Submitted:
15 August 2024
Posted:
21 August 2024
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Data and Methods
2.1. Heart Failure Dataset and Study Design
2.2. Study Variables and Definitions
2.3. Survival Data Analysis
2.4. Shared Frailty AFT Models
2.5. Bayesian AFT Shared Gamma Frailty Models
2.6. Integrated Nested Laplace Approximation Method
2.7. Data Analysis Procedures
3. Results and Discussions
3.1. Results
3.1.1. Descriptive Results
3.1.2. Kaplan-Meier Estimate for Selective Covariates
3.1.3. Bayesian AFT Shared Gamma Frailty Models
3.1.4. Bayesian Model Diagnostics
3.2. Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| HF | Heart Failure |
| AFT | Accelerated Failure Time |
| Cox PH | Cox Proportional Hazard |
| MCMC | Markov Chain Monte Carlo |
| INLA | Integrated Nested Laplace Approximation |
| JUMC | Jimma University Medical Center |
| DIC | Deviance Information Criteria |
| WAIC | Watanabe Akaike Information Criterion |
| KLD | Kullback-Leibler Divergence |
| OPD | Out-Patient Department. |
References
- Ambrosy, A.P., Fonarow, G.C., Butler, J., Chioncel, O., Greene, S.J., Vaduganathan, M., Nodari, S., Lam, C.S., Sato, N., Shah, A.N., et al.: The global health and economic burden of hospitalizations for heart failure: lessons learned from hospitalized heart failure registries.Journal of the American College of Cardiology.2014,63 (12),1123–1133.
- Vos, T., Lim, S.S., Abbafati, C., Abbas, K.M., Abbasi, M., Abbasifard, M., Abbasi-Kangevari, M., Abbastabar, H., Abd-Allah, F., Abdelalim, A., et al.: Global burden of 369 diseases and injuries in 204 countries and territories, 1990–2019: a systematic analysis for the global burden of disease study 2019.Biomedical Signal Processing and Control. 2020, 70, 102–985.
- Yan, T., Zhu, S., Yin, X., Xie, C., Xue, J., Zhu, M., Weng, F., Zhu, S., Xiang, B., Zhou, X., et al.: Burden, trends, and inequalities of heart failure globally, 1990 to 2019: a secondary analysis based on the global burden of disease 2019 study. Journal of the American Heart Association,2023, 12(6), 027852.
- Martin, S.S., Aday, A.W., Almarzooq, Z.I., Anderson, C.A., Arora, P., Avery, C.L., Baker-Smith, C.M., Barone Gibbs, B., Beaton, A.Z., Boehme, A.K., et al.: 2024 heart disease and stroke statistics: a report of us and global data from the american heart association.Circulation, 2024, 149(8), 347–913.
- Hessel, F.P.: Overview of the socio-economic consequences of heart failure. Cardiovascular Diagnosis and Therapy,2021, 11(1), 254.
- Pratley, R., Guan, X., Moro, R.J., Lago, R.: The burden of heart failure.The American Journal of Medicine, 2024, 137(2), 3–8.
- Heidenreich, P., Sandhu, A.: Advances in management of heart failure.bmj,2024, 385.
- Dokainish, H., Teo, K., Zhu, J., Roy, A., AlHabib, K.F., ElSayed, A., Palileo-Villaneuva, L., Lopez-Jaramillo, P., Karaye, K., Yusoff, K., et al.: Global mortality variations in patients with heart failure: results from the international congestive heart failure (inter-chf) prospective cohort study.The Lancet Global Health, 2017, 5(7), 665–672.
- Gtif, I., Bouzid, F., Charfeddine, S., Abid, L., Kharrat, N.: Heart failure disease: an african perspective. Archives of Cardiovascular Diseases,2021, 114(10), 680–690.
- Keates, A.K., Mocumbi, A.O., Ntsekhe, M., Sliwa, K., Stewart, S.: Cardiovascular disease in africa: epidemiological profile and challenges.Nature Reviews Cardiology, 2017, 14(5), 273–293.
- Glezeva, N., Gallagher, J., Ledwidge, M., O’Donoghue, J., McDonald, K., Chipolombwe, J., Watson, C.: Heart failure in sub-saharan africa: review of the aetiology of heart failure and the role of point-of-care biomarker diagnostics.Tropical Medicine and International Health,2015, 20(5), 581–588.
- Oleribe, O.O., Momoh, J., Uzochukwu, B.S., Mbofana, F., Adebiyi, A., Barbera, T., Williams, R., Taylor-Robinson, S.D.: Identifying key challenges facing healthcare systems in africa and potential solutions.International journal of general medicine, 2019, 395–403.
- Bloomfield, G.S., Barasa, F.A., Doll, J.A., Velazquez, E.J.: Heart failure in subsaharan africa. Current cardiology reviews,2013, 9(2), 157–173.
- Damasceno, A., Mayosi, B.M., Sani, M., Ogah, O.S., Mondo, C., Ojji, D., Dzudie, A., Kouam, C.K., Suliman, A., Schrueder, N., et al.: The causes, treatment, and outcome of acute heart failure in 1006 africans from 9 countries: results of the sub-saharan africa survey of heart failure.Archives of internal medicine, 2012, 172(18), 1386–1394.
- Sliwa, K., Mayosi, B.M.: Recent advances in the epidemiology, pathogenesis and prognosis of acute heart failure and cardiomyopathy in africa. Heart,2013, 99(18), 1317–1322.
- Asfaw, E.: Five years clinical characteristics and in hospital outcome of acute heart failure at tertiary care hospital in ethiopia.Ethiopian Medical Journal, 2020, 58(01).
- Tirfe, M., Nedi, T., Mekonnen, D., Berha, A.B.: Treatment outcome and its predictors among patients of acute heart failure at a tertiary care hospital in ethiopia: a prospective observational study. BMC cardiovascular disorders,2020, 20, 1–10.
- Amare, H., Hamza, L., Asefa, H.: Malnutrition and associated factors among heart failure patients on follow up at jimma university specialized hospital, ethiopia.BMC cardiovascular disorders, 2015, 15, 1–6.
- Zeru, M.A.: Assessment of major causes of heart failure and its pharmacologic management among patients at felege hiwot referral hospital in bahir dar, ethiopia. Journal of public health and epidemiology,2018, 10(9), 326–331.
- Moyehodie, Y.A., Muluneh, M.W., Belay, A.T., Fenta, S.M.: Time to death and its determinant factors among patients with chronic heart failure in northwest ethiopia: A retrospective study at selected referral hospitals.Frontiers in Cardiovascular Medicine, 2022, 9, 817074.
- Hailay, A., Kebede, E., Mohammed, K.: Survival during treatment period of patients with severe heart failure admitted to intensive care unit (icu) at gondar university hospital (guh), gondar, ethiopia. American Journal of Health Research,2015, 3(5), 257–269.
- Ahmad, T., Munir, A., Bhatti, S.H., Aftab, M., Raza, M.A.: Survival analysis of heart failure patients: A case study.PloS one, 2017, 12(7), 0181001.
- Ashine, T., Muleta, G., Tadesse, K.: Assessing survival time of heart failure patients: using bayesian approach. Journal of Big Data,2021, 8(1), 156.
- Kalbfleisch, J.D., Prentice, R.L.: The Statistical Analysis of Failure Time Data.John Wiley and Sons, 2011 Jan 25.
- Khanal, S.P., Sreenivas, V., Acharya, S.K.: Accelerated failure time models: an application in the survival of acute liver failure patients in india. Int J Sci Res,2014, 3(6), 161–6.
- Collett, D.: Modelling survival data in medical research,2023 .
- Qi, J.: Comparison of proportional hazards and accelerated failure time models.PhD thesis, University of Saskatchewan, 2009.
- Lee, K.H., Rondeau, V., Haneuse, S.: Accelerated failure time models for semicompeting risks data in the presence of complex censoring. Biometrics,2017, 73(4), 1401–1412.
- Hanagal, D.D.: Modeling Survival Data Using Frailty Models. SpringerInt, 2011 Jan 26.
- Gutierrez, R.G.: Parametric frailty and shared frailty survival models. The Stata Journal,2002, 2(1), 22–44.
- Hougaard, P., Hougaard, P.: Analysis of multivariate survival data 564.2000 .
- Rue, H., Martino, S., Chopin, N.: Approximate bayesian inference for latent gaussian models by using integrated nested laplace approximations.Journal of the Royal Statistical Society Series B: Statistical Methodology, 2009, 71(2), 319–392.
- Berger, J.O.: Statistical Decision Theory and Bayesian Analysis. Springer. 2013.
- Brooks, S.P., Gelman, A.: General methods for monitoring convergence of iterative simulations. Journal of computational and graphical statistics,1998, 7(4), 434–45.
- Ibrahim, J.G., Chen, M.-H., Sinha, D., Ibrahim, J., Chen, M.: Bayesian survival analysis.2001 , 2.
- Ibrahim, J.G., Zhu, H., Tang, N.: Bayesian local influence for survival models.Lifetime Data Analysis, 2011, 17, 43–70.
- Gelman, A., Hwang, J., Vehtari, A.: Understanding predictive information criteria for bayesian models. Statistics and computing,2014, 24, 997–1016.
- Aslanidou, H., Dey, D.K., Sinha, D.: Bayesian analysis of multivariate survival data using monte carlo methods.Canadian Journal of Statistics, 1998, 26(1), 33–48.
- Congdon, P.D.: Applied bayesian hierarchical methods, 2010.
- Kaplan, E.L., Meier, P.: Nonparametric estimation from incomplete observations. Journal of the American statistical association,1958, 53(282), 457–481.
- Aalen, O., Borgan, O., Gjessing, H.: Survival and event history analysis: a process point of view,2008 .
- Cox, D.R.: Regression models and life-tables.Journal of the Royal Statistical Society: Series B (Methodological), 1972, 34(2), 187–202.
- Wei, L.J.: The accelerated failure time model: a useful alternative to the cox regression model in survival analysis. Statistics in medicine,1992, 11(14-15), 1871–1879.
- Bender, R., Augustin, T., Blettner, M.: Generating survival times to simulate cox proportional hazards models.Statistics in medicine, 2005, 24(11), 1713–1723.
- Cox, D.R.: Some remarks on the analysis of survival data. In: Proceedings of the First Seattle Symposium in Biostatistics: Survival Analysis. Springer,1997,pp. 1–9.
- Pan, W.: Using frailties in the accelerated failure time model.Lifetime Data Analysis, 1997, 7, 55–64.
- Kleinbaum, D.G., Klein, M.: Survival Analysis a Self-learning Text. Springer,1996.
- Vaupel, J.W., Manton, K.G., Stallard, E.: The impact of heterogeneity in individual frailty on the dynamics of mortality.Demography, 1979,16(3), 439–454.
- Clayton, D.G.: A model for association in bivariate life tables and its application in epidemiological studies of familial tendency in chronic disease incidence. Biometrika,1978,65(1), 141–151.
- Hougaard, P.: Modelling heterogeneity in survival data.Journal of Applied Probability, 1997, 28(3), 695–701.
- Hougaard, P.: Frailty models for survival data. Lifetime data analysis,1995,1, 255–273.
- Wienke, A.: Frailty Models in Survival Analysis.Chapman and Hall/CRC, 2010.
- Duchateau, L., Janssen, P.: The frailty model. 2008.
- Zhang, J., Peng, Y.: An alternative estimation method for the accelerated failure time frailty model. Computational statistics and data analysis,2007, 51(9), 4413–4423.
- Lambert, P., Collett, D., Kimber, A., Johnson, R.: Parametric accelerated failure time models with random effects and an application to kidney transplant survival.Statistics in medicine, 2004, 23(20), 3177–3192.
- Raman, T., Venkatesan, P.: Accelerated failure time frailty model in survival analysis. International Journal of Science and Technology,2012,2(2), 65–9.
- Bhattacharjee, A.: Application of bayesian approach in cancer clinical trial.World journal of oncology, 2014,5(3), 109.
- Depaoli, S.: The impact of inaccurate “informative” priors for growth parameters in bayesian growth mixture modeling. Structural Equation Modeling: A Multidisciplinary Journal,2014,21(2), 239–252.
- Ganjali, M., Baghfalaki, T.: Bayesian analysis of unemployment duration data in the presence of right and interval censoring.Journal of Reliability and Statistical Studies, 2012, 17–32.
- Hanagal, D.D., Dabade, A.D.: Bayesian estimation of parameters and comparison of shared gamma frailty models. Communications in Statistics-Simulation and Computation,2013,42(4), 910–931.
- Christensen, R., Johnson, W., Branscum, A., Hanson, T.E.: Bayesian ideas and data analysis: an introduction for scientists and statisticians.2010 .
- Santos, C., Achcar, J.: A bayesian analysis for multivariate survival data in the presence of covariates.Journal of Statistical Theory and Applications, 2010,9, 233–253.
- Yin, G., Ibrahim, J.G.: A class of bayesian shared gamma frailty models with multivariate failure time data. Biometrics,2005,61(1), 208–216.
- Yu, B.: Estimation of shared gamma frailty models by a modified em algorithm.Computational statistics and data analysis, 2006,50(2), 463–474.
- Hougaard, P.: Life table methods for heterogeneous populations: distributions describing the heterogeneity. Biometrika,1984,71(1), 75–83.
- Akerkar, R., Martino, S., Rue, H.: Implementing approximate bayesian inference for survival analysis using integrated nested laplace approximations.Prepr Stat Nor Univ Sci Technol, 2010,1, 1–38.
- Chen, D.G and Lio, Y.: Comparative studies on frailties in survival analysis. Communications in Statistics—Simulation and Computation,2008,37(8), 1631–1646.
- Spiegelhalter, D.J., Abrams, K.R., Myles, J.P.: Bayesian approaches to clinical trials and health-care evaluation 13 .2004 .
- Watanabe, S., Opper, M.: Asymptotic equivalence of bayes cross validation and widely applicable information criterion in singular learning theory.Journal of machine learning research, 2010,11(12).
- Chaloner, K.: Bayesian residual analysis in the presence of censoring. Biometrika,1991,78(3), 637–644.
- Hariharaputhiran, S., Peng, Y., Ngo, L., Ali, A., Hossain, S., Visvanathan, R., Adams, R., Chan, W., Ranasinghe, I.: Long-term survival and life expectancy following an acute heart failure hospitalization in australia and new zealand.European Journal of Heart Failure, 2022, 24(9), 1519–1528.
- Abebe, T.B., Gebreyohannes, E.A., Tefera, Y.G., Abegaz, T.M.: Patients with hfpef and hfref have different clinical characteristics but similar prognosis: a retrospective cohort study. BMC cardiovascular disorders,2016, 16, 1–8.
- L´opez-Vilella, R., Guerrero Cervera, B., Donoso Trenado, V., Mart´ınez Dolz, L., Almenar Bonet, L.: Clinical profiling of patients admitted with acute heart failure: a comprehensive survival analysis.Frontiers in Cardiovascular Medicine, 2024,11, 1381514.
- Beghini, A., Sammartino, A.M., Papp, Z., Haehling, S., Biegus, J., Ponikowski, P., Adamo, M., Falco, L., Lombardi, C.M., Pagnesi, M., et al.: 2024 update in heart failure. ESC Heart Failure,2024.
- Martino, S., Akerkar, R., Rue, H.: Approximate bayesian inference for survival models.Scandinavian Journal of Statistics, 2011,38(3), 514–528.
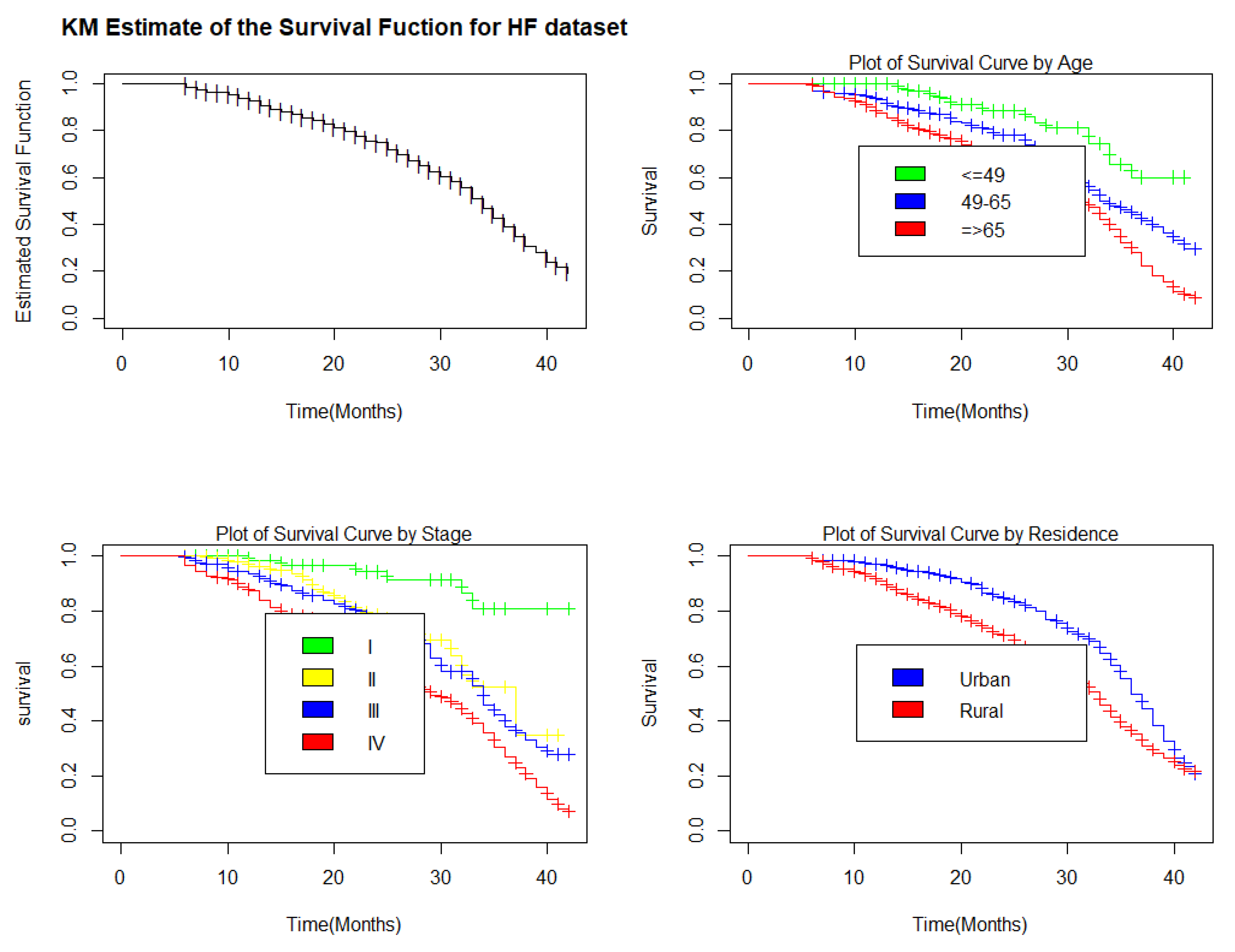
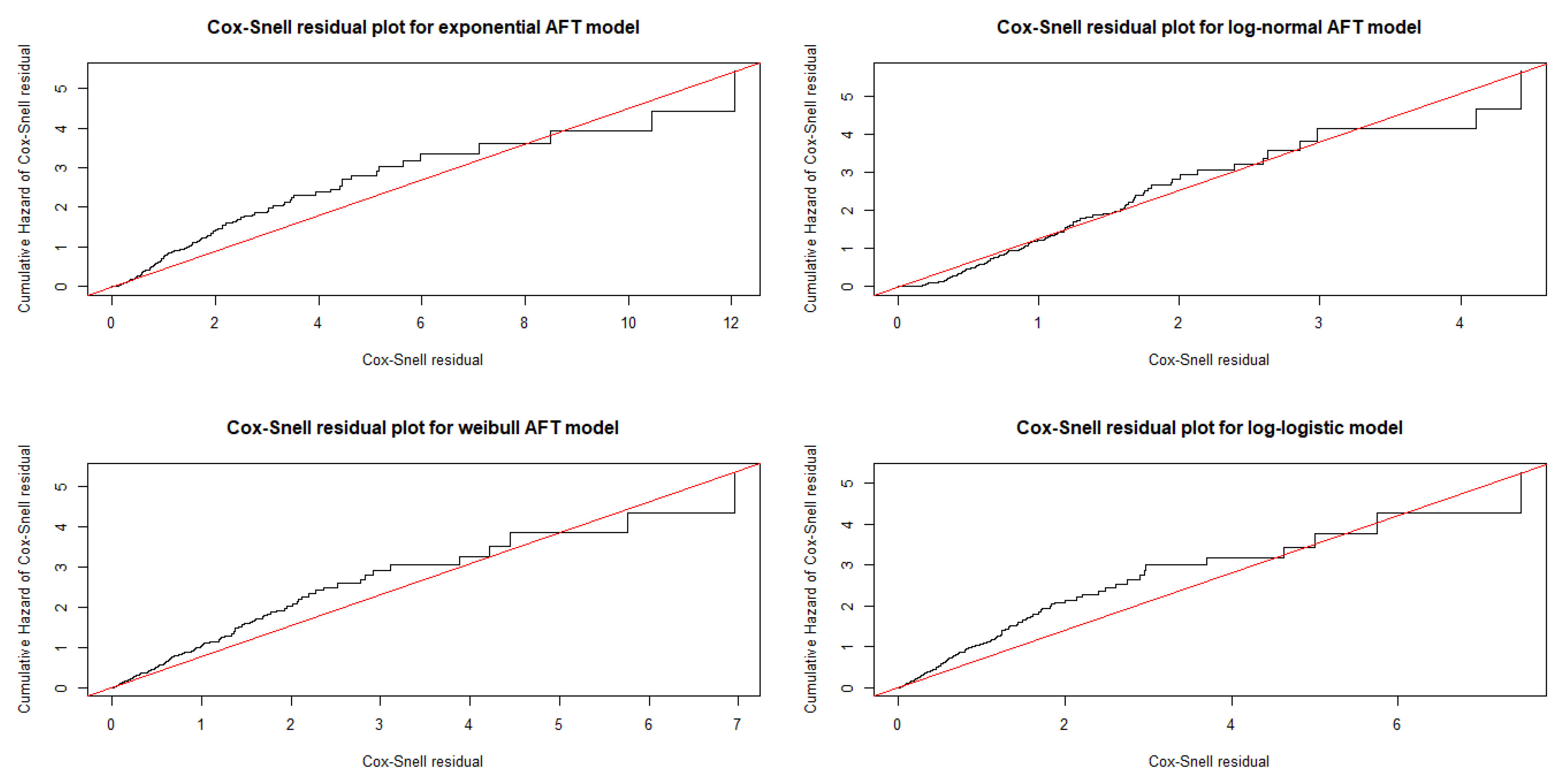
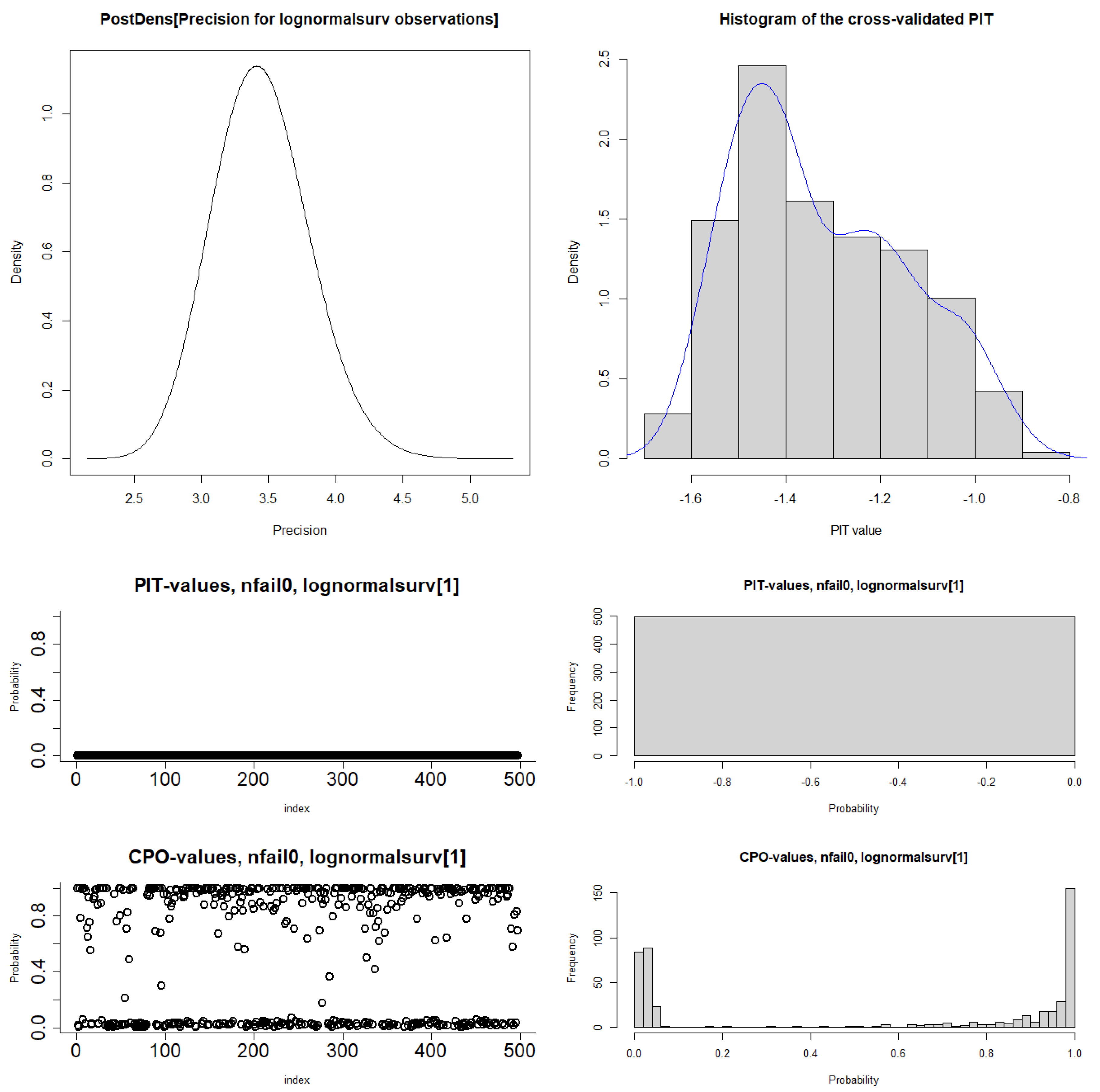
| Covariates | Categories | No. of Censored (%) | No. of Death (%) | Total |
|---|---|---|---|---|
| Sex | Female | 180 (59.80) | 88 (44.90) | 268 (53.92) |
| Male | 121 (40.20) | 108 (55.10) | 229 (46.08) | |
| Age | 119 (39.54) | 18 (9.19) | 137 (27.56) | |
| 49-65 | 101 (33.55) | 61 (31.12) | 162 (32.60) | |
| 81 (26.91) | 117 (59.69) | 198 (39.84) | ||
| Alcohol | No | 202 (67.11) | 113 (57.65) | 315 (63.38) |
| Yes | 99 (32.89) | 83 (42.35) | 182 (36.62) | |
| Residence | Urban | 94 (31.23) | 45 (22.96) | 139 (27.97) |
| Rural | 207 (68.77) | 151 (77.04) | 358 (72.03) | |
| History of HF | New | 128 (42.53) | 64 (32.65) | 192 (38.63) |
| HF patient before | 82 (27.24) | 61 (31.12) | 143 (28.77) | |
| Medical OPD | 91 (30.23) | 71 (36.23) | 162 (32.60) | |
| Chronic kidney disease | No | 270 (89.70) | 72 (36.73) | 342 (68.81) |
| Yes | 31 (10.30) | 124 (63.27) | 155 (31.19) | |
| Hypertension | No | 248 (82.39) | 51 (26.02) | 299 (60.16) |
| Yes | 53 (17.61) | 145 (73.98) | 198 (39.84) | |
| Anemia | No | 256 (85.05) | 73 (37.24) | 329 (66.20) |
| Yes | 45 (14.95) | 123 (62.76) | 168 (33.80) | |
| Diabetes mellitus | Not | 247 (82.06) | 36 (18.37) | 283 (56.94) |
| Type I | 21 (6.98) | 54 (27.55) | 75 (15.09) | |
| Type II | 33 (10.96) | 106 (54.08) | 139 (27.97) | |
| Etiology of HF | IHD | 86 (28.57) | 23 (12.19) | 109 (21.93) |
| RVHD | 59 (19.60) | 53 (26.83) | 112 (22.54) | |
| Cardiomyopathy | 65 (21.59) | 52 (25.61) | 117 (23.54) | |
| HHD | 64 (21.26) | 56 (29.88) | 120 (24.14) | |
| Others | 27 (8.97) | 12 (5.49) | 39 (7.85) | |
| Smoking | No | 280 (93.02) | 114 (58.16) | 394 (79.28) |
| Yes | 21 (6.98) | 82 (41.84) | 103 (20.72) | |
| Treatments | Digoxin | 60 (19.94) | 38 (19.39) | 98 (19.72) |
| Spironolactone | 76 (25.25) | 39 (19.90) | 115 (23.14) | |
| Atorvastatin | 77 (25.58) | 60 (30.61) | 137 (27.56) | |
| Others | 37 (12.29) | 19 (9.69) | 56 (11.27) | |
| Combination | 51 (16.94) | 40 (20.41) | 91 (18.31) | |
| Stages of HF | I | 78 (25.91) | 6 (3.06) | 84 (16.90) |
| II | 81 (26.91) | 27 (13.78) | 108 (21.73) | |
| III | 72 (23.99) | 57 (29.08) | 129 (25.96) | |
| IV | 70 (23.26) | 106 (54.08) | 176 (35.41) |
| Distributions | Bayesian AFT models | Bayesian AFT models with frailty | ||
|---|---|---|---|---|
| DIC | WAIC | DIC | WAIC | |
| Exponential | 1808.093 | 1801.966 | 1799.099 | 1790.696 |
| Log-normal | 1616.099 | 1615.184 | 1608.812 | 1607.838 |
| Weibull | 1642.971 | 1642.486 | 1616.210 | 1617.387 |
| Log-logistic | 1654.036 | 1651.165 | 1619.731 | 1616.926 |
| Covariates | Categories | Pmean () | SD | CrI for | KLD | |
|---|---|---|---|---|---|---|
| Intercept | 5.14 | 0.456 | 0 | |||
| Age | Ref | |||||
| 49–65 | -0.307 | 0.121 | 0.7357 | [0.580, 0.931]* | 0 | |
| -0.361 | 0.116 | 0.6969 | [0.554, 0.873]* | 0 | ||
| History of HF | New | Ref | ||||
| HF patient | 0.183 | 0.103 | 1.2008 | [0.983, 1.469] | 0 | |
| Medical OPD | 0.243 | 0.101 | 1.2751 | [1.046, 1.559]* | 0 | |
| CKD | No | Ref | ||||
| Yes | -0.411 | 0.077 | 0.6630 | [0.569, 0.769]* | 0 | |
| Hypertension | No | Ref | ||||
| Yes | -0.312 | 0.079 | 0.7320 | [0.626, 0.855]* | 0 | |
| Etiology of HF | IHD | Ref | ||||
| RVHD | -0.327 | 0.121 | 0.7211 | [0.568, 0.913]* | 0 | |
| Cardiomyopathy | -0.280 | 0.116 | 0.7558 | [0.602, 0.948]* | 0 | |
| HHD | -0.299 | 0.119 | 0.7416 | [0.586, 0.936]* | 0 | |
| Others | -0.393 | 0.166 | 0.6750 | [0.487, 0.933]* | 0 | |
| Smoking | No | Ref | ||||
| Yes | -0.175 | 0.077 | 0.8395 | [0.721, 0.976]* | 0 | |
| Stages of HF | I | Ref | ||||
| II | -0.457 | 0.173 | 0.6332 | [0.449, 0.888]* | 0 | |
| III | -0.352 | 0.164 | 0.7033 | [0.509, 0.968]* | 0 | |
| IV | -0.446 | 0.160 | 0.6402 | [0.467, 0.875]* | 0 | |
| Diabetes mellitus | Not | Ref | ||||
| Type I | -0.443 | 0.117 | 0.6421 | [0.509, 0.806]* | 0 | |
| Type II | -0.551 | 0.105 | 0.5764 | [0.468, 0.706]* | 0 | |
| Anemia | No | Ref | ||||
| Yes | -0.169 | 0.073 | 0.8445 | [0.729, 0.974]* | 0 | |
| Pre lognormal surv | Shape | 3.45 | 0.364 | 31.5 | [15.958, 67.356]* | |
| Pre Res | Frailty | 0.0264 | 0.0199 | 1.0268 | [1.00598, 1.625]* |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





