Submitted:
10 September 2024
Posted:
12 September 2024
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Materials and Methods
2.1. Population
2.2. Clinical Parameters
2.3. Pathologic Evaluation
2.4. Surgical Procedure
2.5. Functional Analysis
2.6. Oncological Outcomes
2.7. Statistical Analysis
3. Results
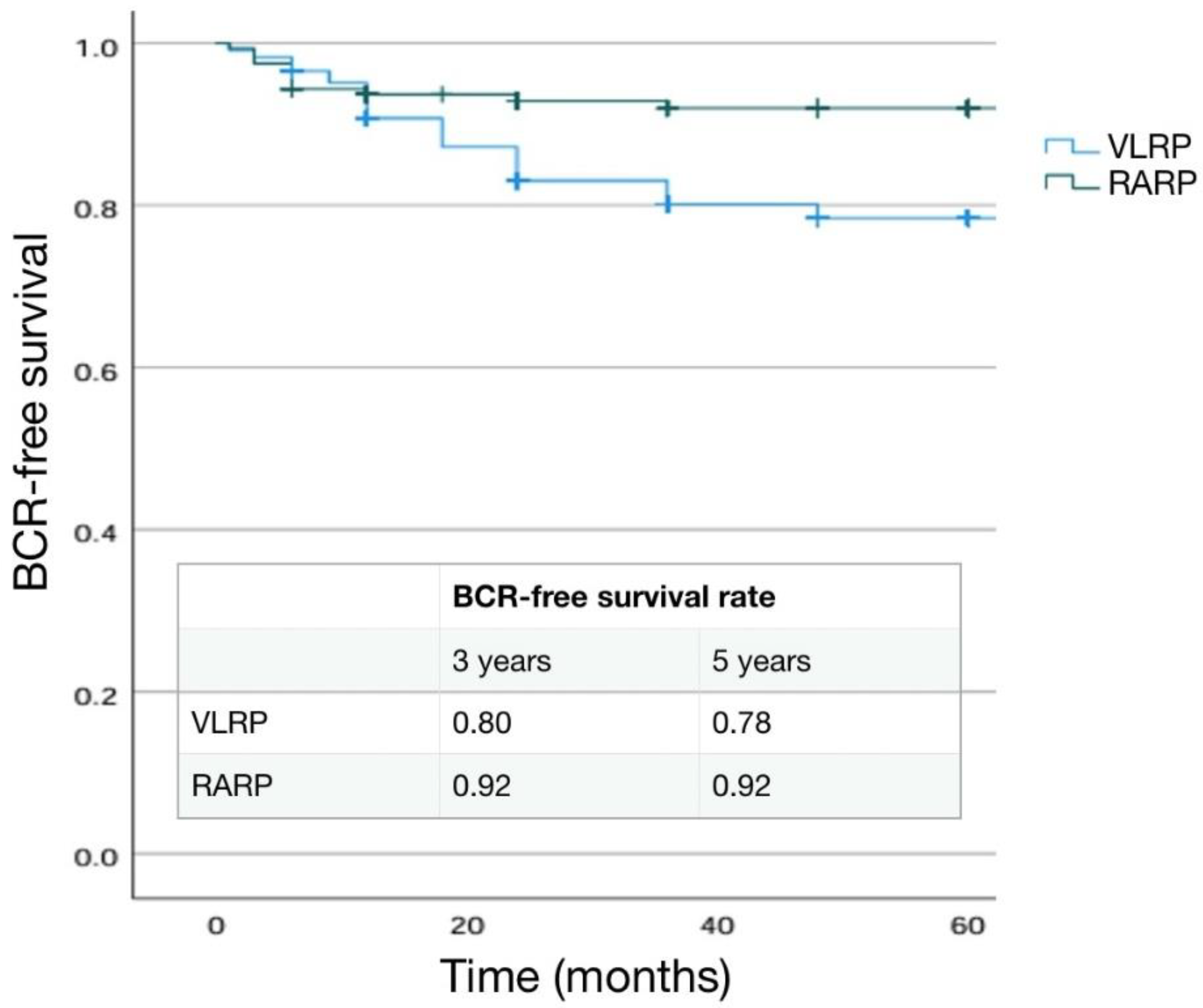
3.1. Differences in Pathological, Oncological and Functional Outcomes according to Surgical Approach
3.1.1. Pathological Outcomes
3.1.2. Functional Outcomes
| Parameter | LRP | RARP | P value |
|---|---|---|---|
|
Postoperative anastomotic leakage No yes |
284(100%) 0 |
157 (98.1%) 3 (1.9%) |
0.245 |
|
Postoperative blood transfusion no yes |
284 (100%) 0 |
158 (98.7%) 2 (1.3%) |
0.294 |
|
Postoperative lymphocele no yes |
278 (97.9%) 6 (2.1%) |
158 (98.7%) 2 (1.3%) |
0.16 |
|
Postoperative anastomotic stricture no yes |
272 (95.8%) 12 (4.2%) |
160 (100%) 0 |
0.51 |
|
Postoperative rectal injury no yes |
824 (100%) 0 |
160 (100%) 0 |
x |
| Postoperative hospitalization (days) | 3.75±0.74;3 (3-7) | 3.11±0.36;3 (3-5) | 0.037 |
| Catheterization time (days) | 11.53±1.45; 12 (8-14) | 9.60±2.06; 10 (6-16) | <0.001 |
| Parameter | LRP | RARP | P value |
|---|---|---|---|
| Postoperative Pelvic floor rehabilitation no yes |
230 (81.0%) 54 (19.0%) |
125 (78.2%) 33 (21.8%) |
0.106 |
| Postoperative PAD test (1 month) (grams) | 163.84±222.0; 50 (0-400) | 68.17± 374.17; 11 (0-404) | 0.68 |
| Postoperative PAD test (3 months (grams) | 75.57±122.20; 20 (0-480) | 14.02±42.09; 0 (0-250) | <0.001 |
| Postoperative PAD test ( 6 months) (grams) | 39.47±76.08; 5 (0-420) | 13.62±28.60; 0 (0-80) | 0.023 |
| Postoperative PAD test ( 12 months) (grams) | 14.76±29.41; 0 (0-100) | 15.33±23.2; 1 (0-50) | 0.964 |
| Parameter | LRP | RARP | P value |
|---|---|---|---|
|
Nerve sparing technique at surgery No Yes Monolateral Bilateral |
223 (78.5%) 61 (21.5%) 27 (43.3%) 34 (56.7%) |
94 (58%) 66 (42%) 25 (31.7%) 41 (68.3%) |
<0.001 0.187 |
| IIEF-5 postoperative ( 6 months) | 9.60±3.78; 9 (6-19) | 10.25±3.94; 9.5 (7-15) | 0.750 |
| IIEF-5 postoperative (12 months) | 10.14±4.77; 10 (5-21) | 18.0±3.75; 18 (18-18) | 0.117 |
3.1.3. Main Significant Differences in Results
3.2. Differences in Pathological, Oncological and Functional Outcomes according to Risk Classes
3.2.1. Pathological Outcomes
3.2.2. Main Significant Differences in Results
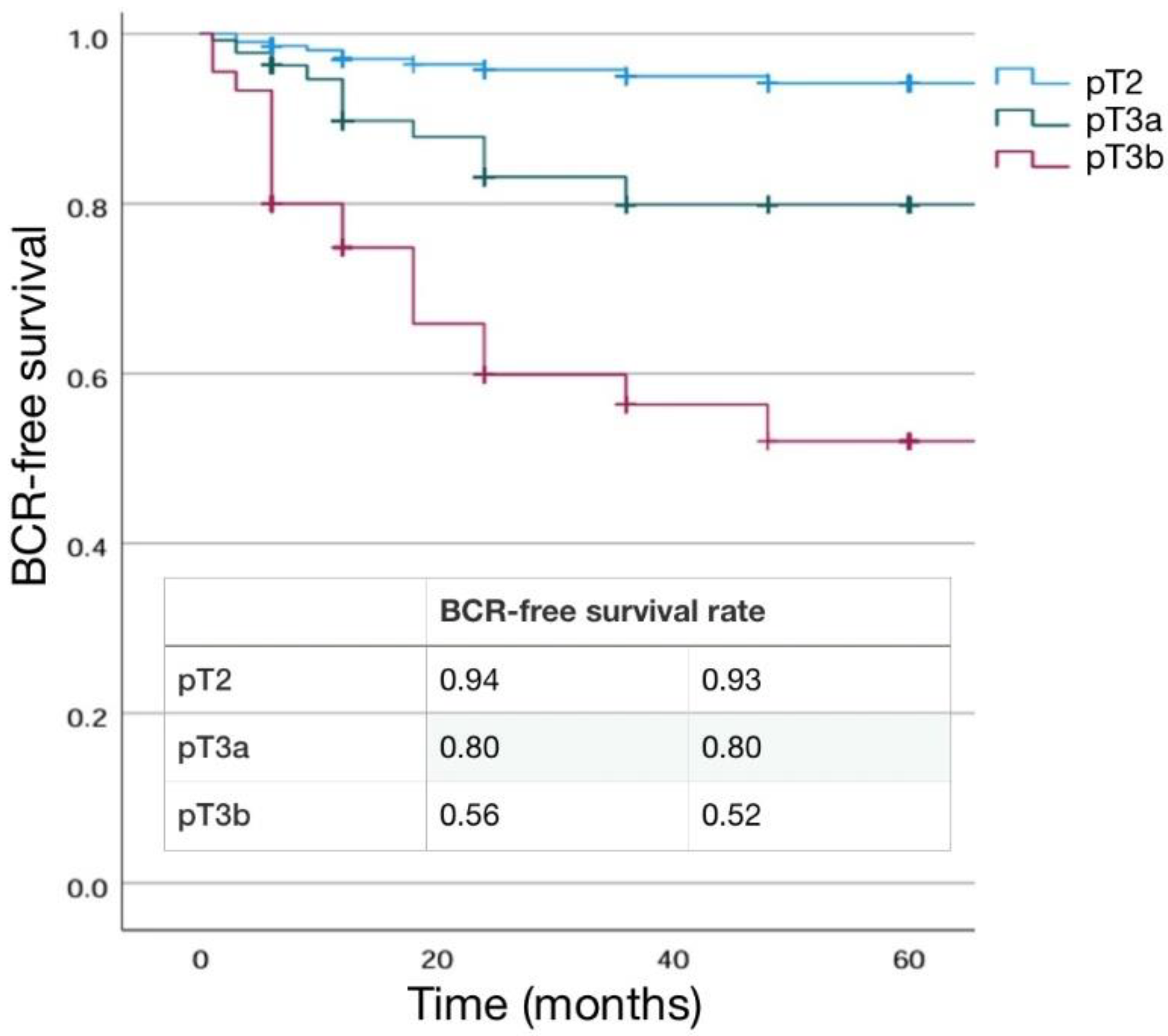
3.3. Differences in Pathological, Oncological and Functional Outcomes according to pT Stage
3.3.1. Pathological Outcomes
3.3.2. Main Significant Differences in Results
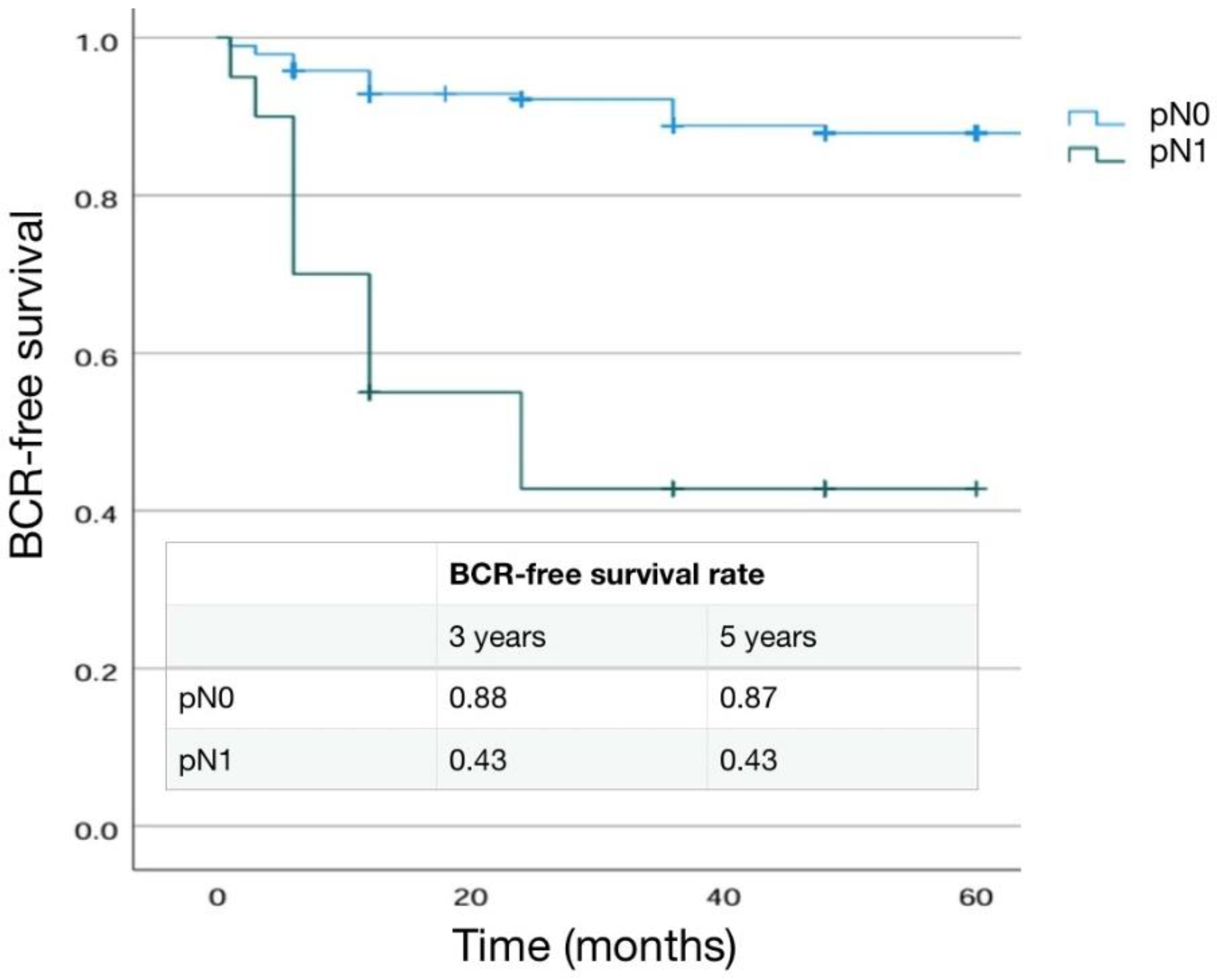
3.4. Differences in Pathological, Oncological and Functional Outcomes according to pN Stage
3.4.1. Pathological Outcomes
3.4.2. Main Significant Differences in Results
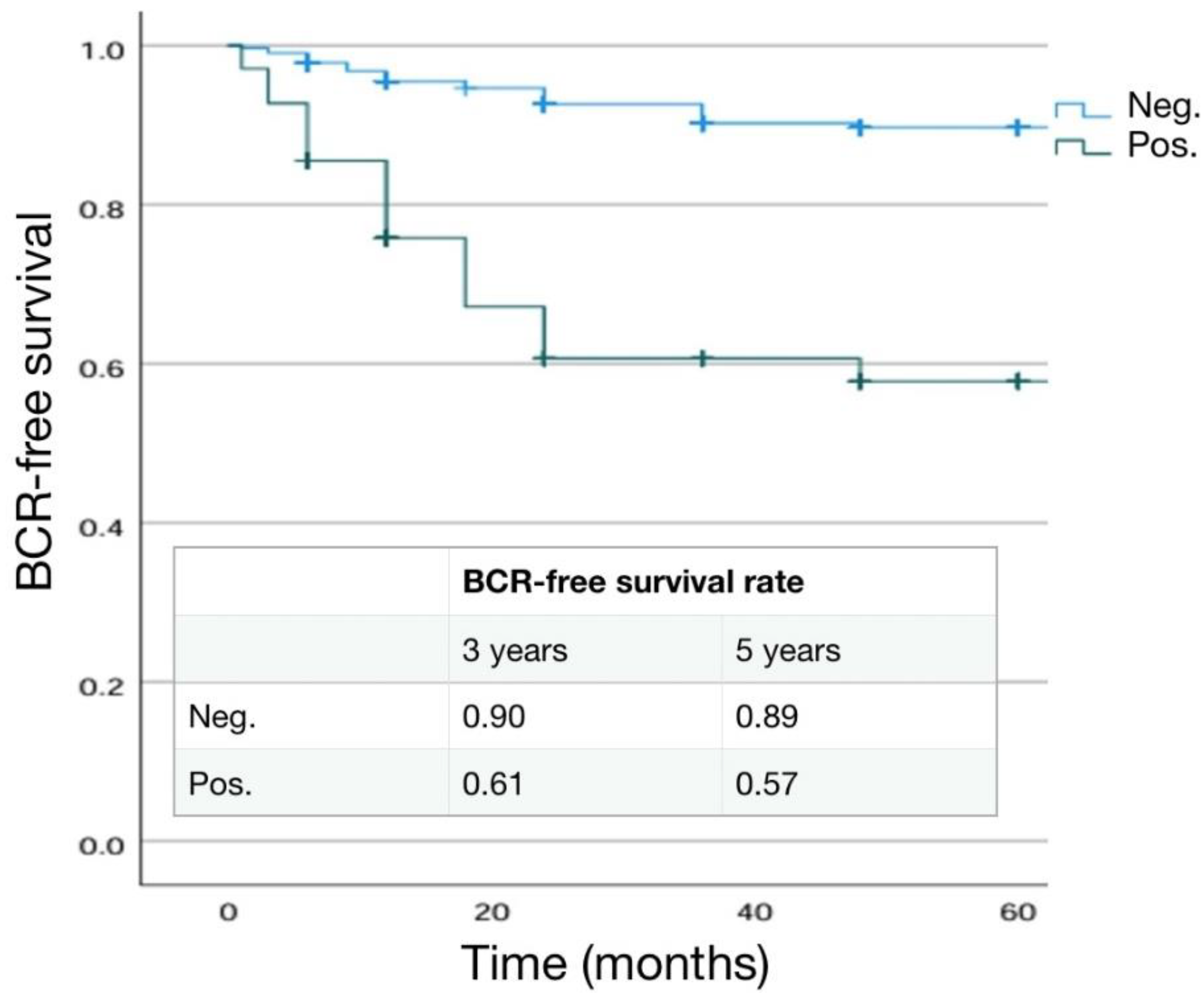
3.5. Differences in Pathological, Oncological and Functional Outcomes according to Surgical Margins
3.5.1. Pathological Outcomes
3.5.2. Main Significant Differences in Results
3.6. Differences in Pathological, Oncological and Functional Outcomes according to Biochemical Recurrence
3.6.1. Pathological Outcomes
3.6.2. Main Significant Differences in Results
3.7. Logistic Regression Analysis
| Univariate | Multivariate | |||||
| Parameter | OR | 95%CI | P value | OR | 95%CI | P value |
| Preoperative PSA | ||||||
| <10 ng/ml | 1.0 | 1.0 | ||||
| ≥ 10 ng/ml | 3.46 | 2.19-5.44 | <0.001 | 1.79 | 0.78-4.0 | 0.16 |
| PIRADS score | ||||||
| 3 | 1.0 | 1.0 | ||||
| 4 | 1.98 | 0.92-4.37 | 0.079 | 1.30 | 0.53-3.15 | 0.50 |
| 5 | 4.84 | 1.97-11.93 | <0.001 | 1.70 | 0.55-5.16 | 0.30 |
| Prostate biopsy + | ||||||
| monolateral | 1.0 | 1.0 | ||||
| bilateral | 1.40 | 0.95-2.07 | 0.087 | 2.0 | 1.48-6.22 | 0.064 |
| Max percentage PC tissue per core | Not included | |||||
| < 25% | 1.0 | |||||
| 25-50% 51-75% >75% |
1.33 1.33 2.0 |
0.76-2.35 0.76-2.35 0.75-5.33 |
0.285 0.285 0.102 |
|||
| ISUP grading | ||||||
| 1 e 2 | 1.0 | 1.0 | ||||
|
3 4-5 |
5.41 10.63 |
3.23-0.97 5.79-19.52 |
<0.001 <0.001 |
2.106.40 | 0.95-5.021.72-24.44 | 0.060.006 |
| Risk Classes | ||||||
| Low | 1.0 | |||||
| Intermediate | 1.20 | 0.87-2.45 | 0.276 | 1.0 | ||
| High | 2.90 | 1.74-4.89 | <0.001 | 2.80 | 1.1-6.2 | 0.04 |
| Univariate | Multivariate | |||||
| Parameter | OR | 95%CI | P value | OR | 95%CI | P value |
| Preoperative PSA | ||||||
| <10 ng/ml | 1.0 | 1.0 | ||||
| ≥ 10 ng/ml | 3.42 | 1.65-7.10 | <0.001 | 2.20 | 0.7-8.69 | 0.15 |
| PIRADS score | Not included | |||||
| 3 | 1.0 | |||||
| 4 | 1.57 | 0.4-6.09 | 0.51 | |||
| 5 | 0.58 | 0.56-5.96 | 0.64 | |||
| Prostate biopsy + | ||||||
| monolateral | 1.0 | 1.0 | ||||
| bilateral | 2.06 | 1.01-4.18 | 0.042 | 1.71 | 0.8-3.54 | 0.1 |
| Max percentage PC tissue per core | Not included | |||||
| < 25% | 1.0 | |||||
| 25-50% 51-75% >75% |
1.0 1.20 1.33 |
0.66-2.15 0.75-2.53 |
0.385 0.102 |
|||
| Risk Classes | ||||||
| Low | 1.0 | |||||
| Intermediate | 1.0 | 1.0 | ||||
| High | 2.90 | 1.74-4.89 | <0.001 | 3.28 | 1.45-7.4 | 0.04 |
| Univariate | Multivariate | |||||
| Parameter | OR | 95%CI | P value | OR | 95%CI | P value |
| Preoperative PSA | ||||||
| <10 ng/ml | 1.0 | 1.0 | ||||
| ≥ 10 ng/ml | 4.27 | 1.65-11.02 | 0.001 | 1.31 | 0.63-2.71 | 0.47 |
| PIRADS score | ||||||
| 3 | 1.0 | 1.0 | ||||
| 4 | 1.07 | 1-1.15 | 0.10 | 2.55 | 1.08-6 | 0.03 |
| 5 | 1.28 | 1.08-1.53 | 0.003 | 3.97 | 1.36-11.54 | 0.01 |
| Prostate biopsy + | ||||||
| monolateral | 1.0 | 1.0 | ||||
| bilateral | 5.20 | 1.48-18.36 | 0.005 | 1.35 | 0.96-1.89 | 0.78 |
| Max percentage PC tissue per core | Not included | |||||
| < 25% | 1.0 | |||||
| 25-50% 51-75% >75% |
1.0 1.0 1.50 |
0.67-3.39 |
0.27 | |||
| Risk classes | ||||||
| Low | 1.0 | |||||
| Intermediate | 1.0 | 1.0 | ||||
| High | 7.32 | 2.06-25.94 | <0,001 | 4.50 | 1.7.11.9 | 0.002 |
| Number of lymph nodes removed at surgery | ||||||
| <10 | 1.0 | 1.0 | ||||
| 10-15 | 3.36 | 0.7-12 | 0.10 | 1.70 | 0.62-11 | 0.5 |
| >15 | 5.69 | 1.78-18.17 | 0.001 | 4.10 | 1.009-26.5 | 0.049 |
| Nomogram risk | ||||||
| ≤7% | 1.0 | 1.0 | ||||
| >7% | 1.30 | 1.07-1.49 | 0.10 | 1.61 | 0.17-15.1 | 0.60 |
| Surgical technique | ||||||
| Laparoscopy | 1.0 | 1.0 | ||||
| Robotic assisted | 1.20 | 0.5-3.15 | 0.60 | 2.0 | 0.34-12.66 | 0.60 |
| ISUP grading | ||||||
| 1 e 2 | 1.0 | 1.0 | ||||
| 3 4-5 |
9.45 9.28 |
1.93-46.32 1.96-43.86 |
0.003<0.001 | 2.215.89 | 3.0-11.523.0-11.52 | 0.005<0.003 |
| pTstage | ||||||
| pT2 | 1.0 | 1.0 | ||||
| pT3a pT3b |
1.08 1.70 |
1.01-1.15 1.28-2.25 |
0.012<0.001 | 1.203.34 | 0.76-1.961.5-7.4 | 0.400.003 |
| Univariate | Multivariate | |||||
| Parameter | OR | 95%CI | P value | OR | 95%CI | P value |
| Preoperative PSA | ||||||
| <10 ng/ml | 1.0 | 1.0 | ||||
| ≥ 10 ng/ml | 3.57 | 2-5.63 | <0.001 | 2.98 | 1-8.8 | 0.04 |
| Prostate volume | Not included | |||||
| <50 cc | 1.0 | |||||
| ≥ 50 cc | 1.04 | 0.47-2.33 | 0.90 | |||
| PIRADS score | ||||||
| 3 | 1.0 | 1.0 | ||||
| 4 | 1.57 | 0.54-4.54 | 0.40 | 0.89 | 0.2-3.2 | 0.80 |
| 5 | 3.32 | 1.08-10.23 | 0.03 | 1.90 | 0.7-9.5 | 0.20 |
| Prostate biopsy + | Not included | |||||
| monolateral | 1.0 | |||||
| bilateral | 1.14 | 0.68-1.09 | 0.60 | |||
| Risk classes | ||||||
| Low | 1.0 | |||||
| Intermediate | 1.0 | 1.0 | ||||
| High | 1.44 | 0.81-2.54 | 0.20 | 4.98 | 1.0-22.8 | 0.40 |
| Surgical technique | ||||||
| Laparoscopic | 1.0 | 1.0 | ||||
| Robotic assisted | 1.27 | 0.7-2.1 | 0.35 | 1.20 | 0.5-1.8 | 0.40 |
| Nerve sparing tecnique at surgery | ||||||
| no | 1.0 | 1.0 | ||||
| yes | 0.80 | 0.46-1.44 | 0.48 | 2.10 | 0.5-7.9 | 0.30 |
| Operative time | Not included | |||||
| ≤ 120 min | 1.0 | |||||
| >120 min | 1.57 | 0.34-7.27 | 0.56 | |||
| pTstage | ||||||
| pT2 | 1.0 | 1.0 | ||||
| pT3a | 2.0 | 1.15-3.52 | 0.01 | 2.0 | 1.1-3.65 | 0.20 |
| pT3b | 4.60 | 2.27-9.32 | <0.001 | 4.60 | 2-10.67 | <0.001 |
| ISUP grading at surgery | ||||||
| 1-2 | 1.0 | 1.0 | ||||
| 3 | 1.65 | 0.90-3.0 | 0.10 | 1.37 | 0.73-2.66 | 0.30 |
| 4-5 | 1.84 | 1.0-3.41 | 0.05 | 1.40 | 0.74-2.70 | 0.30 |
| Univariate | Multivariate | |||||
| Parameter | OR | 95%CI | P value | OR | 95%CI | P value |
| Preoperative PSA | ||||||
| <10 ng/ml | 1.0 | 1.0 | ||||
| ≥ 10 ng/ml | 3.0 | 1.68-5.57 | <0.001 | 4.12 | 1.2-19.21 | 0.007 |
| PIRADS score | ||||||
| 3 | 1.0 | 1.0 | ||||
| 4 | 1.05 | 1-1.09 | 0.06 | 1.02 | 0.9-1.20 | 0.90 |
| 5 | 1.42 | 1.17-1.71 | <0.001 | 1.90 | 0.7-9.50 | 0.20 |
| Prostate biopsy + | ||||||
| monolateral | 1.0 | 1.0 | ||||
| bilateral | 2.64 | 1.37-5.10 | 0.003 | 4.52 | 0.81-25.10 | 0.08 |
| Risk classes | ||||||
| Low | 1.0 | |||||
| Intermediate | 1.0 | 1.0 | ||||
| High | 4.35 | 2.47-8.44 | <0.001 | 9.66 | 1.85-50.32 | 0.007 |
| Surgical technique | ||||||
| Laparoscopic | 1.0 | 1.0 | ||||
| Robotic assisted | 0.38 | 0.19-0.75 | 0.007 | 0.66 | 0.17-2.55 | 0.66 |
| Nerve sparing tecnique at surgery | ||||||
| no | 1.0 | 1.0 | ||||
| yes | 0.24 | 0.09-0.62 | 0.002 | 0.30 | 0.10-2.20 | 0.30 |
| Operative time | ||||||
| ≤ 120 min | 1.0 | 1.0 | ||||
| >120 min | 1.10 | 0.99-1.14 | 0.23 | 0.98 | 0.65-1.62 | 0.90 |
| pTstage | ||||||
| pT2 | 1.0 | 1.0 | ||||
| pT3a | 3.42 | 1.64-7.14 | <0.001 | 1.20 | 0.70-3.0 | 0.40 |
| pT3b | 11.10 | 4.80-25.75 | <0.001 | 2.82 | 0.99-8.21 | 0.06 |
| ISUP grading at surgery | ||||||
| 1-2 | 1.0 | 1.0 | ||||
| 3 | 3.21 | 1.47-7.0 | 0.005 | 1.87 | 0.83-5.72 | 0.190 |
| 4-5 | 6.76 | 3.32-13.70 | <0.001 | 3.73 | 1.50-9.50 | 0.006 |
| Lymphnode involvement | ||||||
| pN0 | 1.0 | 1.0 | ||||
| pN1 | 2.46 | 1.82-5.13 | 0.010 | 8.32 | 1.53-45.0 | 0.014 |
| Number of Lymph nodes removed | ||||||
| <10 | 1.0 | 1.0 | ||||
| 10-15 | 3.18 | 0.99-10.21 | 0.60 | 1.7 | 0.40-3.20 | 0.40 |
| >15 | 2.86 | 1.24-6.60 | 0.010 | 2.2 | 0.80-4.50 | 0.20 |
| Surgical margins | ||||||
| Negative | 1.0 | 1.0 | ||||
| positive | 4.97 | 2.39-10.34 | <0.001 | 7.20 | 1.80-28.30 | 0.004 |
| Surgical margins grade | ||||||
| 3 | 1.0 | 1.0 | ||||
| 4 | 5.0 | 5.57-45.0 | <0.001 | 4.96 | 2.37-10.37 | <0.001 |
| PNI at surgery | ||||||
| negative | 1.0 | 1.0 | ||||
| positive | 4.50 | 1.92-10.55 | <0.001 | 1.50 | 0.63-4.50 | 0.32 |
3.7.1. Predictors for the Risk of Extracapsular Extension
3.7.2. Predictors for the Risk of Upgrading at Surgery
3.7.3. Predictors for the Risk of Lymph Node Involvement
3.7.4. Predictors for the Risk of Positive Surgical Margins
3.7.5. Predictors for the Risk of Biochemical Recurrence
4. Discussion
5. Conclusions
6. Patents
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Bhayani, S, Pavlovich CP, Strup SE, Su LM. Laparoscopic Radical Prostatectomy: a multi-istitutional study of conversion to open surgery. Urology, 2004; 63(1) 99-102. [CrossRef]
- Stolzenburg JU, Holze S, Arthanareeswaran VK, Neuhaus P, Do HM, Haney CM,Dietel A, Truss MC, Stützel KD, Teber D, Hohenfellner M, Rabenalt R, Albers P,Mende M. Robotic-assisted Versus Laparoscopic Radical Prostatectomy: 12-month Outcomes of the Multicentre Randomised Controlled LAP-01 Trial. Eur Urol Focus. 2022 Nov;8(6):1583-1590. [CrossRef]
- Ma J, Xu W, Chen R, Zhu Y, Wang Y, Cao W, Ju G, Ren J, Ye X, He Q, Chang Y,Ren S. Robotic-assisted versus laparoscopic radical prostatectomy for prostate cancer: the first separate systematic review and meta-analysis of randomised controlled trials and non-randomised studies. Int J Surg. 2023 May 1;109(5):1350-1359. [CrossRef]
- Huang X, Wang L, Zheng X, Wang X. Comparison of perioperative, functional,and oncologic outcomes between standard laparoscopic and robotic-assisted radical prostatectomy: a systemic review and meta-analysis. Surg Endosc. 2017 Mar;31(3):1045-1060. [CrossRef]
- Dell'Oglio P, Mottrie A, Mazzone E. Robot-assisted radical prostatectomy vs.open radical prostatectomy: latest evidences on perioperative, functional and oncological outcomes. Curr Opin Urol. 2020 Jan;30(1):73-78. [CrossRef]
- de Oliveira RAR, Guimarães GC, Mourão TC, de Lima Favaretto R, Santana TBM,Lopes A, de Cassio Zequi S. Cost-effectiveness analysis of robotic-assisted versus retropubic radical prostatectomy: a single cancer center experience. J Robot Surg. 2021 Dec;15(6):859-868. [CrossRef]
- European Association of Urology (EAU). EAU Guidelines on Prostate Cancer. 2024 Available at: [EAU Guidelines](https://uroweb.org/guidelines/prostate-cancer).
- Sciarra A, Gentilucci A, Cattarino S, Innocenzi M, Von Heland M, Gentile V, Salciccia S. Laparoscopic versus open radical prostatectomy in high prostate volume cases: impact on oncological and functional results. Int Braz J Urol 2016; 42: 223-33. [CrossRef]
- Okegawa T, Omura S, Samejima M, Fukuhara H. Laparoscopic radical prostatectomy versus robot-assisted radical prostatectomy: comparison of oncological outcomes at a single center. Prostate International 2020 ;8: 16-21. [CrossRef]
- Gacci M, Artibani W, Bassi P, Bertoni F, Bracarda S, Briganti A, Carmignani G, Carmignani L, Conti G, Corvò R, De Nunzio C, Fusco F, Graziotti P, Greco I, Maggi S, Magrini SM, Mirone V, Montironi R, Morgia G, Muto G, Noale M, Pecoraro S, Porreca A, Ricardi U, Russi E, Russo G, Salonia A, Simonato A, Serni S, Tomasini D, Tubaro A, Zagonel V, Crepaldi G; MIRROR-SIU/LUNA Study Group and the Pros-IT CNR Study Group. How radical prostatectomy procedures have changed over the last 10 years in Italy: a comparative analysis based on more than 1500 patients participating in the MIRROR-SIU/LUNA and the Pros-IT CNR study. World J Urol. 2021 May;39(5):1445-1452. [CrossRef]
- Laschena L, Messina E, Flammia RS, Borrelli A, Novelli S, Messineo D, Leonardo C, Sciarra A, Ciardi A, Catalano C, Panebianco V. What the urologist needs to know before radical prostatectomy: MRI effective support to pre-surgery planning. Radiol Med. 2024 Jul;129(7):1048-1061. [CrossRef]
- Salciccia S, Viscuso P, Bevilacqua G, Tufano A, Casale P, De Berardinis E, Di Pierro GB, Cattarino S, Gentilucci A, Lourdes Lia F, Ivan DG, Rosati D, DelGiudice F, Sciarra A, Mariotti G. Comparison of Different Invasive Devices for the Treatment of Urinary Incontinence after Radical Prostatectomy. Adv Urol. 2022 Jun 21;2022:8736249. [CrossRef]
- Di Pierro GB, Salciccia S, Frisenda M, Tufano A, Sciarra A, Scarrone E, Del Giudice F, Asero V, Bevilacqua G, Moriconi M, Carbone A, Pastore A, Signore S, Bove P, Forte F, Emiliozzi P, Tubaro A, De Nunzio C, Canale V. Comparison of Four Validated Nomograms (Memorial Sloan Kettering Cancer Center, Briganti 2012, 2017, and 2019) Predicting Lymph Node Invasion in Patients with High-Risk Prostate Cancer Candidates for Radical Prostatectomy and Extended Pelvic Lymph Node Dissection: Clinical Experience and Review of the Literature. Cancers (Basel). 2023 Mar 9;15(6):1683. [CrossRef]
- Sciarra A, Frisenda M, Maggi M, Magliocca FM, Ciardi A, Panebianco V, Berardinis E, Salciccia S, Di Pierro GB, Gentilucci A, Del Giudice F, Busetto GM, Tufano A. Prospective comparative trial on nerve-sparing radical prostatectomy using a robot-assisted versus laparoscopic technique: expectation versus satisfaction and impact on surgical margins. Cent European J Urol. 2021;74(2):169-177. [CrossRef]
- Valenzi FM, Fuschi A, Al Salhi Y, Sequi MB, Suraci PP, Pacini M, Scalzo S, Rera OA, Antonioni A, Graziani D, Martino G, Candita G, Gianfrancesco F, Zucchi A, Lombardo R, De Nunzio C, Cicione A, Bozzini G, Rengo M, Capodiferro P,Sciarra A, Petrozza V, Carbone A, Pastore AL. Is early continence recovery related to the length of spared urethra? A prospective multicenter study comparing preoperative MRI and histologic specimen measurements after robotic radical prostatectomy. Eur J Surg Oncol. 2024 Jun;50(6):108319. [CrossRef]
- Salciccia S, Sciarra A, Moriconi M, Maggi M, Viscuso P, Rosati D, Frisenda M,Di Pierro GB, Canale V, Bevilacqua G, Nesi G, Del Giudice F, Gentilucci A,Cattarino S, Mariotti G. How to Predict Outcomes from a Biofeedback and Pelvic Floor Muscle Electric Stimulation Program in Patients with Urinary Incontinence after Radical Prostatectomy. J Clin Med. 2021 Dec 27;11(1):127. [CrossRef]
- Lucciola S, Pisciotti ML, Frisenda M, Magliocca F, Gentilucci A, Del Giudice F, Canale V, Scarrone E, Busetto GM, Carrieri G, Cormio L, Carbone A, Pastore A,De Nunzio C, Tubaro A, Leonardo C, Franco G, Di Pierro GB, Salciccia S, Sciarra A, Panebianco V. Predictive role of node-rads score in patients with prostate cancer candidates for radical prostatectomy with extended lymph node dissection:comparative analysis with validated nomograms. Prostate Cancer Prostatic Dis.2023 Jun;26(2):379-387. [CrossRef]
- Salciccia S, Rosati D, Viscuso P, Canale V, Scarrone E, Frisenda M, Catuzzi R, Moriconi M, Asero V, Signore S, De Dominicis M, Emiliozzi P, Carbone A,Pastore AL, Fuschi A, Di Pierro GB, Gentilucci A, Cattarino S, Mariotti G, Busetto GM, Ferro M, De Berardinis E, Ricciuti GP, Panebianco V, Magliocca FM,Del Giudice F, Maggi M, Sciarra A. Influence of operative time and blood loss on surgical margins and functional outcomes for laparoscopic versus robotic-assisted radical prostatectomy: a prospective analysis. Cent European J Urol.2021;74(4):503-515. [CrossRef]
- Fuschi A, Pastore AL, Al Salhi Y, Martoccia A, De Nunzio C, Tema G, Rera OA,Carbone F, Asimakopoulos AD, Sequi MB, Valenzi FM, Suraci PP, Scalzo S, Del Giudice F, Nardecchia S, Bozzini G, Corsini A, Sciarra A, Carbone A. The impact of radical prostatectomy on global climate: a prospective multicentre study comparing laparoscopic versus robotic surgery. Prostate Cancer Prostatic Dis.2024 Jun;27(2):272-278. [CrossRef]
- Sciarra A, Viscuso P, Arditi A, Mariotti G, De Berardinis E, Di Pierro GB,Canale V, Gentilucci A, Maria Busetto G, Maggi M, Eisenberg ML, Vilson F, Chung BI, Ferro M, Salciccia S, Del Giudice F. A biofeedback-guided programme or pelvic floor muscle electric stimulation can improve early recovery of urinary continence after radical prostatectomy: A meta-analysis and systematic review.Int J Clin Pract. 2021 Oct;75(10):e14208. [CrossRef]
- Mariotti G, Salciccia S, Innocenzi M, Gentilucci A, Fasulo A, Gentile V,Sciarra A. Recovery of Urinary Continence After Radical Prostatectomy Using Early vs Late Pelvic Floor Electrical Stimulation and Biofeedback-associated Treatment. Urology. 2015 Jul;86(1):115-20. [CrossRef]
- Mariotti G, Sciarra A, Gentilucci A, Salciccia S, Alfarone A, Di Pierro G,Gentile V. Early recovery of urinary continence after radical prostatectomy using early pelvic floor electrical stimulation and biofeedback associated treatment. J Urol. 2009 Apr;181(4):1788-93. [CrossRef]
- Sciarra A, Gentilucci A, Salciccia S, Von Heland M, Ricciuti GP, Marzio V,Pierella F, Musio D, Tombolini V, Frantellizzi V, Pasquini M, Maraone A,Guandalini A, Maggi M. Psychological and functional effect of different primary treatments for prostate cancer: A comparative prospective analysis. Urol Oncol. 2018 Jul;36(7):340.e7-340.e21. [CrossRef]
- Panebianco V, Salciccia S, Cattarino S, Minisola F, Gentilucci A, AlfaroneA, Ricciuti GP, Marcantonio A, Lisi D, Gentile V, Passariello R, Sciarra A. Use of multiparametric MR with neurovascular bundle evaluation to optimize the oncological and functional management of patients considered for nerve-sparing radical prostatectomy. J Sex Med. 2012 Aug;9(8):2157-66. [CrossRef]
- Sciarra A, Cristini C, Von Heland M, Salciccia S, Gentile V. Randomized trial comparing an anterograde versus a retrograde approach to open radical prostatectomy: results in terms of positive margin rate. Can Urol Assoc J. 2010 Jun;4(3):192-8. [CrossRef]
- Sciarra A, Voria G, Monti S, Mazzone L, Mariotti G, Pozza M, D'Eramo G,Silverio FD. Clinical understaging in patients with prostate adenocarcinoma submitted to radical prostatectomy: predictive value of serum chromogranin A. Prostate. 2004 Mar 1;58(4):421-8. [CrossRef]
- Sciarra A, Gentile V, Voria G, Mariotti G, Seccareccia F, Pastore A, Di Silverio F. Role of radical retropubic prostatectomy in patients with locally advanced prostate cancer: the influence of Gleason score 8-10. Urol Int. 2003;70(3):186-94. [CrossRef]
- Sciarra A, Gentile V, De Matteis A, Dattilo C, Autran Gomez AM, Salciccia S, Di Silverio F. Long-term experience with an anatomical anterograde approach to radical prostatectomy: results in terms of positive margin rate. Urol Int. 2008;80(2):151-6. [CrossRef]
- Sooriakumaran P,Srivastava A,Shariat SF A multinational, multiinstitutional study comparing positive surgical margin rates among 22393 open, laparosocopic and robot assisted radical prostatectomy patients. Eur Urol 2004;66:450-6. [CrossRef]
- Albadine R, Hyndman ME, Chaux A, Jeong JY, Saab S, Tavora F Characteristics of positive surgical margins in robotic assisted radical prostatectomy, open retropubic radical prostatectomy, and laparoscopic radical prostatectomy: a comparative histopathologic study from a single academic center. Hum Pathol. 2012;43:254-60. [CrossRef]
- Hegarty NJ, Kaouk JH. Radical prostatectomy: a comparison of open, laparoscopic and robot-assisted laparoscopic techniques. Can J Urol. 2006;13:56-61.
- Laurila TA, Huang W, Jarrard DF. Robotic-assisted laparoscopic and radical retropubic prostatectomy generate similar positive margin rates in low and intermediate risk patients. Urol Oncol. 2009;27:529-33. [CrossRef]
- Williams SB, D’Amico AV, Weinberg AC, Gu X, Lipsitz SR, Hu JC. Population-based determinants of radical prostatectomy surgical margin positivity. BJU Int. 2011;107:1734-40. [CrossRef]
- Williams SB, Chen MH, D’Amico AV, Weinberg AC, Kacker R, Hirsch MS, et al. Radical retropubic prostatectomy and robotic-assisted laparoscopic prostatectomy: likelihood of positive surgical margin(s). Urology. 2010;76:1097-101. [CrossRef]
- Smith JA Jr, Chan RC, Chang SS, Herrell SD, Clark PE, Baumgartner R, et al. comparison of the incidence and location of positive surgical margins in robotic assisted laparoscopic radical prostatectomy and open retropubic radical prostatectomy. J Urol. 2007;178:2385-9. [CrossRef]
- Pettus JA, Masterson T, Sokol A, Cronin AM, Savage C, Sandhu JS, et al. Prostate size is associated with surgical difficulty but not functional outcome at 1 year after radical prostatectomy. J Urol. 2009;182:949-55. [CrossRef]
| Number cases | 444 |
| Age (years) | 67.49±6.53; 68: (47-73) |
| BMI | 26.05±3.55; 26.0: (18.0- 39.40) |
| Charlson Index | 3.86±1.06; 4: (0-7) |
| Familiarity Yes no |
35 (7.9%) 409(92.1%) |
| Digital Rectal Examination Normal Suspicious |
376 (84.7%) 68 (15.3%) |
| Preoperative total PSA (ng/ml) | 8.61±5.74; 7.30: (3.0-64.0) |
| PSAD | 0.22±0.186; 0.17: (0.1-0.59) |
| Prostate volume (cc) | 48.17±14.79; 45.0: (20.0-120.0) |
|
mMR PIRADS score PIRADS 2 PIRADS 3 PIRADS 4 PIRADS 5 |
(data available in 208 cases) 6 (2.9%) 44 (21.1%) 111 (53.4%) 47 (22.6%) |
| Prostate Tumor size (mm) at mMR | 12.8±6.53; 11.0: (4.0-39.0) |
| Preoperative CT and bone scan No yes |
374 (84.3%) 70 (15.7%) |
| Preoperative PET CT scan no choline PSMA |
434 (97.7%) 6 (1.3%) 4 (1.0%) |
|
Clinical T staging T1 T2a T2b T2c T3a T3b T4 |
12 (2.7%) 30 (6.7%) 192 (43.2%) 173 (39.0%) 30 (6.8%) 7 (1.6%) 0 |
|
Clinical N staging N0 N1 |
440 (99.1%) 4 (0.9%) |
| Number of suspected lymph node at imaging | 3.0±1,4; 3: (2-4) |
| Prostate biopsy type Random Target Target + random |
163(36.7%) 17 (3.8%) 264 (59.5%) |
| Prostate biopsy number of cores | 12.61±3.65;12 (4-27) |
|
Biopsy outcomes % positive samples PC % positive clinical significant PC Max% PC tissue per core |
41.31±26.13; 34: (2-100) 53.47±28.5; 50.0: (8.0-100.0) 58.35±24.7; 50.0: (6.0-100.0) |
| Prostate biopsy laterality + Monoliteral bilateral |
203 (45.7%) 241 (54.3%) |
|
ISUP grading at biopsy 1 2 3 4 5 |
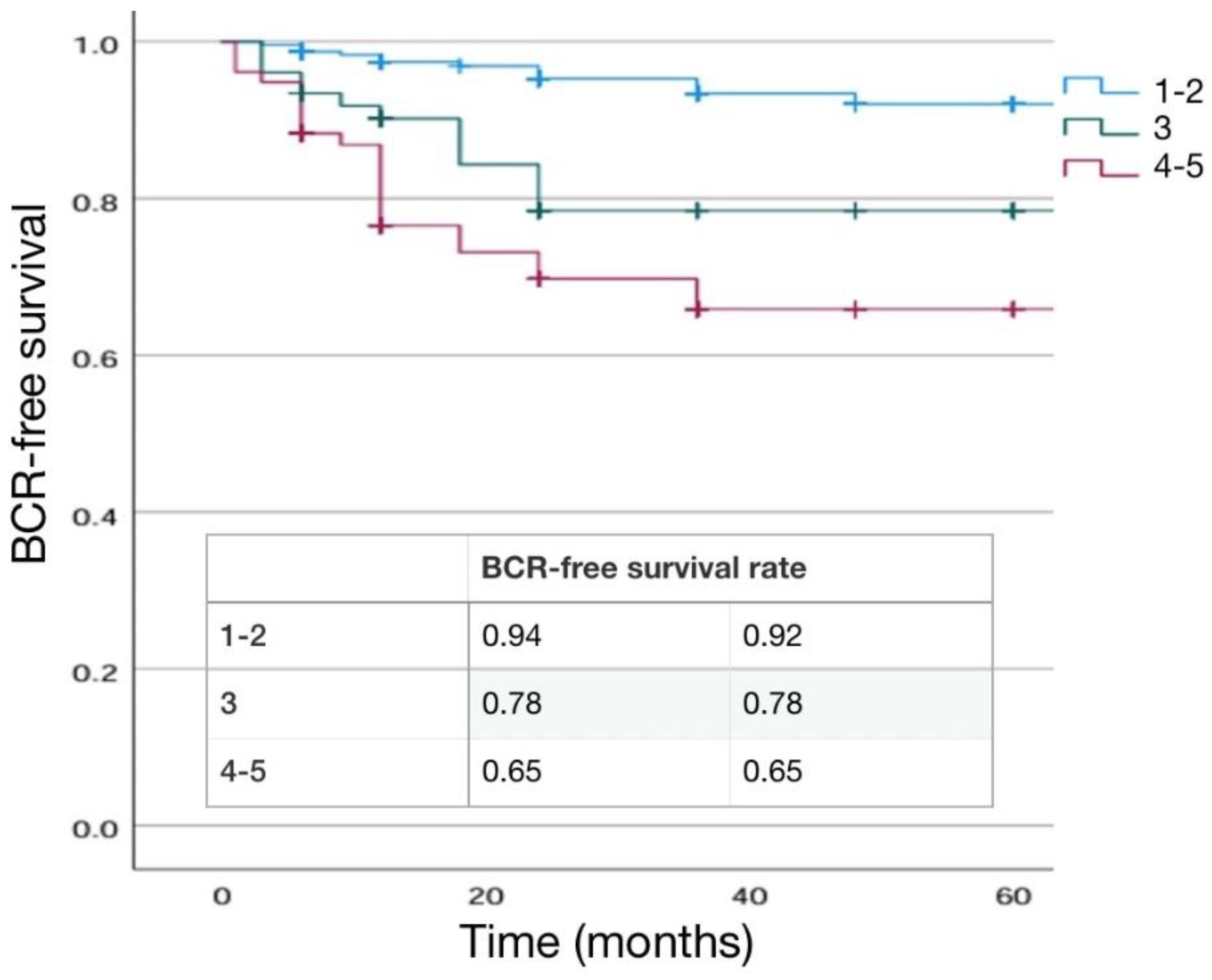
149 (33.6%) 135 (30.4%) 86 (19.4%) 57 (12.8%) 17 (3.8%) |
|
Risk Class ( D’Amico) Low risk Intermediate risk High risk |
142 (32.0%) 200 (45.0%) 102 (23.0%) |
|
Nomograms results (% estimated risk for N+) in intermediate and high risk cases Briganti 2012 Briganti 2019 |
23.40±19.11; 17.5: (2-85) 17.58±31.4; 12.85: (2-95) |
|
Surgical technique at radical prostatectomy Laparoscopic Robotic assisted |
284 (64%) 160 (36%) |
| Operative time (minutes) | 160.05±33.73; 160: (90-300) |
|
Nerve sparing technique at surgery No Yes Monolateral Bilateral |
317 (71.4%) 127 (28.6%) 52 (40.9%) 75 (59.1%) |
|
Extended lymph node dissection no yes |
309 (69.6%) 135 (30.4%) |
|
Pathological stage (T) pT2 pT3a pT3b pT4 |
246 (55.4%) 150 (33.8%) 48 (10.8%) 0 |
|
Pathological stage (N) N0 N+ |
115 (85.2%) 20 (14.8%) |
|
Number Lymph nodes removed at surgery Total cases N+ cases N0 cases |
17.22±6.81; 18: (4-47) 17.95±9.38; 19: (6-47) 17.07±6.24; 18: (4-35) |
| Percentage positive lymph nodes in pN+ cases | 14.80 ±11.60; 10.4: (4.0-26.6) |
|
Site of positive lymphnodes (number of cases) Obturator right Obturator left External iliac right External iliac left Internal iliac right Internal iliac left |
10 (50.0%) 13 (65.0%) 4 (20.0%) 6 (30.0%) 7 (35.0%) 9 (45.0%) |
|
ISUP grading at surgery 1 2 3 4 5 |
92 (20.7%) 183 (41.2%) 89 (20.1%) 48 (10.8%) 32 (7.2%) |
|
Surgical margin at surgery (R) Negative positive |
367 (82.7%) 77 (17.3%) |
|
Positive surgical margin site apex lateral basal posterior multiple |
31 (40.2%) 23 (29.9%) 6 (7.8%) 12 (15.6%) 5 (6.5%) |
|
Positive surgical margin grading 3 4 5 |
55 (71.4%) 21 (27.3%) 1 (1.3%) |
| Positive surgical margin radial distance (mm) | 2.97±1.1; 3 (1-7) |
|
Positive surgical margin Single multiple |
72 (93.5%) 5 (6.5%) |
|
PNI at surgery positive negative |
269 (60.5%) 175 (39.5%) |
|
Cribriform/IDC at surgery positive negative |
20 (4.5%) 424 (95.5%) |
| Postoperative hospitalization (days) | 3.41±0.56;3 (3-7) |
| Catheterization time (days) | 10.27±2.0; 10: (6-16) |
| Postoperative anastomotic leakage no yes |
441 (99.3%) 3 (0.7%) |
| Postoperative blood transfusion no yes |
442 (99.5%) 2 (0.5%) |
| Postoperative lymphocele no yes |
436 (98.2%) 8 (1.8%) |
| Postoperative anastomotic stricture no yes |
432 (97.3%) 12 (2.7%) |
| Postoperative rectal injury no yes |
444 (100%) 0 (0%) |
| Postoperative total PSA (ng/ml)(at 1 month) | 0.09±0.17; 0.02: (0.01-2.0) |
| Postoperative follow-up (months) | 56.4±37.8; 48 (12-120) |
| Biochemical progression No Yes |
391 (88.1%) 53 (11.9%) |
| Time to biochemical progression (months) | 18.67±24.57;12 (1-48) |
| Adjuvant therapy no RT RT+ADT |
391 (88.1%) 37 (8.3%) 16 (3.6%) |
| IIEF-5 preoperative (patients submitted to nerve sparing) | 20.75±5.83; 23: (12-25) |
| Postoperative use of tadalafil No yes |
317 (71.4%) 127 (28.6%) |
| IIEF-5 postoperative (6 months) (patients submitted to nerve sparing) | 9.68±3.74; 9: (6-19) |
| IIEF-5 postoperative (12 months) (patients submitted to nerve sparing) | 10.41±24.10; 8: (5-21) |
| Penile prothesis no yes |
420 (94.6%) 24 (5.4%) |
| Postoperative Pelvic floor rehabilitation no yes |
355 (79.9%) 89 (20.1%) |
|
Postoperative PAD test 1 months (grams) 3 months (grams) 6 months (grams) 12 months (grams) |
99.37±33.0; 20.0: (0-404) 45.14±95.9; 4.0 (0-480) 34.86±70.5; 2.0 (0-420) 14.82±28.7; 0: (0-100) |
| No PADS postoperative status | 407 (91.6%) |
| Artificial Sphincter no yes |
432 (97.3%) 12 (2.7%) |
| LRP | RARP | P value | |
|---|---|---|---|
| Number cases | 284 | 160 | |
| Age (years) | 68.65±6.582; 69: (48-73) | 66.35±5.973; 66 (47-68) | <0.001 |
| BMI | 26.17±11.16; 25.6:(17-39.4) | 25.127±4.317; 25.0 (17-34) | 0.124 |
| Charlson Index | 3.34±1.42; 4 (2-7) | 4.10±0.722; 4 (0-7) | <0.001 |
| Familiarity Yes no |
25 (8.8%) 259 (91.2%) |
10 (6.2%) 150 (93.8%) |
0.217 |
| Digital Rectal Examination Normal Suspicious |
245 (86.3%) 39 (13.7%) |
131 (81.9%) 29 (18.1%) |
0.327 |
| Preoperative total PSA (ng/ml) | 8.79±6.175; 7.5: (3.0-64.0) | 8.23±4.89; 6.9 (3.0-30.0) | 0.328 |
| PSAD | 0.16±0.10; 0.14: (0.04-0.59) | 0.22±0.18; 0.17 (0.07-0.59) | 0.546 |
| Prostate volume (cc) | 49.67±16.07; 49.5: (22-120) | 47.56±14.39; 45 (20-120) | 0.409 |
|
mMR PIRADS score (data available in 208 cases) PIRADS 2 PIRADS 3 PIRADS 4 PIRADS 5 |
3 (4.3%) 21 (30.0%) 33 (47.1%) 13 (18.6%) |
3 (2.2%) 23 (16.7%) 78 (56.5%) 34 (24.6%) |
0.042 |
| Prostate Tumor size (mm) at mMR | 10.76±5.11; 10.0: (6-39) | 13.59±6.94;12.0 (4-38) | 0.10 |
| Preoperative CT and bone scan No yes |
245 (86.3%) 39 (13.7%) |
129(80.6%) 31 (19.4%) |
0.251 |
| Preoperative PET CT scan no choline PSMA |
283 (99.6%) 1 (0.4%) 0 |
151 (94.3%) 5 (3.2%) 4 (2.5%) |
0.117 |
|
Clinical T staging T1 T2a T2b T2c T3a T3b |
9 (3.2) 19 (6.7%) 110 (38.7%) 131 (46.1%) 12 (4.2%) 3 (1.1%) |
3 (1.9%) 11 (6.9%) 82 (51.2%) 42 (26.2%) 18 (11.3%) 4 (2.5%) |
<0.001 |
|
Clinical N staging N0 N1 |
282 (99.3%) 2 (0.7%) |
158 (98.7%) 2 (1.3%) |
0.545 |
| Number of suspected lymph node at imaging | 4±1.4; 4: (3-4) | 2±1.35; 2 (1-3) | 0.64 |
|
Biopsy outcomes % positive samples PC |
40.6±26.26; 29: (8-100) |
41.60±26.09;35 (4-57) |
0.772 |
| Prostate biopsy laterality + Monoliteral bilateral |
102 (35.9%) 182 (64.1%) |
101 (63.1%) 59 (36.9%) |
<0.001 |
|
ISUP grading at biopsy 1 2 3 4 5 |
98 (34.5%) 74 (26.1%) 58 (20.4%) 42 (14.8%) 12 (4.2%) |
51 (31.9%) 61 (38.1%) 28 (17.5%) 15 (9.4%) 5 (3.1%) |
0.610 |
|
Risk Class ( D’Amico) Low risk Intermediate risk High risk |
92 (32.4%) 126 (44.3%) 66 (23.3%) |
50 (31.3%) 74 (46.2%) 36 (22.5%) |
0.849 |
| Operative time (minutes) | 173.33±44.34; 160 (90-300) | 153.21±25.11; 142.5 (90-300) | <0.001 |
|
Nerve sparing technique at surgery No Yes Monolateral Bilateral |
223 (78.5%) 61 (21.5%) 27 (43.3%) 34 (56.7%) |
94 (58.7%) 66 (41.3%) 25 (31.7%) 41 (68.3%) |
<0.001 0.187 |
|
Extended lymph node dissection no yes |
146 (73.2%) 76 (26.8%) |
74 (63.1%) 59 (36.9%) |
0.160 |
|
Pathological stage (T) pT2 pT3a pT3b pT4 |
157 (55.3%) 91 (32.0%) 36 (12.7%) 0 |
89 (55.6%) 59 (36.9%) 12 (7.5%) 0 |
0.910 |
|
Pathological stage (N) N0 N+ |
64(84.2%) 12(15.8%) |
51 (86.4%) 8 (13.6%) |
0.430 |
|
Number Lymph nodes removed at surgery Total cases N+ cases N0 cases |
15.16±7.83; 15: (4-47) 17.45±11.8; 15 (6-47) 14.7±6.8; 14 (4-35) |
19.83±4.78; 20 (6-34) 19.6±7.1; 20 (6-34) 19.8±4.1; 20 (11-31) |
<0.001 |
|
ISUP grading at surgery 1 2 3 4 5 |
73 (25.7%) 98 (34.5%) 54 (19.0%) 37 (13.0%) 22 (7.8%) |
19 (11.9%) 85 (53.1%) 35 (21.9%) 11 (6.9%) 10 (6.2%) |
<0.001 |
|
Surgical margin at surgery (R) Negative positive |
239 (84.1%) 45 (15.9%) |
128 (80.0%) 32 (20.0%) |
0.145 |
|
Positive surgical margin site apex lateral basal posterior multiple |
22 (48.9%) 11 (24.4%) 2 (4.4%) 7 (15.6%) 3 (6.7%) |
9 (28.1%) 12 (37.5%) 4 (12.5%) 5 (15.6%) 2 (6.3%) |
0.037 |
|
Positive surgical margin grading 3 4 5 |
34 (75.6%) 11 (24.4%) 0 (0%) |
21 (65.6%) 10 (31.3%) 1 (3.1%) |
0.225 |
| Positive surgical margin radial distance (mm) | 3.45±0.84; 3 (2-6) | 2.63±1.14; 2.5 (1-7) | 0.008 |
|
Positive surgical margin Single multiple |
42 (93.3%) 3 (6.7%) |
30 (93.7%) 2 (6.3%) |
0.762 |
|
PNI at surgery positive negative |
179 (62.6%) 105 (37.4%) |
90 (56.2%) 70 (43.8%) |
0.10 |
|
Cribriform/IDC at surgery positive negative |
7 (2.5%) 277 (97.5%) |
13 (8.1%) 147 (91.9%) |
0.01 |
| Postoperative hospitalization (days) | 3.75±0.74;3 (3-7) | 3.11±0.36;3 (3-5) | 0.057 |
| Catheterization time (days) | 11.53±1.45; 12 (8-14) | 9.60±2.06; 10 (6-16) | <0.001 |
|
Postoperative anastomotic leakage no yes |
284 (100%) 0 (0%) |
157 (98.1%) 3 (1.9%) |
0.245 |
|
Postoperative blood transfusion no yes |
284 (100%) 0(0%) |
158 (98.7%) 2 (1.3%) |
0.294 |
|
Postoperative lymphocele no yes |
278 (97.9%) 6 (2.1%) |
158 (98.7%) 2 (1.3%) |
0.16 |
|
Postoperative anastomotic stricture no yes |
272 (95.8%) 12 (4.2%) |
160 (100%) 0 (0%) |
0.51 |
|
Postoperative rectal injury no yes |
284 (100%) 0 |
160 (100%) 0 |
-- |
| Postoperative total PSA (ng/ml)(at 1 month) | 0.06±0.2; 0.02: (0.01-2.0) | 0.04±0.92; 0.02 (0.01-1.0) | 0.047 |
| Postoperative follow-up ( years) | 64.4±28.8; 62 (24-120) | 49.2±36.0; 48 (12-120) | 0.385 |
| Biochemical progression No Yes |
243 (85.6%) 41 (14.4%) |
148 (92.5%) 12 (7.5%) |
0.014 |
| Time to biochemical progression (months) | 21.77±26.49; 12 (1-120) | 18.58±13.2; 13 (1-36) | 0.105 |
| Adjuvant therapy no yes |
247 (87.0%) 37 (13.0%) |
143 (90.0%) 16 (10.0%) |
0.55 |
| Adjuvant therapy type -RT -RT + ADT |
27 (73.0%) 10 (27.0%) |
10 (62.5%) 6 (37.5%) |
0.358 |
| IIEF-5 postoperative (6 months) (patients submitted to nerve sparing) | 9.60±3.78; 9 (6-19) | 10.25±3.94; 9.5 (7-15) | 0.750 |
| IIEF-5 postoperative (12 months) (patients submitted to nerve sparing) | 10.14±4.77; 10 (5-21) | 18.0±3.75; 18 (8-18) | 0.117 |
| Postoperative Pelvic floor rehabilitation no yes |
230 (81.0%) 54 (19.0%) |
125 (78.2%) 35 (21.8%) |
0.106 |
|
Postoperative PAD test 1 months (grams) 3 months (grams) 6 months (grams) 12 months (grams) |
163.84±222.0; 50 (0-400) 75.57±122.20; 20 (0-480) 39.47±76.08; 5 (0-420) 14.76±29.41; 0 (0-100) |
68.17± 374.17; 11 (0-404) 14.02±42.09;0 (0-250) 13.62±28.60; 0 (0-80) 15.33±23.2; 1 (0-50) |
0.680 <0.001 0.023 0.964 |
| Low risk | Intermediate Risk | High risk | P value | |
|---|---|---|---|---|
| Number cases | 142 | 200 | 102 | |
| Age (years) | 67.73±6.60; 67: (50-73) | 67.42±6.61; 68 (49-22) | 67.46±6.12; 69 (47-71) | 0.906 |
| BMI | 25.73±3.70; 25.3 (18-37) | 25.84±3.35; 25.4 (18.5- 37) | 26.81±3.63; 26 (21-39.4) | 0.121 |
| Charlson Index | 3.82±1.02; 4 (2-7) | 3.89±1.08; 4 (0-7) | 3.88±1.06; 4 (1-6) | 0.903 |
| Familiarity Yes no |
0 142 (100%) |
19 (9.5%) 181 (90.5%) |
17 (16.7%) 85 (83.3%) |
0.138 |
| Digital Rectal Examination Normal Suspicious |
134 (94.4%) 8 (5.6%) |
176 (88.0%) 24 (12.0%) |
66 (64.7%) 36 (35.3%) |
<0.001 |
| Preoperative total PSA (ng/ml) | 6.73±3.12;6.4 (3.0-19.0) | 8.41±3.91;7.5 (3.0-23.0) | 11.74±9.3;9.3 (3.0-64.0) | <0.001 |
| PSAD | 0.14±0.49;0.13 (0.08-0.26) | 0.23±0.17;0.19 (0.06-0.54) | 0.3±0.3;0.24 (0.11-0.59) | 0.048 |
| Prostate volume (cc) | 47.78±15.48;45 (25-120) | 47.4±13.72;45 (20-87) | 51.53±16.77; 52.5 (25-90) | 0.403 |
|
mMR PIRADS score (data available on 208 cases) PIRADS 2 PIRADS 3 PIRADS 4 PIRADS 5 |
6 (10.7%) 14 (25.0%) 32 (57.1%) 4 (7.2%) |
0 (0%) 26 (26.2%) 58 (58.6%) 15 (15.2%) |
0 (0%) 4 (7.7%) 20 (38.5%) 28 (53.8%) |
<0.001 |
| Prostate Tumor size (mm) at mMR | 10.15±4.78; 9.5 (4.0-30.0) | 11.83±4.66; 10 (5.0-27.0) | 18.25±8.72; 16 (7.0-39.0) | <0.001 |
| Preoperative CT and bone scan No yes |
133 (93.7%) 9 (6.3%) |
88 (87.5%) 25 (12.5) |
66 (64.8%) 36 (35.2%) |
<0.001 |
| Preoperative PET CT scan no choline PSMA |
142 (100%) 0 0 |
195 (97.5%) 4 (2.0%) 1 (0.5%) |
97 (95.1%) 2 (2.0%) 3 (2.9%) |
0.027 |
|
Surgical technique at radical prostatectomy Laparoscopic Robotic assisted |
92 (64.8%) 50 (35.2%) |
126 (63.0%) 74 (37.0%) |
66 (64.7%) 36 (35.3%) |
0.850 |
| Operative time (minutes) | 150.50±27.35;140 (120-300) | 155.0±20.06;157.5 (90-210) | 183.51±50.711; 175 (90-300) | <0.001 |
|
Nerve sparing technique at surgery No Yes Monolateral Bilateral |
73 (51.4%) 69 (48.6%) 19 (27.5%) 50 (72.5%) |
145 (72.5%) 55 (27.5%) 31 (56.4%) 24 (43.6%) |
99 (97.0%) 3 (3.0%) 2 (66.7%) 1 (33.3%) |
<0.001 <0.001 |
|
Extended lymph node dissection no yes |
142 (100%) 0 (0%) |
167 (83.5%) 33(16.5%) |
0 (0%) 102(100%) |
<0.001 |
|
Pathological stage (T) pT2 pT3a pT3b |
110 (77.5%) 28 (19.7%) 4 (2.8%) |
108 (53.0%) 80 (39.9%) 12 (7.1%) |
28 (27.4%) 42 (41.2%) 32 (31.4%) |
<0.001 |
|
Pathological stage (N) N0 N+ |
-- |
26 (73.1%) 7 (26.9%) |
89 (87.2%) 13 (12.8%) |
<0.001 |
|
ISUP grading at surgery 1 2 3 4 5 |
75 (52.8%) 57 (40.2%) 9 (6.3%) 1 (0.7%) 0 (0%) |
14 (7.0%) 109 (54.5%) 58 (29.0%) 12 (6.0%) 7 (3.5%) |
3 (2.9%) 17 (16.7%) 22 (21.6%) 35 (34.5%) 25 (24.5%) |
<0.001 |
|
Surgical margin at surgery (R) Negative positive |
129 (90.8%) 13 (9.2%) |
162(80.8%) 38 (19.2%) |
76 (74.5%) 26 (25.5%) |
0.003 |
|
Positive surgical margin grading 3 4 5 |
13 (100%) 0 (0%) 0 (0%) |
33 (86.8%) 5 (13.2%) 0 (0%) |
9 (34.6%) 16 (61.5%) 1 (3.9%) |
<0.001 |
| Positive surgical margin radial distance (mm) | 2.86±0.945; 3 (2-4) | 2.65±0.89; 2.5 (2-6) | 3.34±1.25; 3 (1-7) | 0.104 |
| Postoperative total PSA (ng/ml)(at 1 month) | 0.03±0.08; 0.02 (0.01-1.0) | 0.04±0.093; 0.02 (0.01-1.0) | 0.12±0.3; 0.03 (0.01-2.0) | <0.001 |
| Biochemical progression -No -Yes |
136 (95.8%) 6 (4.2%) |
183 (91.5%) 17 (8.5%) |
72 (70.6%) 30 (29.4%) |
<0.001 |
| Time to biochemical progression (months) | 19.0±15.83; 15 (6-48) | 23.88±28.22; 18 (1-120) | 15.72±24.12; 6 (1-4120 | 0.576 |
| Adjuvant therapy no yes |
139 (97.9%) 3 (2.1%) |
186 (93.0%) 14 (7.0%) |
66 (64.7%) 36 (35.3%) |
<0.001 |
| Adjuvant therapy type -RT -RT + ADT |
3 (100%) 0 |
13 (92.9%) 1 (7.1%) |
21 (58.3%) 15 (41.7%) |
0.290 |
| pT2 | pT3a | pT3b | P value | |
|---|---|---|---|---|
| Number cases | 246 | 150 | 48 | |
| Age (years) | 66.76±6.45; 67 (48-71) | 68.25±6.587; 68 (47-72) | 68.79±6.75; 68 (54-73) | 0.032 |
| BMI | 25.71±3.56; 25.3 7-37.2) | 26.25±3.41; 25.7 (19-39.4) | 27.21±3.8; 26.1 (19-37) | 0.077 |
| Charlson Index | 3.77±1.08; 4 (0-7) | 4.05±1.01; 4 (0-7) | 3.8±1.05; 4 (1-6) | 0.179 |
| Familiarity Yes no |
14 (5.7%) 232 (94.3%) |
15 (10%) 135 (90%) |
6 (12.5%) 42 (87.5%) |
0.211 |
| Digital Rectal Examination Normal Suspicious |
226 (91.9%) 20 (8.1%) |
118 (78.7%) 32 (21.3%) |
52 (66.7%) 16 (33.3%) |
<0.001 |
| Preoperative total PSA (ng/ml) | 7.64±4.52;6.85 (3.0-48.0) | 8.59±4.77;7.5 (3.0-33.0) | 13.55±10.14: 11.2 (4.0-64.0) | <0.001 |
| PSAD | 0.19±0.15;0.15 (0.1-0.50) | 0.22±0.15;0.18 (0.1-0.59) | 0.35±0.36; 0.23 (0.1-0.50) | 0.112 |
| Prostate volume (cc) | 48.0±4.53; 45 (20-120) | 49.08±14.4;47 (24-90) | 43.91±15.87;40 (25-75) | 0.56 |
|
mMR PIRADS score PIRADS 2 PIRADS 3 PIRADS 4 PIRADS 5 |
6 (5.2%) 31 (26.9%) 61 (53.1%) 17 (14.8%) |
11 (15.1%) 41 (56.2%) 21 (28.7%) |
2 (10.0%) 9 (45.0%) 9 (45.0%) |
0.01 |
| Prostate Tumor size (mm) at mMR | 10.9±4.78; 10 (4-30) | 14.10±5.98; 12 (5-35) | 20.53±11.33; 18 (8-39) | <0.001 |
|
Clinical T staging T1 T2a T2b T2c T3a T3b |
8 (3.2%) 29 (11.8%) 106 (43.1%) 96 (39.0%) 7 (2.9%) 0 |
4 (2.7%) 1 (0.6%) 76 (50.7%) 51 (34.0%) 18 (12.0%) 0 |
0 0 10 (20.8%) 26 (54.2%) 5 (10.4%) 7 (14.6%) |
<0.001 |
|
Biopsy outcomes % positive samples PC |
33.15±21.7.25 (5-100) |
48.11±26.58; 44 (2-100) |
59.66±29.42; 52.5 (10-100) |
<0.01 |
|
ISUP grading at biopsy 1 2 3 4 5 |
113 (45.9%) 77 (31.4%) 34 (13.8%) 20 (8.1%) 2 (0.8%) |
31 (20.7%) 50 (33.3%) 37 (24.7%) 24 (16%) 8 (5.3%) |
5 (10.4%) 8 (16.7%) 15 (31.3%) 13 (27.0%) 7 (14.6%) |
<0.001 |
|
Surgical technique at radical prostatectomy Laparoscopic Robotic assisted |
157 (63.8%) 89 (36.2%) |
91 (60.7%) 59 (39.3%) |
36 (75.0%) 12 (25.0%) |
0.193 |
| Operative time (minutes) | 159.02±35.57;160 (90-300) | 160.42±33.21; 160 (90-300) | 165.28±26.53; 177.5 (100-200) | 0.764 |
|
Pathological stage (N) N0 N+ |
11 (100%) 0 (0%) |
68 (88.9%) 8 (11.1%) |
36 (75.0%) 12 (25.0%) |
<0.01 |
|
ISUP grading at surgery 1 2 3 4 5 |
85 (34.6%) 114 (46.3%) 32 (13.0%) 13 (5.3%) 2 (0.8%) |
7 (4.7%) 65 (43.3%) 39 (26.0%) 27 (18.0%) 12 (8.0%) |
0 4 (8.3%) 18 (37.5%) 8 (16.7%) 18 (37.5%) |
<0.001 |
|
Surgical margin at surgery (R) Negative positive |
218 (88.6%) 28 (11.4%) |
119 (79.2%) 31 (20.8%) |
30 (62.5%) 18 (37.5%) |
<0.001 |
|
Positive surgical margin grading 3 4 5 |
24 (85.7%) 4 (14.3%) 0 (0%) |
25 (80.6%) 5 (16.2%) 1 (3.2%) |
6 (33.3%) 12 (66.7%) 0 (0%) |
0.001 |
| Positive surgical margin radial distance (mm) | 2.72±0.966; 3 (1-4) | 2.67±0.84; 3 (2-5) | 3.71±1.28; 3.5 (2-7) | 0.01 |
|
PNI at surgery positive negative |
103 (41.9%) 143 (58.1%) |
123 (82.0%) 27 (18.0%) |
43 (89.6%) 5 (10.4%) |
<0.001 |
|
Cribriform/IDC at surgery positive negative |
2 (0.8%) 244 (99.2%) |
10 (6.7%) 140 (93.3%) |
8 (16.7%) 40 (83.3%) |
0.001 |
| Postoperative total PSA (ng/ml)(at 1 month) | 0.03±0.31;0.02 (0.01-0.1) | 0.05±0.143; 0.02 (0.01-1.0) | 0.21±0.419; 0.04 (0.01-2.0) | <0.001 |
| Biochemical progression (number of cases and %) -No -Yes |
234 (95.1%) 12 (4.9%) |
127 (84.7%) 23 (13.3%) |
30 (62.5%) 18 (37.5%) |
<0.001 |
| Time to biochemical progression (months) | 34.25±42.26; 15 (3-120) | 16.1±11.38; 12 (1-36) | 11.28±15.18; 2 (1-48) | 0.032 |
| Adjuvant therapy no yes |
242(98.4%) 4 (1.6%) |
133 (88.7%) 17 (11.3%) |
16 (33.3%) 32 (62.7%) |
<0.001 |
| Adjuvant therapy type -RT -RT + ADT |
4 (100%) 0 |
14 (82.4%) 3 (17.6%) |
19 (59.5%) 13 (40.6%) |
0.033 |
| pN0 | pN1 | P value | |
| Number cases | 115 | 20 | |
| Age (years) | 66.16±6; 67 (47-72) | 67.37±6.66;68 (56-71) | 0.40 |
| BMI | 26.1±3.3; 25.1 (19-39.4) | 27.01±3.86;26.65 (19.32.8) | 0.41 |
| Charlson Index | 3.75±1.14; 4 (0-7) | 4±0.6; 4 (3-5) | 0.454 |
| Familiarity Yes no |
3 (1.5%) 201 (98.5%) |
10 (50.0%) 10 (50.0%) |
0.145 |
| Digital Rectal Examination Normal Suspicious |
156 (76.5%) 48 (23.5%) |
9 (45.0%) 11 (55.0%) |
<0.001 |
| Preoperative total PSA (ng/ml) | 8.72±5.29; 7.5 (3.0-48.0) | 16.54±8.83;16 (5.0-30.0) | <0.001 |
| PSAD | 0.22±0.175; 16 (0.1-0.6) | 0.51±0.441;0.33 (0.1-0.7) | 0.012 |
| Prostate volume (cc) | 48.38±15.21; 47 (20-120) | 53.11±21.86;50 (25-90) | 0.386 |
| Prostate Tumor size (mm) at mMR | 11.94±4.77; 10 (5-28) | 27.7±10.47;30 (11-39) | <0.001 |
|
Clinical T staging T1 T2a T2b T2c T3a T3b |
4 (3.5%) 12 (10.4%) 45 (39.1%) 29 (25.2%) 21 (18.3%) 4 (3.5%) |
0 0 3 (15.0%) 8 (40.0%) 6 (30.0%) 3 (15.0%) |
<0.001 |
|
Clinical N staging N0 N1 |
114 (99.1%) 1 (0.9%) |
17 (85.0%) 3 (15.5%) |
<0.001 |
| Number of suspected lymph node at imaging | 2±1; 2 (1-3) | 4±1; 4 (3-4) | 0.21 |
| Nomograms results ( % risk for N+) Briganti 2012 Briganti 2019 |
24.5±17.12; 17: (2-82) 23.4±16.45; 16: (2-82) |
26.4±15.46; 20: (7-85) 26.9±21.36; 21: (4-78) |
0.175 0.143 |
|
Biopsy outcomes % positive samples PC |
38.78±24.76; 30 (2-100) |
72.54±24.7;75 (35-100) |
0.001 |
|
ISUP grading at biopsy 1 2 3 4 5 |
7 (6.1%) 10 (8.7%) 42 (36.5%) 43 (37.4%) 13 (11.3%) |
1 (5.0%) 5 (25.0%) 6 (30.0%) 6 (30.0%) 2 (10.0%) |
0.304 |
|
Surgical technique at radical prostatectomy Laparoscopic Robotic assisted |
64 (55.6%) 51 (44.4%) |
12 (60.0%) 8 (40.0%) |
0.62 |
| Operative time (minutes) | 166.01±34.74; 160 (90-300) | 170±26.06;175 (135-220) | 0.68 |
|
Pathological stage (T) pT2 pT3a pT3b |
11 (9.6%) 68 (59.1%) 36 (31.3%) |
0 8 (40.0%) 12 (60.0%) |
<0.001 |
|
Number Lymph nodes removed at surgery Total cases |
17.07±6.24; 18 (2-35) |
17.95±9.38; 18.5 (6-47) |
0.60 |
|
Site of positive lymphnodes Obturator External iliac Internal iliac |
--- |
20 (100%) 8 (40.0%) 9 (45.0%) |
-- |
|
ISUP grading at surgery 1 2 3 4 5 |
1 (0.9%) 14 (12.2%) 44 (38.2%) 36 (38.3%) 20 (17.4%) |
0 2 (10.0%) 8 (40.0%) 2 (10.0%) 8 (40.0%) |
0.001 |
|
Surgical margin at surgery (R) Negative positive |
83 (72.1%) 32 (27.9%) |
11 (55.0%) 9 (45.0%) |
0.001 |
|
Positive surgical margin grading 3 4 5 |
19 (59.4%) 12 (37.5%) 1 (3.1%) |
4 (44.4%) 5 (55.6%) 0 (0%) |
0.384 |
| Positive surgical margin radial distance (mm) | 3.03±1; 3 (1-7) | 3.79±1.72; 3 (1-7) | 0.136 |
|
PNI at surgery positive negative |
70 (60.9%) 45 (39.1%) |
4 (15.8%) 16 (84.2%) |
0.02 |
|
Cribriform negative positive |
105 (91.3%) 10 (8.7%) |
14 (73.7%) 6 (26.3%) |
<0.001 |
| Postoperative total PSA (ng/ml)(at 1 month) | 0.05±0.19; 0.02 (0.01-2.0) | 0.28±0.376;0.09 (0.01-1.0) | <0.001 |
| Biochemical progression -No -Yes |
94 (81.7%) 21 (18.3%) |
9 (45.0%) 11 (55.0%) |
<0.001 |
| Time to biochemical progression (months) | 28.3±34.6; 12 (1-120) | 4.18±7.37; 3 (1-24) | 0.030 |
| Adjuvant therapy no yes |
85 (73.9%) 30 (26.1%) |
6 (30.0%) 14 (70.0%) |
<0.001 |
| Adjuvant therapy type -RT -RT + ADT |
24 (80.0%) 6 (20.0%) |
4 (28.6%) 10 (71.4%) |
<0.001 |
| Negative SM | Positive SM | P value | |
|---|---|---|---|
| Number cases | 367 | 77 | |
| Age (years) | 67.44±6.62; 68 (47-73) | 67.78±6.14; 69 (52-72) | 0.68 |
| BMI | 25.88±3.44; 25,4 (18.0-39.4) | 26.89±4.04; 26.2 (19-37) | 0.83 |
| Charlson Index | 3.81±1; 4 (0-7) | 4.14±0.93; 4 (1-6) | 0.7 |
| Familiarity Yes no |
23 (6.3%) 344 (93.7%) |
12 (15.6%) 65 (84.4%) |
0.96 |
| Digital Rectal Examination Normal Suspicious |
315 (85.8%) 52 (14.2%) |
61 (79.2%) 16 (20.8%) |
0.01 |
| Preoperative total PSA (ng/ml) | 8.0±1.07; 4 (3.0-7.0) | 11.54±8.8;9.6 (3.0-64.0) | <0.001 |
| PSAD | 0.21±0.14; 0.17 (0.1-0.50) | 0.28±0.318;0.18 (0.2-0.59) | 0.253 |
| Prostate volume (cc) | 47.78±14.65; 445 (20-120) | 49.97±15.77;46.5 (25-90) | 0.452 |
| Prostate Tumor size (mm) at mMR | 11.98±5.66; 10 (4-39) | 16.52±8.67; 14 (5-38) | <0.001 |
|
Clinical T staging T1 T2a T2b T2c T3a T3b |
12 (3.2%) 30 (8.2%) 160 (43.6%) 142 (38.7%) 22 (6.0%) 1 (0.3%) |
0 0 32 (42.1%) 31 (39.5%) 8 (10.5%) 6 (7.9%) |
<0.001 |
|
Clinical N staging N0 N1 |
366 (99.7%) 1 (0.3%) |
74 (96.1%) 3 (3.9%) |
0.002 |
|
Biopsy outcomes % positive samples PC |
38.31±25.167; 30 (5-100) |
54.664±26.78;50 (2-100) |
<0.001 |
|
ISUP grading at biopsy 1 2 3 4 5 |
131 (35.7%) 107 (29.1%) 69 (18.7%) 50 (13.6%) 10 (2.7%) |
18 (23.4%) 28 (36.4%) 17 (22.0%) 7 (9.1%) 7 (9.1%) |
0.14 |
|
Surgical technique at radical prostatectomy Laparoscopic Robotic assisted |
239 (65.1%) 128 (34.9%) |
45 (59.2%) 32 (40.8%) |
0.345 |
| Operative time (minutes) | 160.03±36.02; 160 (90-300) | 160.63±22.39;160 (120-220) | 0.92 |
|
Nerve sparing technique at surgery No Yes Monolateral Bilateral |
259 (70.4%) 108 (29.6%) 42 (38.9%) 66 (61.1%) |
5 8 (74.7%) 19 (25.3%) 10 (52.6%) 9 (47.4%) |
0.450 0.230 |
|
Pathological stage (T) pT2 pT3a pT3b |
218 (59.4%) 119 (32.4%) 30 (8.2%) |
28 (36.4%) 31 (40.2%) 18 (23.4%) |
<0.001 |
|
ISUP grading at surgery 1 2 3 4 5 |
84 (23%) 152 (41.3%) 70 (18.9%) 41 (11.2%) 20 (5.7%) |
8 (10.4%) 31 (40.3%) 19 (24.7%) 7 (9.1%) 12 (15.6%) |
0.006 |
|
Positive surgical margin site apex lateral basal posterior multiple |
31(40.2%) 23 (29.9%) 6 (7.8%) 12 (15.6%) 5 (6.5%) |
-- | |
|
Positive surgical margin grading 3 4 5 |
|
55 (71.4%) 21 (27.3) 1 (1.3%) |
-- |
| Positive surgical margin radial distance (mm) | x | 2.97±1.1; 3 (1-7) | -- |
|
PNI at surgery positive negative |
215 (58.5%) 152 (41.5%) |
54 (70.1%) 23 (29.9%) |
0.05 |
|
Cribriform/IDC at surgery positive negative |
12 (3.3%) 355 (96.7%) |
8 (10.4%) 69 (89.6%) |
0.20 |
| Postoperative total PSA (ng/ml)(at 1 month) | 0.04±0.97; 0.02 (0.01-1.0) | 0.13±3.4;0.03 (0.01-2.0) | <0.001 |
| Biochemical progression -No -Yes |
338 (92.1%) 29 (7.9%) |
53 (68.8%) 24 (31.2%) |
<0.001 |
| Time to biochemical progression (months) | 25.45±29.4; 18 (1-120) | 9.73±11.57; 4.5 (1-48) | 0.022 |
| Adjuvant therapy no yes |
359 (91.6%) 10 (8.4%) |
34 (44.1%) 43 (55.9%) |
<0.001 |
| Adjuvant therapy type -RT -RT + ADT |
4 (40.0%) 6 (60.0%) |
33 (76.7%) 10 (23.3%) |
0.037 |
| No BCR | Yes BCR | P value | |
|---|---|---|---|
| Number cases | 391 | 53 | |
| Age (years) | 67.13±6.588; 68 (47-72) | 68.62±6.7; 70 (49-73) | 0.131 |
| BMI | 25.74±3.43; 25 (18-37) | 27.21±3.61;26.7 (21.4-39.4) | 0.017 |
| Charlson Index | 3.85±1.02; 4 (0-7) | 3.86±1.39; 4 (0-7) | 0,96 |
| Familiarity Yes no |
28 (7.2%) 363 (92.8%) |
7 (13.2%) 46 (86.8%) |
0.12 |
| Digital Rectal Examination Normal Suspicious |
340 (86.9%) 51 (13.1%) |
36 (67.9%) 17 (32.1%) |
<0.001 |
| Preoperative total PSA (ng/ml) | 8.14±5.04; 7.1 (3.0-64.0) | 11.37±7.03;9.65 (4.0-30.0) | <0.001 |
| PSAD | 0.2±0.145; 0.17 (0.1-0.59) | 0.26±0.48;0.28 (0.1-0.47) | 0.007 |
| Prostate volume (cc) | 48.15±14.6; 45 (20-120) | 48.55±18.37;50 (25-90) | 0.933 |
| Prostate Tumor size (mm) at mMR | 11.95±5.14; 10 (4-30) | 23.21±11.18;20 (10-39) | <0.001 |
|
Clinical T staging T1 T2a T2b T2c T3a T3b |
12 (3.1%) 30 (7.7%) 173 (44.2%) 148 (37.8%) 26 (6.7%) 2 (0.5%) |
0 0 19 (35.8%) 25 (47.2%) 4 (7.5%) 5 (9.4%) |
<0.001 |
|
Clinical N staging N0 N1 |
390 (99.7%) 1 (0.3%) |
50 (94.3%) 3 (5.7%) |
<0.001 |
|
Biopsy outcomes % positive samples PC |
38.68±25.2; 30 (2-100) |
59.21±27.57;50 (14-100) |
<0.001 |
|
ISUP grading at biopsy 1 2 3 4 5 |
143 (36.6%) 123 (31.4%) 70 (17.9%) 46 (11.8%) 9 (2.3%) |
6 (11.3%) 12 (22.6%) 16 (30.2%) 11 (20.8%) 8 (15.1%) |
<0.001 |
|
Surgical technique at radical prostatectomy Laparoscopic Robotic assisted |
243 (62.1%) 148 (37.9%) |
41 (77.4%) 12 (22.6%) |
0.034 |
|
Nerve sparing technique at surgery No Yes Monolateral Bilateral |
270 (69.1%) 121 (30.9%) 48 (28.9%) 73 (71.1%) |
47 (88.7%) 6 (11.3%) 4 (80.0%) 2 (20.0%) |
0.002 0.016 |
|
Pathological stage (T) pT2 pT3a pT3b pT4 |
234 (59.8%) 127 (32.5%) 30 (7.7%) 0 |
12 (22.6%) 23 (43.4%) 18 (34.0%) 0 |
<0.001 |
|
Pathological stage (N) N0 N+ |
94 (91.3%) 9 (8.7%) |
21 (65.6%) 11 (34.4%) |
0.014 |
|
ISUP grading at surgery 1 2 3 4 5 |
88 (22.5%) 172 (43.0%) 75 (19.0%) 40(11.4%) 16 (4.1%) |
4 (7.5%) 11 (20.8%) 14 (24.4%) 8 (15.1%) 16 (20.2%) |
P<0.001 |
|
Surgical margin at surgery (R) Negative positive |
338 (86.4%) 53 (13.6%) |
29 (54.7%) 24 (45.3%) |
<0.001 |
|
Positive surgical margin grading 3 4 5 |
45 (84.9%) 7 (13.2%) 1 (1.9%) |
10 (41.7%) 14 (58.3%) 0 |
<0.001 |
| Positive surgical margin radial distance (mm) | 2.67±0.82; 3 (1-4) | 3.75±1.35; 3.5 (2-7) | 0.001 |
|
PNI at surgery positive negative |
225 (57.5%) 166 (42.5%) |
44 (83.0%) 9 (17.0%) |
<0.001 |
|
Cribriform/IDC at surgery positive negative |
15 (3.8%) 376 (91.2%) |
5 (9.4%) 48 (90.6%) |
0.217 |
| Postoperative total PSA (ng/ml)(at 1 month) | 0.03±0.03; 0.02 (0.01-0.1) | 0.24±0.433;0.07 (0.01-2.0) | <0.001 |
| Time to biochemical progression (months) | 18.67±24.57;12 (1-120) | -- | |
| Adjuvant therapy no yes |
359 (91.8%) 32 (8.2%) |
32 (60.4%) 21 (39.6%) |
<0.001 |
| Adjuvant therapy type -RT -RT + ADT |
26 (81.2%) 6 (18.8%) |
11 (55.0%) 10 (45.0%) |
0.042 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





