Submitted:
28 August 2024
Posted:
29 August 2024
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Materials and Methods
2.1. Experimental Area and Soil Characterization
2.2. Field Preparation
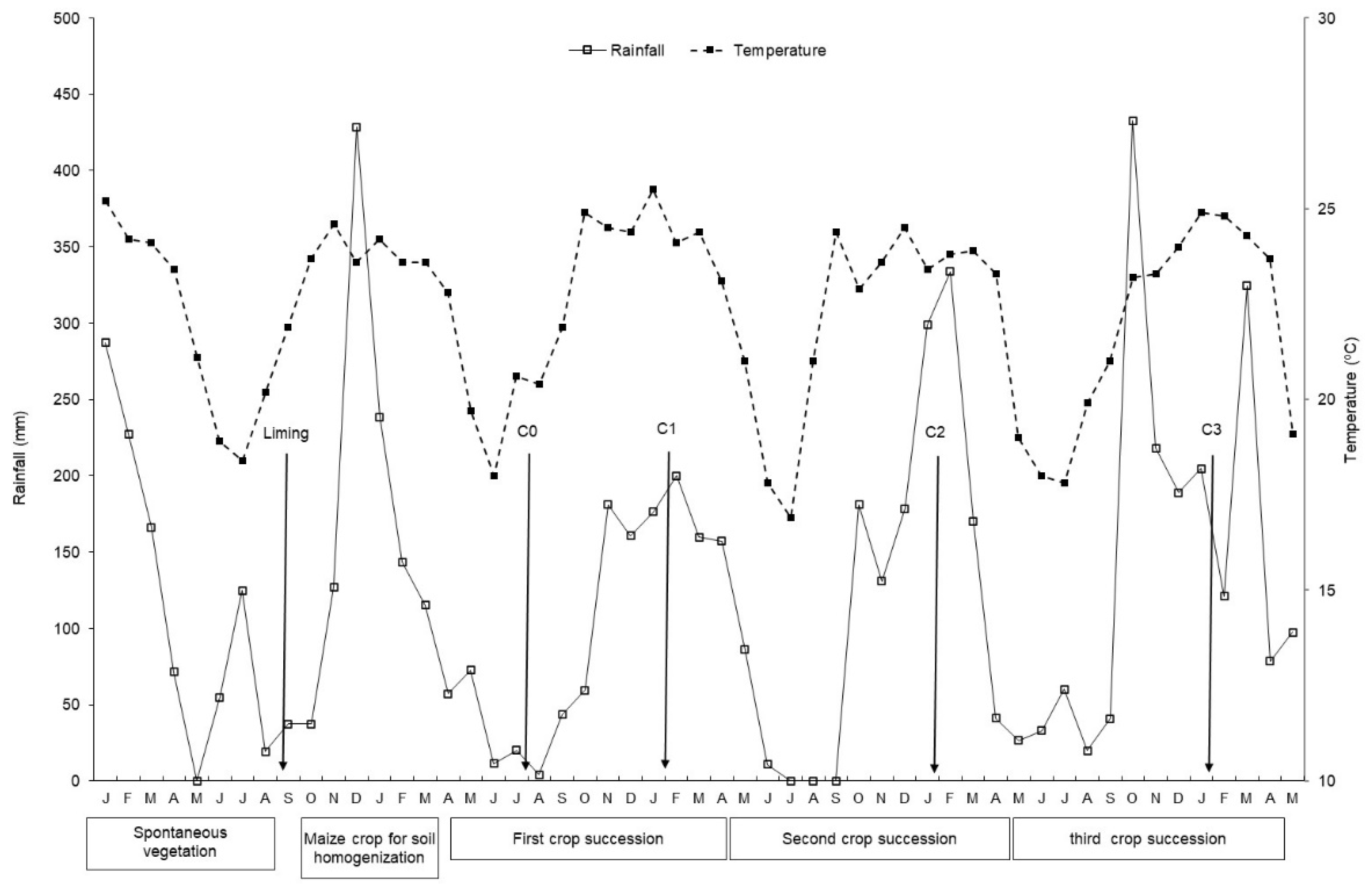
2.3. Experiment Setup and Execution
2.4. Estimated Maize Crop Productivity
2.5. Soil Sampling
2.6. Chemical and Enzymatic Analysis
2.7. Statistical Analysis
3. Results
3.1. Maize Grain Yields Under Green Manure, Fallow and Maize Crop Succession Cycles
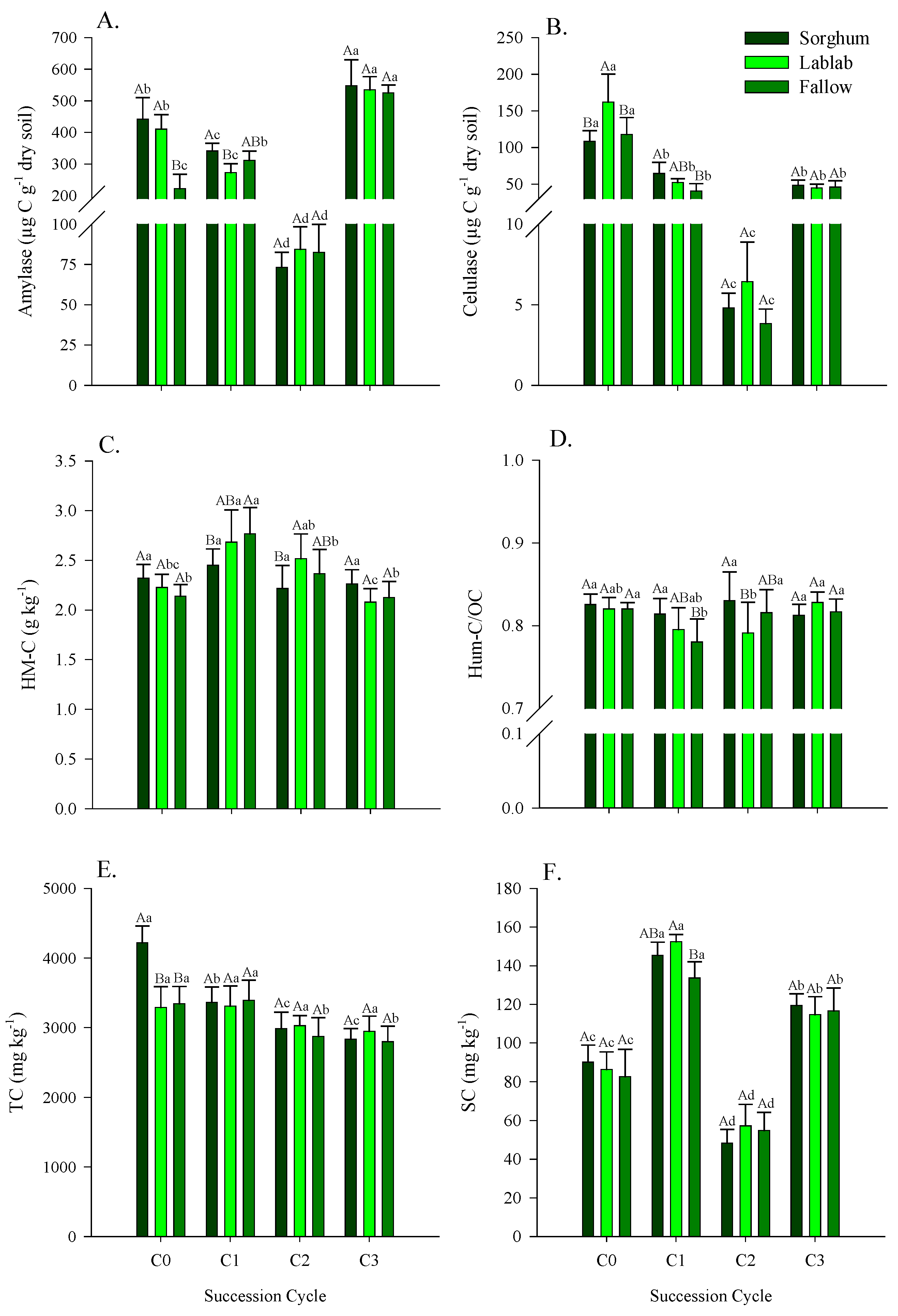
3.2. Enzymatic Activity in Soil Under Green Manure, Fallow and Maize Crop Succession Cycles
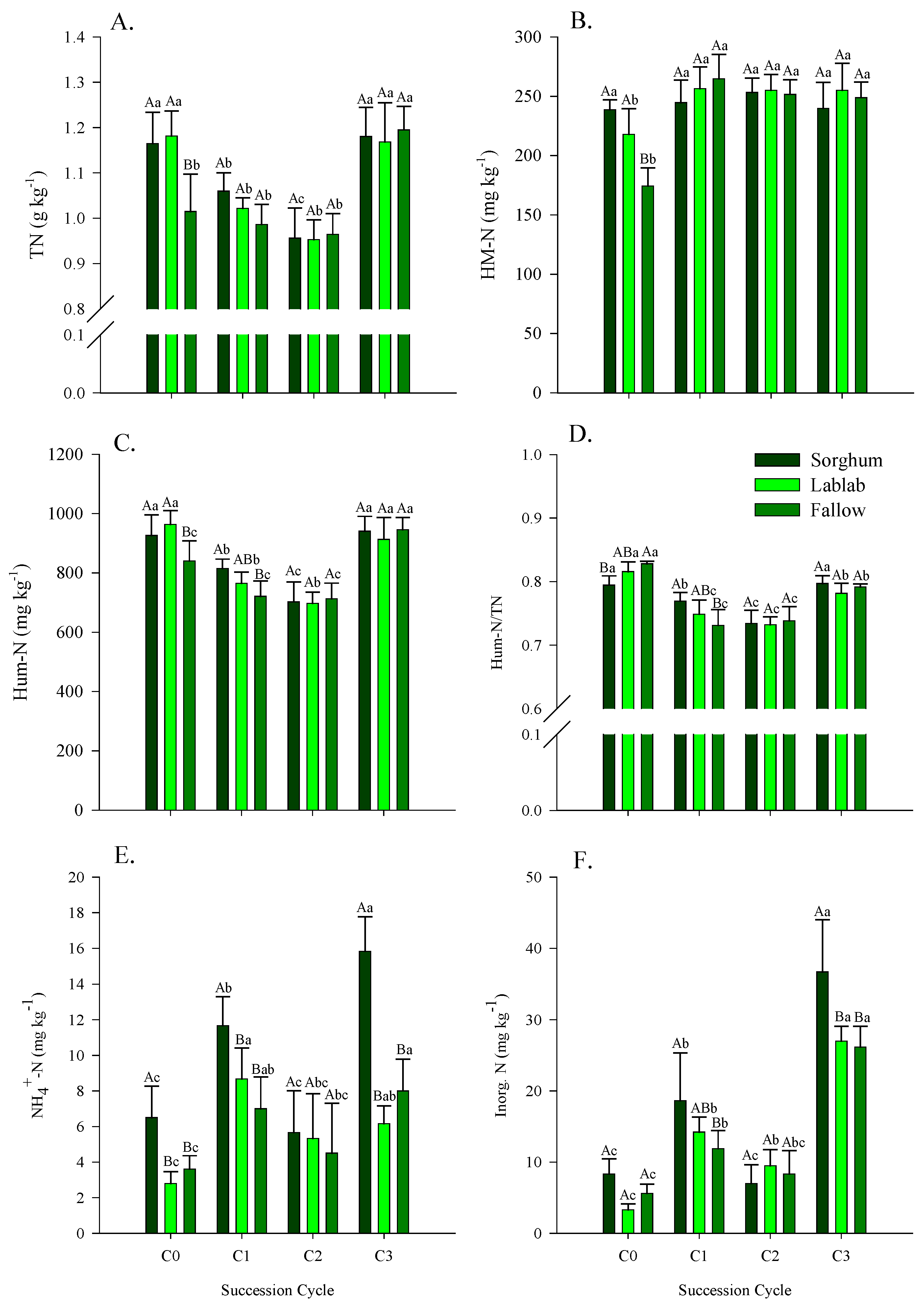
3.3. Carbon and Nitrogen in Soil Under Green Manure, Fallow and Maize Crop Succession Cycles
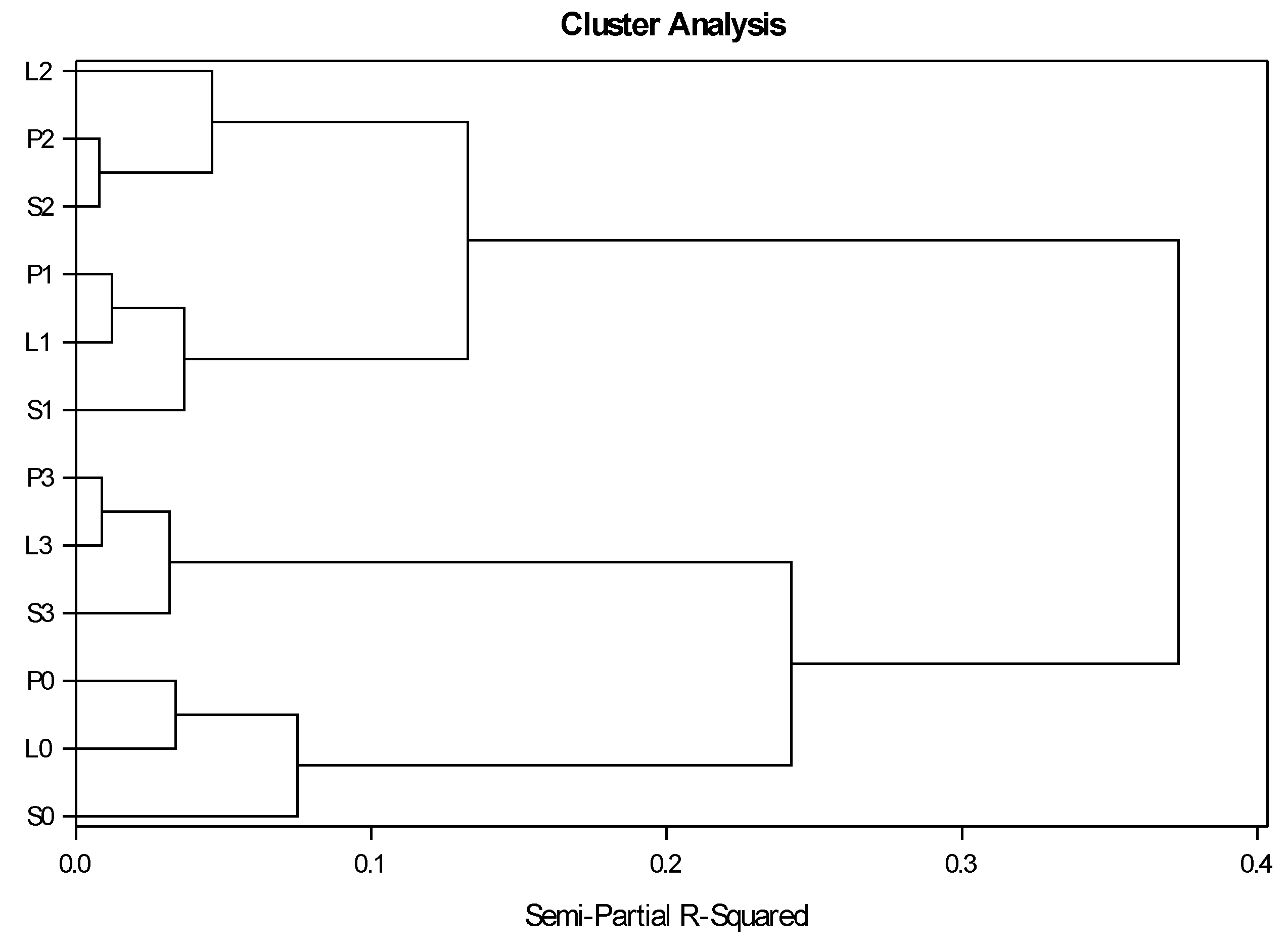
3.4. Similarities of Crop Succession Systems within Succession Cycles
4. Discussion
4.1. Maize Grain Yield Under Green Manure, Fallow and Maize Crop Succession Cycles
4.2. Enzymatic Activity in Soil in Soil Under Green Manure, Fallow and Maize Crop Succession Cycles
4.3. Carbon and Nitrogen in Soil Under Green Manure, Fallow and Maize Crop Succession Cycles
4.4. Performance of Cycles of Crop Succession Systems for Maize Yield
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Islam M, Akter T, Sohel U, Mohammad R, Alam S. Green manuring effects on crop morpho-physiological characters, rice yield and soil properties. Physiol Mol Biol Plants. 2019;25:303–12. [CrossRef]
- Bargaz, A.; Lyamlouli, K.; Chtouki, M.; Zeroual, Y.; Dhiba, D. Soil Microbial Resources for Improving Fertilizers Efficiency in an Integrated Plant Nutrient Management System. Front. Microbiol. 2018, 9, 1606. [CrossRef]
- He, H.-B.; Li, W.-X.; Zhang, Y.-W.; Cheng, J.-K.; Jia, X.-Y.; Li, S.; Yang, H.-R.; Chen, B.-M.; Xin, G.-R. Effects of Italian ryegrass residues as green manure on soil properties and bacterial communities under an Italian ryegrass (Lolium multiflorum L.)-rice (Oryza sativa L.) rotation. Soil Tillage Res. 2019, 196, 104487. [CrossRef]
- Ambrosano EJ, Wutke EB, Tanaka RT, Mascarenhas HAA, Braga NR, Muraoka T. Leguminosas para adubação verde: uso apropriado em rotação de culturas. Coordenadoria de Assistência Técnica Integral, Campinas: 1997, p. 24.
- Yang, T.; Siddique, K.H.; Liu, K. Cropping systems in agriculture and their impact on soil health-A review. Glob. Ecol. Conserv. 2020, 23, e01118. [CrossRef]
- Quintarelli, V.; Radicetti, E.; Allevato, E.; Stazi, S.R.; Haider, G.; Abideen, Z.; Bibi, S.; Jamal, A.; Mancinelli, R. Cover Crops for Sustainable Cropping Systems: A Review. Agriculture 2022, 12, 2076. [CrossRef]
- Thorup-Kristensen, K.; Cortasa, M.S.; Loges, R. Winter wheat roots grow twice as deep as spring wheat roots, is this important for N uptake and N leaching losses? Plant Soil 2009, 322, 101–114. [CrossRef]
- Yang, L.; Bai, J.; Liu, J.; Zeng, N.; Cao, W. Green Manuring Effect on Changes of Soil Nitrogen Fractions, Maize Growth, and Nutrient Uptake. Agronomy 2018, 8, 261. [CrossRef]
- Scavo, A.; Fontanazza, S.; Restuccia, A.; Pesce, G.R.; Abbate, C.; Mauromicale, G. The role of cover crops in improving soil fertility and plant nutritional status in temperate climates. A review. Agron. Sustain. Dev. 2022, 42, 1–25. [CrossRef]
- Balota EL, Chaves JCD. Atividade enzimática e mineralização do carbono e nitrogênio sob solo cultivado com adubos verdes na cultura do cafeeiro. Rev Bras Cienc Solo. 2010;34:1573–83.
- Balota, E.L.; Kanashiro, M.; Filho, A.C.; Andrade, D.S.; Dick, R.P. Soil enzyme activities under long-term tillage and crop rotation systems in subtropical agro-ecosystems. Braz. J. Microbiol. 2004, 35, 300–306. [CrossRef]
- Malhi, S.S.; Nyborg, M.; Goddard, T.; Puurveen, D. Long-term tillage, straw management and N fertilization effects on quantity and quality of organic C and N in a Black Chernozem soil. Nutr. Cycl. Agroecosystems 2011, 90, 227–241. [CrossRef]
- Griffiths, B.S.; Ritz, K.; Bardgett, R.D.; Cook, R.; Christensen, S.; Ekelund, F.; Sørensen, S.J.; Bååth, E.; Bloem, J.; De Ruiter, P.C.; et al. Ecosystem response of pasture soil communities to fumigation-induced microbial diversity reductions: an examination of the biodiversity–ecosystem function relationship. Oikos 2000, 90, 279–294. [CrossRef]
- Zornoza, R.; Acosta, J.; Faz, A.; Bååth, E. Microbial growth and community structure in acid mine soils after addition of different amendments for soil reclamation. Geoderma 2016, 272, 64–72. [CrossRef]
- Matsumoto, L.; Martines, A.; Avanzi, M.; Albino, U.; Brasil, C.; Saridakis, D.; Rampazo, L.; Zangaro, W.; Andrade, G. Interactions among functional groups in the cycling of, carbon, nitrogen and phosphorus in the rhizosphere of three successional species of tropical woody trees. Appl. Soil Ecol. 2004, 28, 57–65. [CrossRef]
- Shamshitov, A.; Kadžienė, G.; Supronienė, S. The Role of Soil Microbial Consortia in Sustainable Cereal Crop Residue Management. Plants 2024, 13, 766. [CrossRef]
- Mummey, D.L.; Stahl, P.D.; Buyer, J.S. Soil microbiological properties 20 years after surface mine reclamation: spatial analysis of reclaimed and undisturbed sites. Soil Biol. Biochem. 2002, 34, 1717–1725. [CrossRef]
- Gómez-Sagasti, M.T.; Alkorta, I.; Becerril, J.M.; Epelde, L.; Anza, M.; Garbisu, C. Microbial Monitoring of the Recovery of Soil Quality During Heavy Metal Phytoremediation. Water, Air, Soil Pollut. 2012, 223, 3249–3262. [CrossRef]
- Bandick, A.K.; Dick, R.P. Field management effects on soil enzyme activities. Soil Biol. Biochem. 1999, 31, 1471–1479. [CrossRef]
- Daunoras, J.; Kačergius, A.; Gudiukaitė, R. Role of Soil Microbiota Enzymes in Soil Health and Activity Changes Depending on Climate Change and the Type of Soil Ecosystem. Biology 2024, 13, 85. [CrossRef]
- Bhaduri, D.; Sihi, D.; Bhowmik, A.; Verma, B.C.; Munda, S.; Dari, B. A review on effective soil health bio-indicators for ecosystem restoration and sustainability. Front. Microbiol. 2022, 13, 938481. [CrossRef]
- Liu, C.-A.; Zhou, L.-M. Soil organic carbon sequestration and fertility response to newly-built terraces with organic manure and mineral fertilizer in a semi-arid environment. Soil Tillage Res. 2017, 172, 39–47. [CrossRef]
- Massenssini, A.M.; Bonduki, V.H.A.; Melo, C.A.D.; Tótola, M.R.; Ferreira, F.A.; Costa, M.D. Relative importance of soil physico-chemical characteristics and plant species identity to the determination of soil microbial community structure. Appl. Soil Ecol. 2015, 91, 8–15. [CrossRef]
- Mukhopadhyay, S.; Masto, R.E.; Cerdà, A.; Ram, L.C. Rhizosphere soil indicators for carbon sequestration in a reclaimed coal mine spoil. CATENA 2016, 141, 100–108. [CrossRef]
- Sobucki, L.; Ramos, R.F.; Meireles, L.A.; Antoniolli, Z.I.; Jacques, R.J.S. Contribution of enzymes to soil quality and the evolution of research in Brazil. Rev. Bras. De Cienc. Do Solo 2021, 45. [CrossRef]
- Uwituze, Y.; Nyiraneza, J.; Fraser, T.D.; Dessureaut-Rompré, J.; Ziadi, N.; Lafond, J. Carbon, Nitrogen, Phosphorus, and Extracellular Soil Enzyme Responses to Different Land Use. Front. Soil Sci. 2022, 2. [CrossRef]
- Brown, S.L.; Chaney, R.L. Use of Amendments to Restore Ecosystem Function to Metal Mining-Impacted Sites: Tools to Evaluate Efficacy. Curr. Pollut. Rep. 2016, 2, 91–102. [CrossRef]
- Rüdisser, J.; Tasser, E.; Peham, T.; Meyer, E.; Tappeiner, U. The dark side of biodiversity: Spatial application of the biological soil quality indicator (BSQ). Ecol. Indic. 2015, 53, 240–246. [CrossRef]
- Marchiori Júnior M, Melo WJ. Carbono, carbono da biomassa microbiana e atividade enzimática em um solo sob mata natural, pastagem e cultura do algodoeiro. Rev Bras Cienc Solo. 1999;23:257–63.
- Roldán, A.; Salinas-García, J.; Alguacil, M.; Díaz, E.; Caravaca, F. Soil enzyme activities suggest advantages of conservation tillage practices in sorghum cultivation under subtropical conditions. Geoderma 2005, 129, 178–185. [CrossRef]
- Hungria, M.; Franchini, J.C.; Brandão-Junior, O.; Kaschuk, G.; Souza, R.A. Soil microbial activity and crop sustainability in a long-term experiment with three soil-tillage and two crop-rotation systems. Appl. Soil Ecol. 2009, 42, 288–296. [CrossRef]
- dos Santos, J.V.; Bento, L.R.; Bresolin, J.D.; Mitsuyuki, M.C.; Oliveira, P.P.A.; Pezzopane, J.R.M.; Bernardi, A.C.d.C.; Mendes, I.C.; Martin-Neto, L. The long-term effects of intensive grazing and silvopastoral systems on soil physicochemical properties, enzymatic activity, and microbial biomass. CATENA 2022, 219. [CrossRef]
- Ross, D.J. EFFECTS OF AIR-DRY, REFRIGERATED AND FROZEN STORAGE ON ACTIVITIES OF ENZYMES HYDROLYSING SUCROSE AND STARCH IN SOILS. Eur. J. Soil Sci. 1965, 16, 86–94. [CrossRef]
- Pancholy, S.K.; Rice, E.L. Soil Enzymes in Relation to Old Field Succession: Amylase, Cellulase, Invertase, Dehydrogenase, and Urease. Soil Sci. Soc. Am. J. 1973, 37, 47–50. [CrossRef]
- Köppen W. Das Geographische System der Klimatologie 1936:44.
- Soil Survey Staff. Keys to soil taxonomy. USDA Natural Resources Conservation Service. 2022;13:410.
- Raij B, Andrade J, Cantarella H, Quaggio JA. Análise Química para Avaliação da Fertilidade de Solos Tropicais. Boletim técnico. 2001:285.
- Teixeira PC, Fontana GKDA, Teixeira WG. Manual de Métodos de Análise de Solo. 3rd ed. Brasília: Embrapa Solos. 2017.
- Cantarella H, Quaggio JA, Mattos Jr D, Boaretto RM, Raij B. Boletim 100: recomendações de adubação e calagem para o estado de São Paulo. 2022.
- Bremner, J.M.; Mulvaney CS. Nitrogen-total. Chemical and microbiological properties, Madison: Methods of soil analysis. Soil Sci Soc Am. 1982:595–624.
- Dabin B. Méthode d’extraction et de fractionnement des matières humiques du sol Application à quelques études pédologiques et agronomiques dans les sols tropicaux. Cah Orston Ser Pédol. 1976;4:287–97.
- Stevenson FJ. The Role of Organic Matter in Modern Agriculture. 2nd ed. New York: Developments in Plant and Soil Sciences; 1982.
- Morse, E.E. Anthrone in Estimating Low Concentrations of Sucrose. Anal. Chem. 1947, 19, 1012–1013. [CrossRef]
- Oser BL. Hawk’s Physiological Chemistry. New York: McGraw-Hill Book Co. Inc., 1965;14 ed.
- Abreu, C.H., Jr.; Muraoka, T.; Lavorante, A.F. Relationship between acidity and chemical properties of brazilian soils. Sci. Agric. 2003, 60, 337–343. [CrossRef]
- Anselmo, J.L.; Sul, C.D.S.F.D.A..P.A.D.C.D.; Bossolani, J.W.; Lazarini, E.; Leal, A.J.F.; Alvarez, R.D.C.F.; Arf, M.V. Maize productivity cultivated as first crop in succession to different cover crops. Oct. 2020 2018, 12, 967–974. [CrossRef]
- Allison, S.D.; Vitousek, P.M. Responses of extracellular enzymes to simple and complex nutrient inputs. Soil Biol. Biochem. 2005, 37, 937–944. [CrossRef]
- Sinsabaugh, R.L.; Belnap, J.; Findlay, S.G.; Shah, J.J.F.; Hill, B.H.; Kuehn, K.A.; Kuske, C.R.; Litvak, M.E.; Martinez, N.G.; Moorhead, D.L.; et al. Extracellular enzyme kinetics scale with resource availability. Biogeochemistry 2014, 121, 287–304. [CrossRef]
- Sainju, U.M.; Liptzin, D.; Dangi, S.M. Enzyme activities as soil health indicators in relation to soil characteristics and crop production. Agrosystems, Geosci. Environ. 2022, 5. [CrossRef]
- Johnson, A.; Hoyt, G. Changes to the Soil Environment under Conservation Tillage. HortTechnology 1999, 9, 380–393. [CrossRef]
- Dotaniya, M.L.; Aparna, K.; Dotaniya, C.K.; Singh, M.; Regar, K.L. Role of Soil Enzymes in Sustainable Crop Production. In Enzymes in Food Biotechnology; Elsevier: Amsterdam, The Netherlands, 2019; pp. 569–589, ISBN 978-0-12-813280-7.
- Lucero, C.T.; Lorda, G.S.; Anzuay, M.S.; Ludueña, L.M.; Taurian, T. Peanut Endophytic Phosphate Solubilizing Bacteria Increase Growth and P Content of Soybean and Maize Plants. Curr. Microbiol. 2021, 78, 1961–1972. [CrossRef]
- Asghar, W.; Kataoka, R. Green manure incorporation accelerates enzyme activity, plant growth, and changes in the fungal community of soil. Arch. Microbiol. 2021, 204, 1–10. [CrossRef]
- Grzyb, A.; Wolna-Maruwka, A.; Niewiadomska, A. Environmental Factors Affecting the Mineralization of Crop Residues. Agronomy 2020, 10, 1951. [CrossRef]
- Junior, C.H.A.; Muraoka, T.; Oliveira, F.C. Carbono, nitrogênio, fósforo e enxofre em solos tratados com composto de lixo urbano. Rev. Bras. De Cienc. Do Solo 2002, 26, 769–780. [CrossRef]
- Stockmann, U.; Adams, M.A.; Crawford, J.W.; Field, D.J.; Henakaarchchi, N.; Jenkins, M.; Minasny, B.; McBratney, A.B.; de Courcelles, V.d.R.; Singh, K.; et al. The knowns, known unknowns and unknowns of sequestration of soil organic carbon. Agric. Ecosyst. Environ. 2013, 164, 80–99. [CrossRef]
- Fontaine, S.; Mariotti, A.; Abbadie, L. The priming effect of organic matter: a question of microbial competition?. Soil Biol. Biochem. 2003, 35, 837–843. [CrossRef]
- Gualberto, A.V.S.; de Souza, H.A.; Sagrilo, E.; Araujo, A.S.F.; Mendes, L.W.; de Medeiros, E.V.; Pereira, A.P.d.A.; da Costa, D.P.; Vogado, R.F.; da Cunha, J.R.; et al. Organic C Fractions in Topsoil under Different Management Systems in Northeastern Brazil. Soil Syst. 2023, 7, 11. [CrossRef]
- Marschner, B.; Brodowski, S.; Dreves, A.; Gleixner, G.; Gude, A.; Grootes, P.M.; Hamer, U.; Heim, A.; Jandl, G.; Ji, R.; et al. How relevant is recalcitrance for the stabilization of organic matter in soils? J. Plant Nutr. Soil Sci. 2008, 171, 91–110. [CrossRef]
- Cotrufo MF, Lavallee JM. Soil organic matter formation, persistence, and functioning: A synthesis of current understanding to inform its conservation and regeneration. Adv Agron. 2022;172:1–66. [CrossRef]
- Pfleger P, Cassol PC, Mafra ÁL. Substâncias húmicas em Cambissolo sob vegetação natural e plantios de pinus em diferentes idades. Ciênc Florest. 2017;27:807–17.
- Souza WJO, Melo WJ. Teores de nitrogênio no solo e nas frações da matéria orgânica sob diferentes sistemas de produção de milho. Rev Bras Cienc Solo. 2000;24:885–96.
- Balota, E.L.; Yada, I.F.U.; Amaral, H.F.; Nakatani, A.S.; Hungria, M.; Dick, R.P.; Coyne, M.S. SOIL QUALITY IN RELATION TO FOREST CONVERSION TO PERENNIAL OR ANNUAL CROPPING IN SOUTHERN BRAZIL. Rev. Bras. De Cienc. Do Solo 2015, 39, 1003–1014. [CrossRef]
- Zhang, K.; Maltais-Landry, G.; Liao, H.-L. How soil biota regulate C cycling and soil C pools in diversified crop rotations. Soil Biol. Biochem. 2021, 156. [CrossRef]
- Villanueva FCA, Boaretto AE, Firme LP, Muraoka T, Franco Filho V do N, Abreu Junior CH. Mudanças químicas e fitodisponibilidade de zinco estimada por método isotópico, em solo tratado com lodo de esgoto. Quim Nova. 2012;35:1348–54.
- Vargas, L.K.; Scholles, D. Nitrogênio da biomassa microbiana, em solo sob diferentes sistemas de manejo, estimado por métodos de fumigação. Rev. Bras. De Cienc. Do Solo 1998, 22, 411–417. [CrossRef]
| Sampling occasion | Resin-P | OC | pH | K+ | Ca2+ | Mg2+ | H+ + Al3+ | CEC | V% |
|---|---|---|---|---|---|---|---|---|---|
| mg dm-3 | g dm-3 | CaCl2 | ------------- mmolc dm-3 ------------- | - % - | |||||
| One month before liming | 21±3 | 11.3±1.5 | 4.6±0.3 | 1.7±0.2 | 10.3±0.9 | 5.7±0.3 | 47±3 | 65±5 | 27±3 |
| Six months after liming | 31±5 | 12.4±1.6 | 5.4±0.4 | 1.7±0.3 | 32.0±2,5 | 11.0±0.5 | 28±2 | 73±6 | 62±5 |
| Management | Before the experiment | First green manure crop (C0) | First maize cycle (C1) |
Second maize cycle (C2) |
Third maize cycle (C3) |
|---|---|---|---|---|---|
| Weed incorporation | August | - | - | - | |
| Soil preparation | August | - | - | - | |
| Manure seeding | - | April | April | April | |
| Manure harvest and dry matter incorporation | - | September | August | September | |
| Maize seeding | October | November | November | November | |
| Leaf diagnosis and soil sampling | - | July | January | January | January |
| Maize harvest and dry matter incorporation | March | March | April | April |
| Treatments | Yield | OC | C-Hum | N-NO3- | C/N ratio |
|---|---|---|---|---|---|
| ---- kg ha-1 ---- | ------------- g kg-1 ------------- | ---- mg kg-1 ---- | - | ||
| Green manure/fallow and maize crop succession | |||||
| Sorghum | 8,713±1,004 A | 13.0±0.99 A | 10.7±1.04 A | 7.74±9.17 A | 12.0±1.59 A |
| Lablab | 8,480±1,036 AB | 12.5±0.89 A | 10.1±0.92 A | 7.76±8.08 A | 11.6±1.67 A |
| Fallow | 7,732±1,003 B | 12.3±1.08 A | 9.9±1.03 A | 7.22±6.75 A | 12.0±1.75 A |
| Succession cycle | |||||
| C0 | - | 12.6±0.94 ab | 10.3±0.86 a | 1.4±1.0 c | 11.3±1.18 b |
| C1 | 8,665±650 a | 13.0±0.66 a | 10.4±0.80 a | 5.8±3.3 b | 12.7±0.54 a |
| C2 | 8,511±923 ab | 12.8±1.44 a | 10.5±1.58 a | 3.1±1.9 bc | 13.4±1.36 a |
| C3 | 7,732±446 b | 11.9±0.50 b | 9.8±0.50 a | 20.0±4.7 a | 10.0±0.86 c |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





