Submitted:
20 August 2024
Posted:
21 August 2024
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Materials and Methods
2.1. Screening and Isolation of EPS-Producing Bacteria from Freshwater Scaled Fish Intestine
2.2. Exopolysaccharide Production, Purification and Quantification
2.3. EPS Characterization of the Purified EPS
2.3.1. Molecular Mass Determination
2.3.2. Functional Groups Determination
2.3.3. Monosaccharide and Oligosaccharide Determination
2.3.4. Scanning Electron Microscopy (SEM) Analysis
2.4. Optimization Condition of EPS Production from the Selected Strain
2.5. Bacterial Identification
2.6. In vitro Biological Activity
2.6.1. Prebiotic Properties
2.6.2. EPS Antioxidant Studies
2.6.3. Antibacterial Activity Studies
2.6.4. Cytotoxicity of EPS by Hemolytic Test
2.7. Experimental Animal
2.7.1. Acclimatization of Oreochromis niloticus Linn.
2.7.2. Experimental Fish Diet Formulation
2.7.3. Experimental Design and Sample Collection
2.8. Growth performance parameters
2.9. Measurement of Immune Response Parameters
2.9.1. Lysozyme Activity
2.9.2. Respiratory Burst Activity
2.9.3. Total Immunoglobulin
2.10. Measurement of Serum Biochemical Parameters
2.11. Challenge Test
2.12. Statistical Analysis
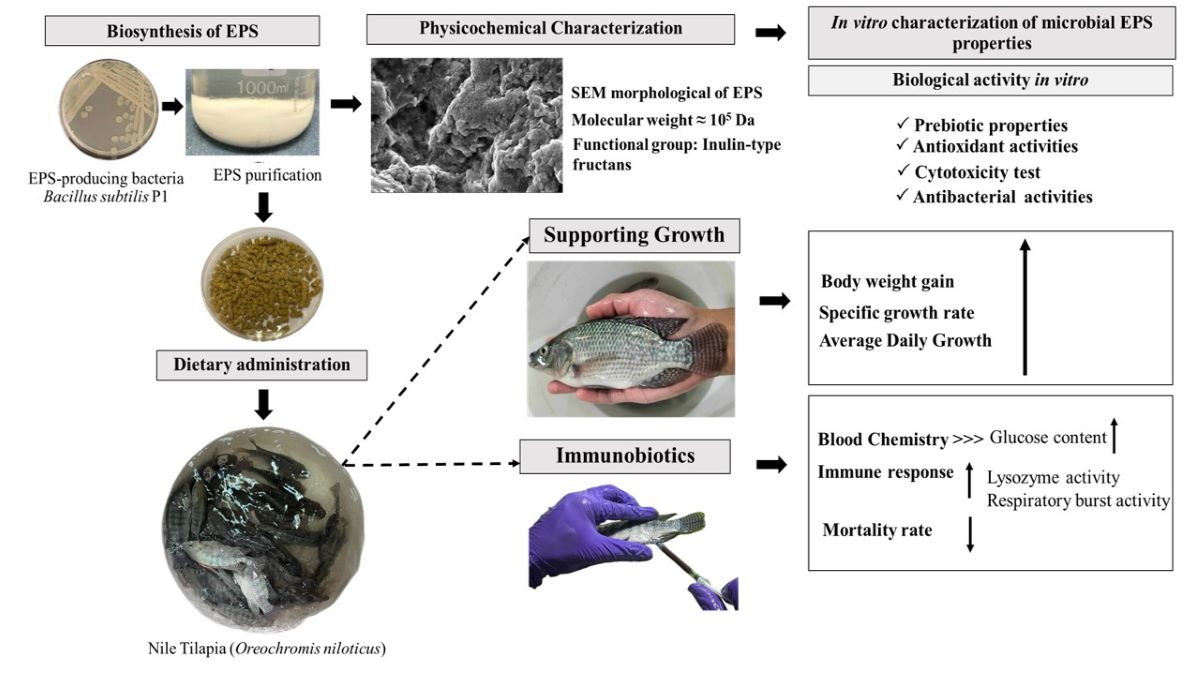
3. Results and Discussion
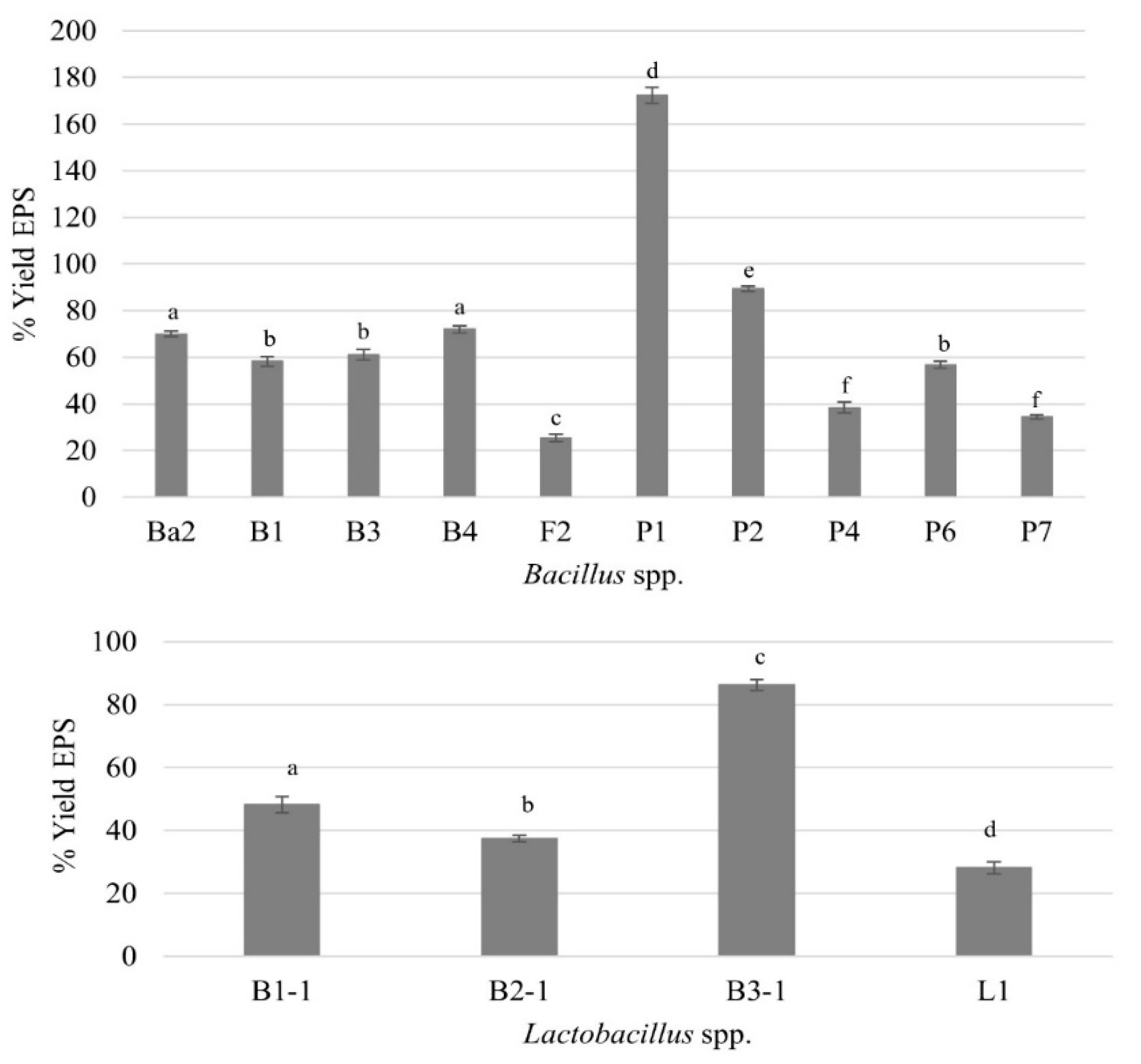
3.1. Screening and Isolation of EPS-Producing Bacteria
3.2. Characterization of the Purified EPS
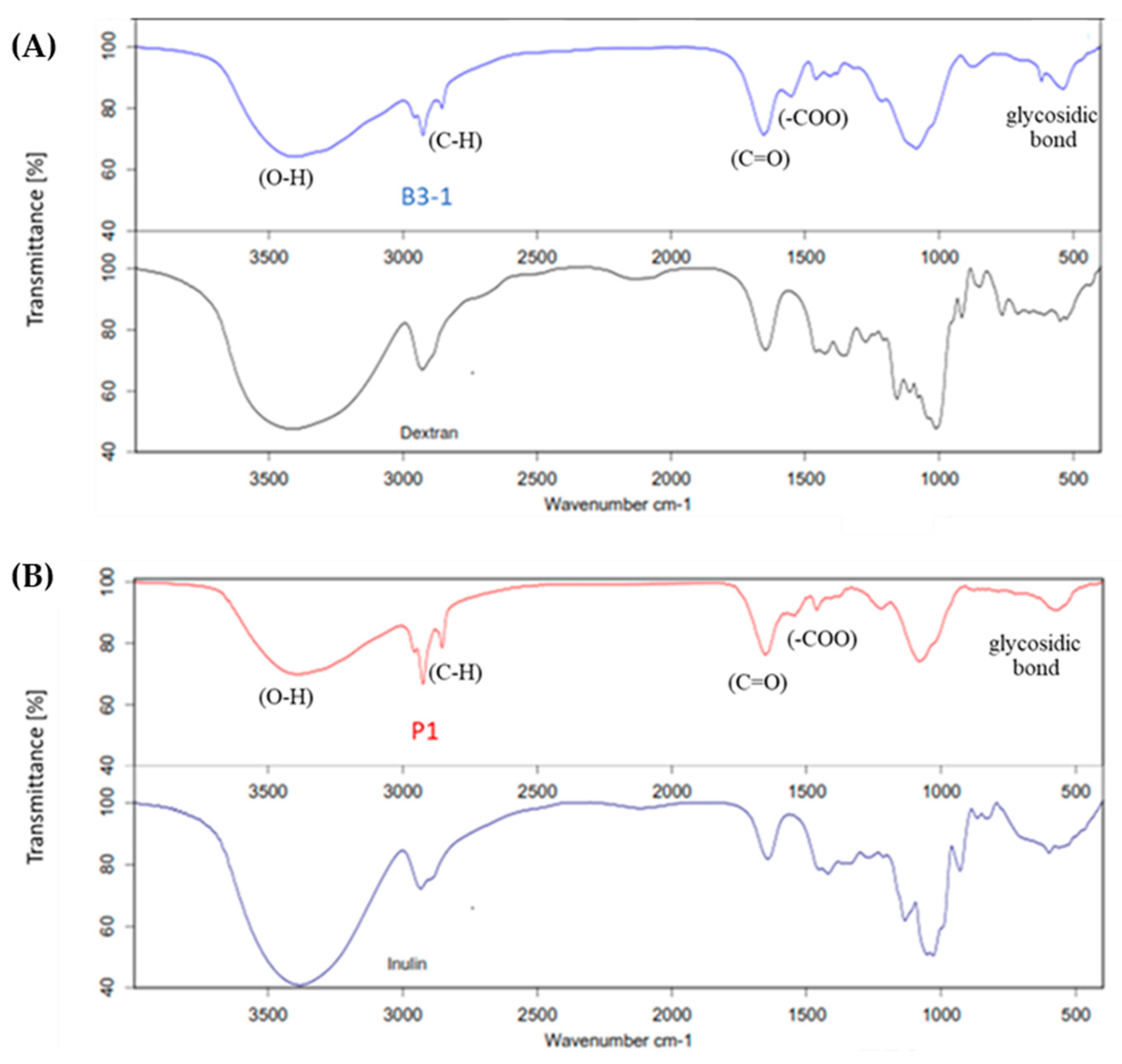
3.2.1. Molecular Mass
3.2.2. Functional Groups of EPS and Its Monosaccharide Compositions
3.3. Optimization Conditions for EPS Production
3.4. Bacterial Identification
3.5. In vitro Biological Activity
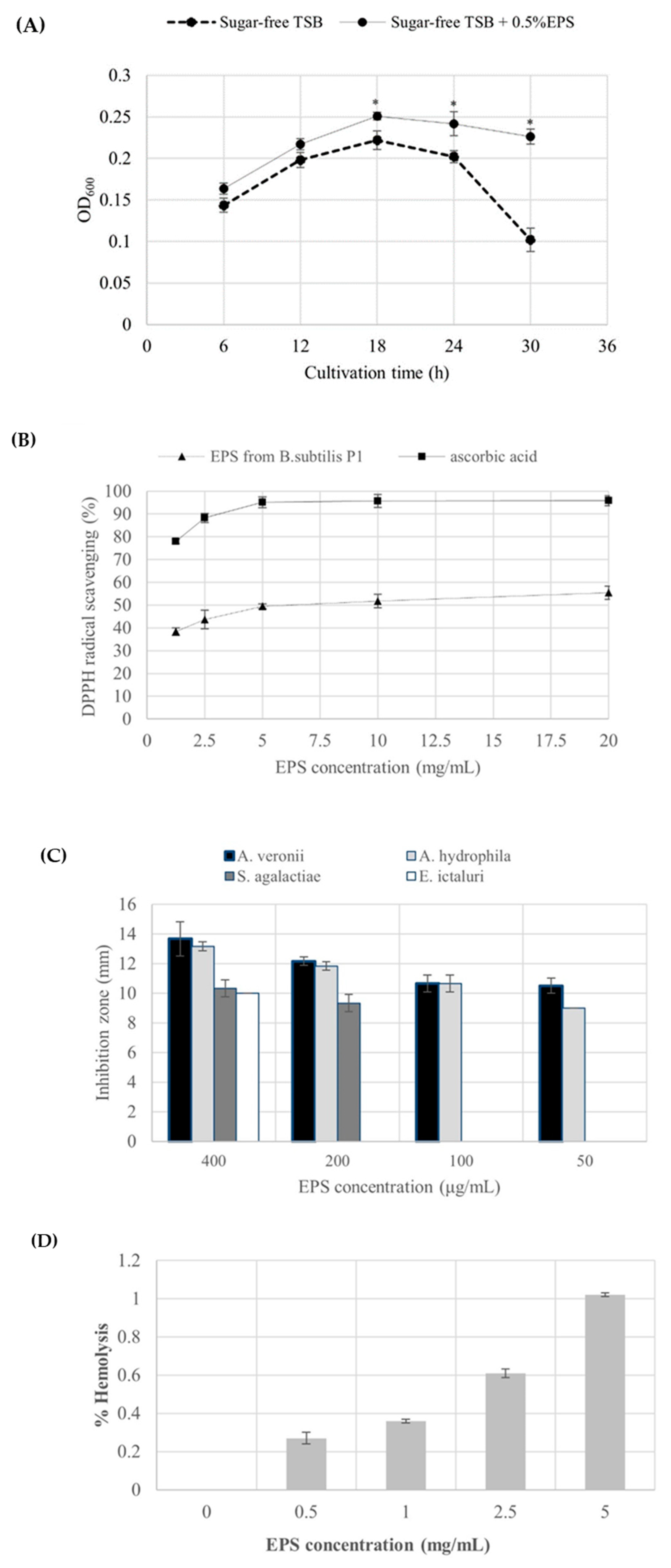
3.5.1. Prebiotic Properties of the Produced EPS
3.5.2. Anti-Oxidant Activity
3.5.3. Anti-Bacterial Activity
3.5.4. Cytotoxicity test
3.6. In Vivo Study of the Effect of EPS Supplementation in Fish Diet on Growth Performance and Immune Response
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- T. Lertwanakarn, T. Purimayata, T. Luengyosluechakul, P. B. Grimalt, A. S. Pedrazzani, M. H. Quintiliano, and W. Surachetpong, “Assessment of Tilapia (Oreochromis spp.) Welfare in the Semi-Intensive and Intensive Culture Systems in Thailand,” Animals, vol. 13, no. 15, p. 2498, 2023. [CrossRef]
- M. D. Ibrahem, M. Fathi, S. Mesalhy, and A. Abd El-Aty, “Effect of dietary supplementation of inulin and vitamin C on the growth, hematology, innate immunity, and resistance of Nile tilapia (Oreochromis niloticus),” Fish & Shellfish Immunology, vol. 29, no. 2, pp. 241–246, 2010. [CrossRef]
- D. Carbone and C. Faggio, “Importance of prebiotics in aquaculture as immunostimulants. Effects on immune system of Sparus aurata and Dicentrarchus labrax,” Fish & Shellfish Immunology, vol. 54, pp. 172–178, 2016. [CrossRef]
- S. H. Hoseinifar, M. Á. Esteban, A. Cuesta, and Y.-Z. Sun, “Prebiotics and fish immune response: a review of current knowledge and future perspectives,” Reviews in Fisheries Science & Aquaculture, vol. 23, no. 4, pp. 315–328, 2015. [CrossRef]
- M. Yousefian and M. S. Amiri, “A review of the use of prebiotic in aquaculture for fish and shrimp,” African Journal of Biotechnology, vol. 8, no. 25, 2009.
- J. C. Camacho-Chab, F. Lango-Reynoso, M. d. R. Castañeda-Chávez, I. Galaviz-Villa, D. Hinojosa-Garro, and B. O. Ortega-Morales, “Implications of extracellular polymeric substance matrices of microbial habitats associated with coastal aquaculture systems,” Water, vol. 8, no. 9, p. 369, 2016. [CrossRef]
- S. Gupta, P. Das, S. Singh, M. Akhtar, D. Meena, and S. Mandal, “Microbial levari, an ideal prebiotic and immunonutrient in aquaculture,” World Aquaculture, vol. 42, no. 1, p. 61, 2011.
- S. You et al., “The promotion mechanism of prebiotics for probiotics: A review,” Frontiers in Nutrition, vol. 9, p. 1000517, 2022. [CrossRef]
- A. I. Netrusov, E. V. Liyaskina, I. V. Kurgaeva, A. U. Liyaskina, G. Yang, and V. V. Revin, “Exopolysaccharides Producing Bacteria: A Review,” Microorganisms, vol. 11, no. 6, p. 1541, 2023. [CrossRef]
- F. Salimi and P. Farrokh, “Recent advances in the biological activities of microbial exopolysaccharides,” World Journal of Microbiology and Biotechnology, vol. 39, no. 8, p. 213, 2023. [CrossRef]
- W. Wang, Y. Ju, N. Liu, S. Shi, and L. Hao, “Structural characteristics of microbial exopolysaccharides in association with their biological activities: a review,” Chemical and Biological Technologies in Agriculture, vol. 10, no. 1, p. 137, 2023. [CrossRef]
- M. Abinaya et al., “Exopolysaccharides-mediated ZnO nanoparticles for the treatment of aquatic diseases in freshwater fish Oreochromis mossambicus,” Toxics, vol. 11, no. 4, p. 313, 2023. [CrossRef]
- N. Sutthi et al., “Dietary Administration Effects of Exopolysaccharide Produced by Bacillus tequilensis PS21 Using Riceberry Broken Rice, and Soybean Meal on Growth Performance, Immunity, and Resistance to Streptococcus agalactiae of Nile tilapia (Oreochromis niloticus),” Animals, vol. 13, no. 20, p. 3262, 2023. [CrossRef]
- P. Ruas-Madiedo, J. Hugenholtz, and P. Zoon, “An overview of the functionality of exopolysaccharides produced by lactic acid bacteria,” International Dairy Journal, vol. 12, no. 2–3, pp. 163–171, 2002. [CrossRef]
- M. Andrew and G. Jayaraman, “Structural features of microbial exopolysaccharides in relation to their antioxidant activity,” Carbohydrate Research, vol. 487, p. 107881, 2020. [CrossRef]
- J. Angelin and M. Kavitha, “Exopolysaccharides from probiotic bacteria and their health potential,” International Journal of Biological Macromolecules, vol. 162, pp. 853–865, 2020. [CrossRef]
- A. Mahdhi et al., “Dietary administration effects of exopolysaccharide from potential probiotic strains on immune and antioxidant status and nutritional value of European sea bass (Dicentrarchus labrax L.),” Research in Veterinary Science, vol. 131, pp. 51–58, 2020. [CrossRef]
- N. Gobi et al., “Dietary supplementation of probiotic Bacillus licheniformis Dahb1 improves growth performance, mucus and serum immune parameters, antioxidant enzyme activity as well as resistance against Aeromonas hydrophila in tilapia Oreochromis mossambicus,” Fish & Shellfish Immunology, vol. 74, pp. 501–508, 2018. [CrossRef]
- W. Zhao et al., “Characterization and antioxidant activity of the exopolysaccharide produced by Bacillus amyloliquefaciens GSBa-1,” 2018. [CrossRef]
- P. Petrova, A. Arsov, I. Ivanov, L. Tsigoriyna, and K. Petrov, “New exopolysaccharides produced by Bacillus licheniformis 24 display substrate-dependent content and antioxidant activity,” Microorganisms, vol. 9, no. 10, p. 2127, 2021. [CrossRef]
- S. A. Moghannem, M. Farag, A. M. Shehab, and M. S. Azab, “Exopolysaccharide production from Bacillus velezensis KY471306 using statistical experimental design,” Brazilian Journal of Microbiology, vol. 49, pp. 452–462, 2018. [CrossRef]
- M. M. Berekaa, “Improved exopolysaccharide production by Bacillus licheniformis strain-QS5 and application of statistical experimental design,” Int J Curr Microbiol App Sci, vol. 3, no. 4, pp. 876–886, 2014.
- R. Gangalla et al., “Optimization and characterization of exopolysaccharide produced by Bacillus aerophilus rk1 and its in vitro antioxidant activities,” Journal of King Saud University-Science, vol. 33, no. 5, p. 101470, 2021. [CrossRef]
- S. A. Razack, V. Velayutham, and V. Thangavelu, “Medium optimization for the production of exopolysaccharide by Bacillus subtilis using synthetic sources and agro wastes,” Turkish Journal of Biology, vol. 37, no. 3, pp. 280–288, 2013. [CrossRef]
- F. C. Mgomi, Y.-R. Yang, G. Cheng, and Z.-Q. Yang, "Lactic acid bacteria biofilms and their antimicrobial potential against pathogenic microorganisms," Biofilm, vol. 100118, 2023. [CrossRef]
- M.-G. Lee et al., "Potential probiotic properties of exopolysaccharide-producing Lacticaseibacillus paracasei EPS DA-BACS and prebiotic activity of its exopolysaccharide," Microorganisms, vol. 10, no. 12, p. 2431, 2022. [CrossRef]
- E. S. Lim, S. J. Nam, O. K. Koo, and J.-S. Kim, "Protective role of Acinetobacter and Bacillus for Escherichia coli O157 in biofilms against sodium hypochlorite and extracellular matrix-degrading enzymes," Food Microbiology, vol. 109, p. 104125, 2023. [CrossRef]
- T. Todhanakasem, A. Sangsutthiseree, K. Areerat, G. M. Young, and P. Thanonkeo, "Biofilm production by Zymomonas mobilis enhances ethanol production and tolerance to toxic inhibitors from rice bran hydrolysate," New Biotechnology, vol. 31, no. 5, pp. 451-459, 2014. [CrossRef]
- C. Liu, J. Lu, L. Lu, Y. Liu, F. Wang, and M. Xiao, "Isolation, structural characterization and immunological activity of an exopolysaccharide produced by Bacillus licheniformis 8-37-0-1," Bioresource Technology, vol. 101, no. 14, pp. 5528-5533, 2010. [CrossRef]
- T. Lin, C. Chen, B. Chen, J. Shaw, and Y. Chen, "Optimal economic productivity of exopolysaccharides from lactic acid bacteria with production possibility curves," Food Science & Nutrition, vol. 7, no. 7, pp. 2336-2344, 2019. [CrossRef]
- M. Ziadi et al., "Evaluation of the efficiency of ethanol precipitation and ultrafiltration on the purification and characteristics of exopolysaccharides produced by three lactic acid bacteria," BioMed Research International, 2018. [CrossRef]
- K. Khongkool, B. Prakit, R. Chiyod, T. Suttibul, and M. Lertworapreecha, "Qualitative analysis of fibre-degrading enzymes production by Bacillus isolated from native swine manures," Burapha Science Journal, pp. 647-659, 2023.
- T. Yılmaz and Ö. Şimşek, "Potential health benefits of ropy exopolysaccharides produced by Lactobacillus plantarum," Molecules, vol. 25, no. 14, p. 3293, 2020. [CrossRef]
- E. H. Zaghloul, M. I. Ibrahim, and H. A. Zaghloul, "Antibacterial activity of exopolysaccharide produced by bee gut-resident Enterococcus sp. BE11 against marine fish pathogens," BMC Microbiology, vol. 23, no. 1, p. 231, 2023. [CrossRef]
- D. W. Wanja et al., "Antibiotic and disinfectant susceptibility patterns of bacteria isolated from farmed fish in Kirinyaga County, Kenya," International Journal of Microbiology, 2020. [CrossRef]
- M. Abinaya et al., "Structural characterization of Bacillus licheniformis Dahb1 exopolysaccharide—antimicrobial potential and larvicidal activity on malaria and Zika virus mosquito vectors," Environmental Science and Pollution Research, vol. 25, pp. 18604-18619, 2018. [CrossRef]
- C. Figueiredo-Silva, A. Lemme, D. Sangsue, and S. Kiriratnikom, "Effect of DL-methionine supplementation on the success of almost total replacement of fish meal with soybean meal in diets for hybrid tilapia (Oreochromis niloticus × Oreochromis mossambicus)," Aquaculture Nutrition, vol. 21, no. 2, pp. 234-241, 2015. [CrossRef]
- J. D. Biller et al., "Lysozyme activity as an indicator of innate immunity of tilapia (Oreochromis niloticus) when challenged with LPS and Streptococcus agalactiae," Revista Brasileira de Zootecnia, vol. 50, 2021. [CrossRef]
- S. A. Stasiak and P. C. Baumann, "Neutrophil activity as a potential bioindicator for contaminant analysis," Fish & Shellfish Immunology, vol. 6, no. 7, pp. 537-539, 1996. [CrossRef]
- L. M. T. Hampton, M. K. S. Jeffries, and B. J. Venables, "A practical guide for assessing respiratory burst and phagocytic cell activity in the fathead minnow, an emerging model for immunotoxicity," MethodsX, vol. 7, p. 100992, 2020. [CrossRef]
- J. D. Biller and L. S. Takahashi, "Oxidative stress and fish immune system: phagocytosis and leukocyte respiratory burst activity," Anais da Academia Brasileira de Ciências, vol. 90, pp. 3403-3414, 2018. [CrossRef]
- M. Sewaka et al., "Efficacy of synbiotic Jerusalem artichoke and Lactobacillus rhamnosus GG-supplemented diets on growth performance, serum biochemical parameters, intestinal morphology, immune parameters and protection against Aeromonas veronii in juvenile red tilapia (Oreochromis spp.)," Fish & Shellfish Immunology, vol. 86, pp. 260-268, 2019. [CrossRef]
- T. Nuryastuti, "Current in vitro assay to determine bacterial biofilm formation of clinical isolates," Journal of the Medical Sciences (Berkala Ilmu Kedokteran), vol. 46, no. 03, 2014. [CrossRef]
- J. A. Amào, P. F. Omojasola, and M. Barooah, "Isolation and characterization of some exopolysaccharide producing bacteria from cassava peel heaps," Scientific African, vol. 4, e00093, 2019. [CrossRef]
- M. Marvasi, P. T. Visscher, and L. Casillas Martinez, "Exopolymeric substances (EPS) from Bacillus subtilis: polymers and genes encoding their synthesis," FEMS Microbiology Letters, vol. 313, no. 1, pp. 1-9, 2010. [CrossRef]
- R. Tallon, P. Bressollier, and M. C. Urdaci, "Isolation and characterization of two exopolysaccharides produced by Lactobacillus plantarum EP56," Research in Microbiology, vol. 154, no. 10, pp. 705-712, 2003. [CrossRef]
- L. Zhang et al., "Antioxidant activity of an exopolysaccharide isolated from Lactobacillus plantarum C88," International Journal of Biological Macromolecules, vol. 54, pp. 270-275, 2013. [CrossRef]
- E. Sánchez-León, E. Huang-Lin, R. Amils, and C. Abrusci, "Production and characterisation of an exopolysaccharide by Bacillus amyloliquefaciens: Biotechnological applications," Polymers, vol. 15, no. 6, p. 1550, 2023. [CrossRef]
- E. Huang-Lin, E. Sánchez-León, R. Amils, and C. Abrusci, "Potential applications of an exopolysaccharide produced by Bacillus xiamenensis RT6 isolated from an acidic environment," Polymers, vol. 14, no. 18, p. 3918, 2022. [CrossRef]
- D. Jurášková, S. C. Ribeiro, and C. C. Silva, "Exopolysaccharides produced by lactic acid bacteria: From biosynthesis to health-promoting properties," Foods, vol. 11, no. 2, p. 156, 2022. [CrossRef]
- S. M. Dănăilă-Gidea et al., "Inulin. Unique among the polyglucides with significant functional properties and biotechnological perspectives," Revista de Biologie, vol. 25, no. 2, pp. 1387-1395, 2020. [CrossRef]
- E. Korcz and L. Varga, "Exopolysaccharides from lactic acid bacteria: Techno-functional application in the food industry," Trends in Food Science & Technology, vol. 110, pp. 375-384, 2021. [CrossRef]
- R. Prete et al., "Lactic acid bacteria exopolysaccharides producers: a sustainable tool for functional foods," Foods, vol. 10, no. 7, p. 1653, 2021. [CrossRef]
- M. A. Ghoneim et al., "Effect of polysaccharide from Bacillus subtilis sp. on cardiovascular diseases and atherogenic indices in diabetic rats," BMC Complementary and Alternative Medicine, vol. 16, no. 1, pp. 1-12, 2016. [CrossRef]
- A. Malick, N. Khodaei, N. Benkerroum, and S. Karboune, "Production of exopolysaccharides by selected Bacillus strains: Optimization of media composition to maximize the yield and structural characterization," International Journal of Biological Macromolecules, vol. 102, pp. 539-549, 2017. [CrossRef]
- R. Daneshazari et al., "Bacillus subtilis isolates from camel milk as probiotic candidates," Scientific Reports, vol. 13, no. 1, p. 3387, 2023. [CrossRef]
- S. K. Nayak, "Multifaceted applications of probiotic Bacillus species in aquaculture with special reference to Bacillus subtilis," Reviews in Aquaculture, vol. 13, no. 2, pp. 862-906, 2021. [CrossRef]
- V. Elisashvili, E. Kachlishvili, and M. L. Chikindas, "Recent advances in the physiology of spore formation for Bacillus probiotic production," Probiotics and Antimicrobial Proteins, vol. 11, pp. 731-747, 2019. [CrossRef]
- L. Zhu, S. Qin, S. Zhai, Y. Gao, and L. Li, "Inulin with different degrees of polymerization modulates composition of intestinal microbiota in mice," FEMS Microbiology Letters, vol. 364, no. 10, p. fnx075, 2017. [CrossRef]
- T. Karirat et al., "Data on exopolysaccharides produced by Bacillus spp. from cassava pulp with antioxidant and antimicrobial properties," Data in Brief, vol. 50, p. 109474, 2023. [CrossRef]
- A.-M. A. Yones, E. I. A.-M. Mohamed, M. A. Ghobashy, and S. S. Marzok, "Effects of dietary inulin as prebiotic on growth performance, immuno-haematological indices and ectoparasitic infection of fingerlings Nile tilapia, Oreochromis niloticus," Egyptian Journal of Histology, vol. 43, no. 1, pp. 88-103, 2020. [CrossRef]
- M. Reyes-Becerril et al., "Single or combined effects of Lactobacillus sakei and inulin on growth, non-specific immunity and IgM expression in leopard grouper (Mycteroperca rosacea)," Fish Physiology and Biochemistry, vol. 40, pp. 1169-1180, 2014. [CrossRef]
- H. Ghafarifarsani et al., "Study on growth enhancement and the protective effects of dietary prebiotic inulin on immunity responses of rainbow trout fry infected with Aeromonas hydrophila," Annals of Animal Science, vol. 21, no. 2, pp. 543-559, 2021. [CrossRef]
- V. Kumar, H. S. Makkar, and K. Becker, "Nutritional, physiological and haematological responses in rainbow trout (Oncorhynchus mykiss) juveniles fed detoxified Jatropha curcas kernel meal," Aquaculture Nutrition, vol. 17, no. 4, pp. 451-467, 2011. [CrossRef]

| Bacterial strain | Mw (Da) | Mn (Da) | PDI | References |
|---|---|---|---|---|
| Lb. plantarum C7 | 3.37 x 104 | 0.85 x 104 | 3.96 | Ziadi et al. [31] |
| Lb.delbrueckii subsp. bulgaricus | 104 to 106 | n.r. | n.r | Jurášková et al. [50] |
| Lactobacillus sp. B3-1 | 8.31 x 105 | 3.38 x 105 | 2.46 | This study |
| B.amyloliquefaciens GSBa-1 | 5.40 x 104 | n.r. | 1.78 | Zhao et al. [19] |
| B. velezensis KY471306 | 1.29 x 105 | 1.14 x 105 | 1.13 | Moghannem et al. [21] |
| B.subtilis | 0.57 to 1.28 x 105 | n.r. | n.r | Marvasi et al. [45] |
| Bacillus sp. P1 | 9.81 x 105 | 6.04 x 105 | 1.62 | This study |
| Source | Sum of Squares | Mean Square | F-value | p-value* |
| Model | 6.268E+05 | 69645.11 | 20.23 | 0.0003 |
| A:sucrose | 33303.93 | 33303.93 | 9.67 | 0.0171 |
| B:yeast extract | 33697.38 | 33697.38 | 9.79 | 0.0166 |
| C:(NH4)2SO4 | 55461.15 | 55461.15 | 16.11 | 0.0051 |
| AB | 8689.97 | 8689.97 | 2.52 | 0.1561 |
| AC | 3863.24 | 3863.24 | 1.12 | 0.3246 |
| BC | 9259.25 | 9259.25 | 2.69 | 0.1450 |
| A² | 59541.62 | 59541.62 | 17.30 | 0.0042 |
| B² | 51357.16 | 51357.16 | 14.92 | 0.0062 |
| C² | 3.325E+05 | 3.325E+05 | 96.59 | < 0.0001 |
| Lack of Fit | 22795.51 | 7598.50 | 23.36 | 0.0054 |
| Pure Error | 1301.35 | 325.34 | - | - |
| R² | 0.9630 | - | - | |
| Adjusted R² | 0.9154 | - | - | - |
| Duration (week) | Experiment | Growth performance parameters | |||||
| Weight gain | Specific growth rate (%) | Average daily gain | Feed conversion rate (g/g) | ||||
| av. per tank (g) | av. per fish (g) | av. per tank (g/day) | av. per fish (g/day) | ||||
| 2 | Control | 527.55±13.35a | 21.10 | 4.83±0.08a | 37.68±0.95a | 1.51 | 1.30±0.06a |
| Treatment | 529.40±19.83a | 21.18 | 4.98±0.14a | 37.81±1.42a | 1.51 | 1.36±0.05a | |
| 4 | Control | 990.28±25.91b | 39.61 | 3.69±0.03b | 35.37±0.93b | 1.41 | 0.66±0.03b |
| Treatment | 1,047.00±11.80c | 41.88 | 3.92±0.07c | 37.40±0.42c | 1.50 | 0.71±0.02b | |
| 6 | Control | 1,403.86±34.17d | 56.15 | 3.03±0.03d | 33.43±0.81d | 1.34 | 0.42±0.04d |
| Treatment | 1,464.57±29.22d | 58.58 | 3.17±0.09d | 34.87±0.70d | 1.40 | 0.43±0.00d | |
| Parameters | Control | Treatment |
|---|---|---|
| Lysozyme activity (U/min) | 3.37±0.13 | 6.88±0.16* |
| Respiratory burst activitya (OD630) | 0.10 | 0.44 |
| Total immunoglobulin (mg/dL) | 3±0 | 3±0 |
| Relative level of protection (%RLP) after 2 weeks challenge test |
0 | 50 |
| Analysis | Parameters | Control | Treatment |
|---|---|---|---|
| Blood chemistry | Blood urea nitrogen (mg/dL) | 3±1 | 3±0 |
| Glucose (mg/dL) | 98.33±15.89a | 118.67±11.59ab | |
| ALT (U/L) | 12.67±7.77 | 15.33±3.51 | |
| AST (U/L) | 68.33±32.59 | 58.67±30.29 | |
| Total bilirubin (mg/dL) | 0.23±0.06 | 0.20±0.00 | |
| Direct bilirubin (mg/dL) | 0.26±0.05 | 0.23±0.05 | |
| Total cholesterol (mg/dL) | 197.33±5.03 | 190.67±16.29 | |
| Total protein (g/dL) | 4.30±0.26 | 4.43±0.06 | |
| Albumin (mg/dL) | 1.47±0.06 | 1.43±0.06 | |
| Proximate chemical compositions (AOAC 2000*) |
Crude protein** | 17.80±0.04 | 19.02±0.10 |
| Crude fat | 0.33±0.01 | 0.83±0.03 | |
| Moisture content | 71.91±0.50 | 69.86±0.20 | |
| Ash | 1.85±0.01 | 2.30±0.02 | |
| Carbohydrate | 8.11±0.02 | 7.99±0.06 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





