Submitted:
23 August 2024
Posted:
27 August 2024
You are already at the latest version
Abstract
Keywords:
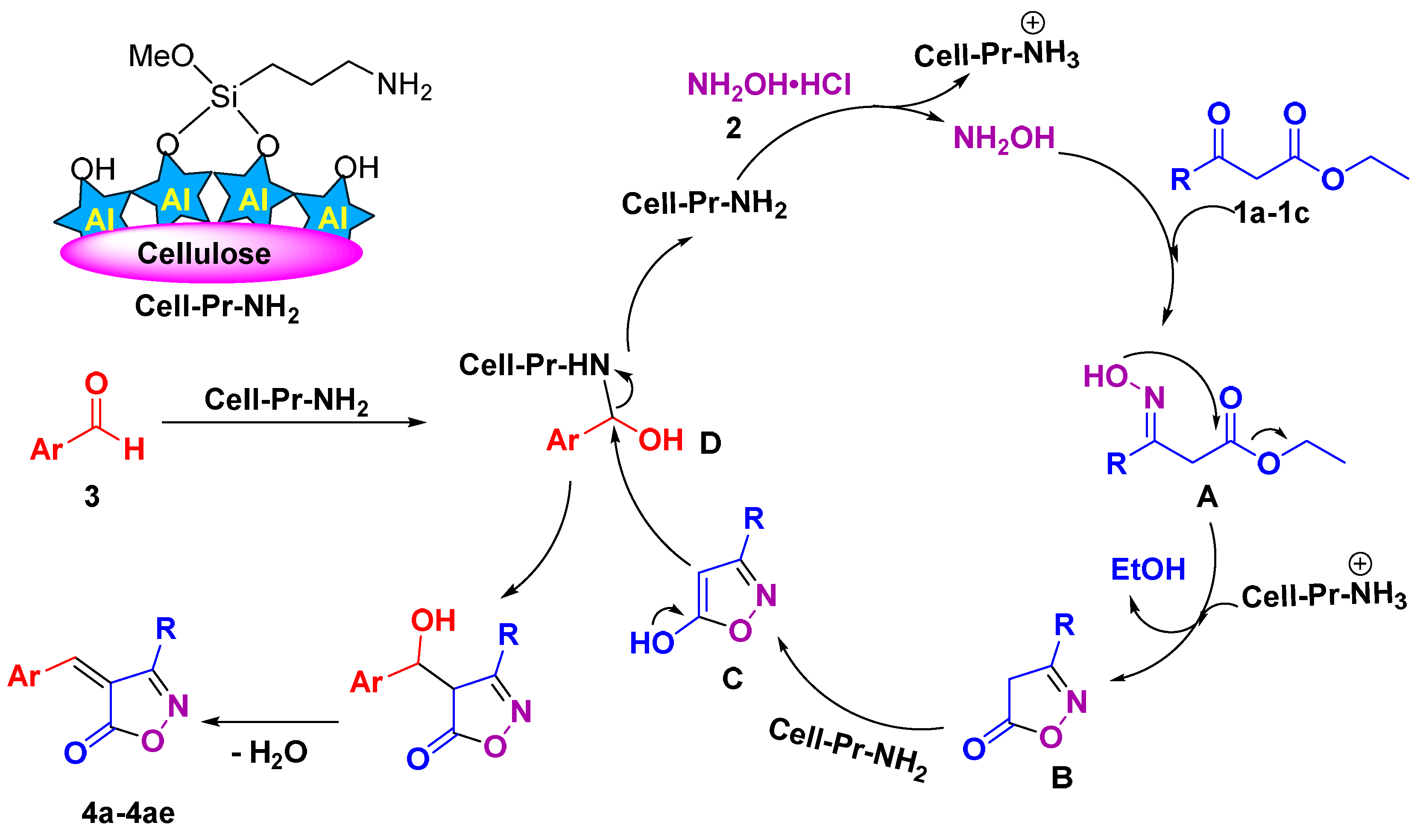
1. Introduction
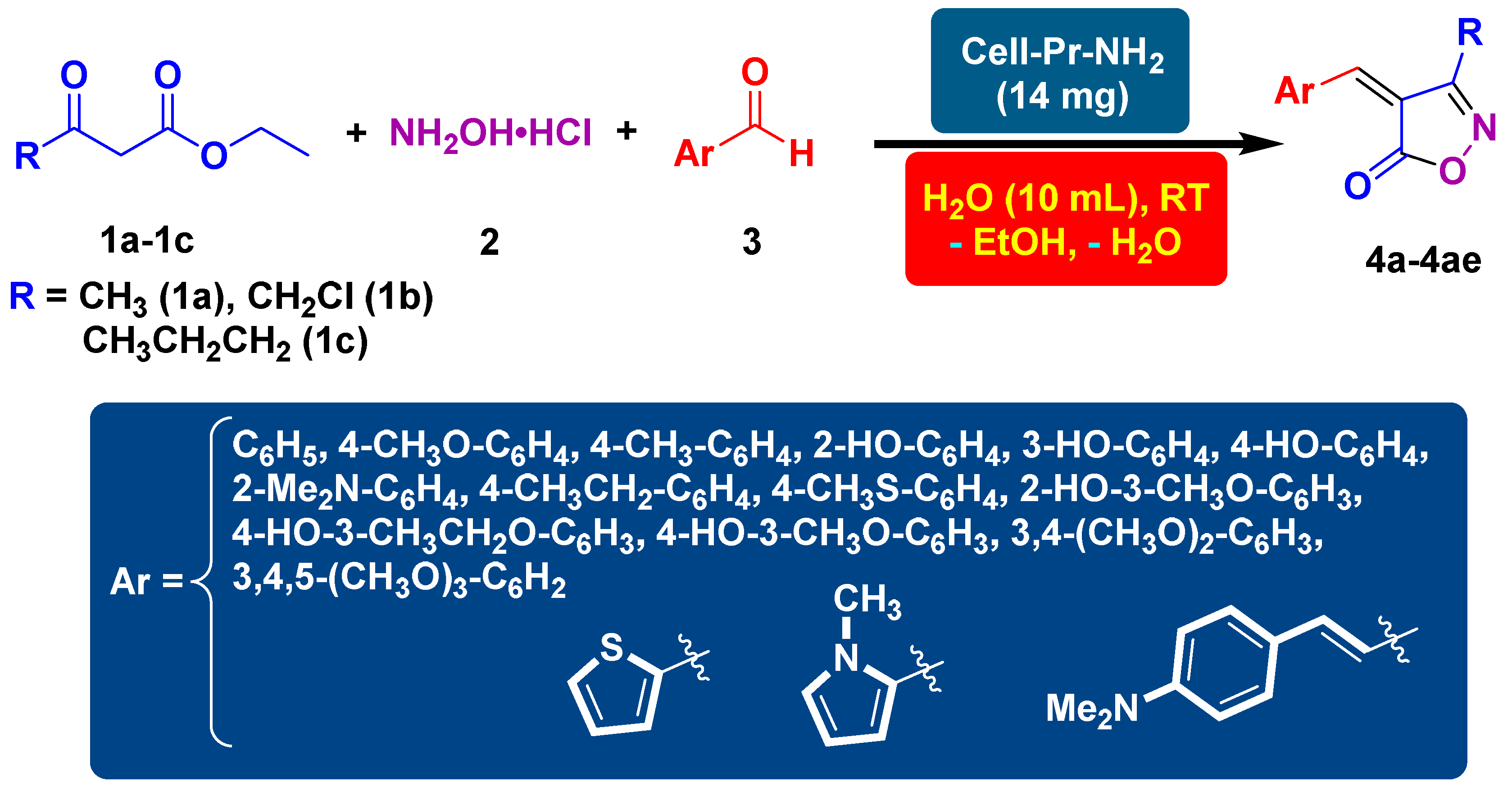
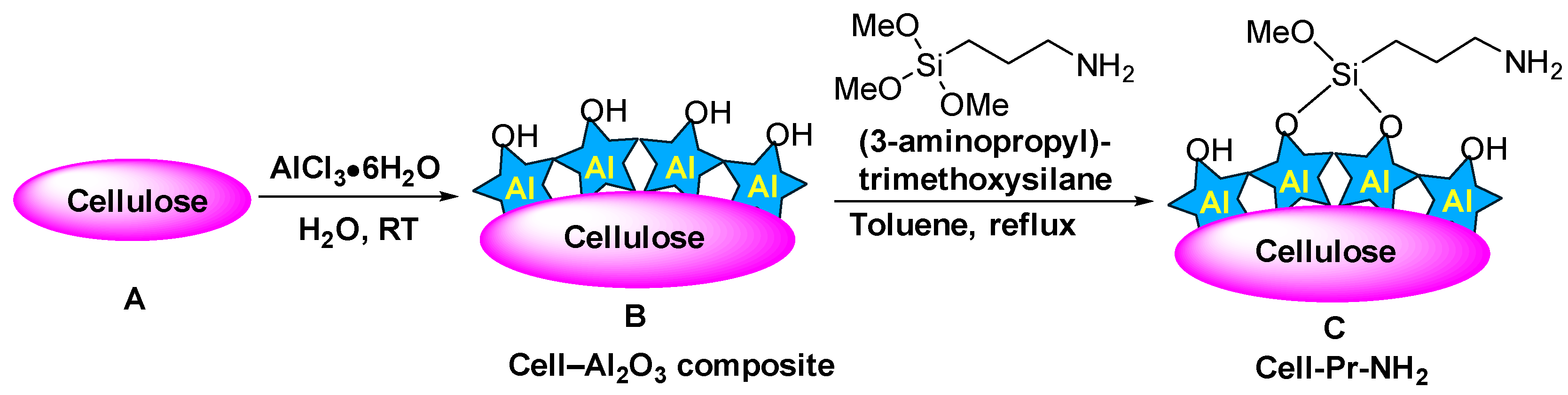
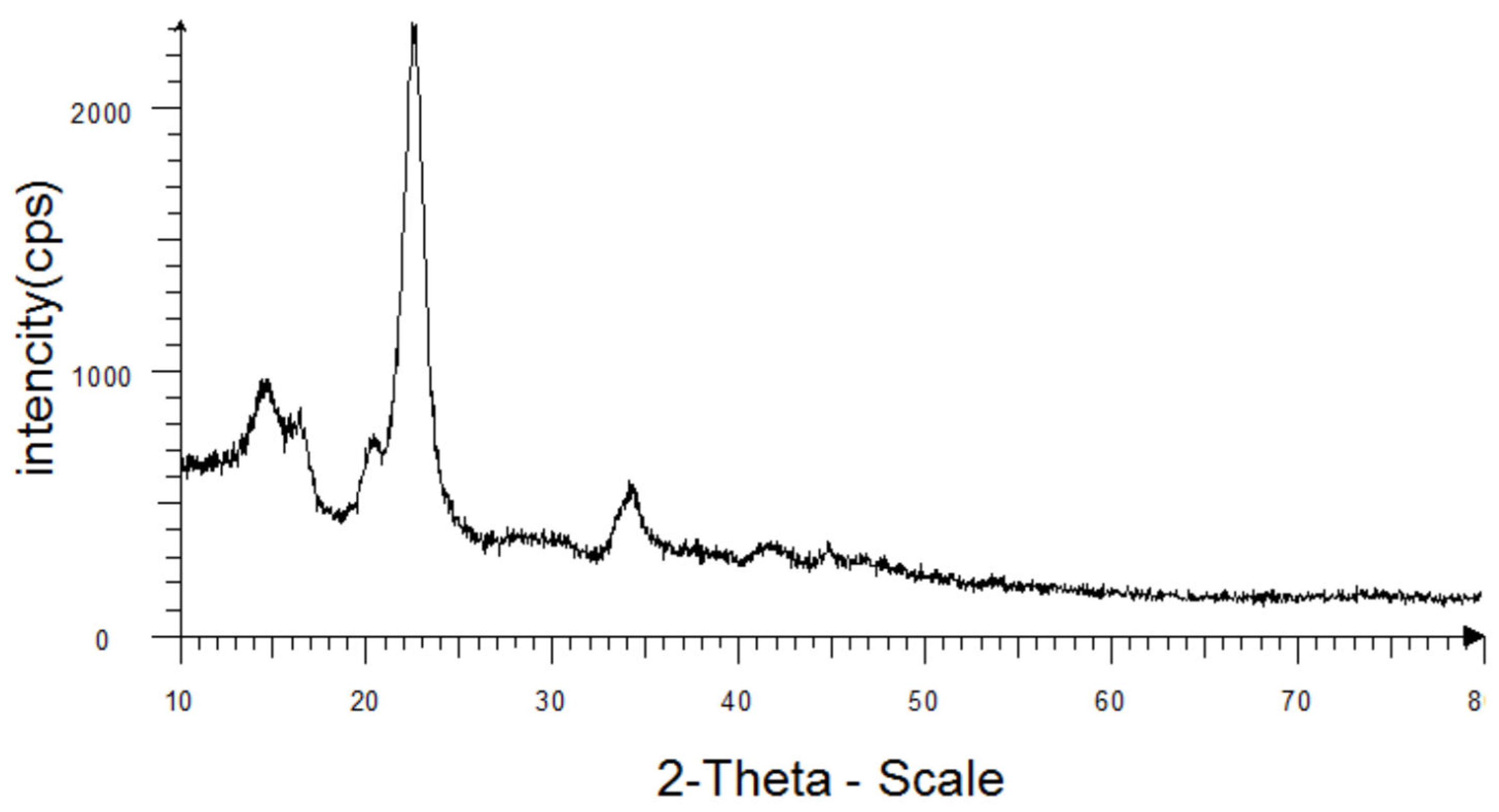
2. Materials and Methods
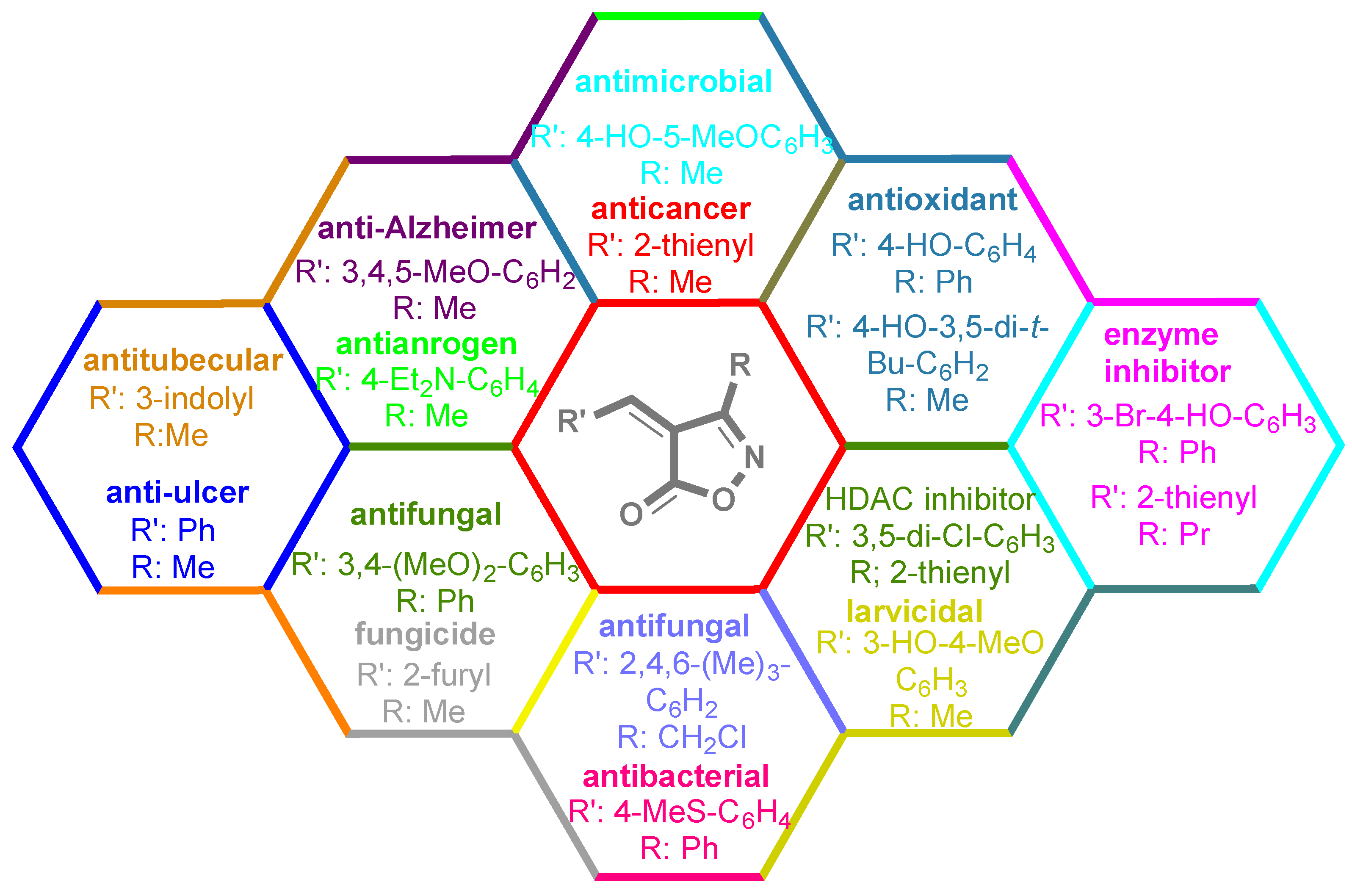
3. Results and Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- (a) Dengale, S.G.; Akolkar, H.N.; Darekar, N.R.; Shaikh, M.H.; Deshmukh, K.K.; Mhaske, S.D.; Karale, B.K.; Raut, D.N.; Khedkar, V.M. Synthesis and Biological Evaluation of 2-(4,5,6,7-Tetrahydrobenzo[c]Isoxazol-3-yl)-4H-Chromen-4-Ones. Polycycl. Aromat. Comp. 2002, 42, 6337-6351. doi:10.1080/10406638.2021.1982733; (b) Thakur, A.; Verma, M.; Bharti, R.; Sharma, R. Oxazole and isoxazole: From one-pot synthesis to medical applications. Tetrahedron 2022, 119, 132813. doi:10.1016/j.tet.2022.132813; (c) Arya, G.C.; Kaur, K.; Jaitak, V. Isoxazole derivatives as anticancer agent: A review on synthetic strategies, mechanism of action and SAR studies. Eur. J. Med. Chem. 2021, 221, 113511. doi:10.1016/j.ejmech.2021.113511; (d) Farooq, S.; Ngaini, Z. Synthesis of Benzalacetophenone-based Isoxazoline and Isoxazole Derivatives. Curr. Org. Chem. 2022, 26, 679-692. doi:10.2174/1385272826666220408120350.
- Pattanayak, P.; Chatterjee, T. Synthesis of (4-Trifluoromethyl)isoxazoles through a Tandem Trifluoromethyloximation/Cyclization/Elimination Reaction of α,β-Unsaturated Carbonyls. J. Org. Chem. 2023, 88, 5420-5430. doi:10.1021/acs.joc.2c03053.
- Zhu, J.; Mo, J.; Lin, H.Z.; Chen, Y.; Sun, H.P. The recent progress of isoxazole in medicinal chemistry. Bioorg. Med. Chem. 2018, 26, 3065-3075. doi:10.1016/j.bmc.2018.05.013.
- Agrawal, N.; Mishra, P. The synthetic and therapeutic expedition of isoxazole and its analogs. Med. Chem. Res. 2018, 27, 1309-1344. doi:10.1007/s00044-018-2152-6.
- Pandhurnekar, C.P.; Pandhurnekar, H.C.; Mungole, A.J.; Butoliya, S. S.; Yadao, B.G. A review of recent synthetic strategies and biological activities of isoxazole. J. Heterocycl. Chem. 2023, 60, 536-565. doi:10.1002/jhet.4586.
- Yang, Y.; Zhao, J.; Zhang, X.; Hu, Z.; Wu, Y. Synthesis and characterization of a new isoxazolone-based nonlinear optical crystal: MPMOI, CrystEngComm 2023, 25, 1313-1318. doi:10.1039/D2CE01299E.
- Razzaq, S.; Minhas, A.M.; Qazi, N.G.; Nadeem, H.; Khan, A.U.; Ali, F.; Hassan, S.S.; Bungau, S. Novel Isoxazole Derivative Attenuates Ethanol-Induced Gastric Mucosal Injury through Inhibition of H+/K+-ATPase Pump, Oxidative Stress and Inflammatory Pathways. Molecules 2022, 27, 5065. doi:10.3390/molecules27165065.
- Kafle, B.; Aher, N.G.; Khadka, D.; Park, H.; Cho, H. Isoxazol-5(4H)one Derivatives as PTP1B Inhibitors Showing an Anti-Obesity Effect. Chem. Asian J. 2011, 6, 2073-2079. doi:10.1002/asia.201100154.
- (a) Sukanya, S.H.; Venkatesh, T.; Kumar R.S. Bodke, Y.D. Facile TiO2 NPs catalysed synthesis of substituted-4-Hydroxy/methoxy benzylidene derivatives as potent antioxidant and anti-tubercular agents. Chem. Data Collect. 2021, 33, 100713. doi:10.1016/j.cdc.2021.100713; (b) Kuchana, M.; Bethapudi, D.R.; Ediga, R.K.; Sisapuram, Y. Synthesis, in-Vitro Antioxidant Activity and In-Silico Prediction of Drug-Likeness Properties of a Novel Compound: 4-(3,5-Di-Tert-Butyl-4-Hydroxybenzylidene)-3-Methylisoxazol-5(4H)-One. J. Appl. Pharm. Sci. 2019, 9, 105-110. doi:10.7324/JAPS.2019.90915.
- Ali, M.; Saleem, U.; Anwar, F.; Imran, M.; Nadeem, H.; Ahmad, B.; Ali, T.; Atta-ur-rehman, Ismail, T. Screening of Synthetic Isoxazolone Derivative Role in Alzheimer’s Disease: Computational and Pharmacological Approach. Neurochem. Res. 2021, 46, 905-920. doi:10.1007/s11064-021-03229-w.
- (a) Kadam, H.K.; Salkar, K.; Naik, A.P.; Naik, M.M.; Salgaonkar, L.N.; Charya, L.; Pinto, K.C.; Mandrekar, V.K.; Vaz, T. Silica Supported Synthesis and Quorum Quenching Ability of Isoxazolones Against Both Gram Positive and Gram-Negative Bacterial Pathogens. ChemistrySelect 2021, 6, 11718-11728. doi:10.1002/slct.202101798; (b) Wang, Y.; Du, D.M. Highly Diastereo- and Enantioselective Synthesis of Isoxazolone-Spirooxindoles via Squaramide-Catalyzed Cascade Michael/Michael Addition Reactions. J. Org. Chem. 2020, 85, 15325-15336. doi:10.1021/acs.joc.0c02150.
- (a) Badiger, K.B.; Khatavi, S.Y.; Kamanna, K. Green synthesis of 3-methyl-4-(hetero)aryl methylene isoxazole-5(4H)-ones using WEOFPA/glycerol: evaluation of anticancer and electrochemical behaviour properties. RSC Med. Chem. 2022, 13, 1367-1377. doi:10.1039/d2md00191h; (b) Bhatt, T.D.; Gojiya, D.G.; Kalavadiya, P.L.; Joshi, H.S. Rapid, Greener and Ultrasound Irradiated One-Pot Synthesis of 4-(Substituted-1H-Pyrazol-4-yl)Methylene)-3-Isopropylisoxazol-5(4H)-ones and Their In Vitro Anticancer Activity. ChemistrySelect 2019, 4, 11125-11129. doi:10.1002/slct.201902164.
- Nongrum, R.; Nongkhlaw, R.; Majaw, S.P.; Kumari, J.; Sriram, D.; Nongkhlaw, R. A nano-organo catalyst mediated approach towards the green synthesis of 3-methyl-4-(phenyl)methylene-isoxazole-5(4H)-one derivatives and biological evaluation of the derivatives as a potent anti-fungal and anti-tubercular agent. Sustain. Chem. Pharm. 2023, 32, 100967. doi:10.1016/j.scp.2023.100967.
- Chande, M.S.; Verma, R.S.; Barve, P.A.; Khanwelkar, R.R.; Vaidya, R.B.; Ajaikumar, K.B. Facile synthesis of active antitubercular, cytotoxic and antibacterial agents: a Michael addition approach. Eur. J. Med. Chem. 2005, 40, 1143-1148. doi:10.1016/j.ejmech.2005.06.004.
- (a) Saleem, A.; Farooq, U.; Bukhari, S.M.; Khan, S.; Zaidi, A.; Wani, T.A.; Shaikh, A.J.; Sarwar, R.; Mahmud, S.; Israr, M.; Khan, F.A.; Shahzad, S.A. Isoxazole Derivatives against Carbonic Anhydrase: Synthesis, Molecular Docking, MD Simulations, and Free Energy Calculations Coupled with In Vitro Studies. Acs Omega 2022, 7, 30359-30368, doi:10.1021/acsomega.2c03600; (b) Kim, S.J.; Yang, J.; Lee, S.; Park, C.; Kang, D.; Akter, J.; Ullah, S.; Kim, Y.J.; Chun, P.; Moon, H.R. The tyrosinase inhibitory effects of isoxazolone derivatives with a (Z)-β-phenyl-α,β-unsaturated carbonyl scaffold,” Bioorg. Med. Chem. 2018, 26, 3882-3889. doi:10.1016/j.bmc.2018.05.047.
- Sampaio, A.B.S.; Mori, M.S.S.; Albernaz, L.C.; Espindola, L.S.; Salvador, C.E.M.; Andrade, C.K.Z.; Continuous Flow Photochemical Synthesis of 3-Methyl-4-arylmethylene Isoxazole-5(4H)-ones through Organic Photoredox Catalysis and Investigation of Their Larvicidal Activity. Catalysts 2023, 13, 518. doi:10.3390/catal13030518.
- (a) Oraby, A.K.; Abdellatif, K.R.A.; Abdelgawad, M.A.; Attia, K.M.; Dawe, L.N.; Georghiou, P.E.; 2,4-Disubstituted Phenylhydrazonopyrazolone and Isoxazolone Derivatives as Antibacterial Agents: Synthesis, Preliminary Biological Evaluation and Docking Studies. ChemistrySelect 2018, 3, 3295-3301. doi:10.1002/slct.201800174; (b) Alizadeh, N.; Kiyani, H.; Albadi, J. Green and three-component synthesis of isoxazolones using natural sunlight and investigating their antibacterial activity. Appl. Chem. 2023, 18, 125-148. doi:10.22075/chem.2022.28043.2093; (C) Wazalwar, S.S.; Banpurkar, A.R.; Perdih, F. Aqueous phase synthesis, crystal structure and biological study of isoxazole extensions of pyrazole-4-carbaldehyde derivatives. J. Mol. Struct. 2017, 1150, 258-267. doi:10.1016/j.molstruc.2017.08.094.
- Gadkari, Y.U.; Jadhav, N.L.; Hatvate, N.T.; Telvekar, V.N. Concentrated Solar Radiation Aided Green Approach for Preparative Scale and Solvent-Free Synthesis of 3-Methyl-4-(hetero)arylmethylene Isoxazole-5(4H)-ones. ChemistrySelect 2020, 5, 12320-12323. doi:10.1002/slct.202003348.
- (a) Uvarova, E.S.; Kutasevich, A.V.; Lipatov, E.S.; Mityanov, V.S. Assembly of isoxazol-5-one with 2-unsubstituted imidazole N-oxides and aldehydes. Org. Biomol. Chem. 2023, 21, 651-659. doi:10.1039/d2ob02157a; (b) Galenko, E.E.; Linnik, S.A.; Khoroshilova, O.V.; Novikov, M.S.; Khlebnikov, A.F. Isoxazole Strategy for the Synthesis of α-Aminopyrrole Derivatives. J. Org. Chem. 2019, 84, 11275-11285. doi:10.1021/acs.joc.9b01634; (c) Galenko, E.E.; Kryukova, M.A.; Novikov, M.S.; Khlebnikov, A.F. An Isoxazole Strategy for the Synthesis of Fully Substituted Nicotinates. J. Org. Chem. 2021, 86, 6888-6896. doi:10.1021/acs.joc.1c00286; (d) Galenko, E.E.; Novikov, M.S.; Khlebnikov, A.F. “[2 + 2] Cycloaddition/Retro-Electrocyclization/Decarboxylation Reaction Sequence: Access to 4-Aminopyridines from Methylideneisoxazolones and Ynamines. J. Org. Chem. 2023, 88, 8854-8864. doi:10.1021/acs.joc.3c00654.
- Sabitha, G.; Reddy, M.M.; Archana, B.; Yadav, J.S. A Convenient Synthesis of Benzopyranacetylenes. Synth. Commun. 1998, 28, 573-581, doi:10.1080/00397919808005928.
- Wan, S.H.; Li, X.A.; Liu, Y.H.; Liu, S.T.N-Allylation versus C-allylation of intermediates from aza-Michael adducts of arylideneisoxazol-5-ones. Org. Biomol. Chem. 2020, 18, 9516-9525. doi:10.1039/D0OB01998D.
- Macchia, A.; Summa, F.F.; Monaco, G.; Eitzinger, A.; Ofial, A.R.; Di Mola, A.; Massa, A. Access to β-Alkylated γ-Functionalized Ketones via Conjugate Additions to Arylideneisoxazol-5-ones and Mo(CO)6-Mediated Reductive Cascade Reactions. Acs Omega 2022, 7, 8808-8818. doi:10.1021/acsomega.1c07081.
- (a) MacChia, A.; Eitzinger, A.; Brière, J.F.; Waser, M.; Massa, A. Asymmetric Synthesis of Isoxazol-5-ones and Isoxazolidin-5-ones. Synthesis 2021, 53, 107-122. doi:10.1055/s-0040-1706483; (b) Martínez-Pardo, P.; Laviós, A.; Sanz-Marco, A.; Vila, C.; Pedro, J.R.; Blay, G. Enantioselective Synthesis of Functionalized Diazaspirocycles from 4-Benzylideneisoxazol-5(4H)-one Derivatives and Isocyanoacetate Esters. Adv. Synth. Catal. 2020, 362, 3564-3569. doi:10.1002/adsc.202000611; (c) da Silva, A.F.; Fernandes, A.A.G.; Thurow, S.; Stivanin, M.L.; Jurberg, I.D.; Isoxazol-5-ones as Strategic Building Blocks in Organic Synthesis. Synthesis 2018, 50, 2473-2489. doi:10.1055/s-0036-1589534.
- Faramarzi, Z.; Kiyani, H. Organocatalyzed Three-Component Synthesis of Isoxazol-5(4H)-Ones under Aqueous Conditions. Heterocycles 2021, 102, 1779-1790. doi:10.3987/COM-21-14488.
- Kapale, S.S.; Gadkari, Y.U.; Chaudhari, H.K. Lipase Catalyzed One-Pot Synthesis of 3-Methyl-4-(Hetero) Arylmethyleneisoxazole-5(4H)-Ones under Aqueous Conditions. Polycycl. Aromat. Comp. 2022, 43, 4856-4865. 10.1080/10406638.2022.2096649,.
- Faramarzi, Z.; Kiyani, H. Steglich’s Base Catalyzed Three-Component Synthesis of Isoxazol-5-Ones. Polycycl. Aromat. Comp. 2023, 43, 3099-3121. doi:10.1080/10406638.2022.2061533.
- Parveen, M.; Aslam, A.; Ahmad, A.; Alam, M.; Silva, M.R.; Silva, P.S.P. A facile & convenient route for the stereoselective synthesis of Z-isoxazol-5(4H)-ones derivatives catalysed by sodium acetate: Synthesis, multispectroscopic properties, crystal structure with DFT calculations, DNA-binding studies and molecular docking studies. J. Mol. Struct. 2020, 1200, 127067. doi:10.1016/j.molstruc.2019.127067.
- Gharehassanlou, S.; Kiyani, H. A Catalytic Three-Component Synthesis of Isoxazol-5(4H)-Ones under Green Conditions. Indian J. Chem. 2022, 61, 515-520. doi:10.56042/ijc.v61i5.63643.
- Barkule, A.B.; Gadkari, Y.U.; Telvekar, V.N. One-Pot Multicomponent Synthesis of 3-Methyl-4-(Hetero)Arylmethylene Isoxazole-5(4H)-Ones Using Guanidine Hydrochloride as the Catalyst under Aqueous Conditions. Polycycl. Aromat. Comp. 2022, 42, 5870-5881. doi:10.1080/10406638.2021.1959353.
- Saikh, F.; Das, J.; Ghosh, S. Synthesis of 3-methyl-4-arylmethylene isoxazole-5(4H)-ones by visible light in aqueous ethanol. Tetrahedron Lett. 2013, 54, 4679-4682. doi:10.1016/j.tetlet.2013.06.086.
- Kiyani, H.; Kanaani, A.; Ajloo, D.; Ghorbani, F.; Vakili, M. N-Bromosuccinimide (NBS)-Promoted, Three-Component Synthesis of α,β-Unsaturated Isoxazol-5(4H)-Ones, and Spectroscopic Investigation and Computational Study of 3-Methyl-4-(thiophen-2-ylmethylene)isoxazol-5(4H)-one. Res. Chem. Intermed. 2015, 41, 7739-7773. doi:10.1007/s11164-014-1857-5.
- Ostadzadeh, H.; H. Kiyani, Synthesis of Isoxazole-5(4H)-Ones Using Citrazinic Acid as an Organocatalyst in Aqueous Conditions. Org. Prep. Proced. Int. 2023, 55, 538-548. doi:10.1080/00304948.2023.2192601.
- Aleaba, G; Asadi, S.K.; Daneshvar, N.; Shirini, F. Introduction of [2,2'-Bipyridine]-1,1'-Diium Perchlorate as a Novel and Highly Efficient Dicationic Brönsted Acidic Organic Salt for the Synthesis of 3-Methyl-4-Arylmethylene Isoxazole-5(4H)-one Derivatives in Water. Polycycl. Aromat. Comp. 2022, 42, 7569-7581. doi:10.1080/10406638.2021.2005641.
- Kiyani, H.; Ghorbani, F. Efficient Tandem Synthesis of a Variety of Pyran-Annulated Heterocycles, 3,4-Disubstituted Isoxazol-5(4H)-ones, and α,β-Unsaturated Nitriles Catalyzed by Potassium Hydrogen Phthalate in Water. Res. Chem. Intermed. 2015, 41, 7847-7882. doi:10.1007/s11164-014-1863-7.
- Daroughezadeh, Z.; Kiyani, H. Efficient and Aqueous Synthesis of 3,4-Disubstituted Isoxazol-5(4H)-one Derivatives Using Piperazine under Green Conditions. Heterocycles 2022, 104, 1625-1640. doi:10.3987/COM-22-14686.
- Kiyani, H.; Ghorbani, F. Potassium Phthalimide as Efficient Basic Organocatalyst for the Synthesis of 3,4-Disubstituted Isoxazol-5(4H)-ones in Aqueous Medium. J. Saudi Chem. Soc. 2017, 21, S112-S119. doi:10.1016/j.jscs.2013.11.002.
- Mosallanezhad, A.; Kiyani, H. Green Synthesis of 3-Substituted-4-Arylmethylideneisoxazol-5(4H)-one Derivatives Catalyzed by Salicylic Acid. Curr. Organocatal. 2019, 6, 28-35. doi:10.2174/2213337206666190214161332.
- Kiyani, H.; Mosallanezhad, A. “Sulfanilic Acid-Catalyzed Synthesis of 4-Arylidene-3-Substituted Isoxazole-5(4H)-ones. Curr. Org. Synth. 2018, 15, 715-722. doi:10.2174/1570179415666180423150259.
- Reihani, N.; Kiyani, H. Three-Component Synthesis of 4-Arylidene-3-Alkylisoxazol-5(4H)-ones in the Presence of Potassium 2,5-Dioxoimidazolidin-1-ide. Curr. Org. Chem. 2021, 25, 950-962. doi:10.2174/1385272825666210212120517.
- Daroughezadeh, Z.; H. Kiyani, Synthesis of Arylideneisoxazol-5-ones Catalyzed by Sodium Cyclamate. Heterocycles 2023, 106, 1187-1197. doi:10.3987/COM-23-14859.
- Delfani, A.M.; Kiyani, H.; Zamani, M. An Expeditious Synthesis of Ethyl-2-(4-(arylmethylene)-5-oxo-4,5-dihydroisoxazol-3-yl)acetate Derivatives. Curr. Org. Chem. 2022, 26, 1575-1584. doi:10.2174/1385272827666221124105402.
- Tahmasabi, S.Z.; Kiyani, H.; Samimi, H.A. Efficient Synthesis of 4-Arylmethylene-3-methylisoxazol-5(4H)-one Derivatives Catalyzed by Malic Acid. Lett. Org. Chem. 2023, 20, 167-174. doi:10.2174/1570178619666220903155012.
- Daroughehzadeh, Z.; Kiyani, H. Arylideneisoxazole-5(4H)-one Synthesis by Organocatalytic Three-Component Hetero-Cyclization. Polycycl. Aromat. Comp. 2024, 44, 3200-3221. doi:10.1080/10406638.2023.2231602.
- Kiyani, H.; Jabbari, M.; Mosallanezhad, A. Efficient Three Component Synthesis of 3,4-Disubstituted isoxazol-5(4H)-ones in Green Media. Jordan J. Chem. 2014, 9, 279-288. doi:10.12816/0025980P.
- Kour, P.; Ahuja, M.; Sharma, P.; Kumar, A.; Kumar, A. An Improved Protocol for the Synthesis of 3,4-Disubstituted Isoxazol-5(4H)-ones through L-Valine-Mediated Domino Three-Component Strategy. J. Chem. Sci. 2020, 132, 108. doi:10.1007/s12039-020-01801-5.
- Kiyani, H.; Ghorbani, F. Boric Acid-Catalyzed Multi-Component Reaction for Efficient Synthesis of 4H-Isoxazol-5-ones in Aqueous Medium. Res. Chem. Intermed. 2015, 41, 2653-2664. doi:10.1007/s11164-013-1411-x.
- Kiyani, H.; Samimi, H.A. Nickel-Catalyzed One-Pot, Three-Component Synthesis of 3,4-Disubstituted Isoxazole-5(4H)-ones in Aqueous Medium. Chiang Mai J. Sci. 2017, 44, 1011-1121. http://cmuir.cmu.ac.th/jspui/handle/6653943832/63929.
- Mosallanezhed, A.; Kiyani, H. KI-Mediated three-component reaction of hydroxylamine hydrochloride with aryl/heteroaryl aldehydes and two β-oxoesters. Orbit. Electron. J. Chem. 2018, 10, 133-139. https://doi.org/10.17807/orbital.v10i2.1134.
- Kiyani, H.; Darbandi, H.; Mosallanezhad, A.; Ghorbani, F. 2-Hydroxy-5-sulfobenzoic acid: an efficient organocatalyst for the three-component synthesis of 1-amidoalkyl-2-naphthols and 3,4-disubstituted isoxazol-5(4H)-ones. Res. Chem. Intermed. 2015, 41, 7561-7579. doi:10.1007/s11164-014-1844-x.
- Ghogare, R.S.; Patankar-Jain, K.; Momin, S.A.H. A Simple and Efficient Protocol for the Synthesis of 3,4-Disubstituted Isoxazol-5(4H)-ones Catalyzed by Succinic Acid Using Water as Green Reaction Medium. Lett. Org. Chem. 2021, 18, 83-87. doi:10.2174/1570178617999200721011300.
- Kundu, T.; Mitra, B.; Ghosh, P. Eucalyptol: An Efficient, Unexplored, Green Media for Transition Metal Free Synthesis of 2,3-Dihydroquinazolin-4(1H)-one Derivatives and Isoxazolone Derivatives. Synth. Commun. 2023, 53, 779-794. doi:10.1080/00397911.2023.2197118.
- Haydari, F.; Kiyani, H. Urea-Catalyzed Multicomponent Synthesis of 4-Arylideneisoxazol-5(4H)-one Derivatives under Green Conditions. Res. Chem. Intermed. 2023, 49, 837-858. doi:10.1007/s11164-022-04907-2.
- Zhang, D.; Liu, C.; Ren, L.; Li, W.; Luan, B.; Zhang, Y. Vitamin B1-Catalyzed Multicomponent Reaction for Efficient Synthesis of an Isoxazolone Compound by Using Ultrasound in a Water and Its Selective Identification of Metal Ions. ChemistrySelect 2023, 8, e202204658. doi:10.1002/slct.202204658.
- Ghorbani, F.; Kiyani, H.; Pourmousavi, S.A. Facile and Expedient Synthesis of α,β-Unsaturated Isoxazol-5(4H)-ones under Mild Conditions. Res. Chem. Intermed. 2020, 46, 943–959. doi:10.1007/s11164-019-03999-7.
- (55) Damghani, F.K.; Kiyani, H.; Pourmousavi, S.A. Green Three-Component Synthesis of Merocyanin Dyes Based on 4-Arylideneisoxazol-5(4H)-ones. Curr. Green Chem. 2020, 7, 217-225. doi:10.2174/2213346107666200122093906; (b) Khoshdel, M.A.; Mazloumi, M.; Zabihzadeh, M.; Shirini Sustainable, F. Scalable, and One-Pot Synthesis of Isoxazol-5(4H)-one and 1,2,4-Triazoloquinazolinone Derivatives Using a Natural Deep Eutectic Solvent. Polycycl. Aromat. Comp. (2023): doi:10.1080/10406638.2023.2254894; (c) Atharifar, H.; Keivanloo, A.; Maleki, B. Greener Synthesis of 3,4-Disubstituted Isoxazole-5(4H)-ones in a Deep Eutectic Solvent. Org. Prep. Proced. Int. 2020, 52, 517-523. doi:10.1080/00304948.2020.1799672.
- Aslanpour, S.; Kiyani, H. Rapid Synthesis of Fully Substituted Arylideneisoxazol-5(4H)-one Using Zinc Oxide Nanoparticles. Res. Chem. Intermed. 2023, 49, 4603-4619. doi:10.1007/s11164-023-05059-7.
- Kiyani, H.; Ghorbani, F. Expeditious Green Synthesis of 3,4-Disubstituted Isoxazole-5(4H)-Ones Catalyzed by Nano-MgO. Res. Chem. Intermed. 2016, 42, 6831-6844. doi:10.1007/s11164-016-2498-7.
- Mosallanezhad, A.; Kiyani, h. Green Synthesis of Arylideneisoxazol-5-Ones Catalyzed by Silicon Dioxide Nanoparticles. Polycycl. Aromat. Comp. (2023): 1-16. doi:10.1080/10406638.2023.2259564.
- Konkala, V.S.; Dubey, P.K. One-pot Synthesis of 3-phenyl-4-pyrazolylmethylene-isoxazol-(5H)-ones Catalyzed by Sodium Benzoate in Aqueous Media under the Influence of Ultrasound Waves: A Green Chemistry Approach. J. Heterocycl. Chem. 2017, 54, 2483-2492. https://doi.org/10.1002/jhet.2848.
- Kulkarni, P. An efficient solvent-free synthesis of 3,4-disubstituted isoxazole-5(4H)-ones using microwave irradiation. J. Indian Chem. Soc. 2021, 98, 100013. doi:10.1016/j.jics.2021.100013.
- Shitre, G.V.; Patel, A.R.; Ghogare, R.S. Green synthesis of 3,4-disubstituted isoxazol-5(4H)-one using Gluconic acid aqueous solution as an efficient recyclable medium. Org. Commun. 2023, 16, 87-97. doi:10.25135/acg.oc.151.2304.2762.
- Mirani-Nezhad, S.; Pourmousavi, S.A.; Zare, E.N.; Heidari, G.; Hosseini, S.; Peyvandtalab, M. Magnetic poly(1,8-diaminonaphthalene)-nickel nanocatalyst for the synthesis of antioxidant and antibacterial isoxazole-5(4H)-ones derivatives. Heliyon 2023, 9, e15886. doi:10.1016/j.heliyon.2023.e15886.
- Liu, Q.; Hou, X. One-Pot Three-Component Synthesis of 3-Methyl-4-Arylmethylene- Isoxazol-5(4H)-Ones Catalyzed by Sodium Sulfide. Phosphor. Sulfur, Silicon Relat. Element. 2012, 187, 448-453. doi:10.1080/10426507.2011.621003.
- Laroum, R.; Debache, A. New eco-friendly procedure for the synthesis of 4-arylmethylene-isoxazol-5(4H)-ones catalyzed by pyridinium p-toluenesulfonate (PPTS) in aqueous medium. Synth. Commun. 2018, 48, 1876-1882, doi:10.1080/00397911.2018.1473440.
- (a) Patil, B.M.; Shinde, S.K.; Jagdale, A.A.; Jadhav, S.D.; Patil, S.S. Fruit Extract of Averrhoa Bilimbi: A Green Neoteric Micellar Medium for Isoxazole and Biginelli-like Synthesis. Res. Chem. Intermed. 2021, 47, 4369-4398. doi:10.1007/s11164-021-04539-y; (b) Popatkar, B.B.; Mane, A.A.; Meshram, G.A. Tomato Fruit Extract: An Environmentally Benign Catalytic Medium for the Synthesis of Isoxazoles Derivatives. Indian J. Chem. 2021, 60B, 1362-1367. doi:123456789/58323B.
- Haidary, F.; Kiyani, H. Green and clean synthesis of 4-arylideneisoxazol-5-ones using NaCl aqueous solution. Sustain. Chem. Environ. 2024, 5, 100066. doi:10.1016/j.scenv.2024.100066.
- Li, X.; Wang, Q.; Zheng, Q.; Kurpiewska, K.; Kalinowska-Tluscik, J.; Dömling, A. Access to Isoquinolin-2(1H)-yl-acetamides and Isoindolin-2-ylacetamides from a Common MCR Precursor. J. Org. Chem. 2022, 87, 14463-14475. doi:10.1021/acs.joc.2c01905.
- Xu, R.; Wang, Z.; Zheng, Q.; Patil, P.; Dömling, A. A Bifurcated Multicomponent Synthesis Approach to Polycyclic Quinazolinones. J. Org. Chem. 2022, 87, 13023-13033. https://doi.org/10.1021/acs.joc.2c01561.
- (a) Kamalifar, S.; Kiyani, H. Facile and Efficient Synthesis of 9-Aryl-1,8-Dioxo-Octahydroxanthenes Catalyzed by Sulfacetamide. Polycycl. Aromat. Comp. 2022, 42, 3675-3693. doi:10.1080/10406638.2021.1872656; (b) Kiyani, H.; Samimi, H.; Ghorbani, F.; Esmaieli, S. One-pot, four-component synthesis of pyrano[2,3-c]pyrazoles catalyzed by sodium benzoate in aqueous medium. Curr. Chem. Lett. 2013, 2, 197-206. doi:10.5267/j.ccl.2013.07.002.
- (a) Pena-Pereira, F.; Lavilla, I.; de la Calle, I.; Romero, V.; Bendicho, C. Detection of gases and organic vapours by cellulose-based sensors. Anal. Bioanal. Chem. 2023, 415, 4039-4060. doi:10.1007/s00216-023-04649-z; (b) Abdelhamid, H.N.; Mathew, A.P. Cellulose-Based Nanomaterials Advance Biomedicine: A Review. Int. J. Mol. Sci. 2022, 23, 5405. doi:10.3390/ijms23105405.
- (a) Karhale, S.; Bhenki, C.; Rashinkar, G.; Helavi, V. Covalently anchored sulfamic acid on cellulose as heterogeneous solid acid catalyst for the synthesis of structurally symmetrical and unsymmetrical 1,4-dihydropyridine derivatives. New J. Chem. 2017, 41, 5133-5141. doi: 10.1039/c7nj00685c; (b) Karhale, S. Grafting of sulphamic acid on functionalized sawdust: A novel solid acid catalyst for the synthesis of 1,8-dioxo-octahydroxanthenes. Res. Chem. Intermed. 2020, 46, 3085-3096. doi:10.1007/s11164-020-04136-5.
- (a) Pharande, P.S.; Rashinkar, G.S.; Pore, D.M. Cellulose Schiff base-supported Pd(II): An efficient heterogeneous catalyst for Suzuki Miyaura cross-coupling. Res. Chem. Intermed. 2021, 47, 4457-4476. doi:10.1007/s11164-021-04528-1; (b) Bezerra, R.D.S.; Leal, R.C.; da Silva, S.M.; Morais, I.S.A.; Marques, H.C.T.; Osajima, A.J.; Meneguin, B.A.; Barud, H.D.S.; da Silva Filho, C.E. Direct Modification of Microcrystalline Cellulose with Ethylenediamine for Use as Adsorbent for Removal Amitriptyline Drug from Environment. Molecules 2017, 22, 2039. doi:10.3390/molecules22112039.
- Qiao, Y.; Teng, J.; Wang, S.; Ma, H. Amine-Functionalized Sugarcane Bagasse: A Renewable Catalyst for Efficient Continuous Flow Knoevenagel Condensation Reaction at Room Temperature. Molecules 2018, 23, 43. doi:10.3390/molecules23010043.
- Abdi, M.M.; Tahir, P.M.; Liyana, R.; Javahershenas. R. A Surfactant Directed Microcrystalline Cellulose/Polyaniline Composite with Enhanced Electrochemical Properties. Molecules 2018, 23, 2470. doi:10.3390/molecules23102470.
- Naz, S.; Ahmad, N.; Akhtar, J.; Ahmad, N.M.; Ali, A.; Zia, M. Management of citrus waste by switching in the production of nanocellulose. IET Nanobiotechnol. 2016, 10, 395-399. doi:10.1049/iet-nbt.2015.0116.
- (a) Safari, J.; Ahmadzadeh, M.; Zarnegar, Z. Sonochemical synthesis of 3-methyl-4-arylmethylene isoxazole-5(4H)-ones by amine-modified montmorillonite nanoclay. Catal. Commun. 2016, 86, 91-95. doi:10.1016/j.catcom.2016.08.018; (b) Mirzazadeh, M.; Mahdavinia, G.H. Fast and Efficient Synthesis of 4-Arylidene-3-phenylisoxazol-5-ones. E-J. Chem. 2012, 9, 425-429. doi:10.1155/2012/562138; (c) Safari, J.; Ahmadzadeh, M.; Zarnegar, Z. Ultrasound-assisted Method for the Synthesis of 3-Methyl-4-arylmethylene Isoxazole-5(4H)-ones Catalyzed by Imidazole in Aqueous Media. Org. Chem. Res. 2016, 2, 134-139. doi:10.22036/ORG.CHEM..2016.15347.

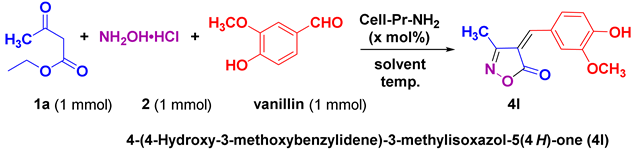 | |||||
| Entry | Solvent | Catalyst (mg) | Temp. (ºC) | Time (min) | Isolated yields (%) |
| 1 | H2O | - | RT | 75 | 40 |
| 2 | H2O | 2 | RT | 75 | 50 |
| 3 | H2O | 4 | RT | 60 | 55 |
| 4 | H2O | 6 | RT | 50 | 65 |
| 5 | H2O | 8 | RT | 45 | 70 |
| 6 | H2O | 10 | RT | 30 | 85 |
| 7 | H2O | 12 | RT | 30 | 88 |
| 81 | H2O | 14 | RT | 25 | 97 |
| 9 | EtOH | 14 | RT | 45 | 65 |
| 10 | CH3COCH3 | 14 | RT | 80 | 20 |
| 11 | CHCl3 | 14 | RT | 80 | trace |
| 12 | DMF | 14 | RT | 80 | Trace |
| 13 | n-Hexane | 14 | RT | 80 | 45 |
| 14 | H2O: EtOH (1:1) | 14 | RT | 50 | 60 |
| 15 | - | 14 | RT | 80 | 35 |
| 16 | H2O | 14 | 50 | 60 | 70 |
| 17 | H2O | 14 | Reflux | 65 | 62 |
| Entry | Compounds structure | Time (min)/isolated yields (%) | Melting points (Lit. [ref.]) |
|---|---|---|---|
| 1 |
 4a 4a
|
40/85 | 140-142 (141-143 [52]) |
| 2 |
 4b 4b
|
35/94 | 176-178 (175-177 [52]) |
| 3 |
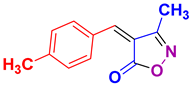 4c 4c
|
45/90 | 136-137 (134-136 [52]) |
| 4 |
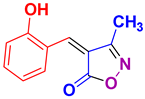 4d 4d
|
30/85 | 200-202 (198-200 [52]) |
| 5 |
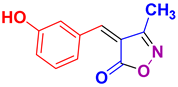 4e 4e
|
37/92 | 202-204 (201-203 [52]) |
| 6 |
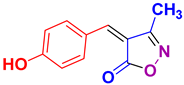 4f 4f
|
40/94 | 211-212 (211-213 [52]) |
| 7 |
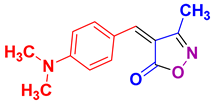 4g 4g
|
40/90 | 225-227 (226-228 [52]) |
| 8 |
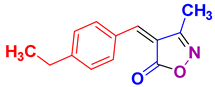 4h 4h
|
55/85 | 87-89 (88-90 [24]) |
| 9 |
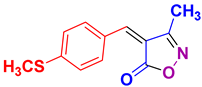 4i 4i
|
45/89 | 128-130 (128-130 [39]) |
| 10 |
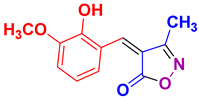 4j 4j
|
35/93 | 218-220 (217-219 [52]) |
| 11 |
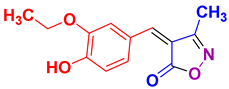 4k 4k
|
27/94 | 135-137 (135-138 [39]) |
| 12 |
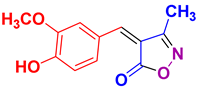 4l 4l
|
25/97 | 213-214 (212-214 [52]) |
| 13 |
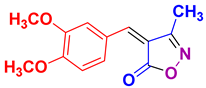 4m 4m
|
30/91 | 127-128 (126-128 [52]) |
| 14 |
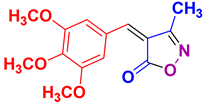 4n 4n
|
40/92 | 170-172 (171-173 [52]) |
| 15 |
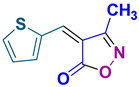 4o 4o
|
45/90 | 146-148 (145-147 [52]) |
| 16 |
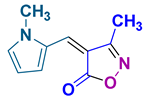 4p 4p
|
40/96 | 212-214 (213-215 [28]) |
| 17 |
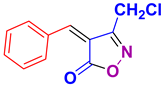 4q 4q
|
45/80 | 182-184 (181-183 [52]) |
| 18 |
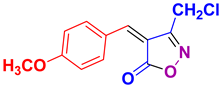 4r 4r
|
38/92 | 176-178 (175-177 [52]) |
| 19 |
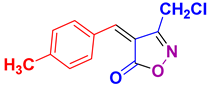 4s 4s
|
45/90 | 178-179 (177-178 [52]) |
| 20 |
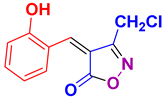 4t 4t
|
30/85 | 198-200 (200-201 [52]) |
| 21 |
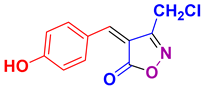 4u 4u
|
40/94 | 184-185 (184-186 [52]) |
| 22 |
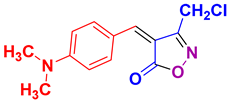 4v 4v
|
40/91 | 179-181 (178-181 [52]) |
| 23 |
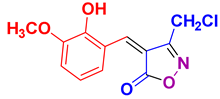 4w 4w
|
45/87 | 167-169 (166-168 [52]) |
| 24 |
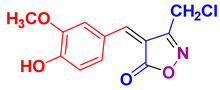 4x 4x
|
25/96 | 144-146 (143-144 [52]) |
| 25 |
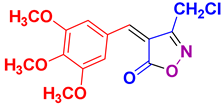 4y 4y
|
45/93 | 128-130 (128-130 [52]) |
| 26 |
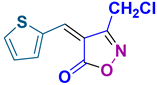 4z 4z
|
45/88 | 137-138 (137-139 [52]) |
| 27 |
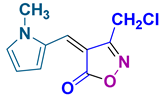 4aa 4aa
|
50/92 | 82-84 (82-83 [28]) |
| 28 |
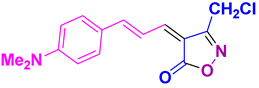 4ab 4ab
|
35/92 | 212-215 (New) |
| 29 |
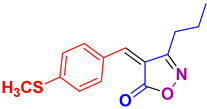 4ac 4ac
|
35/92 | 146-148 (145-148 [39]) |
| 30 |
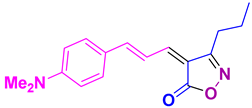 4ad 4ad
|
50/93 | 158-160 (New) |
| 31 |
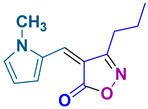 4ae 4ae
|
50/89 | 145-148 (New) |
| Entry | Catalyst (amount)/conditions | Time (min) | Yield (%) | Refs. |
|---|---|---|---|---|
| 1 | Silica-TLC grade (1 g)/H2O, RT | 1440 | 91 | [11a] |
| 2 | Lipase (30 mg)/H2O, RT | 60-120 | 82 | [25] |
| 3 | Guanidine hydrochloride (15 mol%)/H2O, RT | 70 | 88 | [29] |
| 4 | 2,2′-bpy (10 mg)/H2O, reflux | 70 | 80 | [33] |
| 5 | [H2-BiPyr][ClO4]2/H2O, reflux | 30 | 96 | [33] |
| 6 | KHP (10 mol%)/H2O, RT | 45 | 95 | [34] |
| 7 | Salicylic acid (15 mol%)/H2O, RT | 90 | 90 | [37] |
| 81 | Sulfanilic acid (20 mol%)/H2O, RT | 65 | 91 | [38] |
| 9 | Succinic acid (10)/H2O, RT | 90 | 88 | [50] |
| 10 | Eucalyptol (1 mL)/RT | 180 | 84 | [51] |
| 11 | 50 wt % aq. GAAS (5 mL)/70 °C | 45 | 92 | [61] |
| 12 | Na2S·9H2O (5 mol%), EtOH, RT | 90 | 88 | [63] |
| 13 | PPTS (5 mol%)/H2O, reflux | 60 | 65 | [64] |
| 14 | cell-Pr-NH2 (14 mg)/H2O, RT | 35 | 94 | [This work] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





