.; Ospanova, Z.; et al. Effect of Mare’s Milk Prebiotic Supplementation on the Gut Microbiome and the Immune System Following Antibiotic Therapy. Biodiversitas 2020, 21, 5065–5071. [Google Scholar] [CrossRef]
- Li, N.; Xie, Q.; Chen, Q.; Evivie, S.E.; Liu, D.; Dong, J.; Huo, G.; Li, B. Cow, Goat, and Mare Milk Diets Differentially Modulated the Immune System and Gut Microbiota of Mice Colonized by Healthy Infant Feces. J Agric Food Chem 2020, 68, 15345–15357. [Google Scholar] [CrossRef]
- Golzarand, M.; Mirmiran, P.; Jessri, M.; Toolabi, K.; Mojarrad, M.; Azizi, F. Dietary Trends in the Middle East and North Africa: An Ecological Study (1961 to 2007). Public Health Nutr 2012, 15, 1835–1844. [Google Scholar] [CrossRef] [PubMed]
- Eid, N.; Enani, S.; Walton, G.; Corona, G.; Costabile, A.; Gibson, G.; Rowland, I.; Spencer, J.P.E. The Impact of Date Palm Fruits and Their Component Polyphenols, on Gut Microbial Ecology, Bacterial Metabolites and Colon Cancer Cell Proliferation. J Nutr Sci 2014, 3, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Eid, N.; Osmanova, H.; Natchez, C.; Walton, G.; Costabile, A.; Gibson, G.; Rowland, I.; Spencer, J.P.E. Impact of Palm Date Consumption on Microbiota Growth and Large Intestinal Health: A Randomised, Controlled, Cross-over, Human Intervention Study. British Journal of Nutrition 2015, 114, 1226–1236. [Google Scholar] [CrossRef]
- Plummer, E.; Bulach, D.; Carter, G.; Albert, M.J. Gut Microbiome of Native Arab Kuwaitis. Gut Pathog 2020, 12, 1–9. [Google Scholar] [CrossRef]
- Correia, J.M.; Santos, I.; Pezarat-Correia, P.; Silva, A.M.; Mendonca, G.V. Effects of Ramadan and Non-Ramadan Intermittent Fasting on Body Composition: A Systematic Review and Meta-Analysis. Front Nutr 2021, 7, 1–19. [Google Scholar] [CrossRef]
- Song, D.-K.; Kim, Y.-W. Beneficial Effects of Intermittent Fasting: A Narrative Review. Journal of Yeungnam Medical Science 2023, 40, 4–11. [Google Scholar] [CrossRef]
- Visioli, F.; Mucignat-Caretta, C.; Anile, F.; Panaite, S.-A. Traditional and Medical Applications of Fasting. Nutrients 2022, 14, 433. [Google Scholar] [CrossRef]
- Fanti, M.; Mishra, A.; Longo, V.D.; Brandhorst, S. Time-Restricted Eating, Intermittent Fasting, and Fasting-Mimicking Diets in Weight Loss. Curr Obes Rep 2021, 10, 70–80. [Google Scholar] [CrossRef]
- Longo, V.D.; Mattson, M.P. Fasting: Molecular Mechanisms and Clinical Applications. Cell Metab 2014, 19, 181–192. [Google Scholar] [CrossRef]
- Anton, S.D.; Moehl, K.; Donahoo, W.T.; Marosi, K.; Lee, S.A.; Mainous, A.G.; Leeuwenburgh, C.; Mattson, M.P. Flipping the Metabolic Switch: Understanding and Applying the Health Benefits of Fasting. Obesity 2018, 26, 254–268. [Google Scholar] [CrossRef]
- De Cabo, R.; Cabello, R.; Rios, M.; López-Lluch, G.; Ingram, D.K.; Lane, M.A.; Navas, P. Calorie Restriction Attenuates Age-Related Alterations in the Plasma Membrane Antioxidant System in Rat Liver. Exp Gerontol 2004, 39, 297–304. [Google Scholar] [CrossRef] [PubMed]
- Saglam, D.; Colak, G.A.; Sahin, E.; Ekren, B.Y.; Sezerman, U.; Bas, M. Effects of Ramadan Intermittent Fasting on Gut Microbiome: Is the Diet Key? Front Microbiol 2023, 14. [Google Scholar] [CrossRef] [PubMed]
- Ozkul, C.; Yalinay, M.; Karakan, T. Structural Changes in Gut Microbiome after Ramadan Fasting: A Pilot Study. Benef Microbes 2020, 11, 227–233. [Google Scholar] [CrossRef] [PubMed]
- UNICEF UNICEF, WHO, World Bank Group Joint Malnutrition Estimates; 2023.
- Angelakis, E.; Yasir, M.; Bachar, D.; Azhar, E.I.; Lagier, J.C.; Bibi, F.; Jiman-Fatani, A.A.; Alawi, M.; Bakarman, M.A.; Robert, C.; et al. Gut Microbiome and Dietary Patterns in Different Saudi Populations and Monkeys. Sci Rep 2016, 6, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Kisuse, J.; La-ongkham, O.; Nakphaichit, M.; Therdtatha, P.; Momoda, R.; Tanaka, M.; Fukuda, S.; Popluechai, S.; Kespechara, K.; Sonomoto, K.; et al. Urban Diets Linked to Gut Microbiome and Metabolome Alterations in Children: A Comparative Cross-Sectional Study in Thailand. Front Microbiol 2018, 9, 1–16. [Google Scholar] [CrossRef]
- Therdtatha, P.; Shinoda, A.; Nakayama, J. Crisis of the Asian Gut: Associations among Diet, Microbiota, and Metabolic Diseases. Biosci Microbiota Food Health 2022, 41, 83–93. [Google Scholar] [CrossRef]
| Phylum | Order | Family | Genus | Specie | Reference |
|---|---|---|---|---|---|
| Firmicutes | Clostridiales | Clostridiaceae |
Faecalibacterium Clostridium |
[1,2] | |
| Eubacteriaceae | Eubacterium | [3] | |||
| Lachnospiraceae |
Blautia Roseburia Dorea Butyrivibrio |
Blautia obeum | [2,3,4,5] | ||
| Ruminococcaceae |
Ruminococcus Oscillospira |
Ruminococcus gnavus | [3,5,6] | ||
| Lactobacillales | Lactobacillaceae |
Lactobacillus | [7] | ||
| Bacillales | Leuconostocaceae | Weissella | [5] | ||
| Erysipelotrichales | Erysipelatoclostridiaceae | Catenibacterium | [7] | ||
| Veillonellales -Selenomonadales | Veillonellaceae | Megasphaera | [8] | ||
| Bacteroidetes | Bacteroidales | Bacteroidaceae | Bateroides |
Eubacterium callanderi Bacteroides fragilis |
[1,4] |
| Rikenellaceae | Alistipes | [3] | |||
| Prevotellaceae |
Prevotella Alloprevotella |
Prevotella copri Prevotella stercorea |
[1,2] | ||
| Proteobacteria | Bifidobacteriales | Bifidobacteriaceae | Bifidobacterium | Bifidobacterum catenulatum | [2,9] |
| Region | Countries in the Region | Reference |
|---|---|---|
| Central Asia | Kazakhstan, Kyrgyzstan, Tajikistan, Turkmenistan, Uzbekistan | [10] |
| Eastern Asia | China, Hong Kong, Macao (China), Japan, Mongolia, North Korea, South Korea | |
| Southern Asia | Afghanistan, Bangladesh, Bhutan, India, Iran, Maldives, Nepal, Pakistan, Sri Lanka | |
| South-Eastern Asia | Brunei, Cambodia, Indonesia, Laos, Malaysia, Myanmar, Philippines, Singapore, Thailand, Timor-Leste, Vietnam | |
| Western Asia | Armenia, Azerbaijan, Bahrain, Cyprus, Georgia, Iraq, Israel, Jordan, Kuwait, Lebanon, Oman, Qatar, Saudi Arabia, Palestine, Syrian Arab Republic, Turkey, United Arab Emirates |
| Country | Scientific name of seaweed | Food product | Reference |
|---|---|---|---|
| Japan |
Saccharina japonica S. longissima |
Kombu | [21] |
| Porphyra/Pyropia spp. | Nori | ||
| Undaria pinnatifida | Wakame | ||
| Cladosiphon okamuranus | Mozuku | ||
| Sargassum fusiforme | Hijiki | ||
| Ecklonia bicyclis | Arame | ||
| Gelidium amansii | Tengusa | ||
| South Korea | Pyropia spp. | Gim | [22] |
| Nemalion vermiculare | Chamguksunamul | ||
| Gelidium amansii | Umutkasari | ||
| Gloiopeltis furcata | Bultunggasari | ||
| G. tenax | Pulgasari | ||
| G. complanata | Aekipulgasari | ||
| Monostroma complex | Hotparae | ||
| Ulva complex | Parae | ||
| Capsosiphon fulvescens | Maesaengi | ||
| Codium fragile | Cheonggak | ||
| Scytosiphon lomentaria | Korimae | ||
| Ecklonia stolonifera | Kompi | ||
| Undaria pinnatifida | Miyok | ||
| Saccharina japonica | Dasim | ||
| Pelvetia siliquosa | Thumbugi | ||
| Sargassum fusiforme | Tot | ||
| S. fulvellum | Mojaban | ||
| S. Horneri | Kwaengsaegi-mojaban | ||
| World | Ulva lactuca | Sea lettuce | [23] |
| Chondrus crispus | Irish Moss | ||
| Himanthalia elongata | Sea Spaghetti |
| Fermented Product | Fermentative Microorganism | Reference |
|---|---|---|
| Douchi Tempeh Miso Tofu |
Aspergillus oryzae Mucor spp. Rhizopus spp. Fusarium spp. |
[58] |
| Natto Kinema Chungkookjang |
Bacillus | [58] |
| Doenjang |
Bacteria B. subtilis Fungi Rhizopus spp., Mucor spp., Geotrichum spp., and Aspergillus spp. |
[59,60] |
| Fermented soy milk |
Lactiplantibacillus plantarum |
[61] |
| Soya yogurt |
Levilactobacillus brevis L. plantarum |
[62] |
| Dietary Pattern | Characteristics | Reference |
|---|---|---|
| Vegan | Excludes all products of animal origin | Adopted and modified from [150] |
| Lacto-Ovo Vegetarians | Exclude red meat and poultry include dairy and eggs | |
| Raw vegan | Based vegetables, fruits, nuts, seeds, legumes, and grains. | |
| Pescatarian | Exclude red meat and poultry include eggs, dairy and fish | |
| Lacto-vegetarian | Exclude eggs, fish, red meat and poultry include dairy | |
| Semi-vegetarian | Consume red meat, poultry and fish no more than once a week |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





