Submitted:
13 September 2024
Posted:
16 September 2024
You are already at the latest version
Abstract
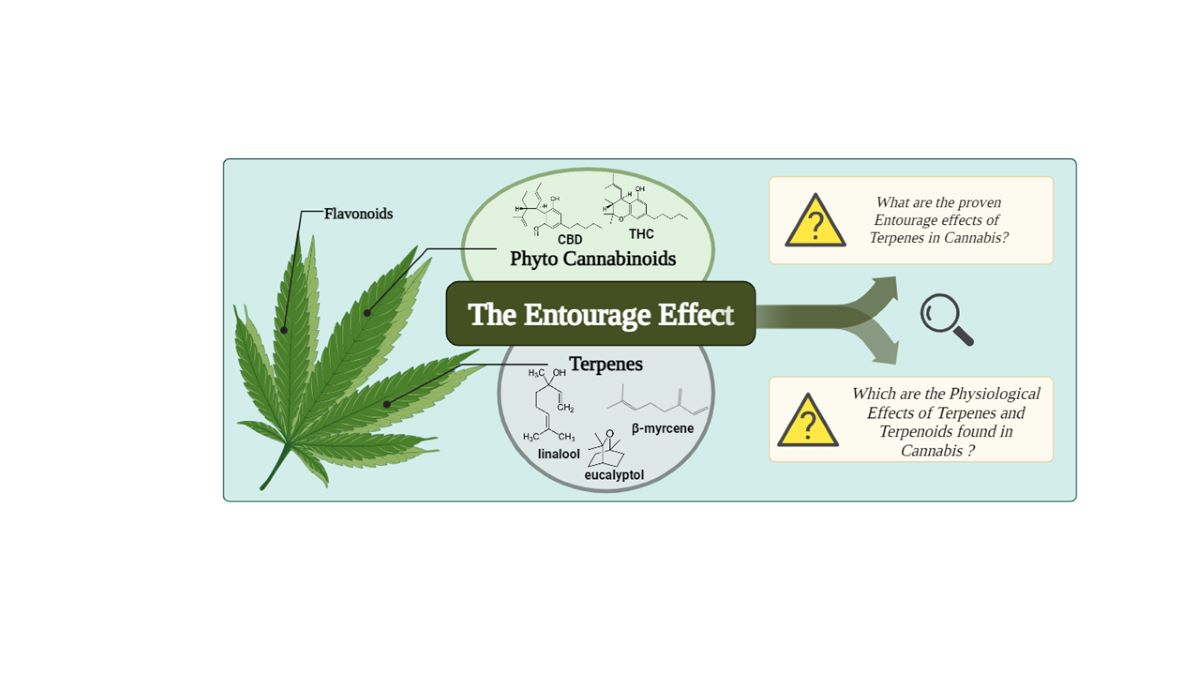
Keywords:
1. Introduction
1.1. The Entourage Effects Concepts
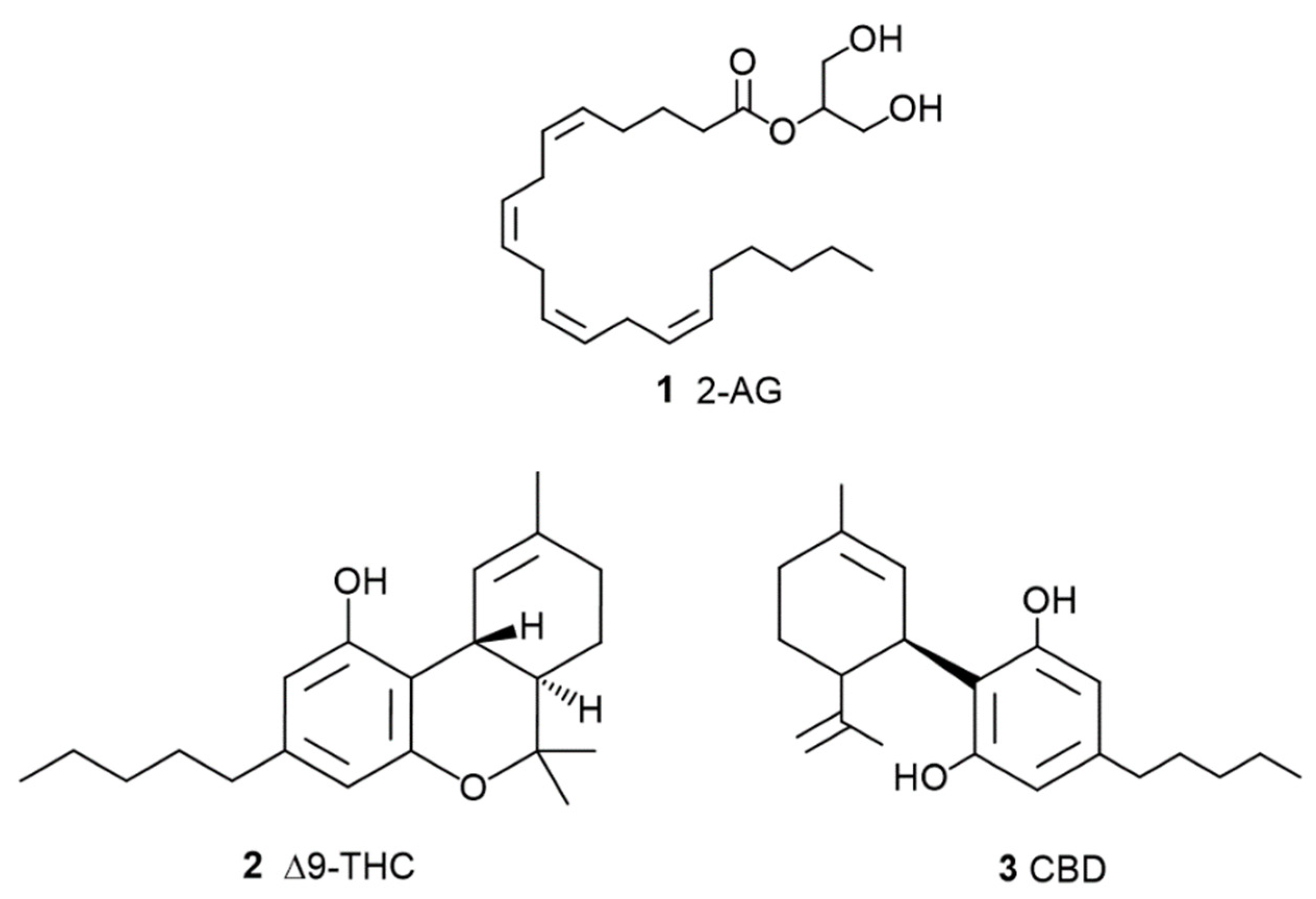
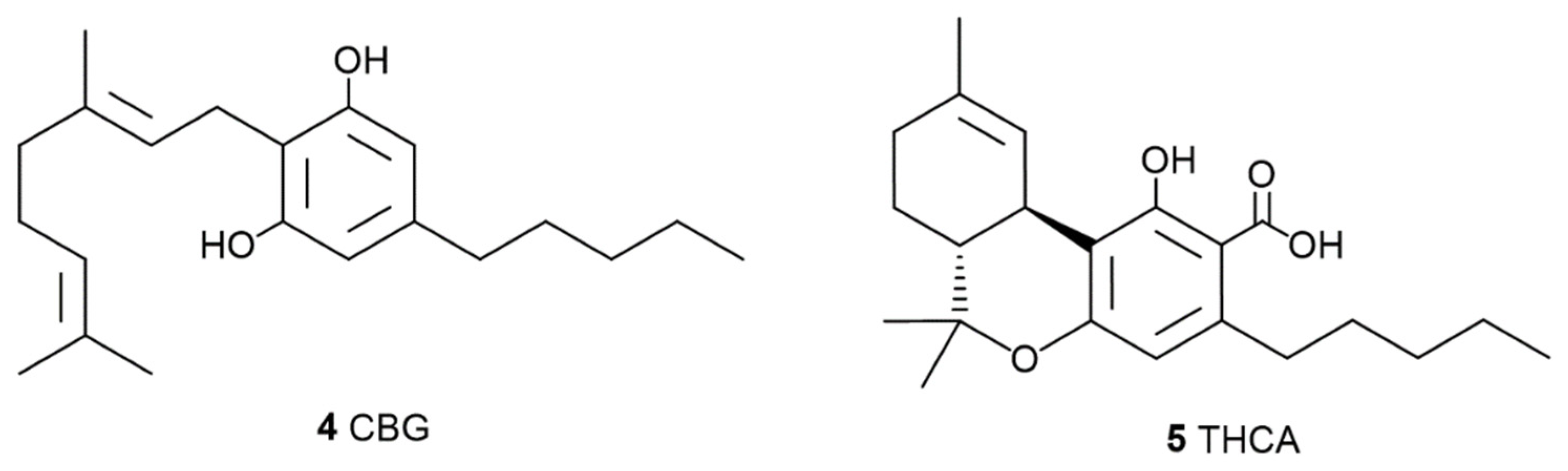
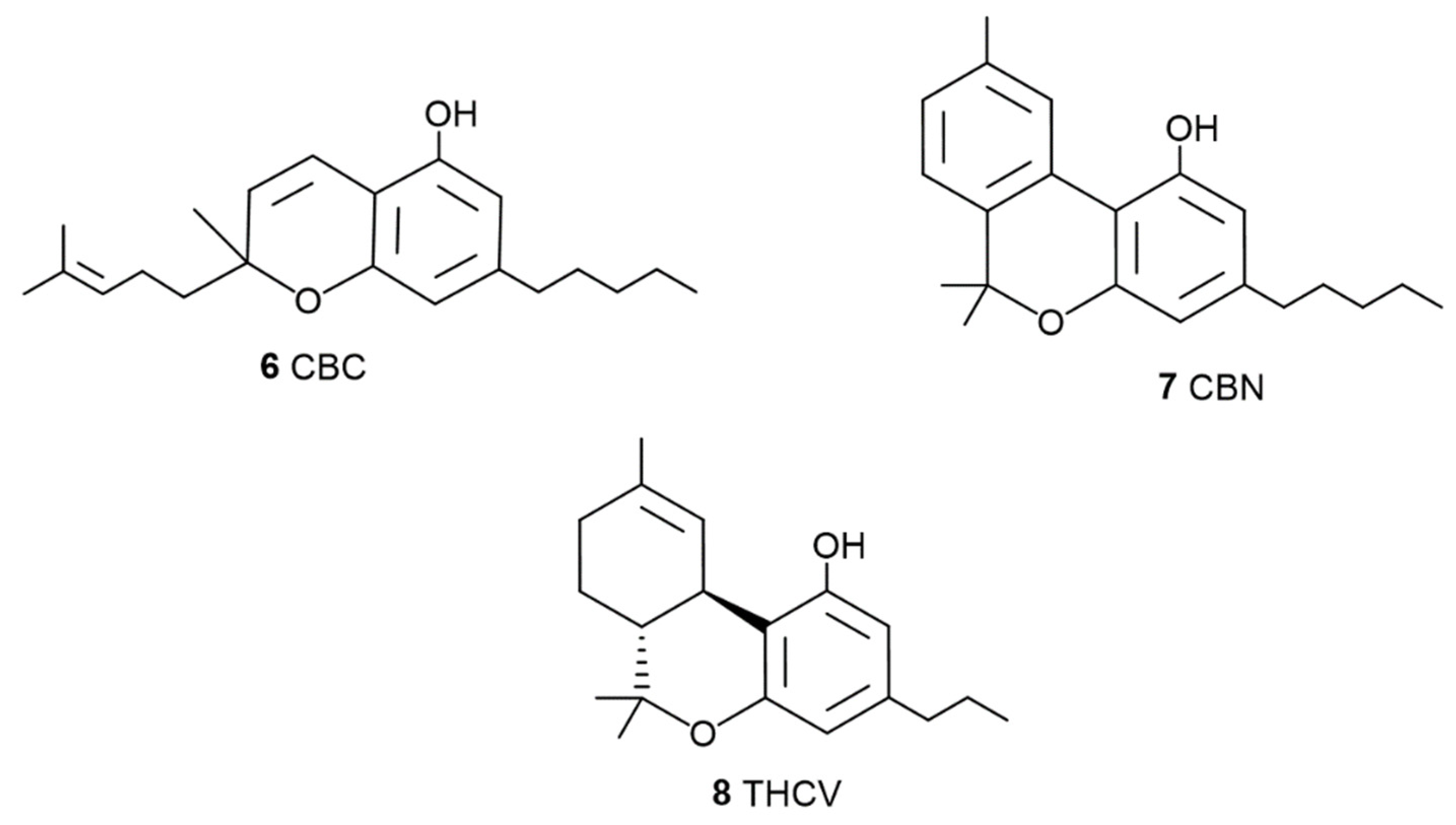
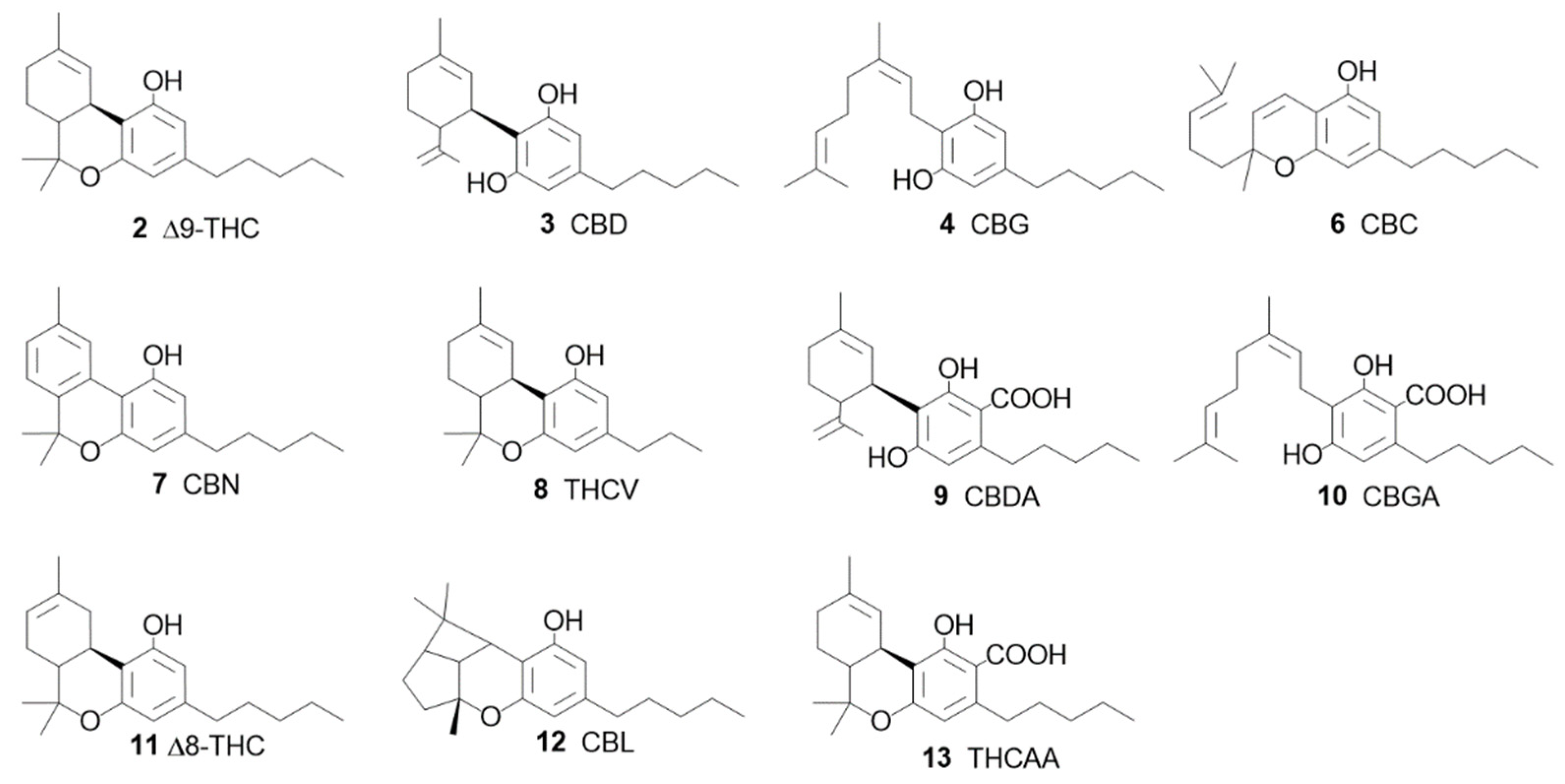
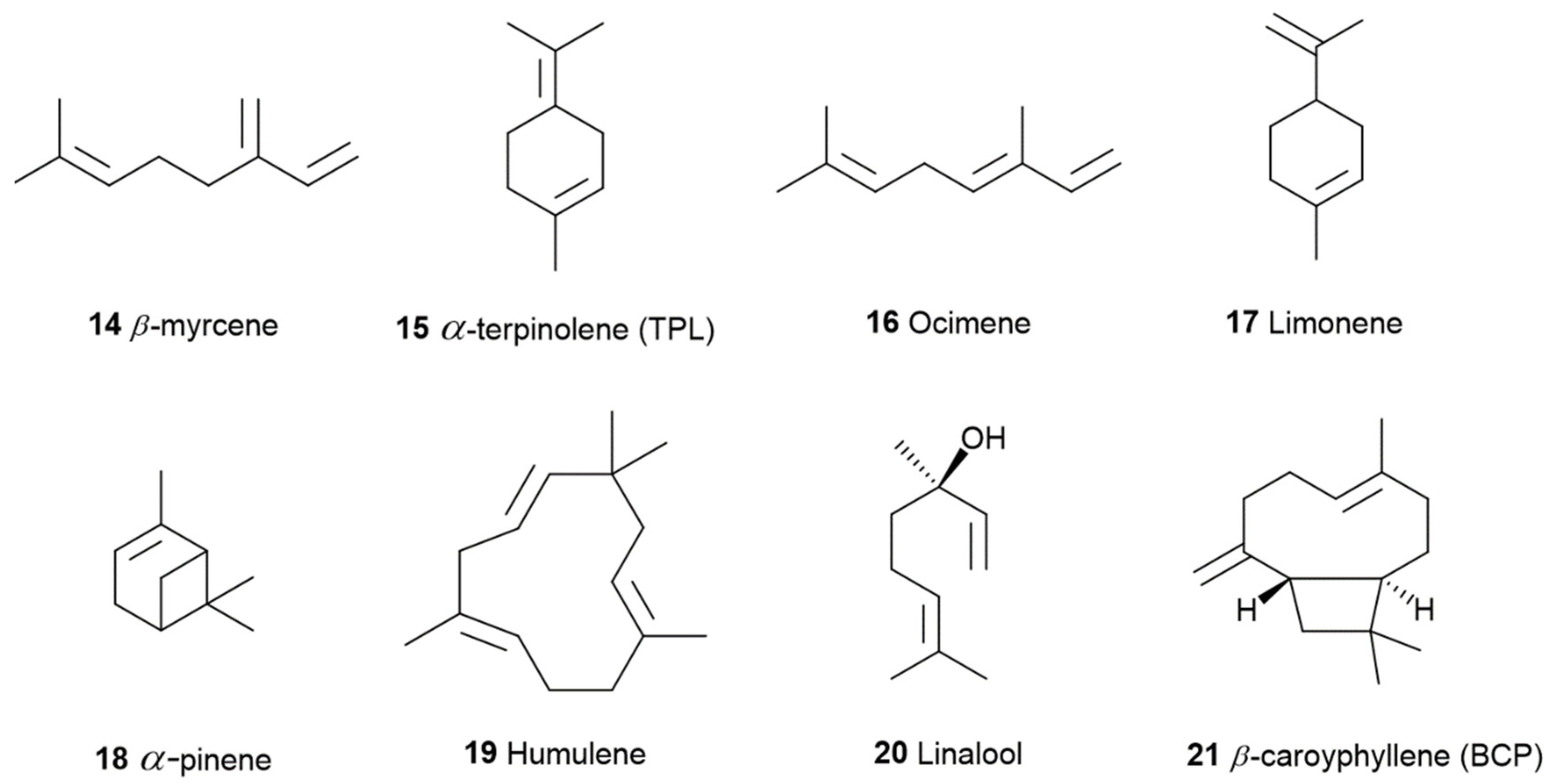
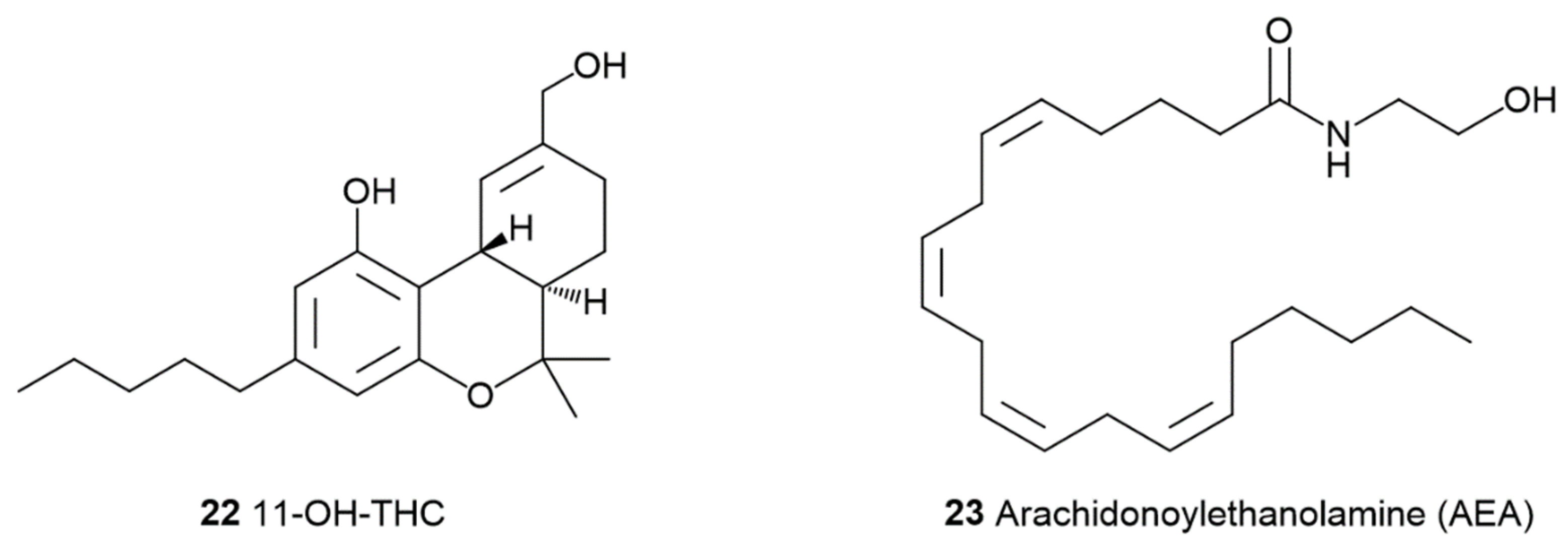
1.2. The Phyto Cannabinoids Entourage
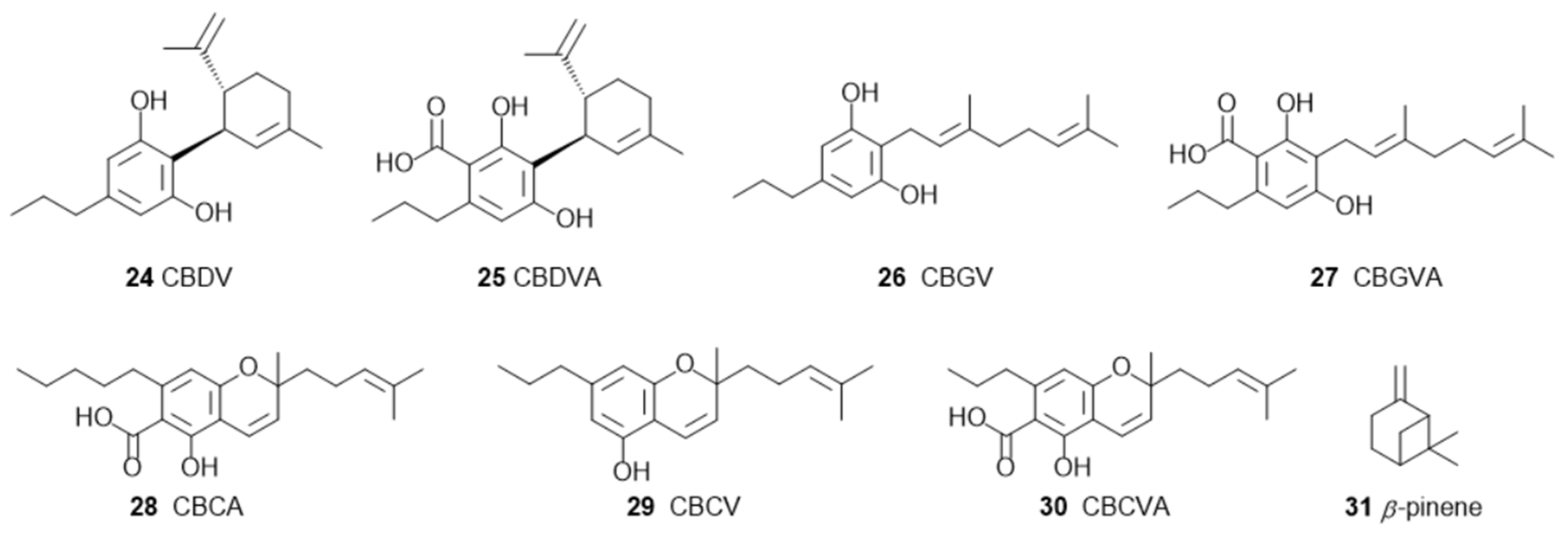
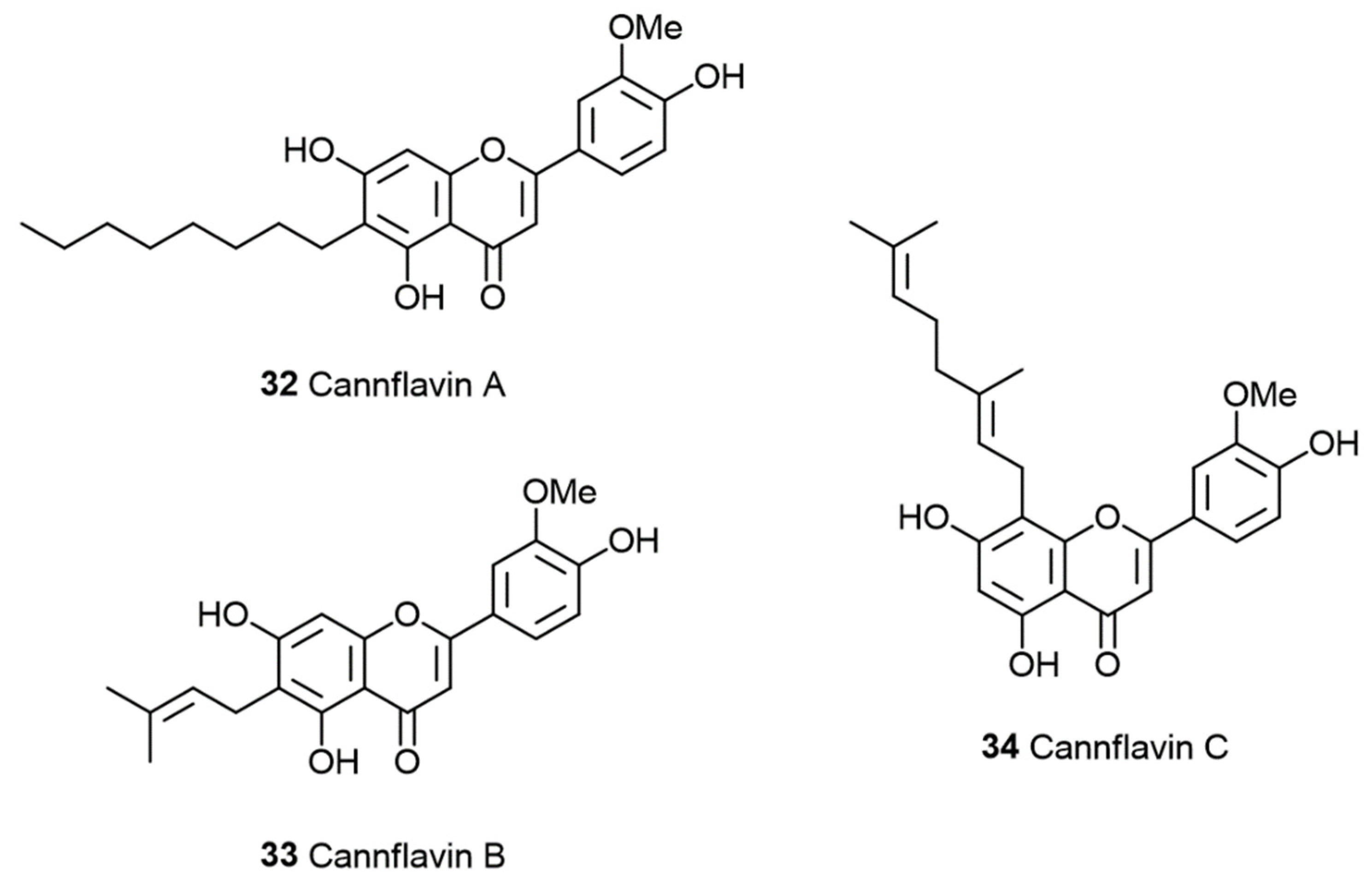
1.3. The Polyphenols Entourage
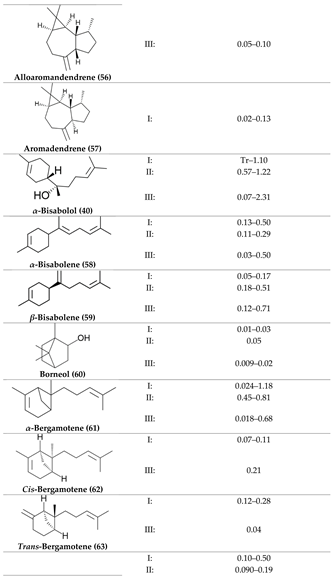
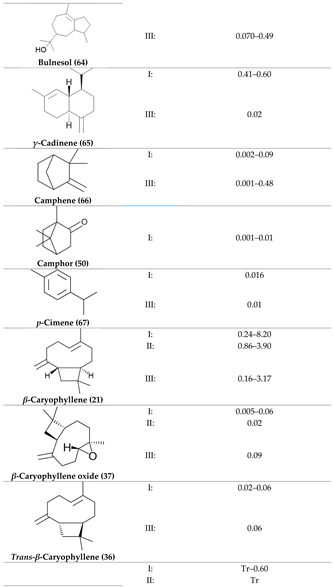
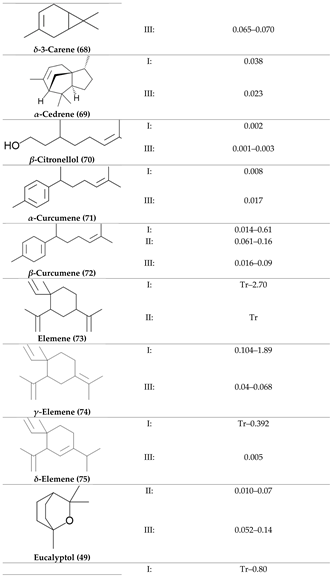
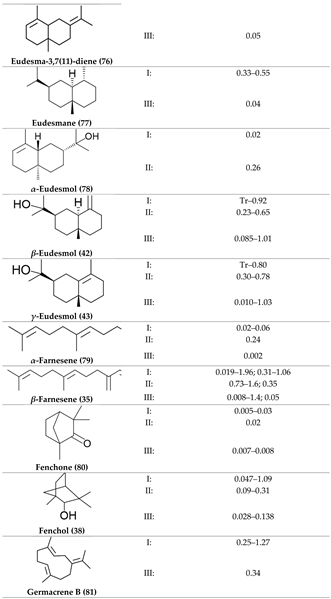
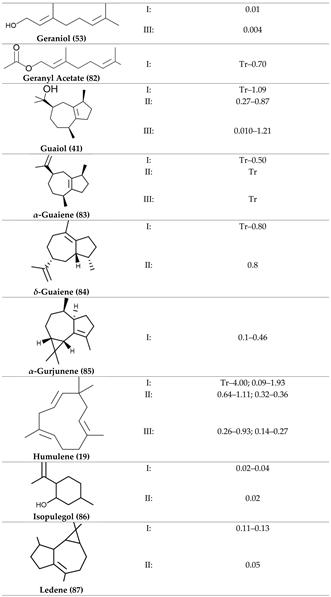
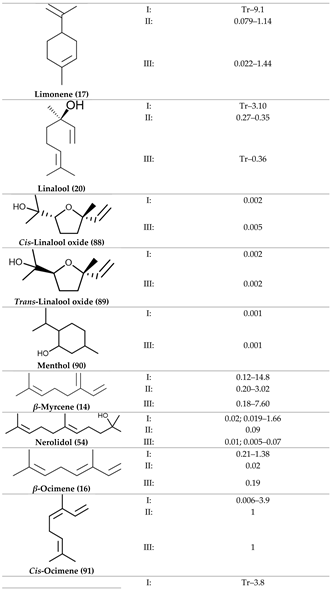
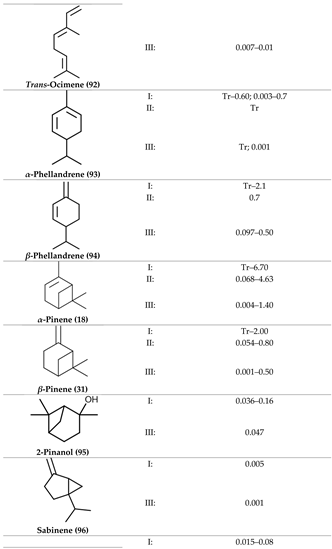
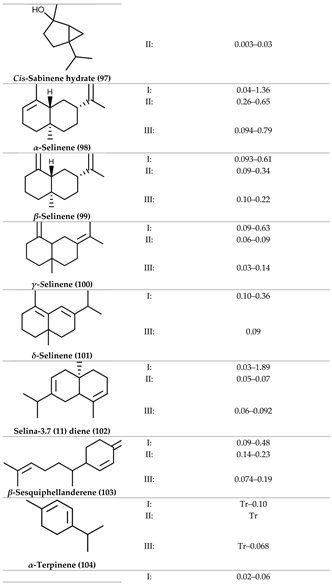
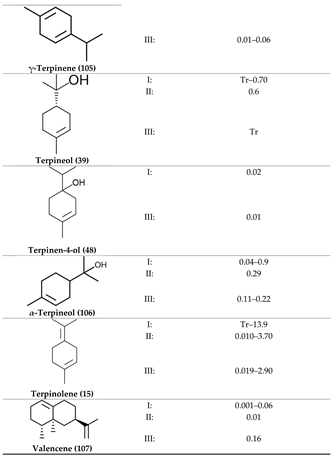
2. Physiological Effects of Terpenes and Terpenoids Found in Cannabis
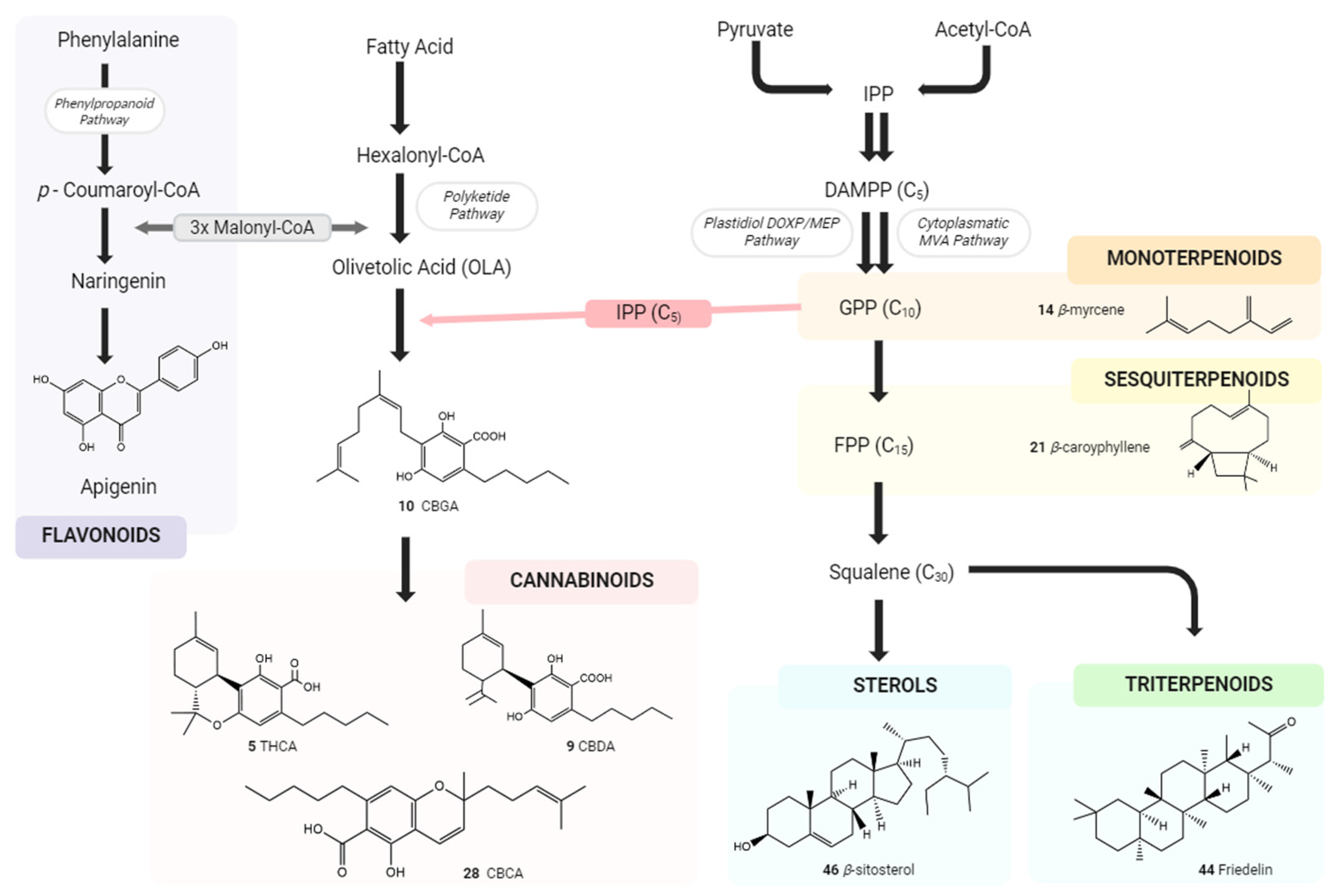
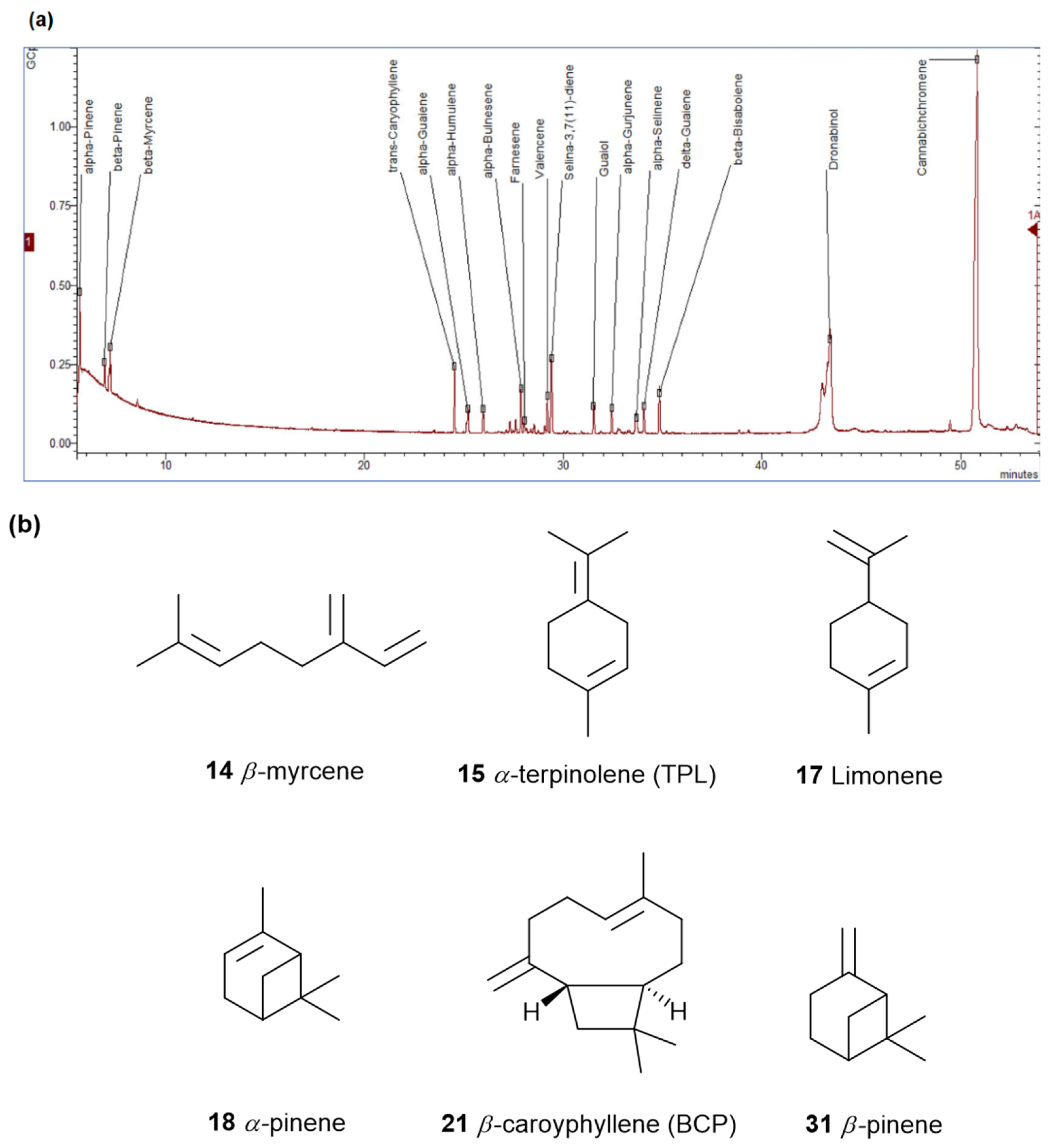
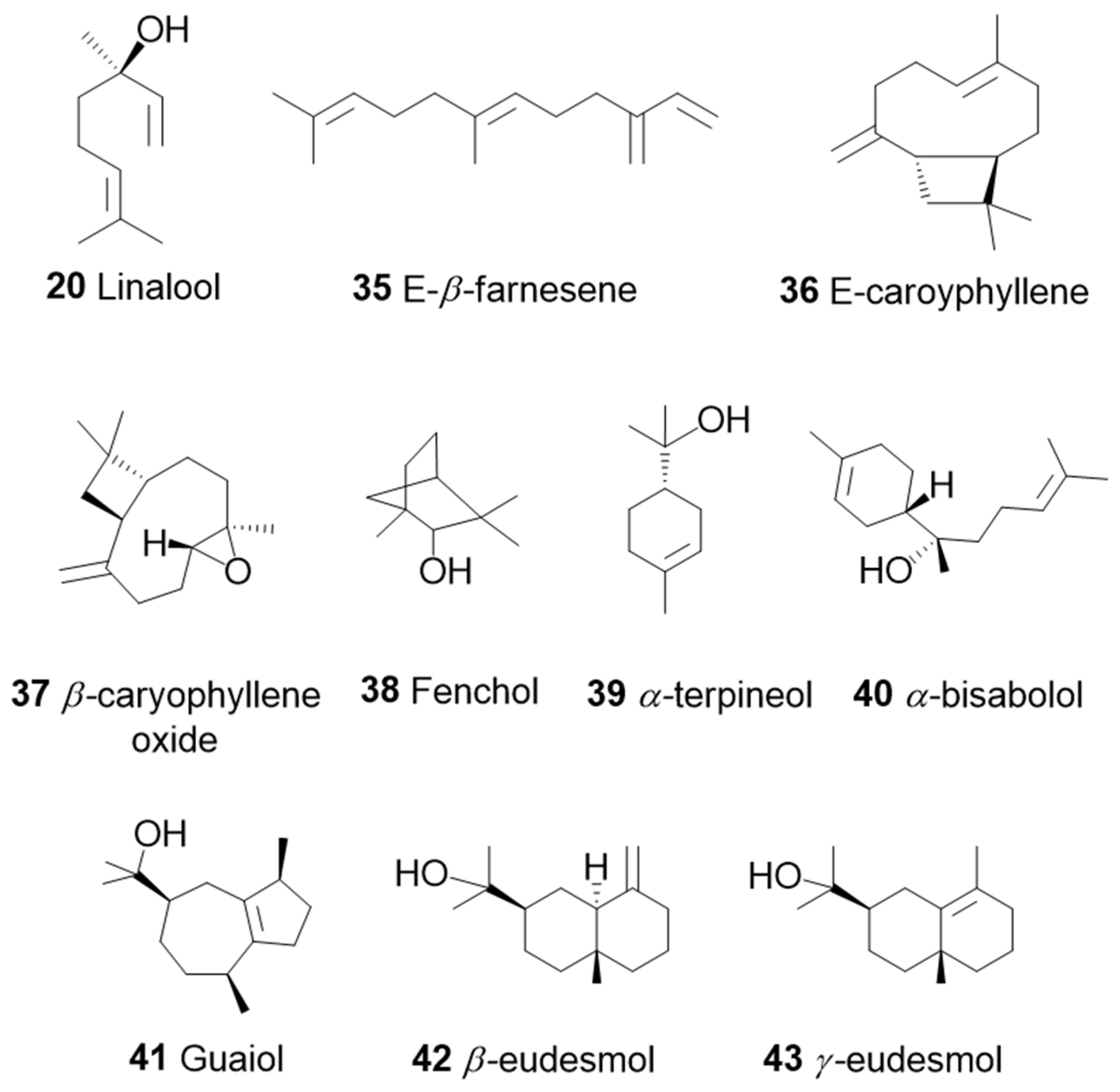
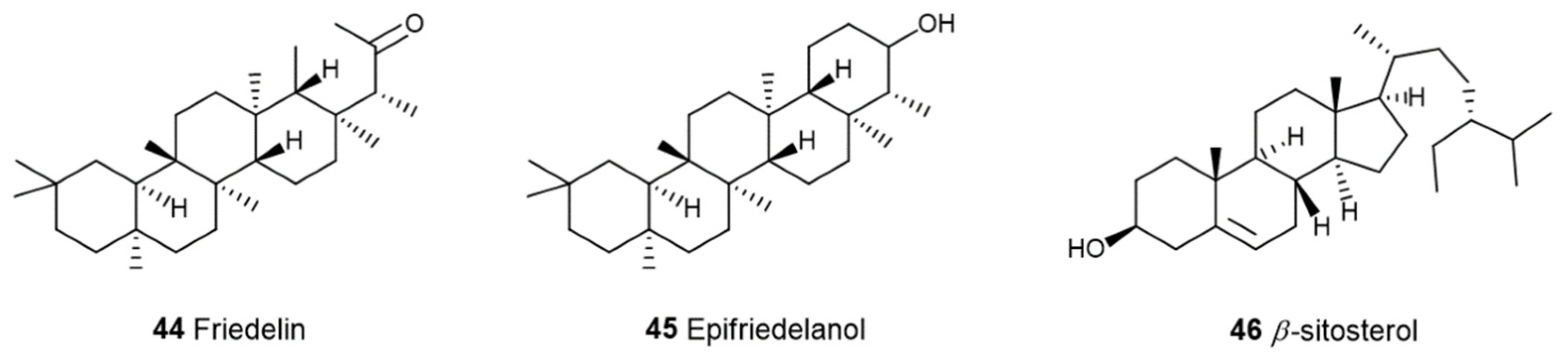
3. The Terpenes Entourage Effects Studied in Cannabis
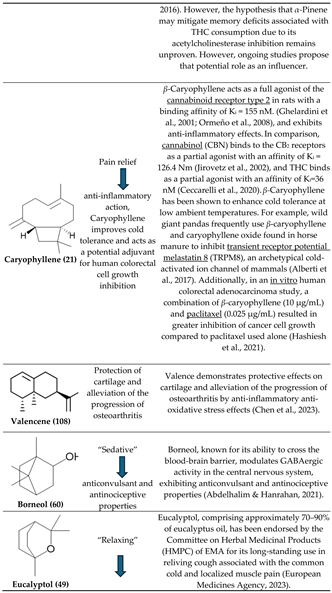
3.1. β-Caryophyllene and Terpinolene
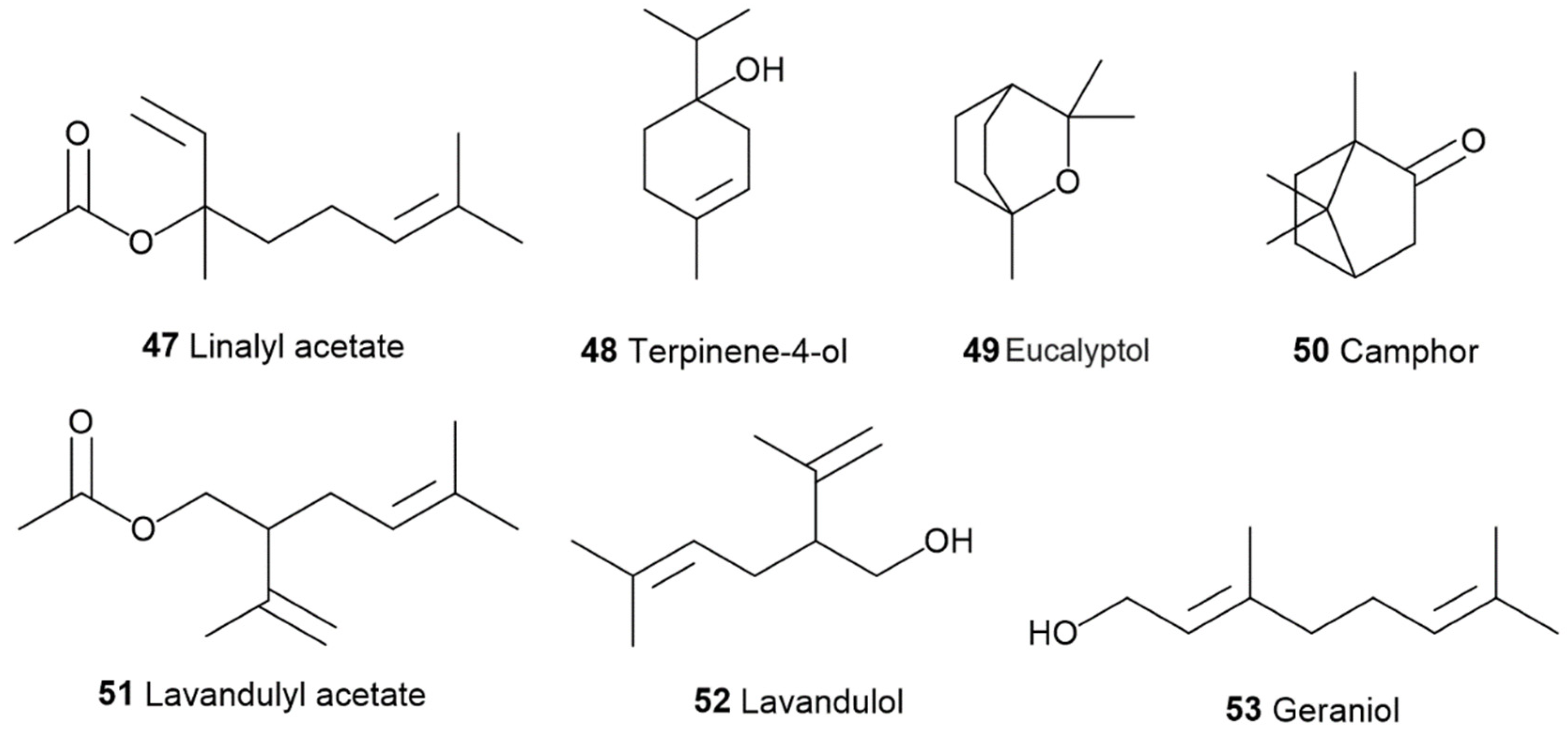
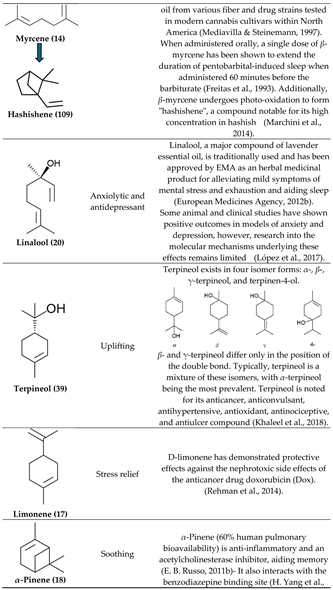
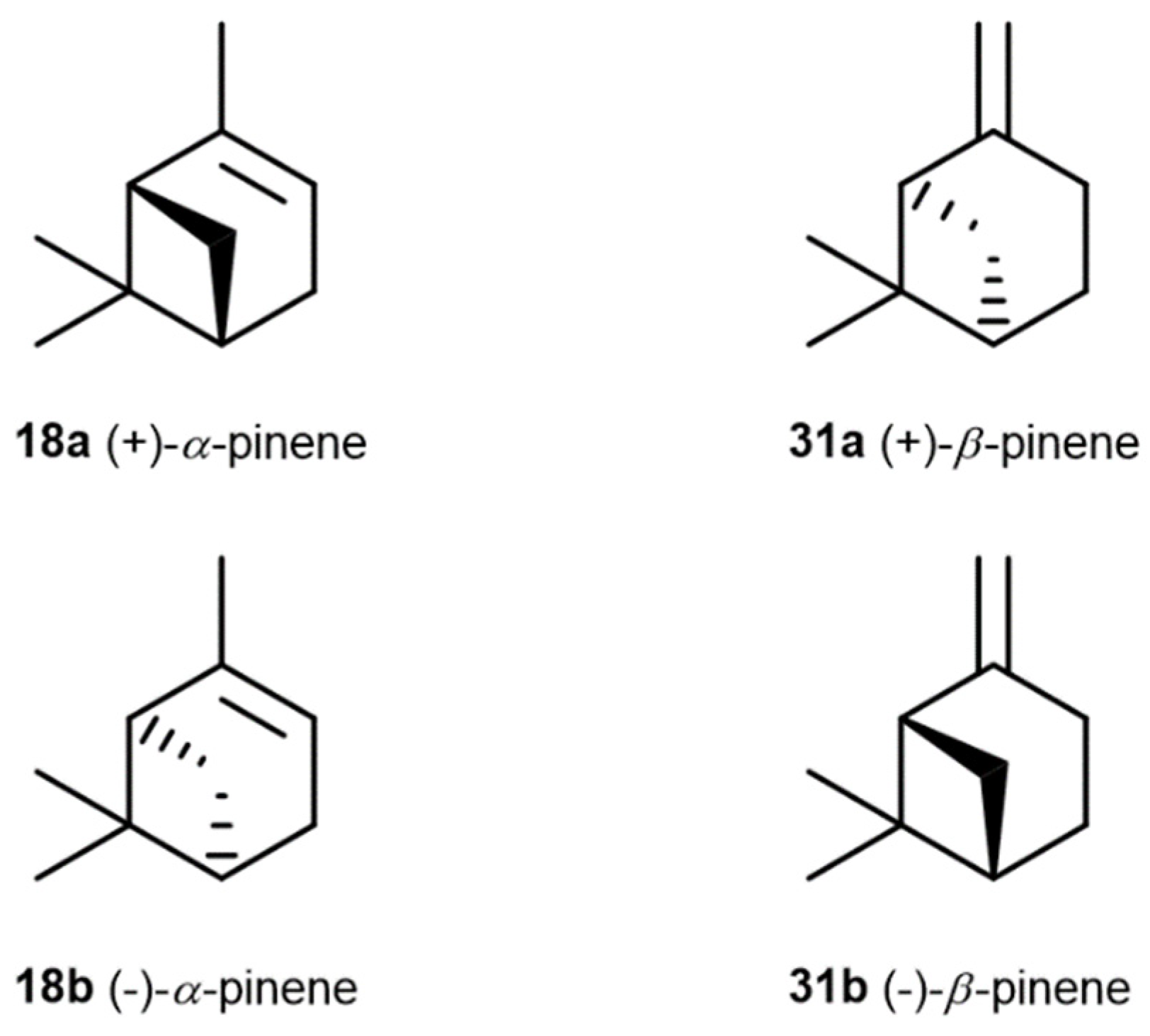
3.2. α- and β-Pinenes
3.3. β-Myrcene
3.4. Bisabolol, D-Limonene, ⍺-Pinene and β-Caryophyllene
3.5. β-Caryophyllene, Humulene, Nerolidol, Linalool, and β-pinene
4. Future Perspectives
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
5. Methodology
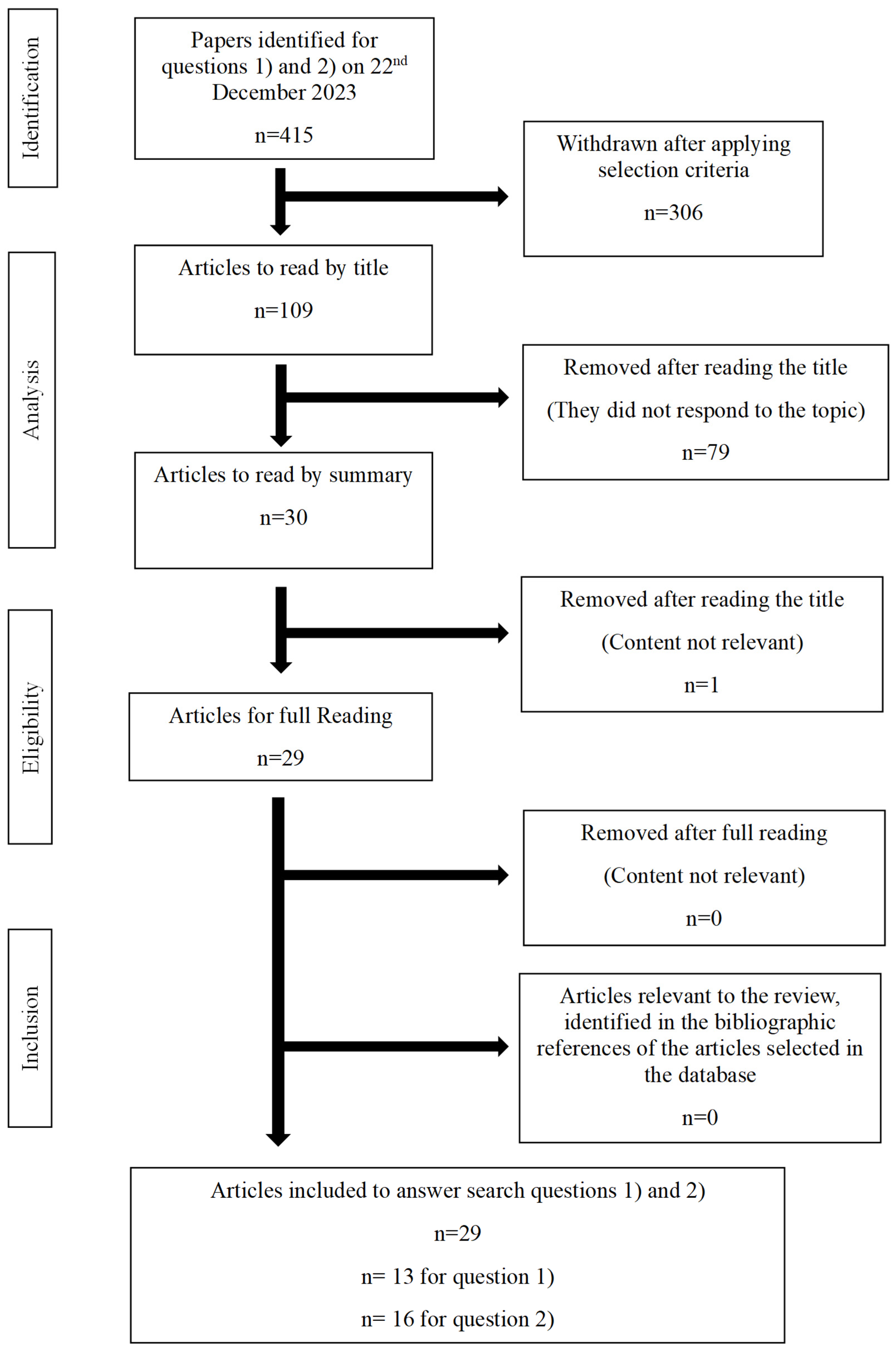
| Terpene | Search Keywords | N. publications |
| Myrcene (relaxing) | (myrcene) AND (cannabis) | 58 None for relaxing 1 no effect |
| Linalool (sedative) | (linalool) AND (cannabis) | 6 31 for sedative effect alone |
| Terpinolene (uplifting) | (terpinolene) AND (cannabis) | 1133 Terpinolene x effect: none uplifting related |
| Limonene (stress relief) | (limonene) AND (cannabis) | 42 3 for stress relief |
| Pinene (soothing) | (pinene) AND (cannabis) | 48 0 soothing |
| Caryophyllene (pain relief) | (caryophyllene) AND (cannabis) | 95 5 for analgesic synergies /cannabis terpenes & CBD |
| Valencene (euphoria) | (valencene) AND (cannabis) | 0 - no study on cannabis |
| Borneol (sedative) | (borneol) AND (cannabis) | 2 with cannabis 10 refs for borneol AND sedative |
| Eucalyptol (relaxing) | (eucalyptol) AND (cannabis) | 6~3/5 on Eucalyptol AND relaxing but in mixtures |
6. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Abdelhalim, A., & Hanrahan, J. (2021). Biologically active compounds from Lamiaceae family: Central nervous system effects (pp. 255–315). [CrossRef]
- Abdollahi, M., Sefidkon, F., Calagari, M., Mousavi, A., & Mahomoodally, M. F. (2020). Impact of four hemp (Cannabis sativa L.) varieties and stage of plant growth on yield and composition of essential oils. Industrial Crops and Products, 155, 112793. [CrossRef]
- Alberti, T., Barbosa, W., Vieira, J., Raposo, N., & Dutra, R. (2017). (−)-β-Caryophyllene, a CB2 Receptor-Selective Phytocannabinoid, Suppresses Motor Paralysis and Neuroinflammation in a Murine Model of Multiple Sclerosis. International Journal of Molecular Sciences, 18(4), 691. [CrossRef]
- Andre, C. M., Hausman, J.-F., & Guerriero, G. (2016). Cannabis sativa: The Plant of the Thousand and One Molecules. Frontiers in Plant Science, 7. [CrossRef]
- Arévalo, R. A., Bertoncini, E. I., Guirado, N., & Chaila, S. (2006). Los términos cultivar o variedad de caña de azúcar (Saccharum spp.). REVISTA CHAPINGO SERIE HORTICULTURA, 12(1), 5–9. https://www.redalyc.org/articulo.oa?id=60912102.
- Bailey, L. H., & Bailey, E. Z. (1976). Hortus third: a concise dictionary of plants cultivated in the United States and Canada (Issue BOOK). MacMillan Publishing Co. http://worldveg.tind.io/record/2320.
- Bautista, J. L., Yu, S., & Tian, L. (2021). Flavonoids in Cannabis sativa: Biosynthesis, Bioactivities, and Biotechnology. ACS Omega, 6(8), 5119–5123. [CrossRef]
- Ben-Shabat, S., Fride, E., Sheskin, T., Tamiri, T., Rhee, M.-H., Vogel, Z., Bisogno, T., De Petrocellis, L., Di Marzo, V., & Mechoulam, R. (1998). An entourage effect: inactive endogenous fatty acid glycerol esters enhance 2-arachidonoyl-glycerol cannabinoid activity. European Journal of Pharmacology, 353(1), 23–31. [CrossRef]
- Berman, P., Futoran, K., Lewitus, G. M., Mukha, D., Benami, M., Shlomi, T., & Meiri, D. (2018). A new ESI-LC/MS approach for comprehensive metabolic profiling of phytocannabinoids in Cannabis. Scientific Reports, 8(1), 14280. [CrossRef]
- Blasco-Benito, S., Seijo-Vila, M., Caro-Villalobos, M., Tundidor, I., Andradas, C., García-Taboada, E., Wade, J., Smith, S., Guzmán, M., Pérez-Gómez, E., Gordon, M., & Sánchez, C. (2018). Appraising the “entourage effect”: Antitumor action of a pure cannabinoid versus a botanical drug preparation in preclinical models of breast cancer. Biochemical Pharmacology, 157, 285–293. [CrossRef]
- Boggs, D. L., Nguyen, J. D., Morgenson, D., Taffe, M. A., & Ranganathan, M. (2018). Clinical and Preclinical Evidence for Functional Interactions of Cannabidiol and Δ9-Tetrahydrocannabinol. Neuropsychopharmacology, 43(1), 142–154. [CrossRef]
- Brickell, C. D., Alexander, C., Cubey, J. J., David, J. C., Hoffman, M. H. A., Leslie, A. C., Malécot, V., & Jin, X. (2009). Internacional code for nomenclature for cultivated plants (International Society for Horticultural Science, Ed.; 9th ed.).
- Britton, E. R., Kellogg, J. J., Kvalheim, O. M., & Cech, N. B. (2018). Biochemometrics to Identify Synergists and Additives from Botanical Medicines: A Case Study with Hydrastis canadensis (Goldenseal). Journal of Natural Products, 81(3), 484–493. [CrossRef]
- Buchbauer, G., Jirovetz, L., Jäger, W., Plank, C., & Dietrich, H. (1993). Fragrance Compounds and Essential Oils with Sedative Effects upon Inhalation. Journal of Pharmaceutical Sciences, 82(6), 660–664. [CrossRef]
- Caesar, L. K., & Cech, N. B. (2019). Synergy and antagonism in natural product extracts: when 1 + 1 does not equal 2. Natural Product Reports, 36(6), 869–888. [CrossRef]
- Casano, S., Grassi, G., Martini, V., & Michelozzi, M. (2011). VARIATIONS IN TERPENE PROFILES OF DIFFERENT STRAINS OF CANNABIS SATIVA L. Acta Horticulturae, 925, 115–121. [CrossRef]
- Cascio, M. G., Zamberletti, E., Marini, P., Parolaro, D., & Pertwee, R. G. (2015). The phytocannabinoid, Δ9-tetrahydrocannabivarin, can act through 5-HT1A receptors to produce antipsychotic effects. British Journal of Pharmacology, 172(5), 1305–1318. [CrossRef]
- Castillo-Arellano, J., Canseco-Alba, A., Cutler, S. J., & León, F. (2023). The Polypharmacological Effects of Cannabidiol. Molecules, 28(7), 3271. [CrossRef]
- Ceccarelli, I., Fiorenzani, P., Pessina, F., Pinassi, J., Aglianò, M., Miragliotta, V., & Aloisi, A. M. (2020). The CB2 Agonist β-Caryophyllene in Male and Female Rats Exposed to a Model of Persistent Inflammatory Pain. Frontiers in Neuroscience, 14. [CrossRef]
- Chacon, F. T., Raup-Konsavage, W. M., Vrana, K. E., & Kellogg, J. J. (2022). Secondary Terpenes in Cannabis sativa L.: Synthesis and Synergy. Biomedicines, 10(12), 3142. [CrossRef]
- Chen, S., Meng, C., He, Y., Xu, H., Qu, Y., Wang, Y., Fan, Y., Huang, X., & You, H. (2023). An in vitro and in vivo study: Valencene protects cartilage and alleviates the progression of osteoarthritis by anti-oxidative stress and anti-inflammatory effects. International Immunopharmacology, 123, 110726. [CrossRef]
- Christensen, C., Rose, M., Cornett, C., & Allesø, M. (2023). Decoding the Postulated Entourage Effect of Medicinal Cannabis: What It Is and What It Isn’t. Biomedicines, 11(8), 2323. [CrossRef]
- Classen, A., Meyer, F. G., Trueblood, E. E., & Heller, J. L. (2001). The Great Herbal of Leonhart Fuchs. De historia stirpium commentarii insignes, 1542. German Studies Review, 24(3), 595. [CrossRef]
- Costa, M. do C., Duarte, P., Neng, N. R., Nogueira, J. M. F., Costa, F., & Rosado, C. (2015). Novel insights for permeant lead structures through in vitro skin diffusion assays of Prunus lusitanica L., the Portugal Laurel. Journal of Molecular Structure, 1079, 327–336. [CrossRef]
- De Petrocellis, L., Ligresti, A., Moriello, A. S., Allarà, M., Bisogno, T., Petrosino, S., Stott, C. G., & Di Marzo, V. (2011). Effects of cannabinoids and cannabinoid-enriched Cannabis extracts on TRP channels and endocannabinoid metabolic enzymes. British Journal of Pharmacology, 163(7), 1479–1494. [CrossRef]
- Dijkshoorn, L., Ursing, B. M., & Ursing, J. B. (2000). Strain, clone and species: comments on three basic concepts of bacteriology. Journal of Medical Microbiology, 49(5), 397–401. [CrossRef]
- Dobetsberger, C., & Buchbauer, G. (2011). Actions of essential oils on the central nervous system: An updated review. Flavour and Fragrance Journal, 26(5), 300–316. [CrossRef]
- dos Santos, É. R. Q., Maia, J. G. S., Fontes-Júnior, E. A., & do Socorro Ferraz Maia, C. (2022). Linalool as a Therapeutic and Medicinal Tool in Depression Treatment: A Review. Current Neuropharmacology, 20(6), 1073–1092. [CrossRef]
- Duggan, P. J. (2021). The Chemistry of Cannabis and Cannabinoids. Australian Journal of Chemistry, 74(6), 369–387. [CrossRef]
- Durbin, D. J., King, J. M., & Stairs, D. J. (2024). Behavioral Effects of Vaporized Delta-8 Tetrahydrocannabinol, Cannabidiol, and Mixtures in Male Rats. Cannabis and Cannabinoid Research, 9(2), 601–611. [CrossRef]
- Elisabetsky, E., Silva Brum, L. F., & Souza, D. O. (1999). Anticonvulsant properties of linalool in glutamate-related seizure models. Phytomedicine, 6(2), 107–113. [CrossRef]
- ElSohly, M. A., Radwan, M. M., Gul, W., Chandra, S., & Galal, A. (2017). Phytochemistry of Cannabis sativa L. (pp. 1–36). [CrossRef]
- Elzinga S, & Fischedick J. (2015). Cannabinoids and Terpenes as Chemotaxonomic Markers in Cannabis. Natural Products Chemistry & Research, 03(04). [CrossRef]
- European Medicines Agency. (2012a). Assessment report on Lavandula angustifolia Miller, aetheroleum and Lavandula angustifolia Miller, flos.
- European Medicines Agency. (2012b). EMA/HMPC/143181/2010 Community Herbal Monograph on Lavandula Angustifolia Miller, Aetheroleum. https://www.ema.europa.eu/en/documents/herbal-monograph/final-community-herbal-monograph-lavandula-angustifolia-miller-aetheroleum_en.pdf.
- European Medicines Agency. (2023). European Union herbal monograph on Eucalyptus globulus Labill.; Eucalyptus polybractea R.T. Baker; Eucalyptus smithii R.T. Baker, aetheroleum EMA/HMPC/320292/2023. draft-european-union-herbal-monograph-eucalyptus-globulus-labill-eucalyptus-polybractea-rt-baker-eucalyptus-smithii-rt-baker-aetheroleum-revision-1_en.pdf.
- European Scientific Cooperative on Phytotherapy. (2009). ESCOP Monographs: The Scientific Foundation for Herbal Medicinal Products. Second Edition, Supplement 2009 (Thieme Medical Publishers, Ed.).
- Ferber, S. G., Namdar, D., Hen-Shoval, D., Eger, G., Koltai, H., Shoval, G., Shbiro, L., & Weller, A. (2020). The “Entourage Effect”: Terpenes Coupled with Cannabinoids for the Treatment of Mood Disorders and Anxiety Disorders. Current Neuropharmacology, 18(2), 87–96. [CrossRef]
- Fischedick, J. T., Hazekamp, A., Erkelens, T., Choi, Y. H., & Verpoorte, R. (2010). Metabolic fingerprinting of Cannabis sativa L., cannabinoids and terpenoids for chemotaxonomic and drug standardization purposes. Phytochemistry, 71(17–18), 2058–2073. [CrossRef]
- Freitas, J. C., Presgrave, O. A., Fingola, F. F., Menezes, M. A., & Paumgartten, F. J. (1993). Effect of beta-myrcene on pentobarbital sleeping time. Brazilian Journal of Medical and Biological Research = Revista Brasileira de Pesquisas Medicas e Biologicas, 26(5), 519–523. http://europepmc.org/abstract/MED/8257941.
- Gallily, R., Yekhtin, Z., & Hanuš, L. O. (2015). Overcoming the Bell-Shaped Dose-Response of Cannabidiol by Using <i>Cannabis</i> Extract Enriched in Cannabidiol. Pharmacology & Pharmacy, 06(02), 75–85. [CrossRef]
- Gao, C., Xin, P., Cheng, C., Tang, Q., Chen, P., Wang, C., Zang, G., & Zhao, L. (2014). Diversity Analysis in Cannabis sativa Based on Large-Scale Development of Expressed Sequence Tag-Derived Simple Sequence Repeat Markers. PLoS ONE, 9(10), e110638. [CrossRef]
- Ghelardini, C., Galeotti, N., Di Cesare Mannelli, L., Mazzanti, G., & Bartolini, A. (2001). Local anaesthetic activity of β-caryophyllene. Il Farmaco, 56(5–7), 387–389. [CrossRef]
- Hanuš, L. O., & Hod, Y. (2020). Terpenes/Terpenoids in <b><i>Cannabis</i></b>: Are They Important? Medical Cannabis and Cannabinoids, 3(1), 25–60. [CrossRef]
- Hashiesh, H. M., Sharma, C., Goyal, S. N., Sadek, B., Jha, N. K., Kaabi, J. Al, & Ojha, S. (2021). A focused review on CB2 receptor-selective pharmacological properties and therapeutic potential of β-caryophyllene, a dietary cannabinoid. Biomedicine & Pharmacotherapy, 140, 111639. [CrossRef]
- Hazekamp, A., & Fischedick, J. T. (2012). Cannabis - from cultivar to chemovar. Drug Testing and Analysis, 4(7–8), 660–667. [CrossRef]
- Heblinski, M., Santiago, M., Fletcher, C., Stuart, J., Connor, M., McGregor, I. S., & Arnold, J. C. (2020). Terpenoids Commonly Found in Cannabis sativa Do Not Modulate the Actions of Phytocannabinoids or Endocannabinoids on TRPA1 and TRPV1 Channels. Cannabis and Cannabinoid Research, 5(4), 305–317. [CrossRef]
- Hillig, K. W. (2004). A chemotaxonomic analysis of terpenoid variation in Cannabis. Biochemical Systematics and Ecology, 32(10), 875–891. [CrossRef]
- Huestis, M. A. (2005). Pharmacokinetics and Metabolism of the Plant Cannabinoids, Δ 9-Tetrahydrocannibinol, Cannabidiol and Cannabinol (pp. 657–690). [CrossRef]
- Ioannidis, K., Tomprou, I., Mitsis, V., & Koropouli, P. (2022). Genetic Evaluation of In vitro Micropropagated and Regenerated Plants of Cannabis sativa L. Using SSR Molecular Markers. Plants, 11(19), 2569. [CrossRef]
- Jenkins, B. W., Moore, C. F., Covey, D., McDonald, J. D., Lefever, T. W., Bonn-Miller, M. O., & Weerts, E. M. (2023). Evaluating Potential Anxiolytic Effects of Minor Cannabinoids and Terpenes After Acute and Chronic Oral Administration in Rats. Cannabis and Cannabinoid Research, 8(S1), S11–S24. [CrossRef]
- Jin, D., Dai, K., Xie, Z., & Chen, J. (2020). Secondary Metabolites Profiled in Cannabis Inflorescences, Leaves, Stem Barks, and Roots for Medicinal Purposes. Scientific Reports, 10(1), 3309. [CrossRef]
- Jirovetz, L., Buchbauer, G., Ngassoum, M. B., & Geissler, M. (2002). Aroma compound analysis of Piper nigrum and Piper guineense essential oils from Cameroon using solid-phase microextraction–gas chromatography, solid-phase microextraction–gas chromatography–mass spectrometry and olfactometry. Journal of Chromatography A, 976(1–2), 265–275. [CrossRef]
- Johnson, A. L., Verbitsky, R., Hudson, J., Dean, R., & Hamilton, T. J. (2023). Cannabinoid type-2 receptors modulate terpene induced anxiety-reduction in zebrafish. Biomedicine & Pharmacotherapy, 168, 115760. [CrossRef]
- Johnson, J. R., Burnell-Nugent, M., Lossignol, D., Ganae-Motan, E. D., Potts, R., & Fallon, M. T. (2010). Multicenter, Double-Blind, Randomized, Placebo-Controlled, Parallel-Group Study of the Efficacy, Safety, and Tolerability of THC:CBD Extract and THC Extract in Patients with Intractable Cancer-Related Pain. Journal of Pain and Symptom Management, 39(2), 167–179. [CrossRef]
- Khaleel, C., Tabanca, N., & Buchbauer, G. (2018). α-Terpineol, a natural monoterpene: A review of its biological properties. Open Chemistry, 16(1), 349–361. [CrossRef]
- Kohlert, C., van Rensen, I., März, R., Schindler, G., Graefe, E. U., & Veit, M. (2000). Bioavailability and Pharmacokinetics of Natural Volatile Terpenes in Animals and Humans. Planta Medica, 66(6), 495–505. [CrossRef]
- Koltai, H., & Namdar, D. (2020). Cannabis Phytomolecule “Entourage”: From Domestication to Medical Use. Trends in Plant Science, 25(10), 976–984. [CrossRef]
- Lamarck, J.-B. de M. de. (1783). Encyclopédie méthodique: Botanique (T.-C. A. (París), C.-J. P. (París) Henri Agasse (Paris), Ed.).
- Laprairie, R. B., Bagher, A. M., Kelly, M. E. M., & Denovan-Wright, E. M. (2015). Cannabidiol is a negative allosteric modulator of the cannabinoid CB 1 receptor. British Journal of Pharmacology, 172(20), 4790–4805. [CrossRef]
- Laws, J. S., & Smid, S. D. (2024). Characterizing cannabis-prevalent terpenes for neuroprotection reveal a role for α and β-pinenes in mitigating amyloid β-evoked neurotoxicity and aggregation in vitro. NeuroToxicology, 100, 16–24. [CrossRef]
- Lee, S., Kim, E. J., Kwon, E., Oh, S. J., Cho, M., Kim, C. M., Lee, W., & Hong, J. (2023). Identification of Terpene Compositions in the Leaves and Inflorescences of Hybrid Cannabis Species Using Headspace-Gas Chromatography/Mass Spectrometry. Molecules, 28(24), 8082. [CrossRef]
- Lewis, M., Russo, E., & Smith, K. (2018). Pharmacological Foundations of Cannabis Chemovars. Planta Medica, 84(04), 225–233. [CrossRef]
- Linck, V. de M., da Silva, A. L., Figueiró, M., Luis Piato, Â., Paula Herrmann, A., Dupont Birck, F., Bastos Caramão, E., Sávio Nunes, D., Moreno, P. R. H., & Elisabetsky, E. (2009). Inhaled linalool-induced sedation in mice. Phytomedicine, 16(4), 303–307. [CrossRef]
- Linnaeus, C. (1753). Species plantarum (Holmiae: Laurentii Salvii, Ed.). [CrossRef]
- López, V., Nielsen, B., Solas, M., Ramírez, M. J., & Jäger, A. K. (2017). Exploring Pharmacological Mechanisms of Lavender (Lavandula angustifolia) Essential Oil on Central Nervous System Targets. Frontiers in Pharmacology, 8. [CrossRef]
- Maccarrone, M., Di Marzo, V., Gertsch, J., Grether, U., Howlett, A. C., Hua, T., Makriyannis, A., Piomelli, D., Ueda, N., & van der Stelt, M. (2023). Goods and Bads of the Endocannabinoid System as a Therapeutic Target: Lessons Learned after 30 Years. Pharmacological Reviews, 75(5), 885–958. [CrossRef]
- Marchini, M., Charvoz, C., Dujourdy, L., Baldovini, N., & Filippi, J.-J. (2014). Multidimensional analysis of cannabis volatile constituents: Identification of 5,5-dimethyl-1-vinylbicyclo [2.1.1]hexane as a volatile marker of hashish, the resin of Cannabis sativa L. Journal of Chromatography A, 1370, 200–215. [CrossRef]
- McDougall, J. J., & McKenna, M. K. (2022). Anti-Inflammatory and Analgesic Properties of the Cannabis Terpene Myrcene in Rat Adjuvant Monoarthritis. International Journal of Molecular Sciences, 23(14), 7891. [CrossRef]
- McPartland, J. M., & Russo, E. B. (2014). Non-Phytocannabinoid Constituents of Cannabis and Herbal Synergy. In Handbook of Cannabis (pp. 280–295). Oxford University Press. [CrossRef]
- Mediavilla, V., & Steinemann, S. (1997). Essential oil of Cannabis sativa L. strains. Journal of the International Hemp Association, 4(2), 80–82.
- Milay, L., Berman, P., Shapira, A., Guberman, O., & Meiri, D. (2020). Metabolic Profiling of Cannabis Secondary Metabolites for Evaluation of Optimal Postharvest Storage Conditions. Frontiers in Plant Science, 11. [CrossRef]
- Morales, P., Hurst, D. P., & Reggio, P. H. (2017). Molecular Targets of the Phytocannabinoids: A Complex Picture (pp. 103–131). [CrossRef]
- Nallathambi, R., Mazuz, M., Namdar, D., Shik, M., Namintzer, D., Vinayaka, A. C., Ion, A., Faigenboim, A., Nasser, A., Laish, I., Konikoff, F. M., & Koltai, H. (2018). Identification of Synergistic Interaction Between Cannabis-Derived Compounds for Cytotoxic Activity in Colorectal Cancer Cell Lines and Colon Polyps That Induces Apoptosis-Related Cell Death and Distinct Gene Expression. Cannabis and Cannabinoid Research, 3(1), 120–135. [CrossRef]
- Namdar, D., Anis, O., Poulin, P., & Koltai, H. (2020). Chronological Review and Rational and Future Prospects of Cannabis-Based Drug Development. Molecules, 25(20), 4821. [CrossRef]
- Niu, J., Straubinger, R. M., & Mager, D. E. (2019). Pharmacodynamic Drug–Drug Interactions. Clinical Pharmacology & Therapeutics, 105(6), 1395–1406. [CrossRef]
- Ormeño, E., Baldy, V., Ballini, C., & Fernandez, C. (2008). Production and Diversity of Volatile Terpenes from Plants on Calcareous and Siliceous Soils: Effect of Soil Nutrients. Journal of Chemical Ecology, 34(9), 1219–1229. [CrossRef]
- Peana, A. T., D’Aquila, P. S., Panin, F., Serra, G., Pippia, P., & Moretti, M. D. L. (2002). Anti-inflammatory activity of linalool and linalyl acetate constituents of essential oils. Phytomedicine, 9(8), 721–726. [CrossRef]
- Pereira da Silva Oliveira, A., do Céu Costa, M., & Pires Bicho, M. (2023). Use of Medicinal Plants: Interindividual Variability of Their Effects from a Genetic and Anthropological Perspective. In Medicinal Plants - Chemical, Biochemical, and Pharmacological Approaches [Working Title]. IntechOpen. [CrossRef]
- Pertwee, R. G. (2008). The diverse CB1 and CB2 receptor pharmacology of three plant cannabinoids: Δ9-tetrahydrocannabinol, cannabidiol and Δ9-tetrahydrocannabivarin. British Journal of Pharmacology, 153(2), 199–215. [CrossRef]
- Peterson, B., Weyers, M., Steenekamp, J. H., Steyn, J. D., Gouws, C., & Hamman, J. H. (2019). Drug Bioavailability Enhancing Agents of Natural Origin (Bioenhancers) that Modulate Drug Membrane Permeation and Pre-Systemic Metabolism. Pharmaceutics, 11(1), 33. [CrossRef]
- Piomelli, D. (2019). Waiting for the Entourage. Cannabis and Cannabinoid Research, 4(3), 137–138. [CrossRef]
- Piomelli, D., & Russo, E. B. (2016). The Cannabis sativa Versus Cannabis indica Debate: An Interview with Ethan Russo, MD. Cannabis and Cannabinoid Research, 1(1), 44–46. [CrossRef]
- Rehman, M. U., Tahir, M., Khan, A. Q., Khan, R., Oday-O-Hamiza, Lateef, A., Hassan, S. K., Rashid, S., Ali, N., Zeeshan, M., & Sultana, S. (2014). D-limonene suppresses doxorubicin-induced oxidative stress and inflammation via repression of COX-2, iNOS, and NFκB in kidneys of Wistar rats. Experimental Biology and Medicine, 239(4), 465–476. [CrossRef]
- Rice, S., & Koziel, J. A. (2015). Characterizing the Smell of Marijuana by Odor Impact of Volatile Compounds: An Application of Simultaneous Chemical and Sensory Analysis. PLOS ONE, 10(12), e0144160. [CrossRef]
- Ross, S. A., & ElSohly, M. A. (1996). The Volatile Oil Composition of Fresh and Air-Dried Buds of Cannabis sativa. Journal of Natural Products, 59(1), 49–51. [CrossRef]
- Russo, E. B. (2011a). Taming THC: potential cannabis synergy and phytocannabinoid-terpenoid entourage effects. British Journal of Pharmacology, 163(7), 1344–1364. [CrossRef]
- Russo, E. B. (2011b). Taming THC: potential cannabis synergy and phytocannabinoid-terpenoid entourage effects. British Journal of Pharmacology, 163(7), 1344–1364. [CrossRef]
- Russo, E. B. (2019). The Case for the Entourage Effect and Conventional Breeding of Clinical Cannabis: No “Strain,” No Gain. Frontiers in Plant Science, 9. [CrossRef]
- Russo, E. B., & Marcu, J. (2017a). Cannabis Pharmacology: The Usual Suspects and a Few Promising Leads. In Advances in Pharmacology (Vol. 80, pp. 67–134). [CrossRef]
- Russo, E. B., & Marcu, J. (2017b). Cannabis Pharmacology: The Usual Suspects and a Few Promising Leads (pp. 67–134). [CrossRef]
- Russo, E., & Guy, G. W. (2006). A tale of two cannabinoids: The therapeutic rationale for combining tetrahydrocannabinol and cannabidiol. Medical Hypotheses, 66(2), 234–246. [CrossRef]
- Santiago, M., Sachdev, S., Arnold, J. C., McGregor, I. S., & Connor, M. (2019). Absence of Entourage: Terpenoids Commonly Found in Cannabis sativa Do Not Modulate the Functional Activity of Δ 9 -THC at Human CB 1 and CB 2 Receptors. Cannabis and Cannabinoid Research, 4(3), 165–176. [CrossRef]
- Sawler, J., Stout, J. M., Gardner, K. M., Hudson, D., Vidmar, J., Butler, L., Page, J. E., & Myles, S. (2015). The Genetic Structure of Marijuana and Hemp. PLOS ONE, 10(8), e0133292. [CrossRef]
- Schultes, R. E., Klein, W. M., Plowman, T., & Lockwood, T. E. (1974). Cannabis: an example of taxonomic neglect. Botanical Museum Leaflets, Harvard University, 23(9), 337–367. http://www.jstor.org/stable/41762285.
- Shen, J., Niijima, A., Tanida, M., Horii, Y., Nakamura, T., & Nagai, K. (2007). Mechanism of changes induced in plasma glycerol by scent stimulation with grapefruit and lavender essential oils. Neuroscience Letters, 416(3), 241–246. [CrossRef]
- Shirley, N., Allgeier, L., LaNier, T., & Coyle, H. M. (2013). Analysis of the NMI01 Marker for a Population Database of scp Cannabis scp Seeds. Journal of Forensic Sciences, 58(s1). [CrossRef]
- Silva Brum, L. F., Emanuelli, T., Souza, D. O., & Elisabetsky, E. (2001). Effects of linalool on glutamate release and uptake in mouse cortical synaptosomes. Neurochemical Research, 26(3), 191–194. [CrossRef]
- Silva, A. C. R. da, Lopes, P. M., Azevedo, M. M. B. de, Costa, D. C. M., Alviano, C. S., & Alviano, D. S. (2012). Biological Activities of a-Pinene and β-Pinene Enantiomers. Molecules, 17(6), 6305–6316. [CrossRef]
- Small, E. (2016). Cannabis: a complete Guide. CRC Press. [CrossRef]
- Small, E., & Cronquist, A. (1976). A pratical and natural taxonomy for cannabis. TAXON, 25(4), 405–435. [CrossRef]
- Soler, S., Gramazio, P., Figàs, M. R., Vilanova, S., Rosa, E., Llosa, E. R., Borràs, D., Plazas, M., & Prohens, J. (2017). Genetic structure of Cannabis sativa var. indica cultivars based on genomic SSR (gSSR) markers: Implications for breeding and germplasm management. Industrial Crops and Products, 104, 171–178. [CrossRef]
- Sommano, S. R., Chittasupho, C., Ruksiriwanich, W., & Jantrawut, P. (2020). The Cannabis Terpenes. Molecules, 25(24), 5792. [CrossRef]
- Sriwichai, T., Junmahasathien, T., Sookwong, P., Potapohn, N., & Sommano, S. R. (2019). Evaluation of the Optimum Harvesting Maturity of Makhwaen Fruit for the Perfumery Industry. Agriculture, 9(4), 78. [CrossRef]
- Stone, N. L., Murphy, A. J., England, T. J., & O’Sullivan, S. E. (2020). A systematic review of minor phytocannabinoids with promising neuroprotective potential. British Journal of Pharmacology, 177(19), 4330–4352. [CrossRef]
- Ternelli, M., Brighenti, V., Anceschi, L., Poto, M., Bertelli, D., Licata, M., & Pellati, F. (2020). Innovative methods for the preparation of medical Cannabis oils with a high content of both cannabinoids and terpenes. Journal of Pharmaceutical and Biomedical Analysis, 186, 113296. [CrossRef]
- Tooker, J. F., & Frank, S. D. (2012). Genotypically diverse cultivar mixtures for insect pest management and increased crop yields. Journal of Applied Ecology, 49(5), 974–985. [CrossRef]
- Turner, C. E., Elsohly, M. A., & Boeren, E. G. (1980). Constituents of Cannabis sativa L. XVII. A Review of the Natural Constituents. Journal of Natural Products, 43(2), 169–234. [CrossRef]
- Usher, G. (1996). The Wordsworth dictionary of botany. Wordsworth Edition .
- Vigil, J. M., Stith, S. S., Brockelman, F., Keeling, K., & Hall, B. (2023). Systematic combinations of major cannabinoid and terpene contents in Cannabis flower and patient outcomes: a proof-of-concept assessment of the Vigil Index of Cannabis Chemovars. Journal of Cannabis Research, 5(1), 4. [CrossRef]
- Vitale, R. M., Iannotti, F. A., & Amodeo, P. (2021). The (Poly)Pharmacology of Cannabidiol in Neurological and Neuropsychiatric Disorders: Molecular Mechanisms and Targets. International Journal of Molecular Sciences, 22(9), 4876. [CrossRef]
- Wanas, A. S., Radwan, M. M., Chandra, S., Lata, H., Mehmedic, Z., Ali, A., Baser, K., Demirci, B., & ElSohly, M. A. (2020). Chemical Composition of Volatile Oils of Fresh and Air-Dried Buds of Cannabis c hemovars, Their Insecticidal and Repellent Activities. Natural Product Communications, 15(5), 1934578X2092672. [CrossRef]
- Wang, Y.-H., Avula, B., ElSohly, M., Radwan, M., Wang, M., Wanas, A., Mehmedic, Z., & Khan, I. (2018). Quantitative Determination of Δ9-THC, CBG, CBD, Their Acid Precursors and Five Other Neutral Cannabinoids by UHPLC-UV-MS. Planta Medica, 84(04), 260–266. [CrossRef]
- Weiss, R. F., & Fintelmann, V. (2000). Herbal Medicine. Thieme. https://books.google.pt/books?id=mF2gFrO0jI8C.
- Wong, H., & Cairns, B. E. (2019). Cannabidiol, cannabinol and their combinations act as peripheral analgesics in a rat model of myofascial pain. Archives of Oral Biology, 104, 33–39. [CrossRef]
- Worth, T. (2019). Unpicking the entourage effect. Nature, 572(7771), S12–S13. [CrossRef]
- Wu, B., Kulkarni, K., Basu, S., Zhang, S., & Hu, M. (2011). First-Pass Metabolism via UDP-Glucuronosyltransferase: a Barrier to Oral Bioavailability of Phenolics. Journal of Pharmaceutical Sciences, 100(9), 3655–3681. [CrossRef]
- Yang, H., Woo, J., Pae, A. N., Um, M. Y., Cho, N.-C., Park, K. D., Yoon, M., Kim, J., Lee, C. J., & Cho, S. (2016). α-Pinene, a Major Constituent of Pine Tree Oils, Enhances Non-Rapid Eye Movement Sleep in Mice through GABA A-benzodiazepine Receptors. Molecular Pharmacology, 90(5), 530–539. [CrossRef]
- Yang, Y., Zhang, Z., Li, S., Ye, X., Li, X., & He, K. (2014). Synergy effects of herb extracts: Pharmacokinetics and pharmacodynamic basis. Fitoterapia, 92, 133–147. [CrossRef]
- Zafar N. (2017). Herbal Bioenhancers: A Revolutionary Concept in Modern Medicine. World J Pharmaceut Res , 16(6), 381–397.
- Zagzoog, A., Mohamed, K. A., Kim, H. J. J., Kim, E. D., Frank, C. S., Black, T., Jadhav, P. D., Holbrook, L. A., & Laprairie, R. B. (2020). In vitro and in vivo pharmacological activity of minor cannabinoids isolated from Cannabis sativa. Scientific Reports, 10(1), 20405. [CrossRef]
- Zhang, J., Yan, J., Huang, S., Pan, G., Chang, L., Li, J., Zhang, C., Tang, H., Chen, A., Peng, D., Biswas, A., Zhang, C., Zhao, L., & Li, D. (2020). Genetic Diversity and Population Structure of Cannabis Based on the Genome-Wide Development of Simple Sequence Repeat Markers. Frontiers in Genetics, 11. [CrossRef]
| Strain | Variety | Cultivar | |
| Definition and Usage | Commonly used in the cannabis community, but scientifically incorrect in the context of plants. Refers to a specific genetic variant or subtype within a bacterial species | A more accurate and appropriate term to describe different Cannabis variants. It is defined as a species’ adaptation due to climate shifts, soil changes, diseases, etc. | A more accurate and appropriate term to describe different Cannabis variants. It is defined as a species’ adaptation due to climate shifts, soil changes, diseases, etc. |
| Characteristics | Primarily used in microbiology for bacteria, viruses, etc. Unique genetic characteristics may be present | Result of adaptations to habitat changes due to accidental factors. Reflects the diversity within the Cannabis species. | Created through deliberate breeding or agricultural techniques. Human intervention is involved in improving uniform traits. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





