Submitted:
14 August 2024
Posted:
14 August 2024
Read the latest preprint version here
Abstract
Keywords:
I. Introduction
II. METHODS
| Experimental Groups | Treatment/Rats (Induced infertility) |
|---|---|
| Group 1 | Distilled water |
| Group 2 | 200mg/kg NS oil + Cyclophosphamide |
| Group 3 | 400mg/kg NS oil + Cyclophosphamide |
| Group 4 | 800mg/kg NS oil + Cyclophosphamide |
| Doses (mg/kg body weight) | Percentage (Mean ± S.E.M) of rats at different phases of the Estrous cycle | |||
|---|---|---|---|---|
| Proestrus | Estrous | Metestrus | Diestrus | |
| Group I (Positive control) | 27.06 ± 4.3 | 25.67 ± 4.2 | 18.53 ± 2.3 | 31.23 ± 2.8 |
| Group II (Negative control) | 37.03 ± 4.7 | 27.30 ± 4.7 | 24.23 ± 2.7 | 17.36 ± 3.1 |
| Group III (200mg/kg) | 47.35 ± 5.3 | 31. 34 ± 5.3 | 18.53 ± 7.3 | 7.52 ± 3.2 |
| Group IV (400mg/kg) | 52.07 ± 2.3 | 36.07 ± 3.2 | 15.63 ± 2.3 | 7.20 ± 5.6 |
| Group V (800mg/kg) | 50.07 ± 3.2 | 37.42 ± 4.3 | 17.73 ± 3.6 | 9.03 ± 1.8 |
| Group | Weight of Uterus, fallopian tubes, and Ovaries Mean weight (g) ± SEM | Body weight/Organ ratio Mean weight (g) ± SEM | P- value |
|---|---|---|---|
| Group I (Positive control) | 0.58 ± 0.04 | 231 ± 5.3 | 0.043 |
| Group II (Negative control) | 0.54 ± 0.04 | 219 ± 4.7ab | 0.042 |
| Group III (200mg/kg) | 0.56 ± 0.04 | 227 ± 5.1 | 0.047 |
| Group IV (400mg/kg) | 0.56 ± 0.04 | 232 ± 4.9 | 0.046 |
| Group V (800mg/kg) | 0.57 ± 0.06 | 233 ± 4.0ab | 0.057 |
| Groups | Estradiol Mean(pg/ml) ± SEM |
Progesterone Mean(pg/ml) ± SEM |
|---|---|---|
| Group I (positive control) | 7.91± 0.13ab | 6.31 ± 0.13b |
| Group II (negative control) | 6.03 ± 0.17 | 5.87 ± 0.14 |
| Group III (200mg/kg) | 7.41 ± 0.14 | 5.61 ± 0.09 |
| Group IV (400mg/kg) | 7.70 ± 0.13 | 6.20 ± 0.10 |
| Group V (800mg/kg) | 6.76 ± 0.14a | 7.46 ± 0.14ab |
| Groups | Cortex volume (µm3) | Medulla volume (µm3) | Total ovary volume (µm3) |
|---|---|---|---|
| Group I (Positive control) | 12.9 ± 2.33 | 8.01 ± 1.23 | 20.93 ± 2.1 |
| Group II (Negative control) | 10.4 ± 2.11a | 6.44 ± 0.50a | 16.79 ± 2.32a |
| Group III (200mg/kg) | 12.48 ± 1.46b | 6.74 ± 1.11b | 19.22 ± 1.86b |
| Group IV (400mg/kg) | 12.77 ± 1.99 | 8.02 ± 0.89 | 20.80 ± 1.72a |
| Group V (800mg/kg) | 12.53 ± 1.37 | 8.41± 1.72 | 19.72 ± 1.62 |
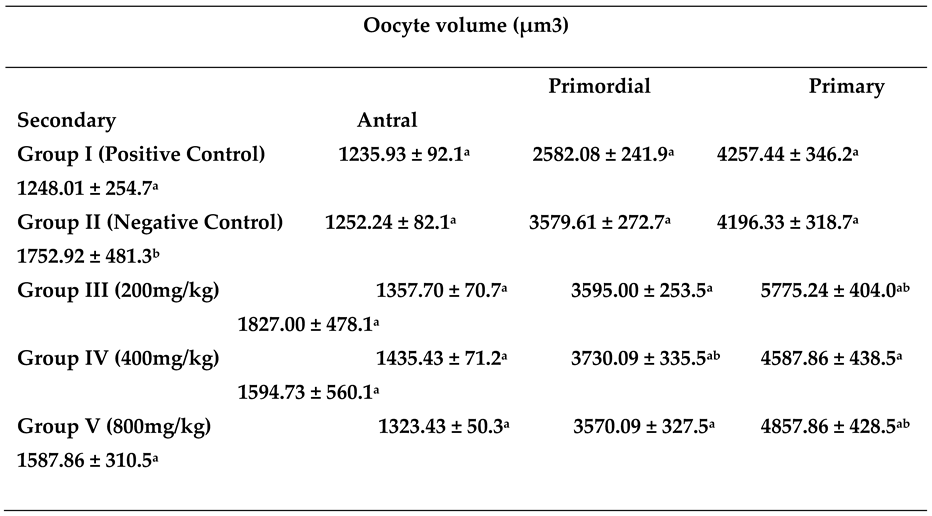 |
- A.
- Group 1 (Positive Control): Ovaries at different developmental stages, with normal histological architecture
- B.
- Group 2. (Negative Control): Shows diminished number of developing follicles and increased number of atretic follicles
- C.
- Group 3. (200mg/kg NS-oil + 0.5 mg/kg Cy): Shows distribution of developing follicles, degenerating follicle and a balloon tissue necrosis on the ovarian surface
- D.
- Group 4 (400mg/kg NS-oil + 0.5 mg/kg Cy): Presence of developing primary follicles and distorted ovarian stroma
- E.
- Group 5 (800mg/kg NS-oil + 0.5 mg/kg Cy): Shows ovary with follicles at different stage of development, and mild necrosis on the surface
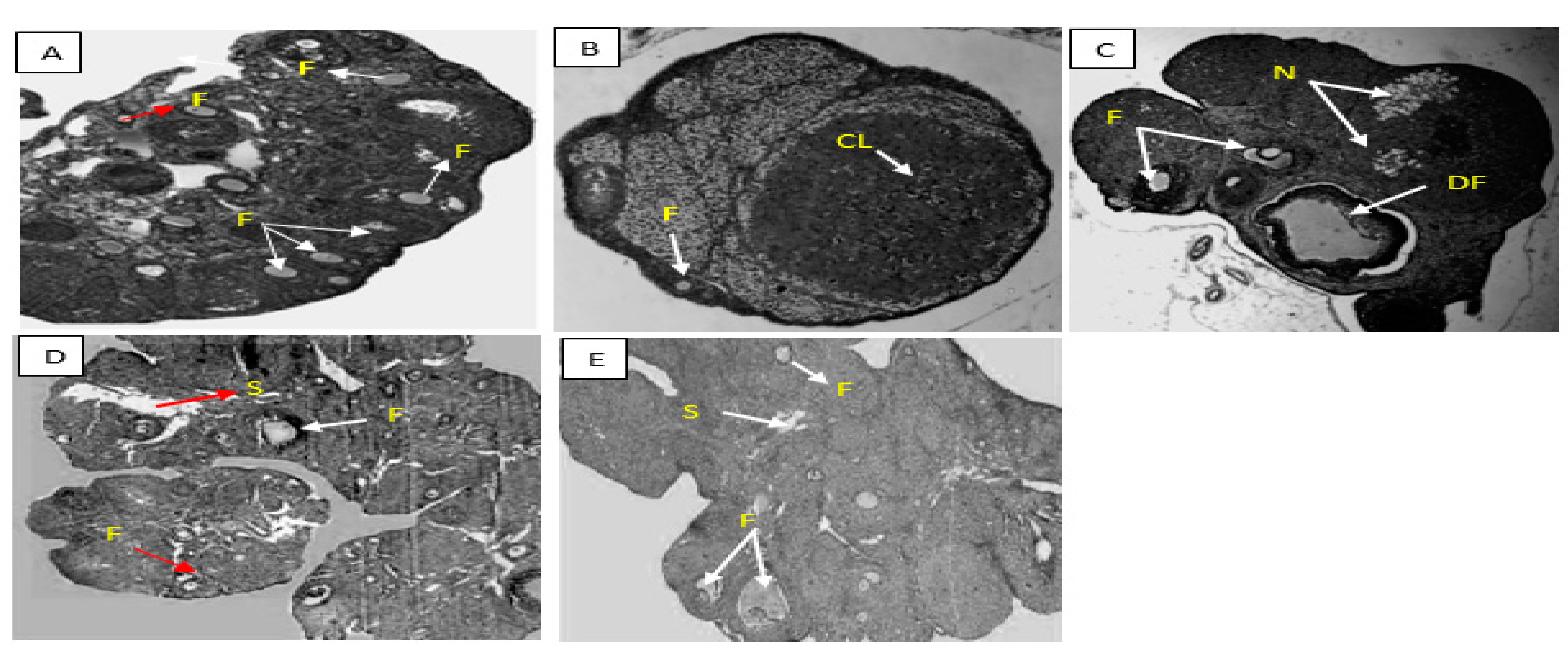
- A.
- Group 1. (Positive Control)
- B.
- Group 2. (Negative Control)
- C.
- Group 3. (200mg/kg NS-oil + 0.5mg/kg Cy)
- D.
- Group 4. (400mg/kg NS-oil + 0.5mg/kg Cy)
- E.
- Group 5. (800mg/kg NS-oil + 0.5mg/kg Cy)
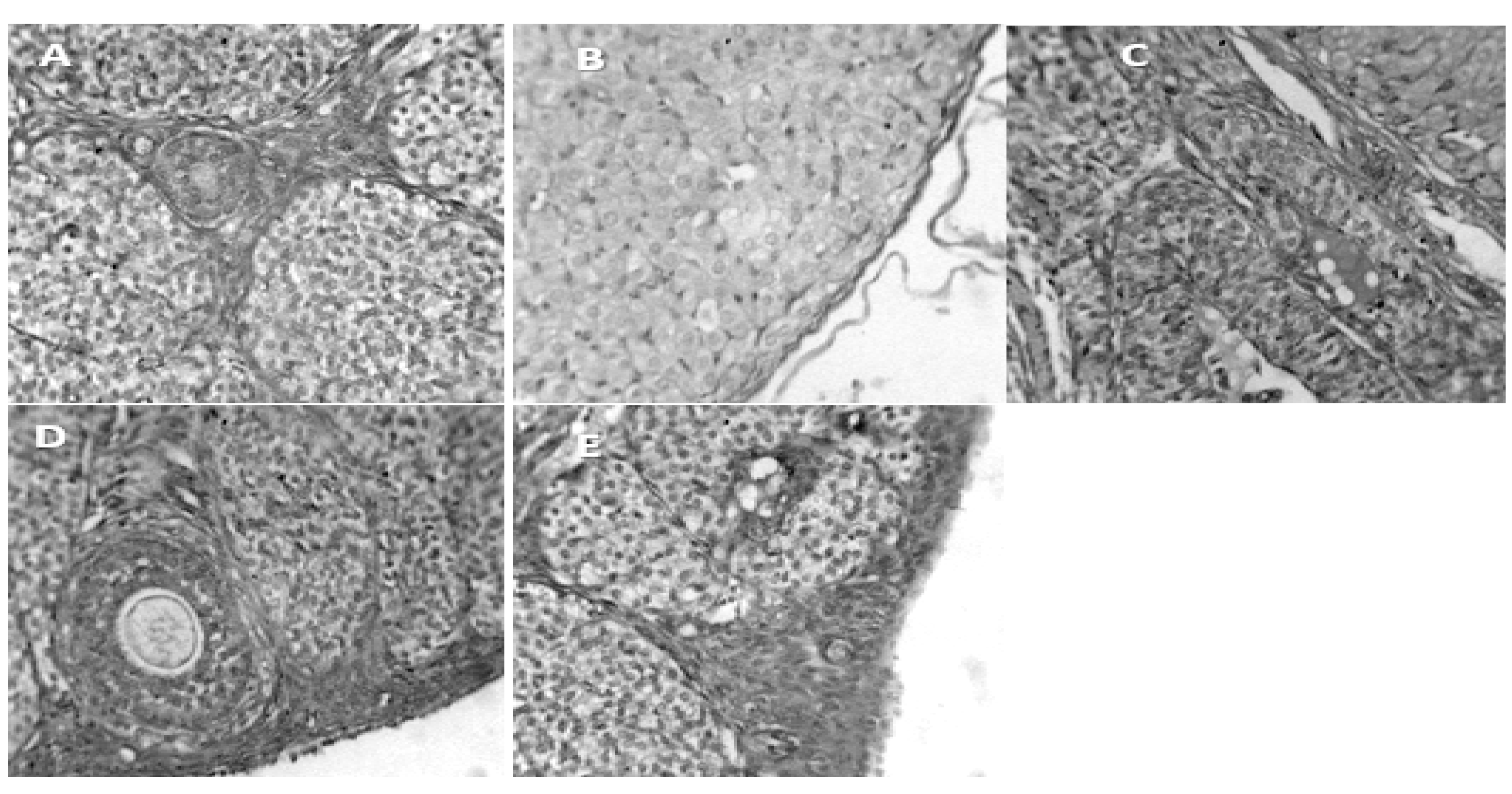
- A.
- Group 1 (Positive Control): Normal appearance of uterine mucous membrane with branching folds, and central lumen (L).
- B.
- Group 2. (Negative Control): Shows increased activity of the mucous membrane of the uterine tube
- C.
- Group 3. (200mg/kg NS-oil + 0.5mg/kg Cy), also shows decrease mucous activity, the muscularis mucosa also shows decrease in diameter
- D.
- Group 4. (400mg/kg NS-oil + 0.5mg/kg Cy), Narrowing of the luminal diameter can be seem in this group
- E.
- Group 5. (800mg/kg NS-oil + 0.5mg/kg Cy). The uterine tube is less intact and numerous distortions can be seen. There is also and increased proliferation of the mucous membrane
References
- WHO 2023.
- | US EPA. (2022). ACUTE ORAL TOXICITY UP-AND-DOWN-PROCEDURE, https://www.epa.gov/pesticide-science-and-assessing-pesticide-risks/acute-oral-toxicity-and-down-procedure.%0A%0A.
- Abbaspour, N. , Hurrell, R., & Kelishadi, R. Review on iron and its importance for human health. Journal of Research in Medical Sciences : The Official Journal of Isfahan University of Medical Sciences 2014, 19, 164–174, https://pubmed.ncbi.nlm.nih.gov/24778671. [Google Scholar] [PubMed]
- Abd El Aziz, A. E. , el Sayed, N. S., & Mahran, L. G. Anti-asthmatic and anti-allergic effects of thymoquinone on airway-induced hypersensitivity in experimental animals. Journal of Applied Pharmaceutical Science 2011, 1, 109–117. [Google Scholar]
- Abd Rani, N. Z. , Husain, K., & Kumolosasi, E. (2018). Moringa Genus: A Review of Phytochemistry and Pharmacology. In Frontiers in Pharmacology (Vol. 9). https://www.frontiersin.org/article/10.3389/fphar.2018. 0010. [Google Scholar]
- Abdelsalam, S. A. E. and E. B. (2018). Some Biological and Pharmacological Effects of the Black Cumin (Nigella sativa): A Concise Review. American Journal of Research Communication, 6(3). www.usa-journals.com.
- Abdulrahman, F. T. J. H. M. A. (20 C.E.). The effects of Nigella sativa oil administration on some physiological and histological values of reproductive aspects of rats: The Iraqi Journal of Veterinary Medicine, 35, 50–60. [CrossRef]
- Abeysinghe, D. T. , Kumara, K. A. H., Kaushalya, K. A. D., Chandrika, U. G., & Alwis, D. D. D. H. Phytochemical screening, total polyphenol, flavonoid content, in vitro antioxidant and antibacterial activities of Sri Lankan varieties of Murraya koenigii and Micromelum minutum leaves. Heliyon 2021, 7, e07449–e07449. [Google Scholar] [CrossRef] [PubMed]
- Adaku, A. Essential Oil from Nigella Sativa Seed Differentially Ameliorates Steroid Genesis, Cellular ATP and Prostate Functions in Anti-Psychotic Drug- Induced Testicular Damage of Rats. 2018, 8, 1–9. 8. [CrossRef]
- Adewoyin, M. , Ibrahim, M., Roszaman, R., Isa, M., Alewi, N., Rafa, A., & Anuar, M. Male Infertility: The Effect of Natural Antioxidants and Phytocompounds on Seminal Oxidative Stress. Diseases 2017, 5, 9. [Google Scholar] [CrossRef]
- Aglave, H. Physiochemical characteristics of sesame seeds. Journal of Medicinal Plants Studies 2018, 6, 64–66. [Google Scholar] [CrossRef]
- Ahmad, A. , Husain, A., Mujeeb, M., Siddiqui, N. A., & Damanhouri, Z. A. (2012). Physicochemical and phytochemical standardization with HPTLC fingerprinting of Nigella sativa L. seeds, 1175–1182.
- Ahmad, A. , Mishra, R. K., Vyawahare, A., Kumar, A., Rehman, M. U., Qamar, W., Khan, A. Q., & Khan, R. Thymoquinone (2-Isoprpyl-5-methyl-1, 4-benzoquinone) as a chemopreventive/anticancer agent: Chemistry and biological effects. Saudi Pharmaceutical Journal 2019, 27, 1113–1126. [Google Scholar] [CrossRef]
- Ahmad, F. , Ali, F., Amir, S., Saad, H. H., Wahab, S., Idreesh, M., Ali, M., & Mohan, S. (2020). Since January 2020 Elsevier has created a COVID-19 resource centre with free information in English and Mandarin on the novel coronavirus COVID- 19. The COVID-19 resource centre is hosted on Elsevier Connect, the company ’ s public news and information . January, 20 January.
- Ahmad, W. , Zeenat, F., & Shaiqua, A. (2017). Therapeutics, Phytochemistry and Pharmacology of an Important Unani Drug Kalonji ( Nigella sativa Linn ): A Review THERAPEUTICS, PHYTOCHEMISTRY AND PHARMACOLOGY OF AN IMPORTANT. July.
- Ajayi, A. F. , & Akhigbe, R. E. Staging of the estrous cycle and induction of estrus in experimental rodents: an update. Fertility Research and Practice 2020, 6, 1–15. [Google Scholar] [CrossRef]
- al Disi, S. S. , Anwar, M. A., & Eid, A. H. Anti-hypertensive herbs and their mechanisms of action: Part I. Frontiers in Pharmacology 2016, 6, 1–24. [Google Scholar] [CrossRef]
- Alanazi, I. O. , Benabdelkamel, H., Alfadda, A. A., AlYahya, S. A., Alghamdi, W. M., Aljohi, H. A., Almalik, A., & Masood, A. Proteomic Analysis of the Protein Expression Profile in the Mature Nigella sativa (Black Seed). Applied Biochemistry and Biotechnology 2016, 179, 1184–1201. [Google Scholar] [CrossRef]
- Alberts B, Johnson A, Lewis J, et al. (2002). Molecular Biology of the Cell. Garland Science. https://www.ncbi.nlm.nih.gov/books/NBK26940/%0A.
- Algandaby, M. M. Quercetin attenuates cisplatin-induced ovarian toxicity in rats: Emphasis on anti-oxidant, anti-inflammatory and anti-apoptotic activities. Arabian Journal of Chemistry 2021, 14, 103191. [Google Scholar] [CrossRef]
- Ali, B. A. (2001). on Blood Glucose in Albino Rats, 242–244.
- Al-Johar, D. , Shinwari, N., Arif, J., Al-Sanea, N., Jabbar, A. A., El-Sayed, R., Mashhour, A., Billedo, G., El-Doush, I., & Al-Saleh, I. Role of Nigella sativa and a number of its antioxidant constituents towards azoxymethane-induced genotoxic effects and colon cancer in rats. Phytotherapy Research 2008, 22, 1311–1323. [Google Scholar] [CrossRef] [PubMed]
- Al-Mamun, M. , & Absar, N. Major nutritional compositions of black cumin seeds-cultivated in Bangladesh and the physicochemical characteristics of its oil. International Food Research Journal 2019, 25, 2634–2639. [Google Scholar]
- Alomar, M. Y. Physiological and histopathological study on the influence of Ocimum basilicum leaves extract on thioacetamide-induced nephrotoxicity in male rats. Saudi Journal of Biological Sciences 2020, 27, 1843–1849. [Google Scholar] [CrossRef] [PubMed]
- Amin, B. , & Hosseinzadeh, H. Black Cumin (Nigella sativa) and Its Active Constituent, Thymoquinone: An Overview on the Analgesic and Anti-inflammatory Effects. Planta Medica 2016, 82, 8–16. [Google Scholar] [CrossRef] [PubMed]
- Amin, B. , & Hosseinzadeh, H. Black Cumin (Nigella sativa) and Its Active Constituent, Thymoquinone: An Overview on the Analgesic and Anti-inflammatory Effects. Planta Medica 2016, 82, 8–16. [Google Scholar] [CrossRef] [PubMed]
- Andrade, G. M. , Collado, M. del, Meirelles, F. V., da Silveira, J. C., & Perecin, F. Intrafollicular barriers and cellular interactions during ovarian follicle development. Animal Reproduction 2019, 16, 485–496. [Google Scholar] [CrossRef]
- Angad, G. , Veterinary, D. E. v, & Sciences, A. (2015). HISTOMORPHOCHEMICAL AND ULTRASTRUCTURAL CHARACTERIZATION OF HYPOTHALAMO-HYPOPHYSEAL-OVARIAN AXIS IN INDIAN BUFFALO ( Bubalus bubalis ) ( Minor Subject : Veterinary Physiology ) By Department of Veterinary Anatomy College of Veterinary Science GURU ANGAD D.
- Antoniadis, V. , Shaheen, S. M., Levizou, E., Shahid, M., Niazi, N. K., Vithanage, M., Ok, Y. S., Bolan, N., & Rinklebe, J. A critical prospective analysis of the potential toxicity of trace element regulation limits in soils worldwide: Are they protective concerning health risk assessment? - A review. Environment International 2019, 127, 819–847. [Google Scholar] [CrossRef] [PubMed]
- Arnal, J. , Lenfant, F., Metivier, R., Flouriot, G., Henrion, D., Adlanmerini, M., Fontaine, C., Gourdy, P., Chambon, P., Katzenellenbogen, B., & Katzenellenbogen, J. MEMBRANE AND NUCLEAR ESTROGEN RECEPTOR ALPHA ACTIONS : FROM TISSUE SPECIFICITY TO MEDICAL IMPLICATIONS Figure 2, 2022, 1045–1087. 2022. [Google Scholar] [CrossRef]
- ARN.eBook. (n.d.).
- Arroyo, A. , Kim, B., & Yeh, J. Luteinizing Hormone Action in Human Oocyte Maturation and Quality: Signaling Pathways, Regulation, and Clinical Impact. Reproductive Sciences 2020, 27, 1223–1252. [Google Scholar] [CrossRef] [PubMed]
- Article, O. The enhancing effects of alcoholic extract of Nigella sativa seed on fertility potential, plasma gonadotropins and testosterone in male rats. 2012, 10, 355–362. 10.
- Assi, M. A. , Hezmee, M., Noor, M., Farhana Bachek, N., Ahmad, H., Haron, A. W., Sabri, M., Yusoff, M., & Rajion, M. A. The Various Effects of Nigella Sativa on Multiple Body Systems in Human and Animals. PJSRR Pertanika Journal of Scholarly Research Reviews 2016, 2, 1–19. [Google Scholar]
- Atanasov, A. G. , Zotchev, S. B., Dirsch, V. M., Orhan, I. E., Banach, M., Rollinger, J. M., Barreca, D., Weckwerth, W., Bauer, R., Bayer, E. A., Majeed, M., Bishayee, A., Bochkov, V., Bonn, G. K., Braidy, N., Bucar, F., Cifuentes, A., D’Onofrio, G., Bodkin, M., … Taskforce, the I. N. P. S. Natural products in drug discovery: advances and opportunities. Nature Reviews Drug Discovery 2021, 20, 200–216. [Google Scholar] [CrossRef]
- Atata, J. A. , Esievo, K. A. N., Adamu, S., Abdulsalam, H., Avazi, D. O., & Ajadi, A. A. Haemato-biochemical studies of dogs with haemorrhage-induced dehydration. Comparative Clinical Pathology 2019, 28, 129–135. [Google Scholar] [CrossRef]
- Atiku, I. A. Cephalic index in relation to academic performance among students of basic medical sciences, bayero university kano, nigeria. 2018, 4, 404–415. 4.
- Badar, A. , Kaatabi, H., Bamosa, A., Al-Elq, A., Abou-Hozaifa, B., Lebda, F., Alkhadra, A., & Al-Almaie, S. Effect of Nigella sativa supplementation over a one-year period on lipid levels, blood pressure and heart rate in type-2 diabetic patients receiving oral hypoglycemic agents: Nonrandomized clinical trial. Annals of Saudi Medicine 2017, 37, 56–63. [Google Scholar] [CrossRef] [PubMed]
- Bagnjuk, K. , & Mayerhofer, A. Human luteinized granulosa cells—a cellular model for the human corpus luteum. Frontiers in Endocrinology 2019, 10, 1–7. [Google Scholar] [CrossRef]
- Bailey, E. J. , Chang, A. B., & Thomson, D. In children with prolonged cough, does treatment with antibiotics have a better effect on cough resolution than no treatment? Part B: Clinical commentary. Paediatrics and Child Health 2008, 13, 514. [Google Scholar] [CrossRef] [PubMed]
- Bakathir, H. A. , & Abbas, N. A. Detection of the antibacterial effect of nigella sativa ground seedswith water. African Journal of Traditional, Complementary and Alternative Medicines 2011, 8, 159–164. [Google Scholar] [CrossRef]
- Balali-Mood, M. , Naseri, K., Tahergorabi, Z., Khazdair, M. R., & Sadeghi, M. (2021). Toxic Mechanisms of Five Heavy Metals: Mercury, Lead, Chromium, Cadmium, and Arsenic. In Frontiers in Pharmacology (Vol. 12). https://www.frontiersin.org/article/10.3389/fphar.2021. 6439. [Google Scholar]
- Bashir, M. U. , & Qureshi, H. J. No Title Analgesic effect of Nigella sativa seeds extract on experimentally induced pain in albino mice. Journal of the College of Physicians and Surgeons--Pakistan, JCPSP 2010, 20, 464–467. [Google Scholar] [PubMed]
- Benefits, H. , Pharmacology, M., Dash, R., Sikder, M. H., Rahman, S., Timalsina, B., & Munni, Y. A. (2021). Black Cumin (Nigella sativa L.): A Comprehensive Review on Phytochemistry, Health Benefits, Molecular Pharmacology, and Safety. Nutrients.
- Bieberich, E. Synthesis, Processing, and Function of N-glycans in N-glycoproteins. Advances in Neurobiology 2014, 9, 47–70. [Google Scholar] [CrossRef]
- Blaik, P. No 主観的健康感を中心とした在宅高齢者における 健康関連指標に関する共分散構造分析Title. Gospodarka Materiałowa i Logistyka, 2013, 26, 185–197. [Google Scholar]
- Blanc, L. , & Wolfe, L. C. (2016). Chapter 9 - General Considerations of Hemolytic Diseases, Red Cell Membrane, and Enzyme Defects. In P. Lanzkowsky, J. M. Lipton, & J. D. Fish (Eds.), Lanzkowsky’s Manual of Pediatric Hematology and Oncology (Sixth Edition) (Sixth Edit, pp. 134–158). Academic Press. [CrossRef]
- Boskabady, M. H. , Keyhanmanesh, R., Khamneh, S., & Ebrahimi, M. A. The effect of Nigella sativa extract on tracheal responsiveness and lung inflammation in valbuminsensitized guinea pigs. Clinics 2011, 66, 879–887. [Google Scholar] [CrossRef]
- Bridgewater, P. , Upadhaya, S., Poudyal, B., Kunwar, R. M., Bussmann, R. W., & Paniagua-Zambrana, N. Y. (2020) Nigella sativa L. Ranunculaceae BT - Ethnobotany of the Himalayas( R. Kunwar, H. Sher, & R. W. Bussmann, Eds.; pp. 1–10). Springer International Publishing. [CrossRef]
- Brunt, V. E. , Miner, J. A., Meendering, J. R., Kaplan, P. F., & Minson, C. T. Cutaneous Thermal Hyperemia But Not Reactive Hyperemia. 2012, 18, 347–355. [Google Scholar] [CrossRef]
- Bruyn, G. W. Human central nervous system. Journal of the Neurological Sciences 1989, 92, 117. [Google Scholar] [CrossRef]
- BUREAU OF PUBLIC PROCUREMENT BIDDERS ’ CORRESPONDENCE DETAILS TEMPLATE Ministry : Name of Procuring Entity : Title of Procurement : (n.d.).
- Butt, M. S. , & Sultan, M. T. (2010). Nigella sativa : Reduces the Risk of Various Maladies Nigella sativa : Reduces the Risk. 8398. [CrossRef]
- Caligioni, C. S. Assessing Reproductive Status/Stages in Mice. Current Protocols in Neuroscience 2009, 48, A.4I.1–A4I8. [Google Scholar] [CrossRef]
- Campinas, U. E. de, & Campinas, U. E. de. (2002). DETERMINATION OF THE ESTROUS CYCLE PHASES OF RATS : SOME HELPFUL CONSIDERATIONS. 62, 609–614.
- Casarini, L. , & Crépieux, P. (2019). Molecular Mechanisms of Action of FSH (2019). Molecular Mechanisms of Action of FSH. Frontiers in Endocrinology. 10. [CrossRef]
- Castellini, C. , Mattioli, S., Signorini, C., Cotozzolo, E., Noto, D., Moretti, E., Brecchia, G., Dal Bosco, A., Belmonte, G., Durand, T., De Felice, C., & Collodel, G. (2019). Effect of Dietary n-3 Source on Rabbit Male Reproduction. Oxidative Medicine and Cellular Longevity, 3279670. [CrossRef]
- Chaklader, M. R. , Fotedar, R., Howieson, J., Siddik, M. A. B., & Foysal, M. J. (2020). The ameliorative effects of various fish protein hydrolysates in poultry by-product meal based diets on muscle quality, serum biochemistry and immunity in juvenile barramundi, Lates calcarifer. Fish & Shellfish Immunology, 104, 567–578. [CrossRef]
- Chassagne, F. , Samarakoon, T., Porras, G., Lyles, J. T., Dettweiler, M., Marquez, L., Salam, A. M., Shabih, S., Farrokhi, D. R., & Quave, C. L. (2021). A Systematic Review of Plants With Antibacterial Activities: A Taxonomic and Phylogenetic Perspective. Frontiers in Pharmacology, 11. [CrossRef]
- Chauvin, S. , Cohen-Tannoudji, J., & Guigon, C. J. (2022). Estradiol Signaling at the Heart of Folliculogenesis: Its Potential Deregulation in Human Ovarian Pathologies. International Journal of Molecular Sciences, 23. [CrossRef]
- ChiauMingj, Md. S. A. A. HingGohefZannatUrbigMd. M. R. (2021). A review of ethnobotany, phytochemistry, antimicrobial pharmacology and toxicology of Nigella sativa L. Biomedicine & Pharmacotherapy, Volume 143(November 2021, 112182).
- Choi, Y. J. , Kim, N. N., Habibi, H. R., & Choi, C. Y. (2016). Effects of gonadotropin inhibitory hormone or gonadotropin-releasing hormone on reproduction-related genes in the protandrous cinnamon clownfish, Amphiprion melanopus. General and Comparative Endocrinology, 235, 89–99. [CrossRef]
- Choudhury, H. , Pandey, M., Hua, C. K., Mun, C. S., Jing, J. K., Kong, L., Ern, L. Y., Ashraf, N. A., Kit, S. W., Yee, T. S., Pichika, M. R., Gorain, B., & Kesharwani, P. An update on natural compounds in the remedy of diabetes mellitus: A systematic review. Journal of Traditional and Complementary Medicine 2018, 8, 361–376. [Google Scholar] [CrossRef] [PubMed]
- Chu, Y. L. , Xu, Y. R., Yang, W. X., & Sun, Y. Aging-V10I3-101391 2018, 10, 305–321. [Google Scholar]
- Clark, A. R. , & Stokes, Y. M. (2011). Follicle structure influences the availability of oxygen to the oocyte in antral follicles M. (2011). Computational and Mathematical Methods in Medicine. 2011. [CrossRef]
- Clarke, H. J. Regulation of germ cell development by intercellular signaling in the mammalian ovarian follicle. Wiley Interdisciplinary Reviews: Developmental Biology 2018, 7, 1–33. [Google Scholar] [CrossRef]
- Comish, P. B. , Drumond, A. L., Kinnell, H. L., Anderson, R. A., Matin, A., Meistrich, M. L., & Shetty, G. Fetal cyclophosphamide exposure induces testicular cancer and reduced spermatogenesis and ovarian follicle numbers in mice. PLoS ONE, 2014, 9. [CrossRef]
- Contreras-Zentella, M. L. , & Hernández-Muñoz, R. Is Liver Enzyme Release Really Associated with Cell Necrosis Induced by Oxidant Stress? Oxidative Medicine and Cellular Longevity 2016, 2016, 3529149. [Google Scholar] [CrossRef] [PubMed]
- Coskun, D. , Britto, D. T., Shi, W., & Kronzucker, H. J. How Plant Root Exudates Shape the Nitrogen Cycle. Trends in Plant Science 2017, 22, 661–673. [Google Scholar] [CrossRef]
- Cruz, G. , Fernandois, D., & Paredes, A. H. Ovarian function and reproductive senescence in the rat: Role of ovarian sympathetic innervation. Reproduction 2017, 153, R59–R68. [Google Scholar] [CrossRef]
- da Broi, M. G. , Giorgi, V. S. I., Wang, F., Keefe, D. L., Albertini, D., & Navarro, P. A. Influence of follicular fluid and cumulus cells on oocyte quality: clinical implications. Journal of Assisted Reproduction and Genetics 2018, 35, 735–751. [Google Scholar] [CrossRef] [PubMed]
- da Broi, M. G. , Giorgi, V. S. I., Wang, F., Keefe, D. L., Albertini, D., & Navarro, P. A. Influence of follicular fluid and cumulus cells on oocyte quality: Clinical implications. Journal of Assisted Reproduction and Genetics 2018, 35, 735–751. [Google Scholar] [CrossRef]
- Darkwah, W. K. , Kadri, A., Adormaa, B. B., & Aidoo, G. Cephalometric study of the relationship between facial morphology and ethnicity: Review article. Translational Research in Anatomy 2018, 12, 20–24. [Google Scholar] [CrossRef]
- David Lazer, Ryan Kennedy, Gary King, & Vespignani Alessandro. (2014). The Parable of Google Flu: Traps in Big Data Analysis. Science, 343(March), 1203–1205. www.sciencemag.orgSCIENCEVOL34314MARCH2014.
- Department Of General Histology General Embriology Introduction. (n.d.).
- Dey, S. , Samanta, P., Pal, S., Mukherjee, A. K., Kole, D., & Ghosh, A. R. Integrative assessment of biomarker responses in teleostean fishes exposed to glyphosate-based herbicide (Excel Mera 71). Emerging Contaminants 2016, 2, 191–203. [Google Scholar] [CrossRef]
- Dharmalingam, K. , Birdi, A., Tomo, S., Sreenivasulu, K., Charan, J., Yadav, D., Purohit, P., & Sharma, P. Trace Elements as Immunoregulators in SARS-CoV-2 and Other Viral Infections. Indian Journal of Clinical Biochemistry : IJCB 2021, 36, 416–426. [Google Scholar] [CrossRef]
- Diederich, L. , Iv, T. C. S. K., Kuhn, V., & Kramer, C. M. Red Blood Cell Function and Dysfunction 2017, 26, 718–742. [Google Scholar] [CrossRef]
- Dollah, M. A. , Parhizkar, S., Latiff, L. A., & bin Hassan, M. H. Toxicity effect of nigella sativa on the liver function of rats. Advanced Pharmaceutical Bulletin 2013, 3, 97–102. [Google Scholar] [CrossRef]
- Dubey, P. N. , Singh, B., Mishra, B. K., Kant, K., & Solanki, R. K. Nigella (Nigella sativa): A high value seed spice with immense medicinal potential. Indian Journal of Agricultural Sciences 2016, 86, 967–979. [Google Scholar]
- Ebrahimi, M. , & Akbari Asbagh, F. Pathogenesis and causes of premature ovarian failure: an update. International Journal of Fertility & Sterility 2011, 5, 54–65. [Google Scholar]
- EFFECT ON REPRODUCTIVE SYSTEM. (n.d.).
- Effenberger, K. , Breyer, S., & Schobert, R. Terpene Conjugates of the Nigella sativa Seed-Oil Constituent Thymoquinone with Enhanced Efficacy in Cancer Cells 2010, 7, 129–139. [Google Scholar]
- Eid, A. M. , Elmarzugi, N. A., Ayyash, L. M. A., Sawafta, M. N., & Daana, H. I. A Review on the Cosmeceutical and External Applications of Nigella sativa 2017, 2017. [Google Scholar]
- Eleawa, S. M. , Alkhateeb, M. A., Alhashem, F. H., Bin-Jaliah, I., Sakr, H. F., Elrefaey, H. M., Elkarib, A. O., Alessa, R. M., Haidara, M. A., Shatoor, A. S., & Khalil, M. A. Resveratrol reverses cadmium chloride-induced testicular damage and subfertility by downregulating p53 and Bax and upregulating gonadotropins and Bcl-2 gene expression. Journal of Reproduction and Development 2014, 60, 115–127. [Google Scholar] [CrossRef] [PubMed]
- Elements, T. B. , Rodríguez-Álvarez, M., Paz, S., Hardisson, A., González-Weller, D., Rubio, C., & Gutiérrez, Á. J. (2011). Assessment of Toxic Metals (Al, Cd, Pb) and Trace Elements (B, Ba, Co, Cr, Cu, Fe, Mn, Mo, Li, Zn, Ni, Sr, V) in the Common Kestrel (. Biological Trace Element Research. [CrossRef]
- El-hack, M. E. A. , & Alagawany, M. Review Article Nutritional, Healthical and Therapeutic Efficacy of Black Cumin ( Nigella sativa ) in Animals, Poultry and Humans. International Journal of Pharmacology 2016, 12, 232–248. [Google Scholar] [CrossRef]
- Elkareem, M. A. , Abd, M. A. M., Rahman, E., Khalil, N. S. A., & Amer, A. S. Antioxidant and cytoprotective effects of Nigella sativa L. seeds on the testis of monosodium glutamate challenged rats. Scientific Reports 2021, 1–16. [Google Scholar] [CrossRef]
- El-kholy, A. , Eraky, M., & Omar, G. (2018). Print ISSN : 1110 - 208X Online ISSN : 2357 - 0016. January. [CrossRef]
- Elliott, S. (2010a). E c e i p t. Training.
- Elliott, S. (2010b). N v o i c e. Science.
- Elmasry, T. A. , Al-Shaalan, N. H., Tousson, E., El-Morshedy, K., & Al-Ghadeer, A. Star anise extracts modulation of reproductive parameters, fertility potential and DNA fragmentation induced by growth promoter equigan in rat testes. Brazilian Journal of Pharmaceutical Sciences 2018, 54, 1–10. [Google Scholar] [CrossRef]
- El-tohamy, M. M. , Ws, E., & Ri, E. (2015). The Beneficial Effects of Nigella sativa, Raphanus sativus and Eruca sativa Seed Cakes to Improve Male Rabbit Fertility, Immunity and Production The Beneficial Effects of Nigella sativa, Raphanus sativus and Eruca sativa Seed Cakes to Improve Male Rabb. October.
- Erhirhie, E. O. , Ihekwereme, C. P., & Ilodigwe, E. E. Advances in acute toxicity testing: strengths, weaknesses and regulatory acceptance. Interdisciplinary Toxicology 2018, 11, 5–12. [Google Scholar] [CrossRef] [PubMed]
- Evaluation of Cep. (n.d.).
- FACULTY OF MEDICINE Course Code : ANA4315. 2019, 2019.
- Farooqui, Z. , Ahmed, F., Rizwan, S., Shahid, F., Khan, A. A., & Khan, F. No TitleProtective effect of Nigella sativa oil on cisplatin induced nephrotoxicity and oxidative damage in rat kidney. Biomedicine & Pharmacotherapy = Biomedecine & Pharmacotherapie 2017, 85, 7–15. [Google Scholar] [CrossRef]
- Farooqui, Z. , Ahmed, F., Rizwan, S., Shahid, F., Khan, A. A., & Khan, F. Protective effect of Nigella sativa oil on cisplatin induced nephrotoxicity and oxidative damage in rat kidney. Biomedicine & Pharmacotherapy = Biomedecine & Pharmacotherapie 2017, 85, 7–15. [Google Scholar] [CrossRef]
- Feng, W. , Li, M., Hao, Z., & Zhang, J. (2019). Analytical Methods of Isolation and Identification. In V. Rao, D. Mans, & L. Rao (Eds.), Phytochemicals in Human Health. IntechOpen. [CrossRef]
- Fiorentini, D. , Cappadone, C., Farruggia, G., & Prata, C. Magnesium: Biochemistry, Nutrition, Detection, and Social Impact of Diseases Linked to Its Deficiency. Nutrients 2021, 13, 1136. [Google Scholar] [CrossRef]
- Food, I. (2018). Major nutritional compositions of black cumin seeds – cultivated in Bangladesh and the physicochemical characteristics of its oil. 25(December), 2634–2639.
- Forouzanfar, F. , Sedigheh, B., Bazzaz, F., & Hosseinzadeh, H. (n.d.). Black cumin ( Nigella sativa ) and its constituent ( thymoquinone ): a review on antimicrobial effects. 7.
- Frezza, C. , Sciubba, F., Vincenti, F., Montesano, C., Venditti, A., Enrica, M., Cocco, D., & Bianco, A. (2020). Ac ce pt us. Plant Biosystems - An International Journal Dealing with All Aspects of Plant Biology. [CrossRef]
- Gany, Z. S. A. , & Mahdi, M. F. Cytotoxic Assay of Nigella sativa Leaf Callus Extract (Thymol) on Hep-2 Cell Line Using ELISA Assay. Iraqi J Pharm Sci 2008, 17, 63–66. [Google Scholar]
- Gharby, S. , Harhar, H., Guillaume, D., Roudani, A., Boulbaroud, S., Ibrahimi, M., Ahmad, M., & Sultana, S. Chemical investigation of Nigella sativa L. seed oil produced in Morocco. Journal of the Saudi Society of Agricultural Sciences 2015, 14, 172–177. [Google Scholar] [CrossRef]
- Gholamnezhad, Z. , Havakhah, S., & Hossein, M. Preclinical and clinical effects of Nigella sativa and its constituent, thymoquinone : A review. Journal of Ethnopharmacology 2016, 190, 372–386. [Google Scholar] [CrossRef]
- Gholamnezhad, Z. , Shakeri, F., Saadat, S., Ghorani, V., & Boskabady, M. H. Clinical and experimental effects of Nigella sativa and its constituents on respiratory and allergic disorders. Avicenna Journal of Phytomedicine 2019, 9, 195–212. [Google Scholar] [CrossRef] [PubMed]
- Goff, J. P. Invited review: Mineral absorption mechanisms, mineral interactions that affect acid–base and antioxidant status, and diet considerations to improve mineral status. Journal of Dairy Science 2018, 101, 2763–2813. [Google Scholar] [CrossRef] [PubMed]
- Griffin, J. , Emery, B. R., Huang, I., Peterson, C. M., & Carrell, D. T. (2006a). Comparative analysis of follicle morphology and oocyte diameter in four mammalian species (mouse, hamster, pig, and human). Journal of Experimental & Clinical Assisted Reproduction, 3, 2. [CrossRef]
- Griffin, J. , Emery, B. R., Huang, I., Peterson, C. M., & Carrell, D. T. (2006b). Comparative analysis of follicle morphology and oocyte diameter in four mammalian species (mouse, hamster, pig, and human). Journal of Experimental and Clinical Assisted Reproduction, 3(February 2006). [CrossRef]
- Hadi, M. Y. , Mohammed, G. J., & Hameed, I. H. (2016). Analysis of bioactive chemical compounds of Nigella sativa using gas chromatography-mass spectrometry. 8(February), 8–24. [CrossRef]
- Hanafy, M. S. , & Hatem, M. E. (1991). (1991). No TStudies on the antimicrobial activity of Nigella sativa seed (black cumin).itle. Journal of Ethnopharmacology, 34(2-3)(275–278). [CrossRef]
- Hannan, M. A. , Zahan, M. S., Sarker, P. P., Moni, A., Ha, H., & Uddin, M. J. (2021). Protective effects of black cumin (Nigella sativa) and its bioactive constituent, thymoquinone against kidney injury: An aspect on pharmacological insights. International Journal of Molecular Sciences, 22(16). [CrossRef]
- Hannan, Md. A. , Rahman, Md. A., Sohag, A. A. M., Uddin, Md. J., Dash, R., Sikder, M. H., Rahman, Md. S., Timalsina, B., Munni, Y. A., Sarker, P. P., Alam, M., Mohibbullah, Md., Haque, Md. N., Jahan, I., Hossain, Md. T., Afrin, T., Rahman, Md. M., Tahjib-Ul-Arif, Md., Mitra, S., … Kim, B. (2021). Black Cumin (Nigella sativa L.): A Comprehensive Review on Phytochemistry, Health Benefits, Molecular Pharmacology, and Safety. Nutrients, 13(6). [CrossRef]
- Harakeh, S. , Almuhayawi, M. S., Akefe, I. O., Saber, S. H., al Jaouni, S. K., Alzughaibi, T., Almehmadi, Y., Ali, S. S., Bharali, D. J., & Mousa, S. (2022). Novel Pomegranate-Nanoparticles Ameliorate Cisplatin-Induced Nephrotoxicity and Improves Cisplatin Anti-Cancer Efficacy in Ehrlich Carcinoma Mice Model. Molecules, 27(5). [CrossRef]
- Haseena, S. , Aithal, M., Das, K. K., & Saheb, S. H. (2015). 7(8), 2015.
- Hassan, E. , El-Neweshy, M., Hassan, M., & Noreldin, A. (2019). Thymoquinone attenuates testicular and spermotoxicity following subchronic lead exposure in male rats: Possible mechanisms are involved. Life Sciences, 230, 132–140. [CrossRef]
- Hassan, S. T. S. , & Šudomová, M. (2020). Comment on: Effects of nigella sativa on type-2 diabetes mellitus: A systematic review. International Journal of Environmental Research and Public Health, 17(5). [CrossRef]
- Hatzirodos, N. , Hummitzsch, K., Irving-Rodgers, H. F., & Rodgers, R. J. (2014). Transcriptome profiling of the theca interna in transition from small to large antral ovarian Follicles. PLoS ONE, 9(5). [CrossRef]
- Holesh JE, Bass AN, L. M. (n.d.). Physiology, Ovulation. Treasure Island (FL): StatPearls Publishing; 2023. https://www.ncbi.nlm.nih.gov/books/NBK441996/.
- Hongyan Yang, Yan Xie, Dongyu Yang, & Decheng Ren. Oxidative stress-induced apoptosis in granulosa cells involves. Oncotarget 2017, 8, 25310–25322. [Google Scholar] [CrossRef]
- Huang, Q. , Chen, S., Chen, J., Shi, Q., & Lin, S. (2022). Therapeutic options for premature ovarian insufficiency : an updated review. Reproductive Biology and Endocrinology, 1–16. [CrossRef]
- Hubscher, C. H. , Brooks, D. L., & Johnson, J. R. A quantitative method for assessing stages of the rat estrous cycle. Biotechnic \& Histochemistry 2005, 80, 79–87. [Google Scholar] [CrossRef]
- Hulisz, D. Efficacy of Zinc Against Common Cold Viruses: An Overview. Journal of the American Pharmacists Association 2004, 44, 594–603. [Google Scholar] [CrossRef] [PubMed]
- Hummitzsch, K. , Irving-Rodgers, H. F., Hatzirodos, N., Bonner, W., Sabatier, L., Reinhardt, D. P., Sado, Y., Ninomiya, Y., Wilhelm, D., & Rodgers, R. J. A New Model of Development of the Mammalian Ovary and Follicles. PLoS ONE, 2013, 8. [CrossRef]
- Ijaz, M. U. , Shahzadi, S., Samad, A., Ehsan, N., Ahmed, H., Tahir, A., Rehman, H., & Anwar, H. Dose-Dependent Effect of Polystyrene Microplastics on the Testicular Tissues of the Male Sprague Dawley Rats. Dose-Response 2021, 19, 1–11. [Google Scholar] [CrossRef]
- Internal organs 2.pdf. (2019). . 2062.
- International, A. , Reviewed, P., & Journal, S. (n.d.). of Biological S ciences.
- Intervention, Y. O. F. (n.d.). Staff Development and Training Intervention Project Staff Development and Training Intervention Project, 1–8.
- Ishtiaq, S. , Ashraf, M., Hayat, M. Q., & Asrar, M. (2013). Phytochemical Analysis of Nigella sativa and its Antibacterial Activity against Clinical Isolates Identified by Ribotyping Pakistan Council for Phytochemical Analysis of Nigella sativa and its Antibacterial Activity against Clinical Isolates Identified by. November.
- Ishunina, T. A. , Kruijver, F. P. M., Balesar, R., & Swaab, D. F. Differential expression of estrogen receptor α and β immunoreactivity in the human supraoptic nucleus in relation to sex and aging. Journal of Clinical Endocrinology and Metabolism 2000, 85, 3283–3291. [Google Scholar] [CrossRef] [PubMed]
- It, E. H. A. T. I. S. (n.d.). Human factors & ergonomics.
- Jaarin, K. , Foong, W. D., Yeoh, M. H., Kamarul, Z. Y. N., Qodriyah, H. M. S., Azman, A., Zuhair, J. S. F., Juliana, A. H., & Kamisah, Y. Mechanisms of the antihypertensive effects of Nigella sativa oil in L-NAME-induced hypertensive rats. Clinics 2015, 70, 751–757. [Google Scholar] [CrossRef] [PubMed]
- Jankowska, K. Premature ovarian failure. Przeglad Menopauzalny = Menopause Review 2017, 16, 51–56. [Google Scholar] [CrossRef] [PubMed]
- Jasim, W. K. , Hassan, M. S., & Keam, G. G. Study the effect of Nigella Sativa on thyroid function and reproductive hormone of female rat. J Contemp Med Sci | 2016, 2, 67–69. [Google Scholar]
- Jian, Z. , Liu, R., Zhu, X., Smerin, D., Zhong, Y., Gu, L., Fang, W., & Xiong, X. (2019). The Involvement and Therapy Target of Immune Cells After Ischemic Stroke. Frontiers in Immunology 10. [CrossRef]
- Jiang, Y. , Shen, M., Chen, Y., Wei, Y., Tao, J., & Liu, H. Melatonin represses mitophagy to protect mouse granulosa cells from oxidative damage. Biomolecules 2021, 11. [Google Scholar] [CrossRef]
- Jones, A. S. K. , & Shikanov, A. Follicle development as an orchestrated signaling network in a 3D organoid. Journal of Biological Engineering 2019, 13, 2. [Google Scholar] [CrossRef]
- Jones, A. S. K. , & Shikanov, A. Follicle development as an orchestrated signaling network in a 3D organoid. Journal of Biological Engineering 2019, 13, 1–12. [Google Scholar] [CrossRef]
- Jurban, S. Using Multi Sensory Approach for Teaching English Skills and Its Effect on Students ’. European Scientific Journal 2011, 8, 50–61. [Google Scholar]
- Kadry, H. , Noorani, B., & Cucullo, L. A blood–brain barrier overview on structure, function, impairment, and biomarkers of integrity. Fluids and Barriers of the CNS 2020, 17, 69. [Google Scholar] [CrossRef] [PubMed]
- Kamal, A. , Arif, J. M., & Ahmad, I. Z. Potential of Nigella sativa L. seed during different phases of germination on inhibition of bacterial growth. E3 Journal of Biotechnology and Pharmaceutical Research 2010, 1, 9–13. [Google Scholar]
- Kamil, Z. H. (2013). Spectacular Black Seeds ( Nigella sativa ) : Medical Importance Review. 10.
- Karbalay-Doust, S. , & Noorafshan, A. Stereological estimation of ovarian oocyte volume, surface area and number: Application on mice treated with nandrolone decanoate. Folia Histochemica et Cytobiologica 2012, 50, 275–279. [Google Scholar] [CrossRef] [PubMed]
- Kenealy, B. P. , & Terasawa, E. (2012). Rapid direct action of estradiol in GnRH neurons: Findings and implications. Frontiers in Endocrinology, 3(JAN), 1–8. [CrossRef]
- Khalid, O. M. , Eltayeb, M., Ali, I. A., & Musa, O. A. (2019). Effect of Oral Nigella Sativa on Fasting Blood Glucose in Adults. International Journal of Diabetes & Metabolic Disorders, 4. [CrossRef]
- Kim, E. , Cai, L., & Hyun, S. H. (2021). Effects of Stem Cell Factor/c-Kit Signaling on In Vitro Maturation of Porcine Oocytes and Subsequent Developmental Competence After Fertilization. Frontiers in Veterinary Science, 8(October). [CrossRef]
- Kim, J. , & You, S. Extended adverse effects of cyclophosphamide on mouse ovarian function. BMC Pharmacology & Toxicology 2021, 22, 3. [Google Scholar] [CrossRef]
- Kolahdooz, M. , Nasri, S., Modarres, S. Z., Kianbakht, S., & Huseini, H. F. (2014). Effects of Nigella sativa L. seed oil on abnormal semen quality in infertile men: A randomized, double-blind, placebo-controlled clinical trial. European Journal of Integrative Medicine. [CrossRef]
- Kooti, W. , Hasanzadeh-noohi, Z., Sharafi-ahvazi, N., Asadi-samani, M., & Ashtary-larky, D. Phytochemistry, pharmacology, and therapeutic uses of black seed ( Nigella sativa ). Chinese Journal of Natural Medicines 2016, 14, 732–745. [Google Scholar] [CrossRef] [PubMed]
- Kordowitzki, P. , Sokołowska, G., Wasielak-Politowska, M., Skowronska, A., & Skowronski, M. T. (2021). Pannexins and connexins: Their relevance for oocyte developmental competence. International Journal of Molecular Sciences, 22(11). [CrossRef]
- Koshak, A. , Koshak, E., & Heinrich, M. (2017). Medicinal benefits of Nigella sativa in bronchial asthma: A literature review. Saudi Pharmaceutical Journal : SPJ : The Official Publication of the Saudi Pharmaceutical Society, 25(8)(1130–1136). [CrossRef]
- Kozat, S. (2017). Methods of Diagnosing in Liver Diseases for Dog and Cats. 10(2), 36–46.
- Kumar, A. , Siddiqi, N. J., Alrashood, S. T., Khan, H. A., Dubey, A., & Sharma, B. (2021). Protective effect of eugenol on hepatic inflammation and oxidative stress induced by cadmium in male rats. Biomedicine & Pharmacotherapy, 139, 111588. [CrossRef]
- Kumar, R. , Zakharov, M. N., Khan, S. H., Miki, R., Jang, H., Toraldo, G., Singh, R., Bhasin, S., & Jasuja, R. (2011). The Dynamic Structure of the Estrogen Receptor. 2011. [CrossRef]
- Kumar, S. , Metz, D. C., Ellenberg, S., Kaplan, D. E., & Goldberg, D. S. Risk Factors and Incidence of Gastric Cancer After Detection of Helicobacter pylori Infection: A Large Cohort Study. Gastroenterology 2020, 158, 527–536.e7. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S. , VK, J., George, J., & Mukkadan, J. Bleeding time and Clotting Time in Healthy Male and Female College Students of Karukutty Village, Kerala. Health Prospect 2013, 12, 7–9. [Google Scholar] [CrossRef]
- Lala V, Goyal A, M. da. (2022). Liver Function Tests. StatPearls Publishing; 2022. https://www.ncbi.nlm.nih.gov/books/NBK482489/.
- LaPlante, C. D. , Bansal, R., Dunphy, K. A., Jerry, D. J., & Vandenberg, L. N. Oxybenzone alters mammary gland morphology in mice exposed during pregnancy and lactation. Journal of the Endocrine Society 2018, 2, 903–921. [Google Scholar] [CrossRef]
- Lazic, S. E. , Semenova, E., & Williams, D. P. Determining organ weight toxicity with Bayesian causal models: Improving on the analysis of relative organ weights. Scientific Reports 2020, 10, 6625. [Google Scholar] [CrossRef]
- Lee, S. , Nambi, R. W., Won, S., Katya, K., & Bai, S. C. (2016). Dietary selenium requirement and toxicity levels in juvenile Nile tilapia, Oreochromis niloticus. Aquaculture 464, 153–158. [CrossRef]
- Leong, X. F. , Rais Mustafa, M., & Jaarin, K. (2013). Erratum: Nigella sativa and Its Protective Role in Oxidative Stress and Hypertension (Evidence-based Complementary and Alternative Medicine). Evidence-Based Complementary and Alternative Medicine. [CrossRef]
- Leong, X.-F. , Choy, K. W., & Alias, A. Anti-Inflammatory Effects of Thymoquinone in Atherosclerosis: A Mini Review. Frontiers in Pharmacology 2021, 12, 1–8. [Google Scholar] [CrossRef]
- Liu, M. , Li, M., Liu, J., Wang, H., Zhong, D., Zhou, H., & Yang, B. Elevated urinary urea by high-protein diet could be one of the inducements of bladder disorders. Journal of Translational Medicine 2016, 14, 53. [Google Scholar] [CrossRef]
- Livezey, M. , Kim, J. E., & Shapiro, D. J. A New Role for Estrogen Receptor α in Cell Proliferation and Cancer : Activating the Anticipatory Unfolded Protein Response, 2018, 9. [CrossRef]
- Lorke, D. A new approach to practical acute toxicity testing. Archives of Toxicology 1983, 54, 275–287. [Google Scholar] [CrossRef] [PubMed]
- Mahboubi, M. Natural therapeutic approach of Nigella sativa (Black seed) fixed oil in management of Sinusitis. Integrative Medicine Research 2018, 7, 27–32. [Google Scholar] [CrossRef] [PubMed]
- Mahdavi, R. , Heshmati, J., & Namazi, N. (2015). Ac ce p te d t. Perspectives in Medicine. [CrossRef]
- Mahdy, A. K. H. , Pavani, K. C., Baron, E., & da Silva, F. M. (2017). Studies on Gene Expression and Developmental Competence of Bovine Embryos Produced Under Different Conditions of Heat Stress. In M. Abubakar (Ed.), Trends and Advances in Veterinary Genetics. IntechOpen. [CrossRef]
- Majeed, A. , Minhas, W. A., Mehboob, N., Farooq, S., Hussain, M., Alam, S., & Rizwan, M. S. Iron application improves yield, economic returns and grain-Fe concentration of mungbean. PLOS ONE 2020, 15, e0230720. [Google Scholar] [CrossRef]
- Malgorzata, D. , Malgorzata, G., Malgorzata, K.-S., & Tabarowski, Z. (2016). The Primordial to Primary Follicle Transition — A Reliable Marker of Ovarian Function. In R. P. Carreira (Ed.), Insights from Animal Reproduction. IntechOpen. [CrossRef]
- Mansour, S. W. , Sangi, S., Harsha, S., Khaleel, M. A., & Ibrahim, A. R. N. Sensibility of male rats fertility against olive oil, Nigella sativa oil and pomegranate extract. Asian Pacific Journal of Tropical Biomedicine 2013, 3, 563–568. [Google Scholar] [CrossRef] [PubMed]
- Mar, O. , El-naggar, T., Pilar, G., Arce, C., & Carretero, E. (2010). Nigella sativa L. Seed Extract Modulates the Neurotransmitter Amino Acids Release in Cultured Neurons In Vitro. 2010. [CrossRef]
- Marshall, W. F. , Young, K. D., Swaffer, M., Wood, E., Nurse, P., Kimura, A., Frankel, J., Wallingford, J., Walbot, V., Qu, X., & Roeder, A. H. K. What determines cell size? BMC Biology 2012, 10, 101. [Google Scholar] [CrossRef]
- Mascarenhas, M. N. , Flaxman, S. R., Boerma, T., Vanderpoel, S., & Stevens, G. A. National, Regional, and Global Trends in Infertility Prevalence Since 1990: A Systematic Analysis of 277 Health Surveys. PLoS Medicine 2012, 9, 1–12. [Google Scholar] [CrossRef]
- Mathur, M. , Gaur, J., Sharma, R., & Haldiya, K. Antidiabetic Properties of a Spice Plant Nigella sativa. Journal Of Endocrinology And Metabolism 2011, 1, (1–8). [Google Scholar]
- Mathur, V. , & Sharma, M. (2021). Therapeutic use of nigella sativa on metabolic disorders: A review. May.
- Maulidiani, M. , Sheikh, B. Y., Mediani, A., Sze, L., Sa, I., Abas, F., & Lajis, N. H. (2015). Phytochemistry Letters Differentiation of Nigella sativa seeds from four different origins and their bioactivity correlations based on NMR-metabolomics approach. 13, 308–318. [CrossRef]
- Medical, R. (n.d.). a l e s e c e i p t, 3–5.
- Medicine, F. O. F. (2008). Faculty of Medicine. Encyclopedia of Public Health, 427–427. [CrossRef]
- MedlinePlus Medical Encyclopedia. (2022). Medline. Medlineplus.gov. https://medlineplus.gov/ency/article/002062.htm.
- Mehranjani, M. S. , Noorafshan, A., Hamta, A., Momeni, H. R., Abnosi, M. H., Mahmoodi, M., Anvari, M., & Hazaveh, M. Effects of vitamin E on ovarian tissue of rats following treatment with p-nonylphenol: A stereological study. Iranian Journal of Reproductive Medicine 2010, 8, 1–9. [Google Scholar]
- Michel, J. , Abd Rani, N. Z., & Husain, K. (2020). A Review on the Potential Use of Medicinal Plants From Asteraceae and Lamiaceae Plant Family in Cardiovascular Diseases. Frontiers in Pharmacology 11. [CrossRef]
- Mills, C. C. , Kolb, E. A., & Sampson, V. B. Development of Chemotherapy with Cell-Cycle Inhibitors for Adult and Pediatric Cancer Therapy. Cancer Research 2018, 78, 320–325. [Google Scholar] [CrossRef] [PubMed]
- Mohammed, N. K. , Yazid, M., Manap, A., Tan, C. P., Muhialdin, B. J., Alhelli, A. M., Shobirin, A., & Hussin, M. (2016). The Effects of Different Extraction Methods on Antioxidant Properties, Chemical Composition, and Thermal Behavior of Black Seed ( Nigella sativa L.) Oil. 2016.
- Morris AL, M. SS. (2022). Biochemistry, Nutrients. (Updated 20). Treasure Island (FL): StatPearls Publishing. https://www.ncbi.nlm.nih.gov/books/NBK554545/.
- Mosaed, M. M. (n.d.-a). Anatomy of jejunum, ileum and large intestine Jejunum and Ileum.
- Mosaed, M. M. (n.d.-b). Anatomy of the liver and biliary system.
- Mosbah, R. , Yousef, M. I., Maranghi, F., & Mantovani, A. (2014). and Industrial Health. [CrossRef]
- Motta, P. M. , Makabe, S., & Nottola, S. A. The ultrastructure of human reproduction. 1. The natural history of the female germ cell: Origin, migration and differentiation inside the developing ovary. Human Reproduction Update 1997, 3, 281–295. [Google Scholar] [CrossRef] [PubMed]
- Munday, A. J. , & Hunt, J. B. University of Southampton. Tribology 1970, 3, 106–107. [Google Scholar] [CrossRef]
- Muthai, K. U. , Karori, M. S., Muchugi, A., Indieka, A. S., Dembele, C., Mng’omba, S., & Jamnadass, R. Nutritional variation in baobab (Adansonia digitata L.) fruit pulp and seeds based on Africa geographical regions. Food Science & Nutrition 2017, 5, 1116–1129. [Google Scholar] [CrossRef]
- Muthai, K. U. , Mbuthia, |, Karori, S., Muchugi, A., Indieka, A. S., Dembele, | Catherine, Mng’omba, S., & Jamnadass, | Ramni. Nutritional variation in baobab (Adansonia digitata L.) fruit pulp and seeds based on Africa geographical regions. Food Sci Nutr 2017, 5, 1116–1129. [Google Scholar] [CrossRef]
- National, G. , & Pillars, H. (n.d.). No 主観的健康感を中心とした在宅高齢者における 健康関連指標に関する共 分散構造分析 Title.
- Nelson, K. S. , Bwala, D. A. A., & Nuhu, E. J. Nigerian veterinary journal. Nigerian Veterinary Journal 2015, 36, 1299–1317. [Google Scholar]
- Nie, H. , Zhang, R., Yu, X., Zhang, Y., Yan, P., Li, E., Wang, R., & Wu, X. (2021). Molecular cloning, immunological characterization, and expression analysis of gonadotropin-releasing hormone (GnRH) in the brain of the Chinese alligator during different stages of reproductive cycle. Gene, 789, 145672. [CrossRef]
- Nigella sativa, L. (n.d.).
- Nuclear, I. (2009). Determination of essential elements in milk and urine of camel and in nigella sativa Seeds. 42.
- OCDE OECD GUIDELINE FOR THE TESTING OF CHEMICALS. (1997). TG 471: Bacterial Reverse Mutation Test. OECD Guideline for Testing of Chemicals, July, 1–13.
- Ojah, A. , & Borthakur, M. K. (2021). UTTAR PRADESH JOURNAL OF ZOOLOGY : EFFECT OF METHANOLIC EXTRACT OF Persicaria hydropiper ROOT ON ESTROUS CYCLE AND REPRODUCTIVE EFFECT OF METHANOLIC EXTRACT OF Persicaria hydropiper ROOT ON ESTROUS CYCLE AND REPRODUCTIVE HORMONES IN FEMALE ALBINO MICE. June.
- Oluwasola, A. , Olayaki, L. A., & Ayinde, T. O. Effects of Melatonin on Estrous Cycle Changes Induced by Ethanolic Extract of Cannabis-sativa in Female Wistar Rats 2019, 19, 76–81. [Google Scholar]
- Orisaka, M. , Tajima, K., Tsang, B. K., & Kotsuji, F. Oocyte-granulosa-theca cell interactions during preantral follicular development. Journal of Ovarian Research 2009, 2, 1–7. [Google Scholar] [CrossRef]
- Palacios, O. A. , Bashan, Y., & de-Bashan, L. E. Proven and potential involvement of vitamins in interactions of plants with plant growth-promoting bacteria—an overview. Biology and Fertility of Soils 2014, 50, 415–432. [Google Scholar] [CrossRef]
- Parhizkar, S. , Latiff, L. A., & Parsa, A. (2016). Effect of Nigella sativa on reproductive system in experimental menopause rat model. 6(1), 95–103. [PubMed]
- Paterni, I. , Granchi, C., Katzenellenbogen, J. A., & Minutolo, F. (2014). Estrogen receptors alpha (ERα) and beta (ERβ): Subtype-selective ligands and clinical potential. Steroids, 90(June), 13–29. [CrossRef]
- Patrizio, M. C. I. and P. (2015). new thinking on gender, reproductive technologies and global movements in the 21st century. Human Reproduction Update Advance Access Published, Vol.0.
- Perše, M. , & Večerić-Haler, Ž. (2018). Cisplatin-induced rodent model of kidney injury: Characteristics and challenges. BioMed Research International, 2018. [CrossRef]
- Physiology, R. , & Section, B. (2008). organs of female rats ch Ar of ive. 6(1), 7–11.
- Ping, N. C. , Hashim, N. H., & Hasan Adli, D. S. (2014). Effects of Nigella sativa (Habbatus sauda) oil and nicotine chronic treatments on sperm parameters and testis histological features of rats. Evidence-Based Complementary and Alternative Medicine, 2014. [CrossRef]
- Porwal, M. , Khan, N. A., & Maheshwari, K. K. (2017). Evaluation of Acute and Subacute Oral Toxicity Induced by Ethanolic Extract of Marsdenia tenacissima Leaves in Experimental Rats. Scientia Pharmaceutica, 85. [CrossRef]
- Potential, P. , & Nigella, O. F. (2018). THERAPEUTIC & PHARMACOLOGICAL POTENTIAL OF NIGELLA. 7(12), 153–158. [CrossRef]
- Protective, M. L. S. (2016). thymoquinone against toxicity induced by the Protective effects of Nigella sativa oil and thymoquinone against toxicity induced by the anticancer drug cyclophosphamide. 4845. [CrossRef]
- Rachid, M. , Zouhir, D., & Alberto, M. toxicity in male rats Protective effect of Nigella sativa oil against acetamiprid induced reproductive toxicity in male rats. Drug and Chemical Toxicology 2017, 0, 1–7. [Google Scholar] [CrossRef]
- Radusheva, P. , Pashev, A., Uzunova, G., Nikolova, K., Gentscheva, G., Perifanova, M., & Marudova, M. Comparative Physicochemical Analysis of Oils Derived From Nigella Sativa and Coriandrum Sativum L. Journal of Chemical Technology and Metallurgy 2021, 56, 1175–1180. [Google Scholar]
- Rai, S. , Singh, P. K., Mankotia, S., Swain, J., & Satbhai, S. B. (2021). Iron homeostasis in plants and its crosstalk with copper, zinc, and manganese. Plant Stress 1, 100008. [CrossRef]
- Rakowska, P. D. , Tiddia, M., Faruqui, N., Bankier, C., Pei, Y., Pollard, A. J., Zhang, J., & Gilmore, I. S. (2021). Antiviral surfaces and coatings and their mechanisms of action. Communications Materials, 2. [CrossRef]
- Ramadori, P. , Klag, T., Malek, N. P., & Heikenwalder, M. Platelets in chronic liver disease, from bench to bedside. JHEP Reports 2019, 1, 448–459. [Google Scholar] [CrossRef] [PubMed]
- Reference2. (n.d.).
- References442. (n.d.).
- Regulation, C. (2021). Whole Transcriptome Analysis : Implication to Estrous.
- Review, A. A. , Dalli, M., Bekkouch, O., Azizi, S., Azghar, A., Gseyra, N., & Kim, B. (2022). Nigella sativa L. Phytochemistry and Pharmacological.
- Review, P. (2013). review on therapeutic potential of N igella sativa : A miracle herb. 3(5), 337–352. [CrossRef]
- Robles, H. (2014). Phosphorus. Encyclopedia of Toxicology: Third Edition, 920–921. [CrossRef]
- Röllin, H. B. , & Nogueira, C. M. C. A. (2011). Manganese: Environmental Pollution and Health Effects, (J. O. B. T.-E. of E. H. Nriagu, Ed.; pp. 617–629). Elsevier. [CrossRef]
- Rondanelli, M. , Miccono, A., Lamburghini, S., Avanzato, I., Riva, A., Allegrini, P., Faliva, M. A., Peroni, G., Nichetti, M., & Perna, S. (2018). Self-Care for Common Colds: The Pivotal Role of Vitamin D, Vitamin C, Zinc, and Echinacea in Three Main Immune Interactive Clusters (Physical Barriers, Innate and Adaptive Immunity) Involved during an Episode of Common Colds-Practical Advice on Dosages an. Evidence-Based Complementary and Alternative Medicine : ECAM, 2018, 5813095. [CrossRef]
- Rose Khavari Nicholas Dias, Y. P. 乳鼠心肌提取 HHS Public Access. Physiology & Behavior 2017, 176, 139–148. [Google Scholar] [CrossRef]
- Rosenfield, R. L. , & Ehrmann, D. A. The Pathogenesis of Polycystic Ovary Syndrome (PCOS): The Hypothesis of PCOS as Functional Ovarian Hyperandrogenism Revisited. Endocrine Reviews 2016, 37, 467–520. [Google Scholar] [CrossRef]
- Safa Mohammed El Mobasher Omar Saleh, B. , & Khadiga Abbas Abdelatti, S. (2004). ﺍﻟﺮﺣﻴﻢ ﺍﻟﺮﺣﻤﻦ ﺍﷲ ﺑﺴﻢ Effect of Black Seed (Nigella Sativa) Supplementation on Rabbits Performance and Some Blood Parameters. January.
- Samarakoon, S. R. , Thabrew, I., Galhena, P. B., Silva, D. de, & Tennekoon, K. H. (2011). A comparison of the cytotoxic potential of standardized aqueous and ethanolic extracts of a polyherbal mixture comprised of Nigella sativa ( seeds ), Hemidesmus indicus ( roots ) and Smilax glabra ( rhizome ). 2. [CrossRef]
- Sanfins, A. , Rodrigues, P., & Albertini, D. F. (2018). GDF-9 and BMP-15 direct the follicle symphony The oocyte-a key player in ovarian function, 20–29. [CrossRef]
- Sativa, N. , Ranunculaceae, L., & Muhammad, B. (2016). CHEMICAL CONTENTS AND CHARACTERIZATION OF THE BIOLOGICALLY ACTIVE COMPOUNDS OF.
- Schiffer, L. , Barnard, L., Baranowski, E. S., Gilligan, L. C., Taylor, A. E., Arlt, W., Shackleton, C. H. L., & Storbeck, K. H. (2019). Human steroid biosynthesis, metabolism and excretion are differentially reflected by serum and urine steroid metabolomes: A comprehensive review. Journal of Steroid Biochemistry and Molecular Biology, 194(July), 105439. [CrossRef]
- Sciences, V. (2016). Review Article. 4.
- Scudiero, R. , & Verderame, M. Gene expression profile of estrogen receptors alpha and beta in rat brain during aging and following high fat diet. Comptes Rendus - Biologies 2017, 340, 372–378. [Google Scholar] [CrossRef]
- Seeds, L. , Standardization, I. T. S. P., Nehar, S., & Rani, P. (2011). HPTLC STUDIES ON ETHANOLIC EXTRACT OF NIGELLA SATIVA. 1, 105–108.
- Selvaraju, A. , Kaviya, S., & Dhanraj, K. M. (2019). Phytochemical analysis and anthelmintic potential of Nigella sativa against the trematode , Cotylophoron cotylophorum. 8(3), 3161–3166.
- Series, P. I. (2012). Abnormal Uterine Bleeding A Guide for Patients PATIENT INFORMATION SERIES.
- Shahin, Y. R. , Elguindy, N. M., Abdel Bary, A., & Balbaa, M. The protective mechanism of Nigella sativa against diethylnitrosamine-induced hepatocellular carcinoma through its antioxidant effect and EGFR/ERK1/2 signaling. Environmental Toxicology 2018, 33, 885–898. [Google Scholar] [CrossRef]
- Shomar, B. (2012). Major and trace elements in Nigella sativa provide a potential mechanism for its healing effects. 6(34), 4836–4843. [CrossRef]
- Silveira, D. , Prieto-Garcia, J. M., Boylan, F., Estrada, O., Fonseca-Bazzo, Y. M., Jamal, C. M., Magalhães, P. O., Pereira, E. O., Tomczyk, M., & Heinrich, M. (2020). COVID-19: Is There Evidence for the Use of Herbal Medicines as Adjuvant Symptomatic Therapy? Frontiers in Pharmacology, 11(September), 1–44. [CrossRef]
- Søndergaard, C. R. , Olsson, M. H. M., Rostkowski, M., & Jensen, J. H. Improved Treatment of Ligands and Coupling Effects in Empirical Calculation and Rationalization of pKa Values. Journal of Chemical Theory and Computation 2011, 7, 2284–2295. [Google Scholar] [CrossRef]
- Stowasser, M. , & Gordon, R. D. Primary aldosteronism: Changing definitions and new concepts of physiology and pathophysiology both inside and outside the kidney. Physiological Reviews 2016, 96, 1327–1384. [Google Scholar] [CrossRef] [PubMed]
- Sun, M. (2012). Cash Receipt. September 2012.
- Sun, Y. , Liu, Y., Ma, X., & Hu, H. (2021). The Influence of Cell Cycle Regulation on Chemotherapy. International Journal of Molecular Sciences, 22. [CrossRef]
- System, M. R. (n.d.). Male Reproductive System Male Reproductive System - side, 1-23.
- Tansaz, M. Tansaz, M. (2018). 2 , 4 , 5 ,. 9(11), 4716–4722. [CrossRef]
- Tavakkoli, A. , Mahdian, V., Razavi, B. M., & Hosseinzadeh, H. Review on clinical trials of black seed (Nigella sativa ) and its active constituent, thymoquinone. Journal of Pharmacopuncture 2017, 20, 179–193. [Google Scholar] [CrossRef]
- Tokmakov, A. A. , Stefanov, V. E., & Sato, K. I. (2020). Dissection of the Ovulatory Process Using ex vivo Approaches. Frontiers in Cell and Developmental Biology, 8(December). [CrossRef]
- Toma, C. , & Simu, G. M. (2010). Chemical composition of the Tunisian Nigella sativa. note II. Profile on fatty oil CHEMICAL COMPOSITION OF THE TUNISIAN NIGELLA SATIVA. NOTE II. PROFILE ON FATTY OIL. October 2015.
- Udu, R. , Oyweri, J., & Gathirwa, J. (2021). Antimalarial Activity of Nigella sativa L. Seed Extracts and Selection of Resistance in Plasmodium berghei ANKA in a Mouse Model. Journal of Pathogens, 2021, 6165950. [CrossRef] [PubMed]
- Unconfirmed 125575.crdownload. (n.d.).
- USMAN BALA, ISAH M. DAZAR, M. M. J. A. F., & YAKUBU. (2020). Ameliorative effect of Nigella sativa oil on lead acetate induced hepatotoxicity on adult wistar rats. Bima Journal of Science and Technology, vol 4(2).
- Uzochukwu, C. (2020). theRepository at St. Cloud State NIGELLA SATIVA SUPPLEMENTATION EFFECTS ON GROWTH, ORGAN DEVELOPMENT AND REPRODUCTION IN SPRAGUE-.
- Valadabadi, S. A. , & Farahani, H. A. (2011). Investigation of biofertilizers influence on quantity and quality characteristics in Nigella sativa L. 3(March). 88–92.
- van Tran, L. , Malla, B. A., Kumar, S., & Tyagi, A. K. Polyunsaturated Fatty Acids in Male Ruminant Reproduction - A Review. Asian-Australasian Journal of Animal Sciences 2017, 30, 622–637. [Google Scholar] [CrossRef]
- Verghese. 基因的改变NIH Public Access. Bone 2011, 23, 1–7. [Google Scholar] [CrossRef]
- Vision, O. (2017). Chapter One. Commentary on Genesis, 25–77. [CrossRef]
- Wang, N. , Zhao, F., Lin, P., Zhang, G., Tang, K., Wang, A., & Jin, Y. Knockdown of XBP1 by RNAi in mouse granulosa cells promotes apoptosis, inhibits cell cycle, and decreases estradiol synthesis. International Journal of Molecular Sciences 2017, 18, 1–13. [Google Scholar] [CrossRef]
- Wang, W. , Mai, K., Zhang, W., Xu, W., Ai, Q., Liufu, Z., & Li, H. (2012). Dietary selenium requirement and its toxicity in juvenile abalone Haliotis discus hannai Ino. Aquaculture, 330–333, 42–46. [CrossRef]
- Weiss, G. , & Carver, P. L. Role of divalent metals in infectious disease susceptibility and outcome. Clinical Microbiology and Infection 2018, 24, 16–23. [Google Scholar] [CrossRef]
- WHO. (2020). No Title. In wikepedia. https://www.who.int/news-room/fact-sheets/detail/infertility.
- Williams, C. J. , & Erickson, G. F. (2000). Morphology and Physiology of the Ovary, /: Inc., South Dartmouth (MA). http, 2789. [Google Scholar]
- Winterhager, E. , & Kidder, G. M. Gap junction connexins in female reproductive organs: Implications for women’s reproductive health. Human Reproduction Update 2015, 21, 340–352. [Google Scholar] [CrossRef]
- World Health Organization. (2019). WHO global report on traditional and complementary medicine 2019. In World Health Organization. https://apps.who.int/iris/bitstream/handle/10665/312342/9789241515436-eng.pdf?ua=1.
- Xu, X. , Du, X., Wang, F., Sha, J., Chen, Q., Tian, G., Zhu, Z., Ge, S., & Jiang, Y. (2020). Effects of Potassium Levels on Plant Growth, Accumulation and Distribution of Carbon, and Nitrate Metabolism in Apple Dwarf Rootstock Seedlings. In Frontiers in Plant Science (Vol. 11). https://www.frontiersin.org/article/10.3389/fpls.2020. 0090. [Google Scholar]
- Yang, X. , Guo, Y., He, J., Zhang, F., Sun, X., & Yang, S. (2017). Estrogen and estrogen receptors gastrointestinal epithelial secretion in the modulation of. 8(57), 97683–97692.
- Yao, J. , Chen, P., Apraku, A., Zhang, G., Huang, Z., & Hua, X. (2019). Hydrolysable Tannin Supplementation Alters Digestibility and Utilization of Dietary Protein, Lipid, and Carbohydrate in Grass Carp (Ctenopharyngodon idellus). Frontiers in Nutrition 6, 183. [CrossRef]
- Yaşar, P. , Ayaz, G., Damla, S., Gizem, U., & Mesut, G. (2017). Molecular mechanism of estrogen – estrogen receptor signaling. March 2016, 4–20. [CrossRef]
- Yaseen, D. , Sabbah, M., Al-Asmar, A., Altamimi, M., Famiglietti, M., Giosafatto, C. V. L., & Mariniello, L. (2021). Functionality of Films from Nigella sativa Defatted Seed Cake Proteins Plasticized with Grape Juice: Use in Wrapping Sweet Cherries. In Coatings (Vol. 11, Issue 11). [CrossRef]
- Yee, C. , Dickson, K., Muntasir, M. N., & Ma, Y. (2022). Three-Dimensional Modelling of Ovarian Cancer : From Cell Lines to Organoids for Discovery and Personalized Medicine. 10(February), 1–26. [CrossRef]
- Yimer, E. M. , Tuem, K. B., Karim, A., Ur-rehman, N., & Anwar, F. (2019). Nigella sativa L. ( Black Cumin ): A Promising Natural Remedy for Wide Range of Illnesses. 2019.
- Zhang, Q.-W. , Lin, L.-G., & Ye, W.-C. Techniques for extraction and isolation of natural products: a comprehensive review. Chinese Medicine 2018, 13, 20. [Google Scholar] [CrossRef]
- Zhou, J. , Peng, X., & Mei, S. Autophagy in ovarian follicular development and Atresia. International Journal of Biological Sciences 2019, 15, 726–737. [Google Scholar] [CrossRef] [PubMed]
- Zubaida A. Hawsawi, MBBS; Basil A. Ali, PhD; Abdullah, O. Bamosa, P. (2001). EFFECT OF NIGELLA SATIVA (BLACK SEED) AND THYMOQUINONE ON BLOOD GLUCOSE IN ALBINO RATS. Annals o f Saudi Medicine, 21.
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).





