Submitted:
17 September 2024
Posted:
19 September 2024
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Photocatalysts Preparation
2.3. Materials Characterization
3. Results and discussions
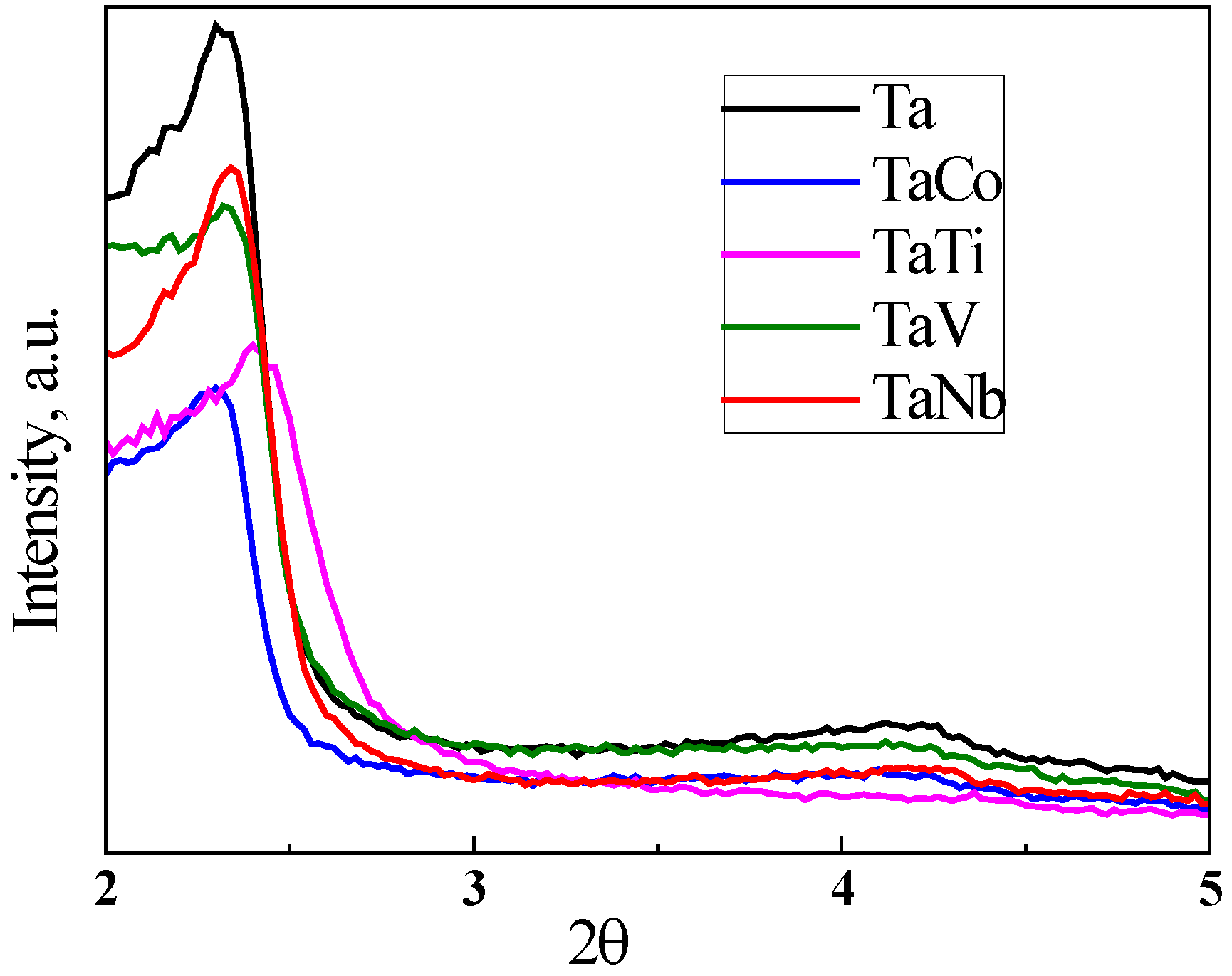
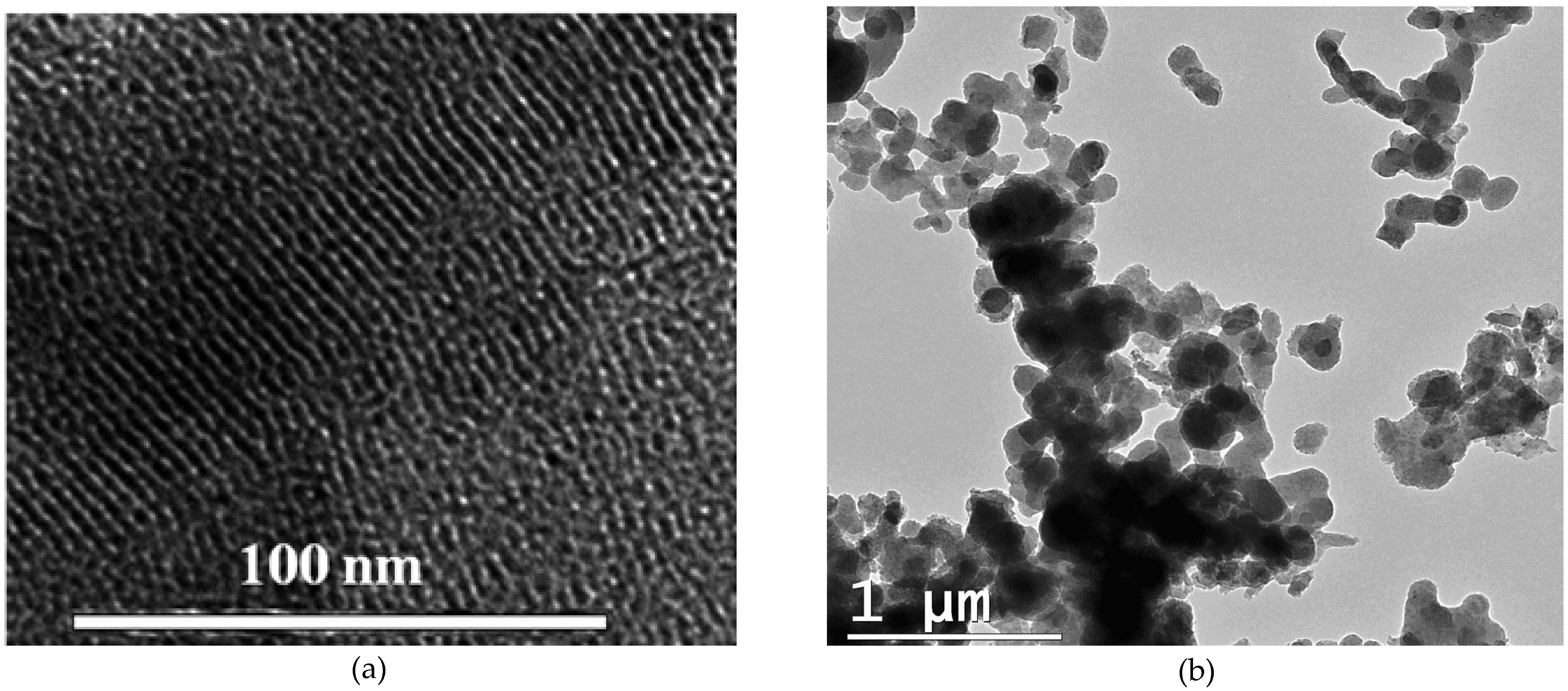
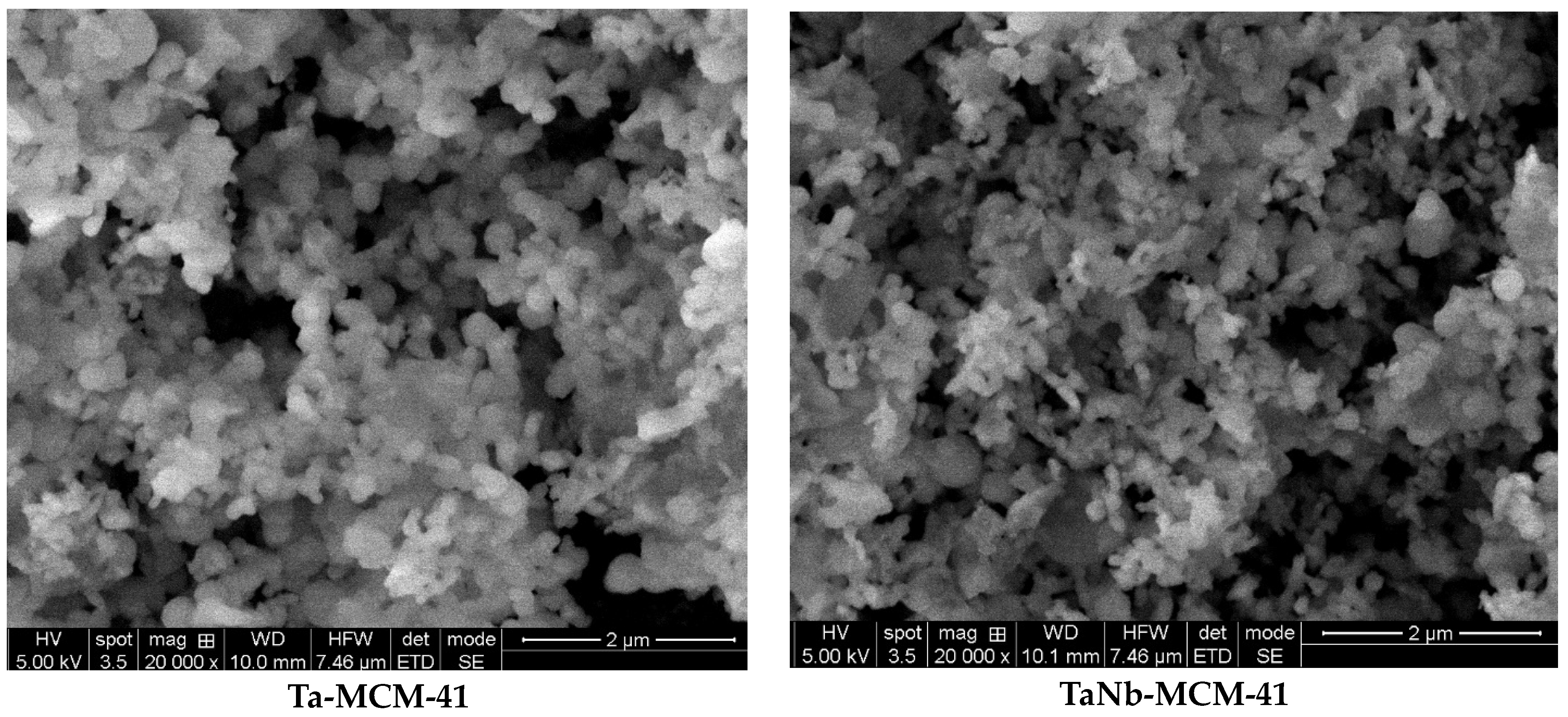
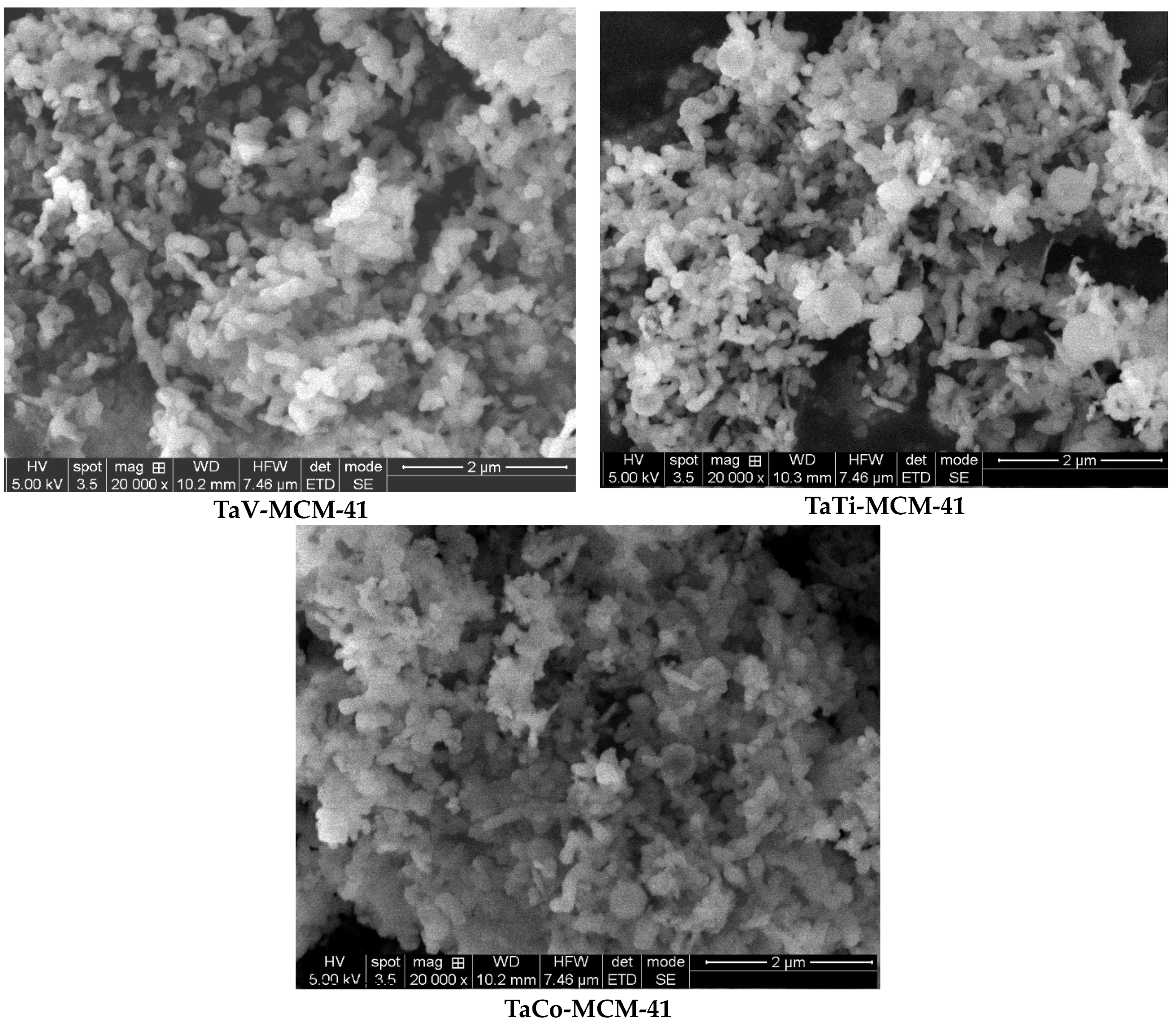
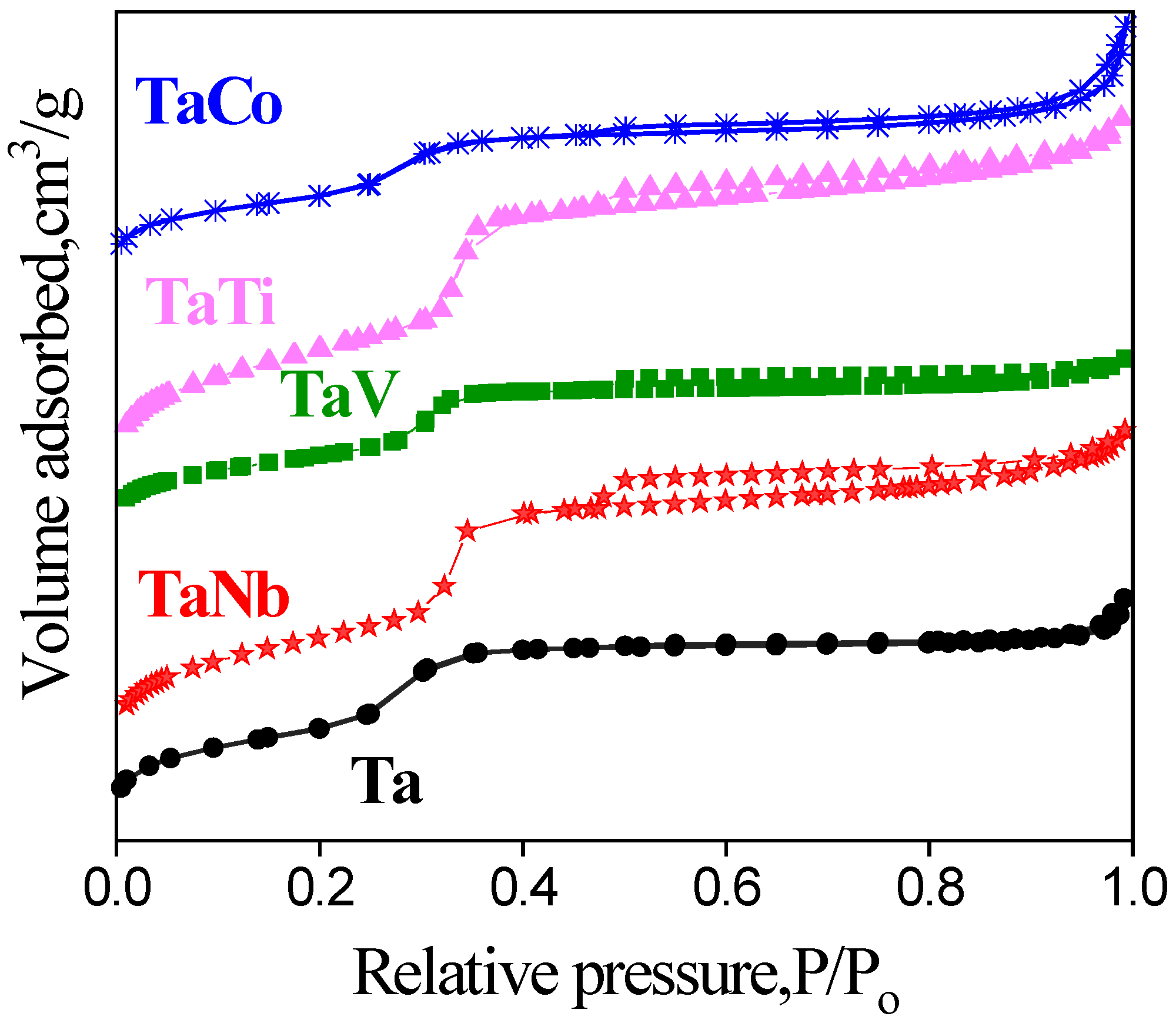
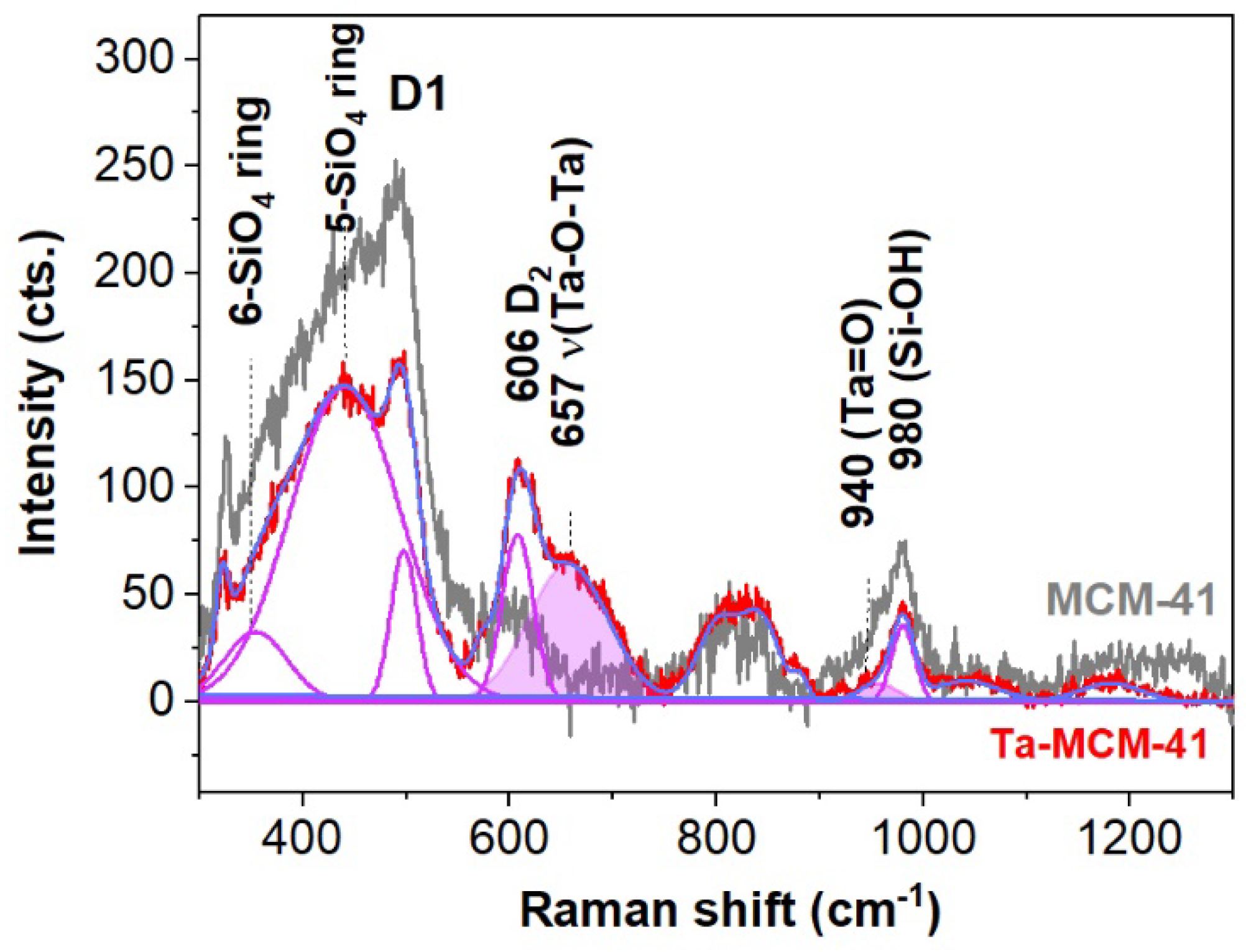
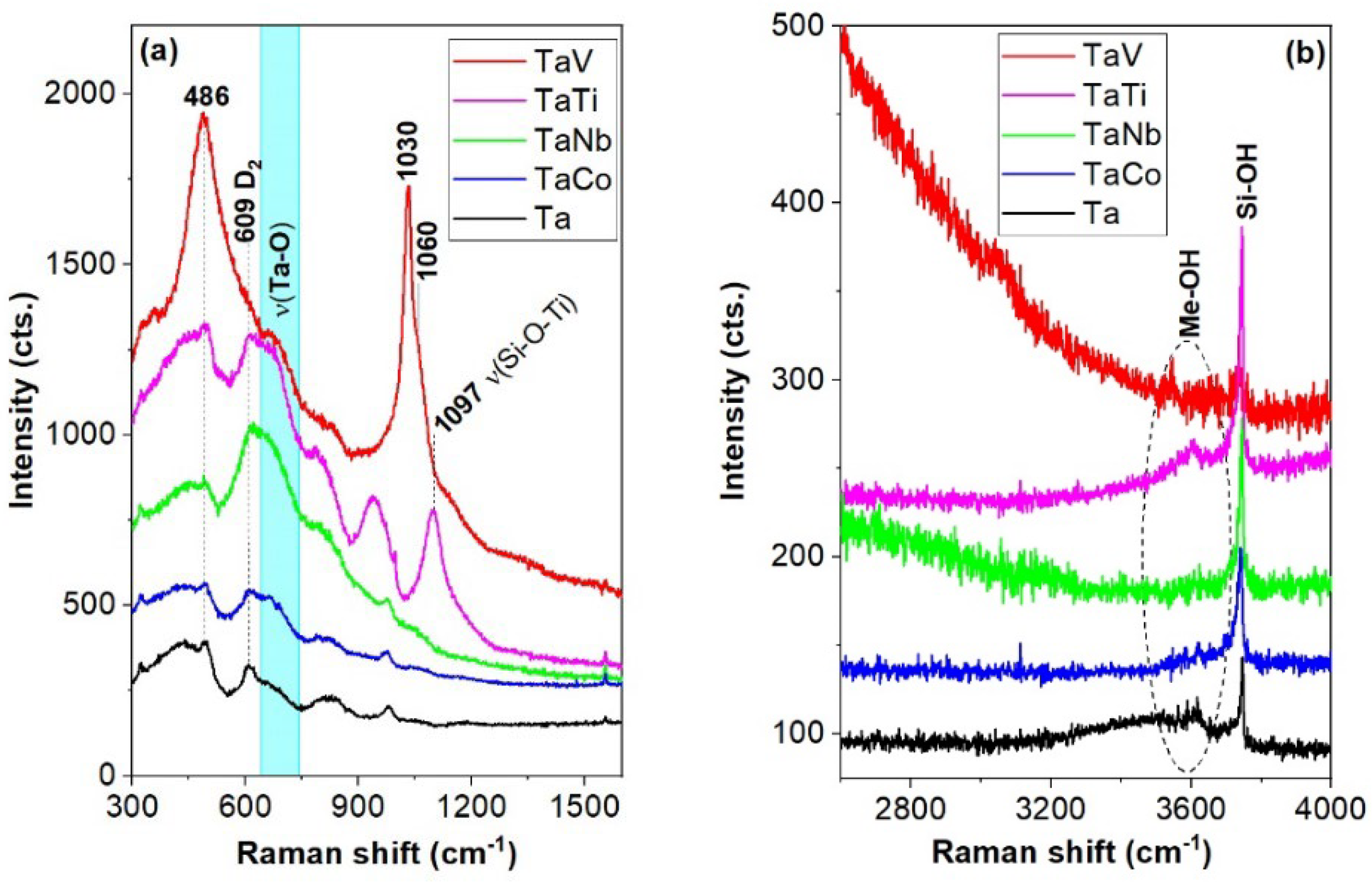
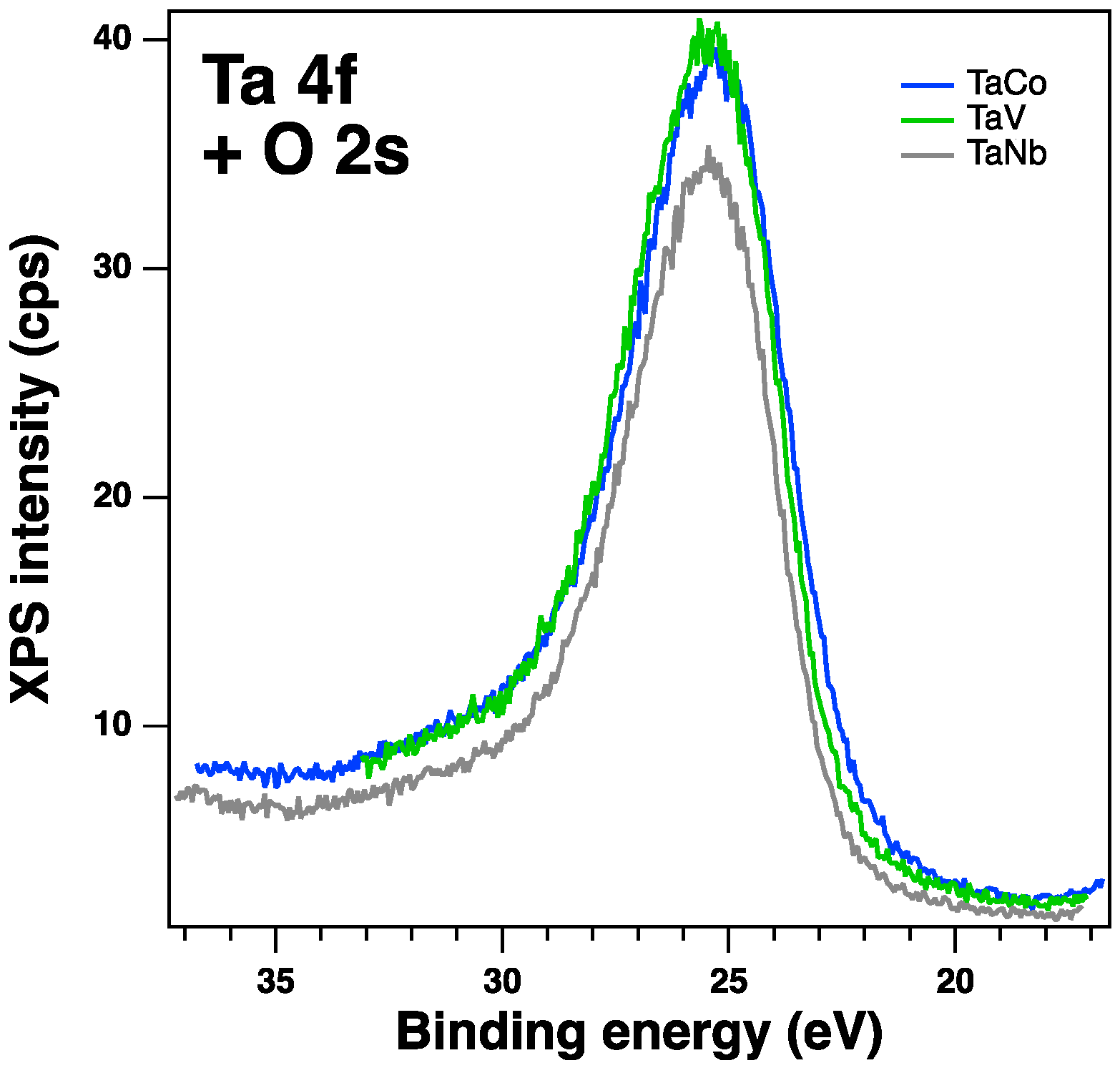
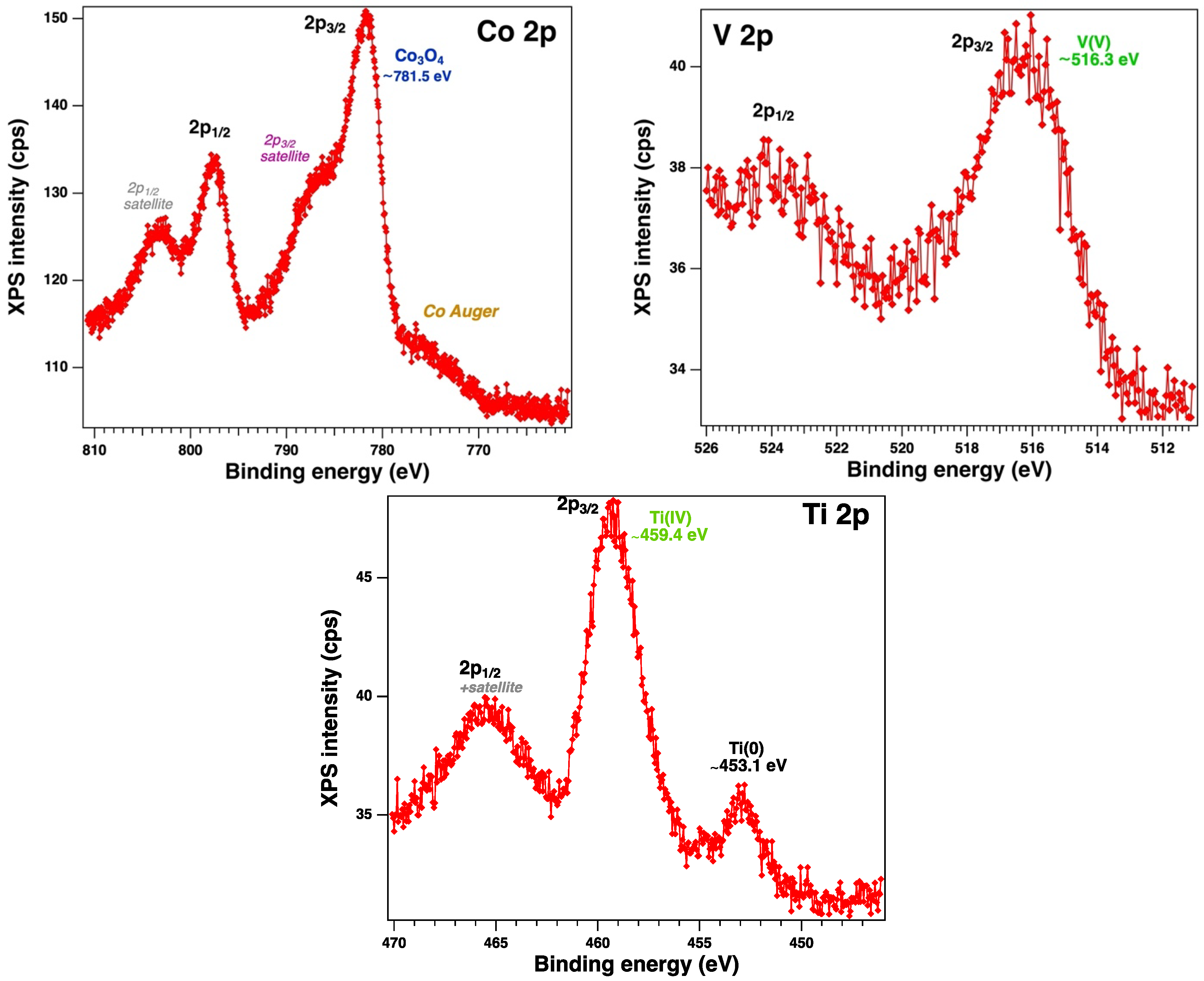
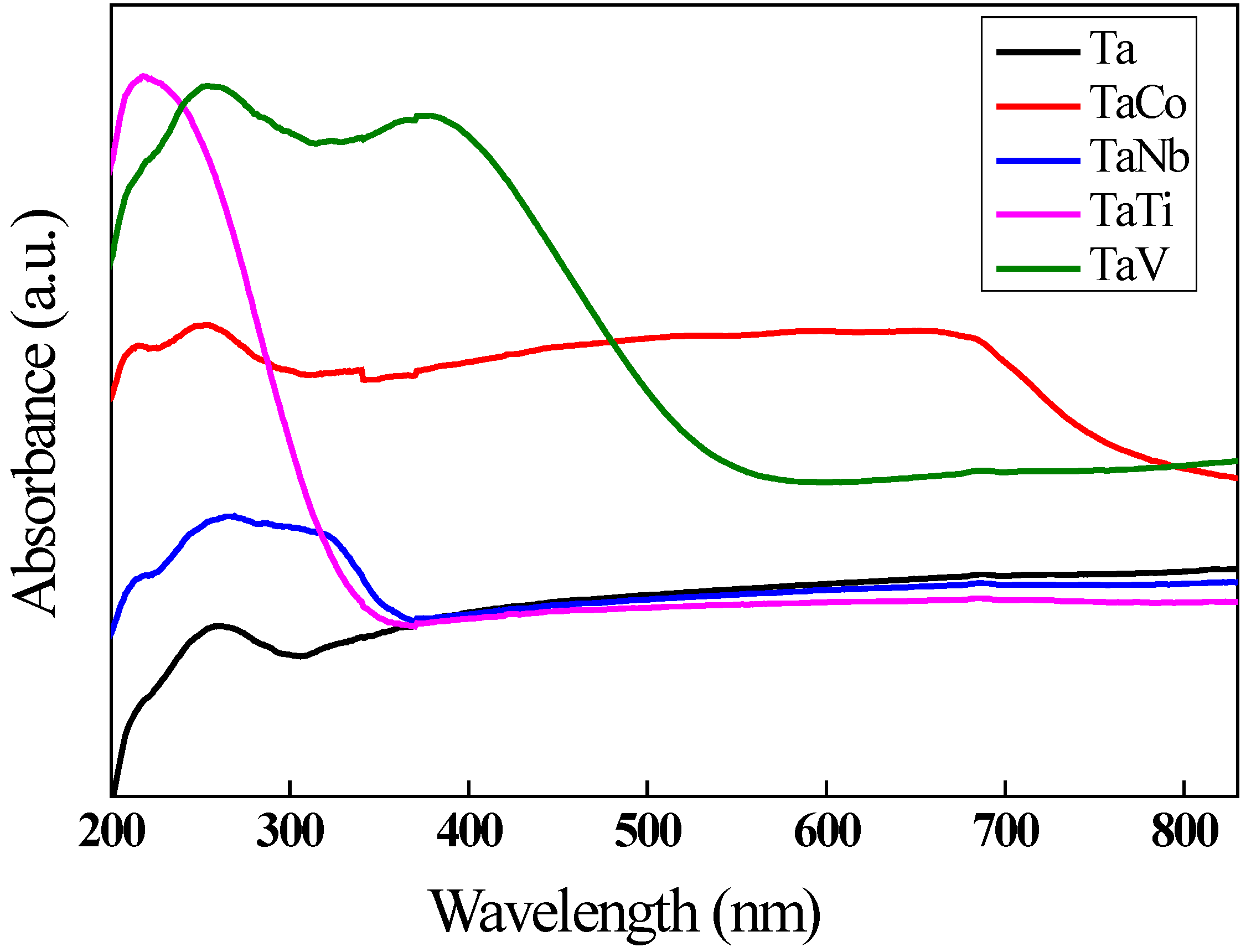
3.1. Characterization of materials
| Peak position (cm-1) | Assignments | Ref. | |||||
|---|---|---|---|---|---|---|---|
| Ta | TaNb | TaTi | TaCo | TaV | <650 cm-1 n-SiO4 rings | 34, 35 | |
| 355 | δ (O-V-O) | 36,37 | |||||
| 360 | 348 | 375 | 362 | 5,6,7-SiO4 rings | 34 | ||
| 441 | 447 | 435 | 5,6,7-SiO4 rings and Eg modes of the extra-framework rutile (445 cm-1) | 34, 35 | |||
| 497 | 496 | 497 | 495 | 488 | D1 modes (4- SiO4 rings), bending modes of the framework Ti-O-Si speciation | 34, 35 | |
| 608 | 608 | 607 | 612 | 609 | D2 modes (3- SiO4 rings) | 34 | |
| 615 | 616 | υ(Nb-O-Nb) polymerized Nb species (607-650 cm-1) and A1g modes in extra-framework rutile (612 cm-1) | [48] and 35 | ||||
| 660 | 685 | 670 | υ(Ta-O) in TaO6 and Co3O4 (690 cm-1) | 38, 39 | |||
| 689 | Nb2O5 | 48 | |||||
| 707 | |||||||
| 801 | 798 | 796 | υs modes of the siloxane bridges Si-O-Si | 34 | |||
| 828 | 830 | νs modes of the siloxane bridges Si-O-Si | 34 | ||||
| 958 | 945 | υs (Si-O-Ti/Nb) | 41 | ||||
| 980 | 978 | 985 | 973 | υ (Si-OH), ν(Si-NBO) in Q2 units, υ(Nb=O) of isolated NbO4 and TaOx species (965-980 cm-1) | 34,44,48 and 18 | ||
| 1031 | (SiO)3V=O stretching modes | 26 | |||||
| 1060 | Shorter V=O bonds | 47 | |||||
| 1050 | 1056 | 1065 | Q4 units in silica framework | 34,44 | |||
| 1097 | υas (Si-O-Ti) with Ti4+ | 35 | |||||
| 1185 | 1164 | 1187 | |||||
| >3500 cm-1 (hydroxyl stretching modes) | |||||||
| 3617 | 3602 | 3623 | 3547 | Me-OH and free H2O | 41 | ||
| 3746 | 3744 | 3744 | 3744 | 3738 | Isolated Si-OH in MCM-41 | 42 | |
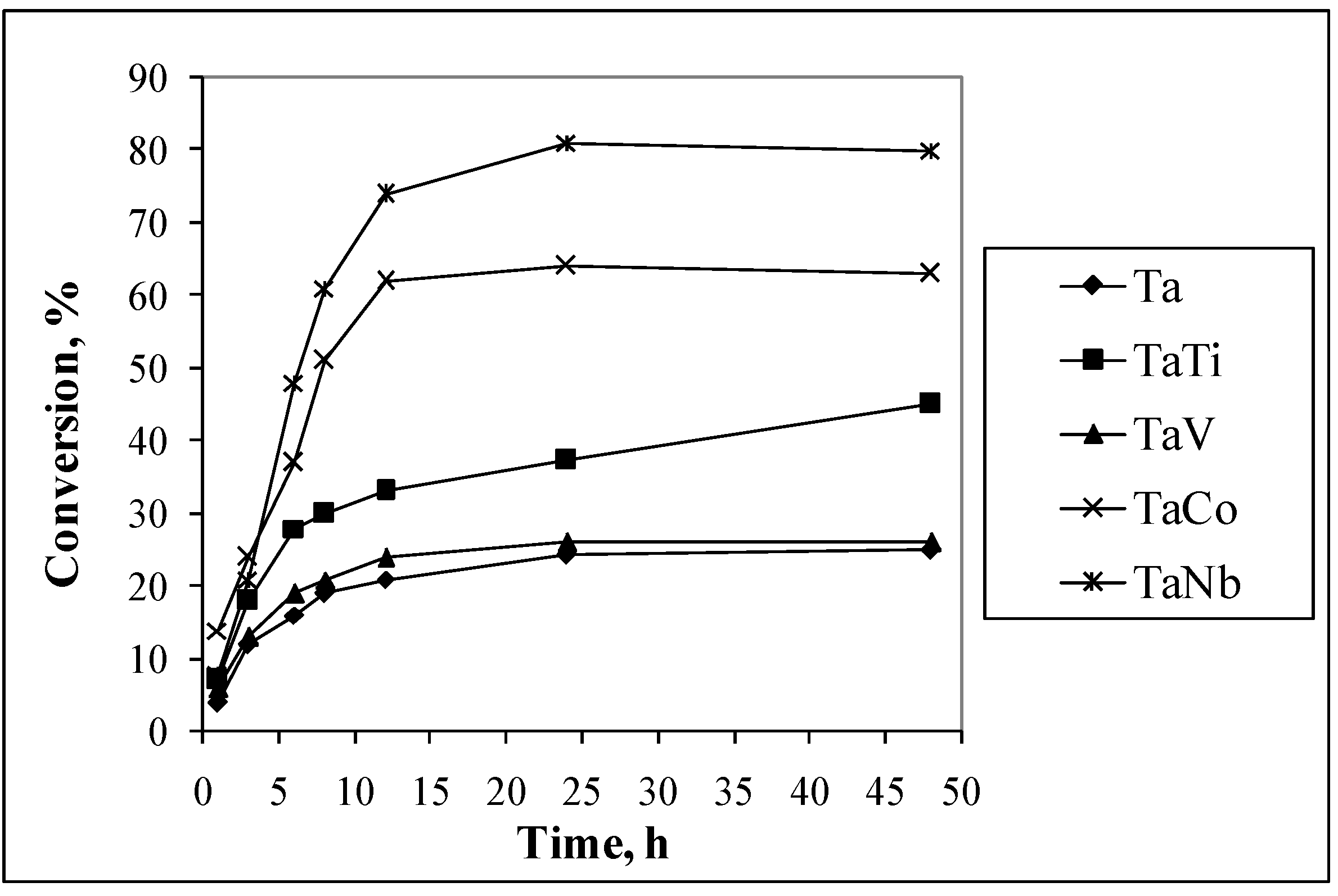
3.2. Catalytic Properties
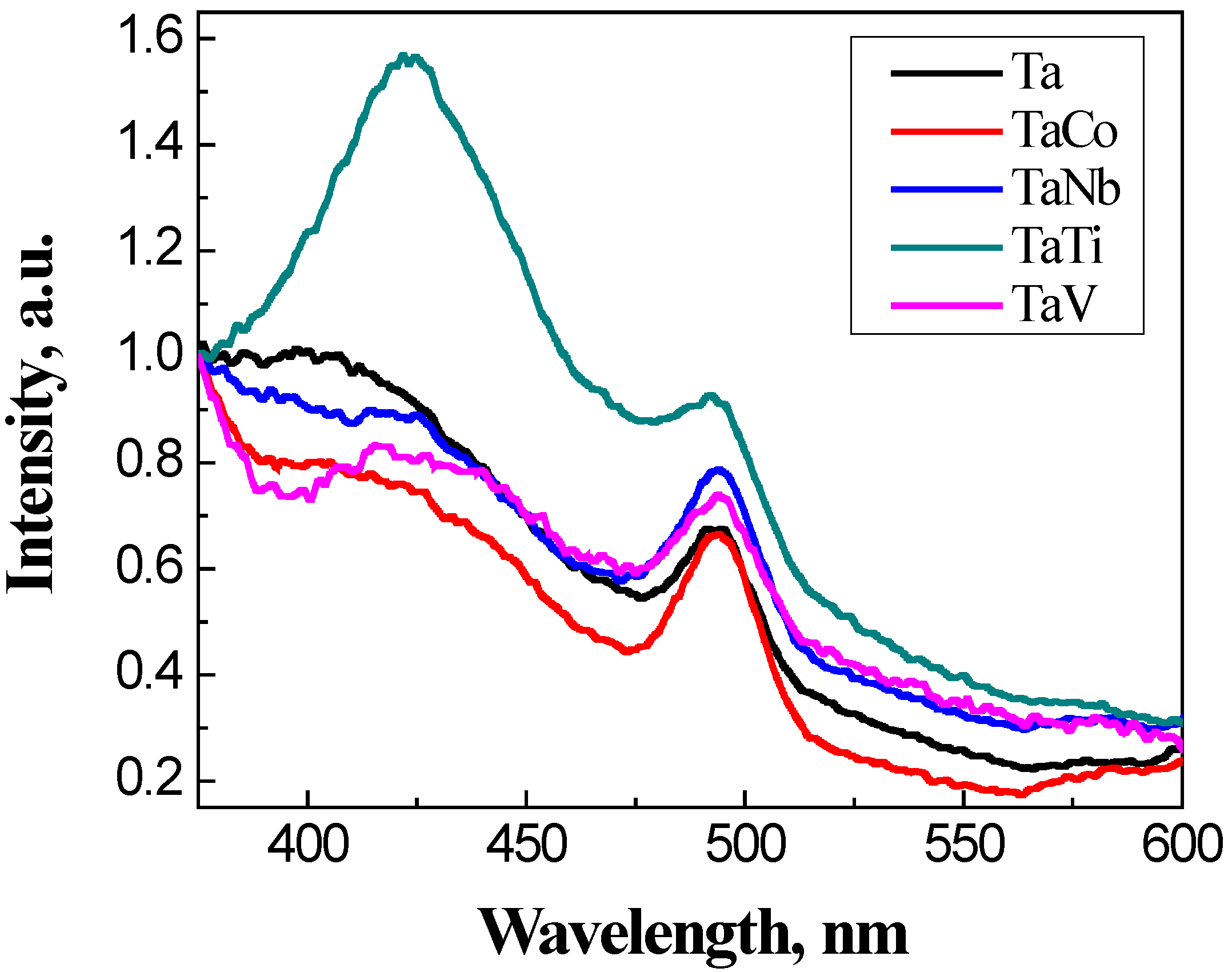
3.3. Photocatalytic Properties
4. Conclusions
Supplementary Materials
Author Contributions
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Morais, L.A.; Castro, F. L.; Fernandes, G.J.T.; Araujo, M.D.S.; Farias, M.F.; Guedes, A.P.M.A.; Fernandes Jr., V.J.; Araujo, A.S. Synthesis and Characterization of MCM-41 Nanomaterials Containing Titanium and Application for Catalytic Oxidation of BTEX. Catalysis Research 2023, 3, 17. [CrossRef]
- Hachemaoui, M.; Molina, C.B.; Belver, C.; Bedia, J.; Mokhtar, A.; Hamacha, R.; Boukoussa, B. Metal-Loaded Mesoporous MCM-41 for the Catalytic Wet Peroxide Oxidation (CWPO) of Acetaminophen. Catalysts 2021, 11, 219. [CrossRef]
- Peng, W.; Cai, L.; Lu, Y.; Zhang, Y. Preparation of Mn-Co-MCM-41 Molecular Sieve with Thermosensitive Template and Its Degradation Performance for Rhodamine B. Catalysts 2023, 13, 991. [CrossRef]
- Sahoo, D.P.; Rath, D.; Nanda, B.; Parida, K.M. Transition metal/metal oxide modified MCM-41 for pollutant degradation and hydrogen energy production: a review. RSC Adv. 2015, 5, 83707-83724. [CrossRef]
- Schlichter, S.; Sapag, K.; Dennehy, M.; Alvarez, M. Metal-based mesoporous materials and their application as catalysts for the degradation of methyl orange azo dye. J. Environ. Chem. Eng. 2017, 5, 5207-5214. [CrossRef]
- Todorova, S.; Parvulescu, V.; Kadinov, G.; Tenchev, K.; Somacescu, S.; Su, B.-L. Metal states in cobalt- and cobalt-vanadium-modified MCM-41 mesoporous silica catalysts and their activity in selective hydrocarbons oxidation. Micropor. Mesopor. Mat. 2008, 113, 22–30. [CrossRef]
- Kilos, B.; M. Nowak, A.I.; Ziolek M.; J.C. Volta, J.C. The role of niobium in the gas- and liquid-phase oxidation on metallosilicate MCM-41-type materials. J. Catal. 2004, 224, 314-325. [CrossRef]
- Parvulescu, V.; Tablet, C.; Anastasescu, C.; Su, B.L. Activity and stability of bimetallic Co (V, Nb, La)-modified MCM-41 catalysts. Catal. Today 2004, 93-95, 307-313. [CrossRef]
- Parvulescu, V.; Anastasescu, C.; Su, B.L. Vanadium incorporated mesoporous silicates as catalysts for oxidation of alcohols and aromatics. J. Mol. Catal. A: Chem. 2003, 198, 249-261. [CrossRef]
- Genel, S.; Durak, H.; Genel Y.; Catalytic effect of metal powder and MCM-41/metal catalysts on the pyrolysis of cellulose. Environ. Prog. Sustain. 2024, 43, 14225. [CrossRef]
- Parvulescu, V.; Anastasescu, C.; Su, B. L. Bimetallic Ru-(Cr, Ni, or Cu) and La-(Co or Mn) incorporated MCM-41 molecular sieves as catalysts for oxidation of aromatic hydrocarbons. J. Mol. Catal. A: Chem. 2004, 211, 143-148. [CrossRef]
- Parvulecu, V. 2-Catalytic behavior of metal active sites from modified silicas in oxidation of organic compounds. In Redox, 2019, editor Khattak R., IntechOpen, pp 1-25. [CrossRef]
- Fernandes de Oliveira, T.; Pereira de Souza, C.; Lopes-Moriyama, A. L.; Pereira da Silva M. L. In situ modification of MCM-41 using niobium and tantalum mixed oxide from columbite processing for methylene blue adsorption: Characterization, kinetic, isotherm, thermodynamic and mechanism study. Mater. Chem. Phys. 2023, 294, 127011. [CrossRef]
- Dubiel, W.; Kowalczyk, A.; Jankowska, A.; Michalik, M.; Mozgawa, W.; Kobielusz, M.; Macyk, W.; Chmielarz, L. Silica-titania mesoporous silicas of MCM-41 type as effective catalysts and photocatalysts for selective oxidation of diphenyl sulfide by H2O2. Green Process. Synth. 2023, 12, 20230052. Doi: 10.1515/gps-2023-0052.
- Arellano, U.; Wang, J.A.; Chen, L.F.; Asomoza, M.; Guzmán, A.; Solís, S.; Estrella, A.; Cipagauta, S.; Noreña, L.E. Transition metal oxides dispersed on Ti-MCM-41 hybrid core-shell catalysts for the photocatalytic degradation of Congo red colorant. Catal. Today 2020, 349, 128-140. [CrossRef]
- Nguyen, V.H.; Lin, S.D.; Wu, J. C.-S. Synergetic photo-epoxidation of propylene over V-Ti/MCM-41 mesoporous photocatalysts. J. Catal. 2015, 331, 217–227. [CrossRef]
- Brutchey, R. L.; Lugmair, C.G.; Schebaum, L.O.; Tilley, T.D. Thermolytic conversion of a bis(alkoxy)tris(siloxy)tantalum(V) single-source molecular precursor to catalytic tantala–silica materials. J. Catal. 2005, 229, 72–81. [CrossRef]
- Jehng, J.M.; Tung, W.C.; Huang, C.H.; Wachs, I.E. Structural characteristics and reactivity properties of the tantalum modified mesoporous silicalite (MCM-41) catalysts. Microporous Mesoporous Mat. 2007, 99, 299–307. [CrossRef]
- Oliveira, T.F.; Pereira da Silva, M.L.; Lopes-Moriyama, A.L.; Pereira de Souza, C. Facile preparation of ordered mesoporous Nb, Ta-MCM-41 by hydrothermal direct synthesis using columbite ore as metal source. Ceram. Int. 2021, 47, 29509–29514. [CrossRef]
- Lee, B.; Yamashita, T.; Lu, D.; Kondo, J.N.; Domen, K. Single-Crystal Particles of Mesoporous Niobium-Tantalum Mixed Oxide. Chem. Mater. 2002, 14, 867-875. [CrossRef]
- Ziolek, M.; Nowak, I.; Characterization techniques employed in the study of niobium and tantalum-containing materials. Catal. Today 2003, 78, 543-553. [CrossRef]
- Takahara, Y.; Kondo, J.N.; Lu, D.; Domen, K. Synthesis and application for overall water splitting of transition metal-mixed mesoporous Ta oxide. Solid State Ion. 2002, 151, 305-311. [CrossRef]
- Yang, X.; Roy, A.; Alhabradi, M.; Alruwaili, M.; Chang, H.; Tahir, A.A. Fabrication and Characterization of Tantalum–Iron Composites for Photocatalytic Hydrogen Evolution. Nanomaterials 2023, 13, 2464. [CrossRef]
- Oliveira, T.F.; de Souza, C.P.; Lopes-Moriyama, A.L.; Acid leaching and thermal treatments in the obtaining of mixed oxides of Nb and Ta from ferrocolumbite. Miner. Eng. 2020, 147,106157. [CrossRef]
- Talukdar, H.; Saikia, G.; Das, A.; Sultana, S.Y.; Islam, N.S. Organic-solvent-free oxidation of styrene, phenol and sulfides with H2O2 over eco-friendly niobium and tantalum based heterogeneous catalysts. J. Ind. Eng. Chem. 2023, 121, 249–263. [CrossRef]
- Paul, R.; Kavinarmatha, K.; Parthiban S. Tantalum doped titanium dioxide nanoparticles for efficient photocatalytic degradation of dyes. J. Mol. Struct. 2023, 1277, 134869. [CrossRef]
- Fadhlia, M.; Khedhera, I.; José M. Fraile J.M. Modified Ta/MCM-41 catalysts for enantioselective oxidation of thioanisole. J. Mol. Catal. A: Chem. 2015, 410, 140–148. [CrossRef]
- Fadhli, M.; Khedher, I.; Fraile, J.M. Comparison of Ta-MCM-41 and Ti-MCM-41 as catalysts for the enantioselective epoxidation of styrene with TBHP. C R Chim. 2017, 20, 827-832. [CrossRef]
- Cimpeanu, V.; Pârvulescu, V.; Pârvulescu, V.I.; Capron, M.; Grange, P.; Thompson, J.M.; Hardacre, C. Selective oxidation of a pyrimidine thioether using supported tantalum catalysts. J. Catal. 2005, 235, 184-194. [CrossRef]
- Shindhal, T.; Rakholiya, P.; Varjani, S.; Pandey, A.; Ngo, H.H.; Guo, W.; Ng, H.Y. Taherzadeh, M.J. A critical review on advances in the practices and perspectives for the treatment of dye industry wastewater. Bioengineered 2021, 12, 70–87. [CrossRef]
- Makuła, P.; Pacia, M.; Macyk,W. How to correctly determine the band gap energy of modified semiconductor photocatalysts based on UV−Vis spectra. J. Phys. Chem. Lett. 2018, 9, 6814–6817. [CrossRef]
- Teodorescu, C.M.; Esteva, J.M.; Karnatak, R.C.; El Afif, A. An approximation of the Voigt I profile for the fitting of experimental X-ray absorption data. Nucl. Instrum. Meth. Phys. Res. A 1994, 345, 141–147. [CrossRef]
- Al Ebraheem, J.S.; Alkhoder, M.N.A.; Tulaimat, R.H. Synthesis and characterization of mesoporous V–Mo-MCM-41 nanocatalysts: Enhancing efficiency in oxalic acid synthesis. Heliyon 2024, 10, 24652. [CrossRef]
- Thu, J.; Dutta, P.K.; Kresge, C.T. Raman spectroscopic studies of the synthesis of faujasitic zeolites: Comparison of two silica sources. Zeolites 1991, 11, 672-679. [CrossRef]
- Jin, S.; Feng, Z.; Fan, F.; Li, C. UV Raman Spectroscopic Characterization of Catalysts and Catalytic Active Sites. Catal Lett 2015, 145, 468–481. [CrossRef]
- Lewandowska, A.E.; Banares, M.A.; Tielens, F.; Che, M.; Dzwigaj, S. Different Kinds of Tetrahedral V Species in Vanadium-Containing Zeolites Evidenced by Diffuse Reflectance UV-vis, Raman, and Periodic Density Functional Theory. J. Phys. Chem. C 2010, 114, 19771–19776. [CrossRef]
- Zhang, Q.; Wang, Y.; Ohishi, Y.; Shishido, T.; Takehira, K. Vanadium-Containing MCM-41 for Partial Oxidation of Lower Alkanes. J. Catal. 2002, 202, 308–318. [CrossRef]
- Wetherall, K.M.; Doughty, P.; Mountjoy, G.; Bettinelli, M.; Speghini, A.; Casula, M.F.; Cesare-Marincola, F.; Locci, E.; Newport, R. J. The atomic structure of niobium and tantalum containing borophosphate glasses. J. Phys.: Condens. Matter 2009, 21, 375106. [CrossRef]
- Aspromonte, S.G.; Sastre, Á.; Boix, A.V.; Cocero, M.J.; Alonso, E. Cobalt oxide nanoparticles on mesoporous MCM-41 and Al-MCM-41 by supercritical CO2 deposition. Micropor. Mesopor. Mater. 2012, 148, 53-61. [CrossRef]
- Yu, J.; Feng, Z.; Xu, L.; Li, M.; Xin, Q.; Liu, Z.; Li, C. Ti-MCM-41 Synthesized from Colloidal Silica and Titanium Trichloride: Synthesis, Characterization, and Catalysis. Chem. Mater. 2001, 13, 994-998. [CrossRef]
- Nitsche, D.; Hess, C. Structure of Isolated Vanadia and Titania: A Deep UV Raman, UV−Vis, and IR Spectroscopic Study. J. Phys. Chem. C 2016, 120, 1025−1037. [CrossRef]
- Malfait, B.; Moréac, A.; Jani, A.; Lefort, R.; Huber, P.; Fröba, M.; Morineau, D. Structure of Water at Hydrophilic and Hydrophobic Interfaces: Raman Spectroscopy of Water Confined in Periodic Mesoporous (Organo)Silicas. J. Phys. Chem. C, 2022, 126, 3520-3531. [CrossRef]
- Kondratenko, E.V.; Cherian, M.; Baerns, M.; Su, D.; Schlögl, R.; Wang, X.; Wachs, I.E. Oxidative dehydrogenation of propane over V/MCM-41 catalysts: comparison of O2 and N2O as oxidants. J. Catal. 2005, 234, 131–142. [CrossRef]
- Petrescu, S.; Constantinescu, M.; Anghel, E.M.; Atkinson, I.; Olteanu, M.; Zaharescu, M. 52. Structural and physico-chemical characterization of some soda lime zinc alumino-silicate glasses. J Non-Crystall Solids 2022, 358, 3280-3288. [CrossRef]
- Lee, E.L.; Wachs, I.E. In Situ Spectroscopic Investigation of the Molecular and Electronic Structures of SiO2 Supported Surface Metal Oxides, J. Phys. Chem. C 2007, 111, 14410-14425. [CrossRef]
- Liu, W.-S.; Liao, M.-W.; Huang, S.-H.; Reyes, Y. L. A.; Chen, H.-Y.T.; T.-P. Perng, T.-P. Formation and characterization of gray Ta2O5 and its enhanced photocatalytic hydrogen generation activity. Int. J. Hydr Energ 2020, 45, 16560-16568. [CrossRef]
- Xiong, G.; Li, C.; Li, H.; Xin, Q.; Feng, Z. Direct spectroscopic evidence for vanadium species in V-MCM-41 molecular sieve characterized by UV resonance Raman spectroscopy. Chem. Commun., 2000, 677–678. [CrossRef]
- Gao, X.; Wachs, I.E.; Wong, M.S.; Ying, J.Y. Structural and Reactivity Properties of Nb–MCM-41: Comparison with That of Highly Dispersed Nb2O5/SiO2 Catalysts. J. Catal. 2001, 203, 18–24. [CrossRef]
- Suib, S. L.; Prěch, J.; Szaniawska, E.; Čejka, J. Recent Advances in Tetra- (Ti, Sn, Zr, Hf) and Pentavalent (Nb, V, Ta) Metal-Substituted Molecular Sieve Catalysis. Chem. Rev. 2023, 123, 877−917. [CrossRef]
- Wachs, I.E.; Chen,Y.; Jehng,J.-M.; Briand, L.E.; Tanaka, T. Molecular structure and reactivity of the Group V metal oxides. Catal. Today 2003, 78, 13–24. [CrossRef]
- Pedersen, C.S.; Chang, J.H.; Li, Y.; Pryds, N.; Garcia Lastra, J. M. Phase separation in amorphous tantalum oxide from first principles. APL Mater. 2020, 8, 071108. [CrossRef]
- Qiyang Lu, G. How to Correctly Analyze 2p X-ray Photoelectron Spectra of 3d Transition-Metal Oxides: Pitfalls and Principles, ACS Nano 2024, 18, 13973−13982. [CrossRef]
- Prokopenko, V.B.; Gurin, V.S.; Alexeenko, A.A.; Kulikauskas, V.S.; D L Kovalenko, D.I. Surface segregation of transition metals in sol–gel silica films. J. Phys. D: Appl. Phys. 2000, 33, 3152–3155. [CrossRef]
- Jiangfeng, L.; Xiaoling, L.; Junying, L.; Ximing P., Wang, J.; Huang, Z.; Yin, G.; Effects of incorporated vanadium and its chemical states on morphology and mesostructure of mesoporous bioactive glass particles. Microp. Mesop. Mater. 2021, 319, 111061. [CrossRef]
- Chen, J.Y.; Leng, Y.X.; Zhang, X.; Yang, P.; Sun, H.; Wang, J.; Wan, G.J.; Zhao, A.S.; Huang, N.; Chu, P.K. Effect of tantalum content of titanium oxide film fabricated by magnetron sputtering on the behavior of cultured human umbilical vein endothelial cells (HUVEC). Nucl. Instrum. Methods Phys. Res. B 2006, 242, 26–29. [CrossRef]
- Martinez, M.; Chourasia, A.R. Characterization of Ti/SnO2 Interface by X-ray Photoelectron, Spectroscopy. Nanomater. 2022, 12, 202. [CrossRef]
- Suib, S. L.; Prěch, J.; Szaniawska, E.; Čejka, J. Recent Advances in Tetra- (Ti, Sn, Zr, Hf) and Pentavalent (Nb, V, Ta) Metal-Substituted Molecular Sieve Catalysis, Chem. Rev. 2023, 123, 877−917. [CrossRef]
- Dupin, J.-C.; Danielle Gonbeau,D.; Vinatier, P.; Levasseur, A. Systematic XPS studies of metal oxides, hydroxides and peroxides. Phys. Chem. Chem. Phys. 2000, 2, 1319-1324. [CrossRef]
- Chao, K. J.; Wu, C. N.; Chang, H. Incorporation of Vanadium in Mesoporous MCM-41 and Microporous AFI Zeolites. J. Phys. Chem. B 1997, 101, 6341-6349. [CrossRef]
- Galacho, C.; Ribeiro Carrott, M.; Carrott, Peter; Cansado, I. Hydrothermal Stability of Ordered Mesoporous Titanosilicate Materials Prepared at Room Temperature. Adv. Mater. Research 2010, 107, 63-70. [CrossRef]
- Garcia, L. M. P.;Tavares, M. T. S.; Andrade Neto, N. F.; Nascimento, R. M.; · Paskocimas, C. A.; ·Longo, E.; Bomio, M. R. D.; Motta, F. V. Photocatalytic activity and photoluminescence properties of TiO2, In2O3,TiO2/In2O3 thin films multilayer. J. Mater. Sci: Mater Electron 2018, 29, 6530–6542. [CrossRef]
- Chen, Y.; Zhou, X.; Zha, X.; He, X.; Gu, X. Crystallite structure, surface morphology and optical properties of In2O3– TiO2 composite thin films by sol–gel method. Mater. Sci. Eng. B. 2008, 151, 179–186. [CrossRef]
- Liu, Y.; Xu, L.; Xin, Y.; Liu, F.; Xuan, J.; Guo, M.; Duan, T. IrO2-Ta2O5 Anode for Oxygen Evolution with TaOx Interlayer Prepared by Thermal Decomposition in Inert Atmosphere. J. Electrochem. Soc. 2022, 169, 046516. Doi: 10.1149/1945-7111/ac65bd.
- Thornburg, N.E.; Thompson, A.B.; Notestein J.M.; Periodic Trends in Highly Dispersed Groups IV and V Supported Metal Oxide Catalysts for Alkene Epoxidation with H2O2. ACS Catal. 2015, 5, 5077−5088. [CrossRef]
- Parvulescu, V.I; Visinescu, C.; Parvulescu, V.; Marcu, V.; Levy, F. Comparative photocatalytic behavior of Ta catalysts prepared by d.c.-sputtering, sol–gel and grafting in acetone degradation. Catal. Today 2006, 118, 433–439. [CrossRef]
- Hassan, N.S.; Jalil, A.A.; Hitam, C.N.C.; Sawal, M.H.; Rahim, M.N.S.; Hussain, I.; Jusoh, N.W.C.; Saravanan, R.; Prasetyoko, D. Enhanced photooxidative desulphurization of dibenzothiophene over fibrous silica tantalum: Influence of metal-disturbance electronic band structure. Int. J. Hydrog. Energy 2023, 48, 6575-6585. [CrossRef]
- Emeline, A.V.; Zhang, X.; Murakami, T.; Fujishima, A. Activity and selectivity of photocatalysts in photodegradation of phenols. J. Hazardous Mat. 2012, 211– 212, 154– 160. [CrossRef]
- Dionysiou, D. D.; Suidan, M. T.; Baudin, I.; Laıné, J.-M. Effect of hydrogen peroxide on the destruction of organic contaminants-synergism and inhibition in a continuous-mode photocatalytic reactor. Appl.Catal. B: Environ 2004, 50, 259–269. Doi: 10.1016/j.apcatb.2004.01.022.
- Cheng, Y.; Cao, T.; Xiao, Z.; Zhu, H.; Yu, M. Photocatalytic Treatment of Methyl Orange Dye Wastewater by Porous Floating Ceramsite Loaded with Cuprous Oxide. Coatings 2022, 12, 286. [CrossRef]
- Din, S.T.U.; Xie, W.-F.; Yang, W. Synthesis of Co3O4 Nanoparticles-Decorated Bi12O17Cl2 Hierarchical Microspheres for Enhanced Photocatalytic Degradation of RhB and BPA. Int. J. Mol. Sci. 2022, 23, 15028. [CrossRef]
- Petcu, G.; Anghel, E.M.; Atkinson, I.; Culita, D.C.; Apostol, N.G.; Kuncser, A.; Papa, F.; Baran, A.; Blin, J.-L.; Parvulescu, V. Composite Photocatalysts with Fe, Co, and Ni Oxides on Supports with Tetracoordinated Ti Embedded into Aluminosilicate Gel during Zeolite Y Synthesis. Gels 2024, 10, 129. [CrossRef]
- Makota, O.; Dutková, E.; Briancin, J.; Bednarcik, J.; Lisnichuk, M.; Yevchuk, I.; Melnyk, I. Advanced Photodegradation of Azo Dye Methyl Orange Using H2O2-Activated Fe3O4@SiO2@ZnO Composite under UV Treatment. Molecules 2024, 29, 1190. [CrossRef]
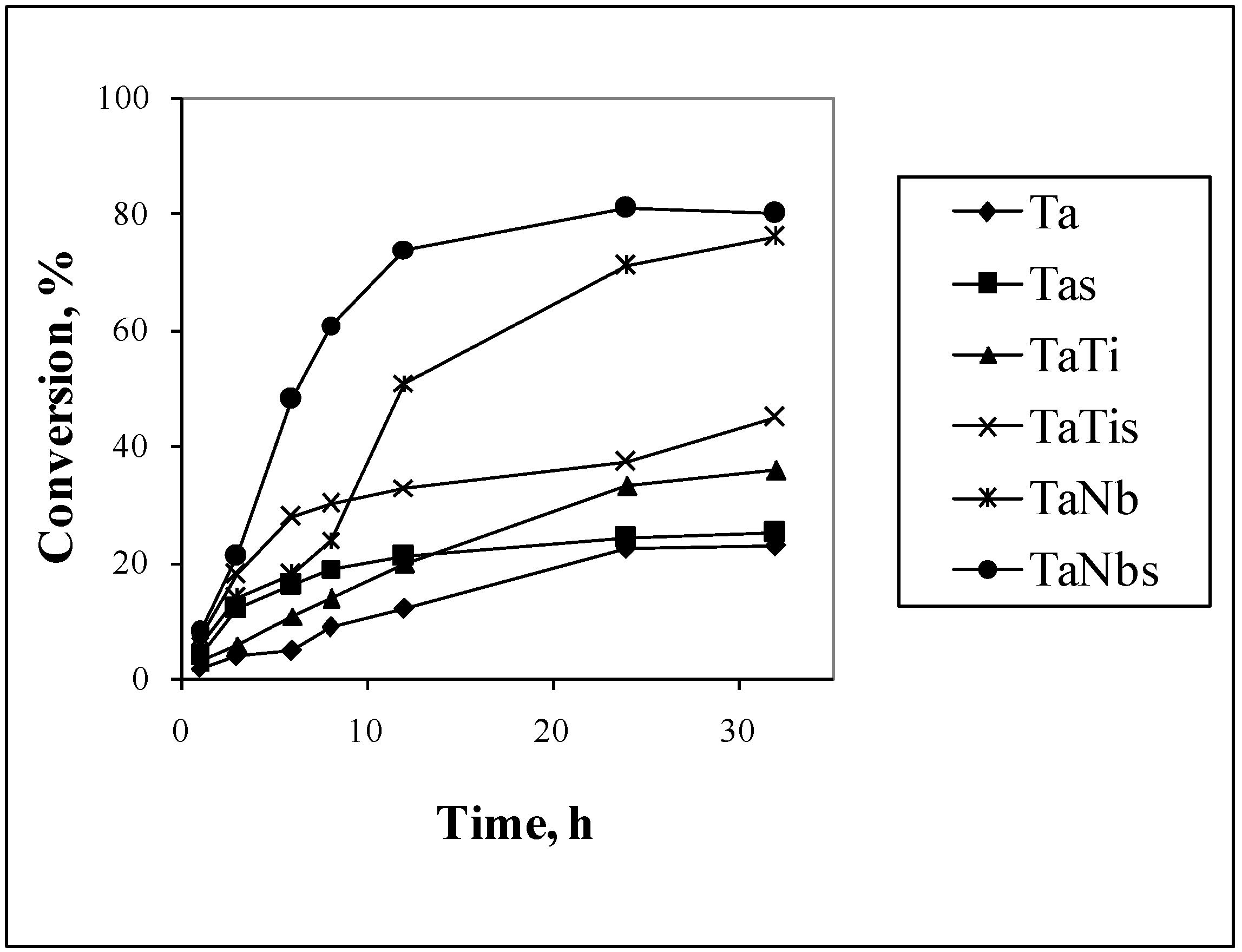
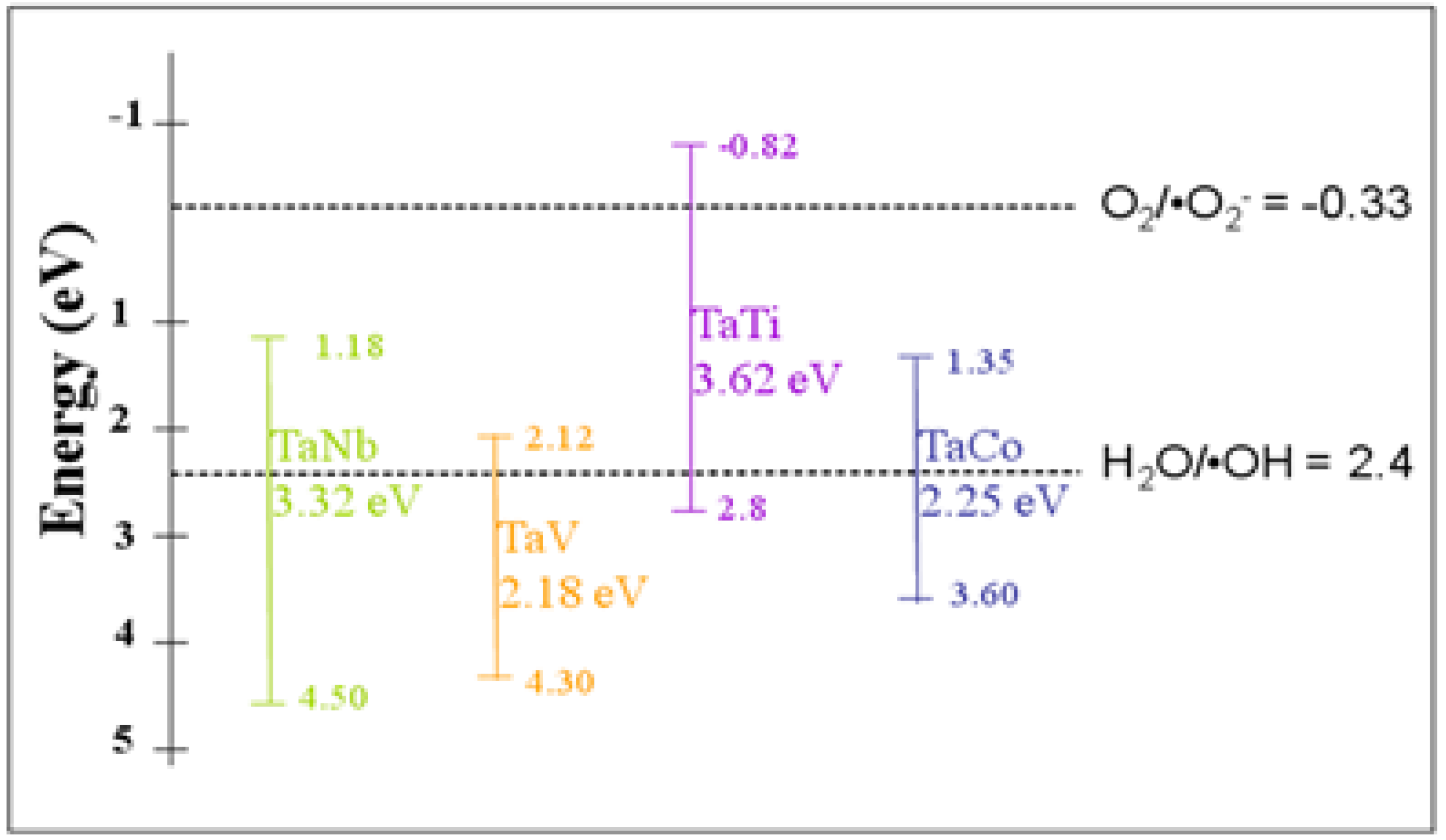
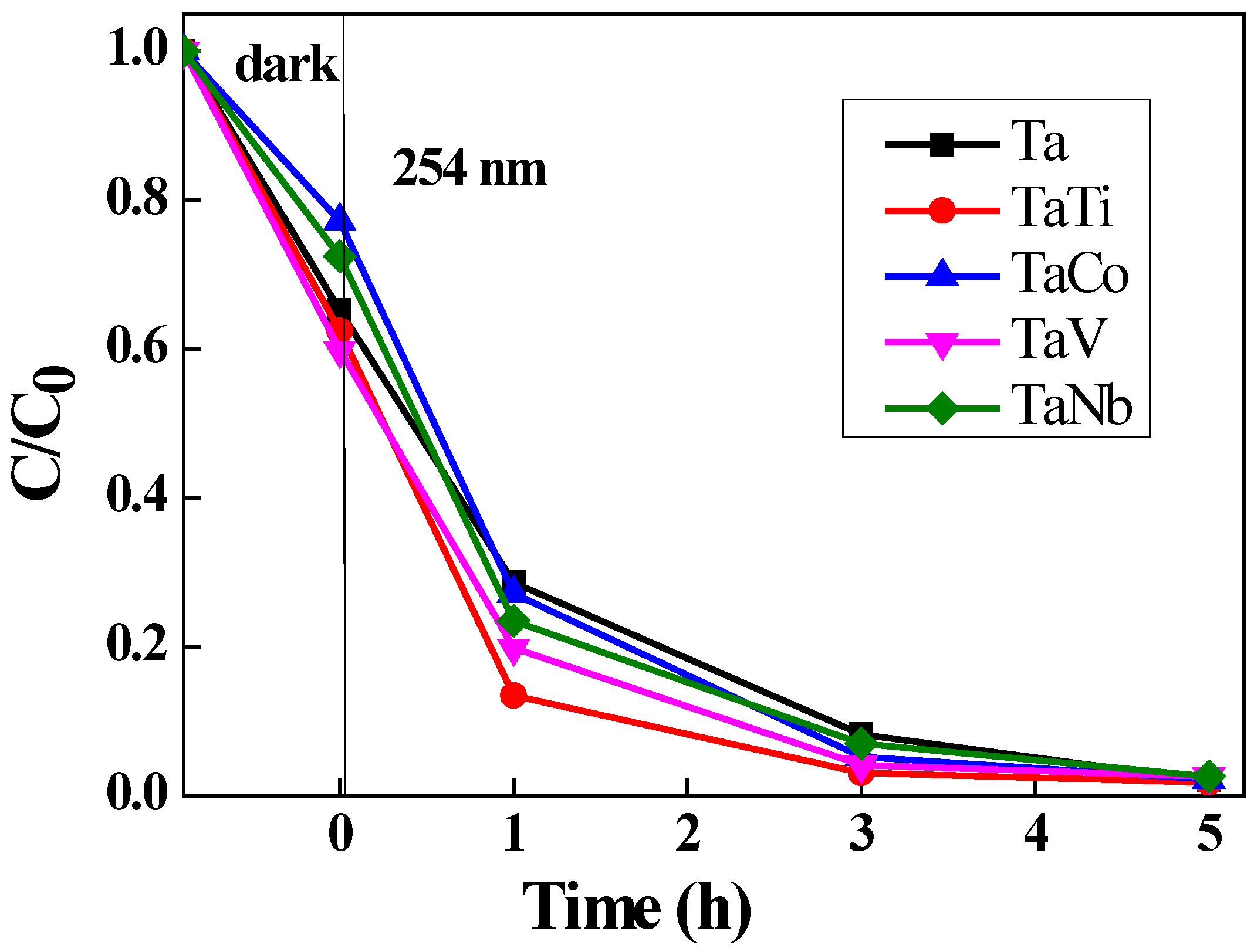
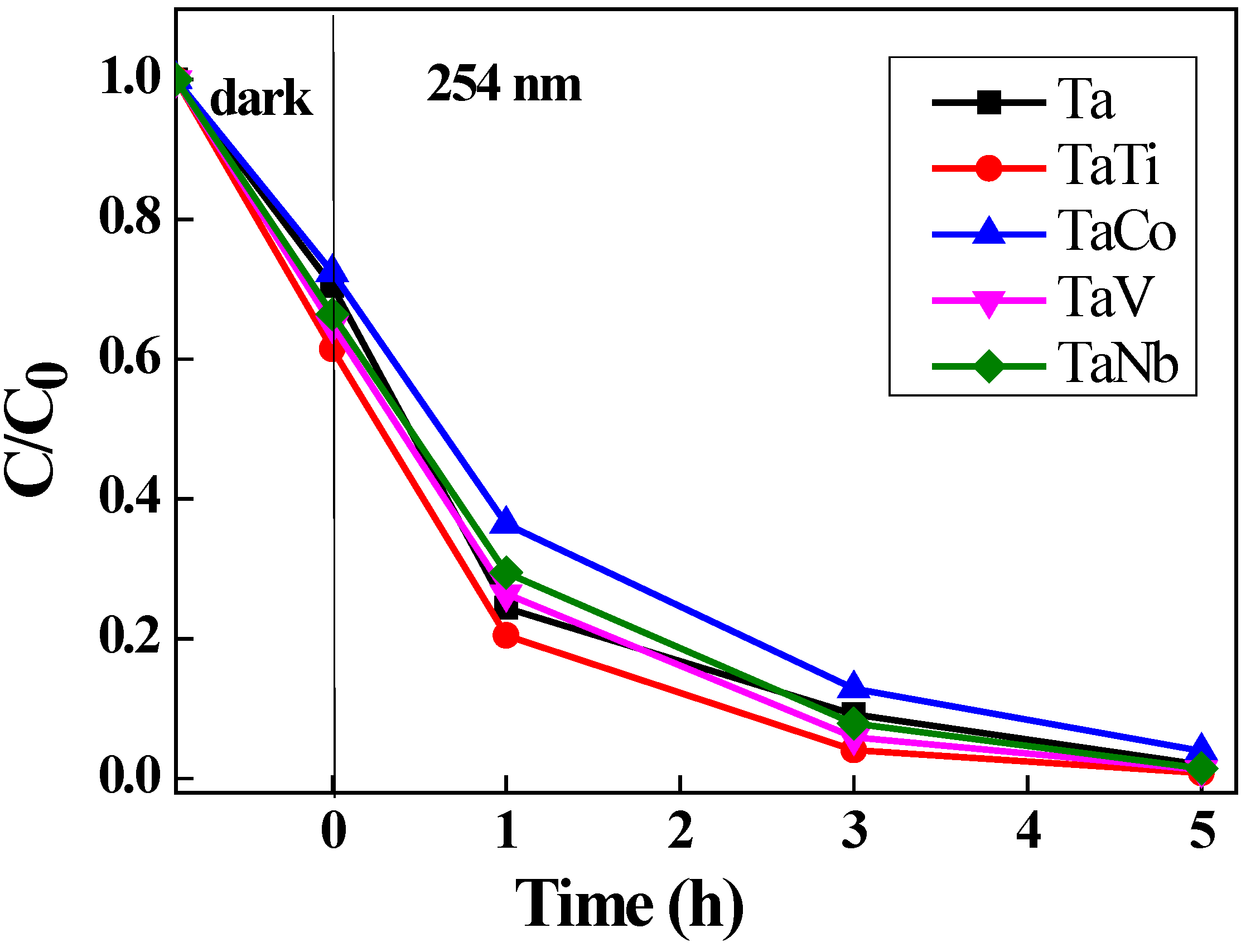
| Sample | Sample code | Ta, % | Nb, % | V, % | Ti, % | Co, % | Ta/Meb |
|---|---|---|---|---|---|---|---|
| Ta-MCM-41 | Ta | 5.36 | - | - | - | - | - |
| TaNb-MCM-41 | TaNb | 5.36 | 3.28 | - | - | - | 0.829 |
| TaV-MCM-41 | TaV | 5.55 | - | 1.96 | - | - | 0.789 |
| TaTi-MCM-41 | TaTi | 5.34 | - | - | 4.23 | - | 0.329 |
| TaCo-MCM-41 | TaCo | 3.33 | - | - | - | 2.44 | 0.445 |
| Sample | Ta | TaNb | TaV | TaTi | TaCo |
|---|---|---|---|---|---|
| SBET (m2/g) | 867 | 856 | 862 | 826 | 817 |
| V BJH (cm3/g) | 0.877 | 0.942 | 0.894 | 1.012 | 1.025 |
| D BJH (nm) | 2.9 | 3.1 | 2.8 | 3.0 | 3.9 |
| Eg (eV) | 5.64 | 3.32 | 2.18 | 3.62 | 2.25 |
| Sample | 1,4 Cyclohexadiene | Cyclohexene | Styrene | |||
|---|---|---|---|---|---|---|
| C (%) | SHOL (%) | C (%) | SHO (%) | C (%) | SSO (%) | |
| Ta | 25.2 | 91.2 | 28.1 | 25.2 | 48.1 | 21.2 |
| Ta Nb | 81.1 | 85.2 | 65.7 | 71.2 | 76.4 | 65.0 |
| TaV | 36.1 | 84.5 | 32.4 | 78.9 | 89.0 | 66.8 |
| TaTi | 37.4 | 98.6 | 56,3 | 89.4 | 95.0 | 62.6 |
| TaCo | 64.0 | 89.0 | 64.2 | 84.4 | 78.2 | 71.2 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





