Submitted:
09 August 2024
Posted:
09 August 2024
You are already at the latest version
Abstract
Keywords:
Introduction
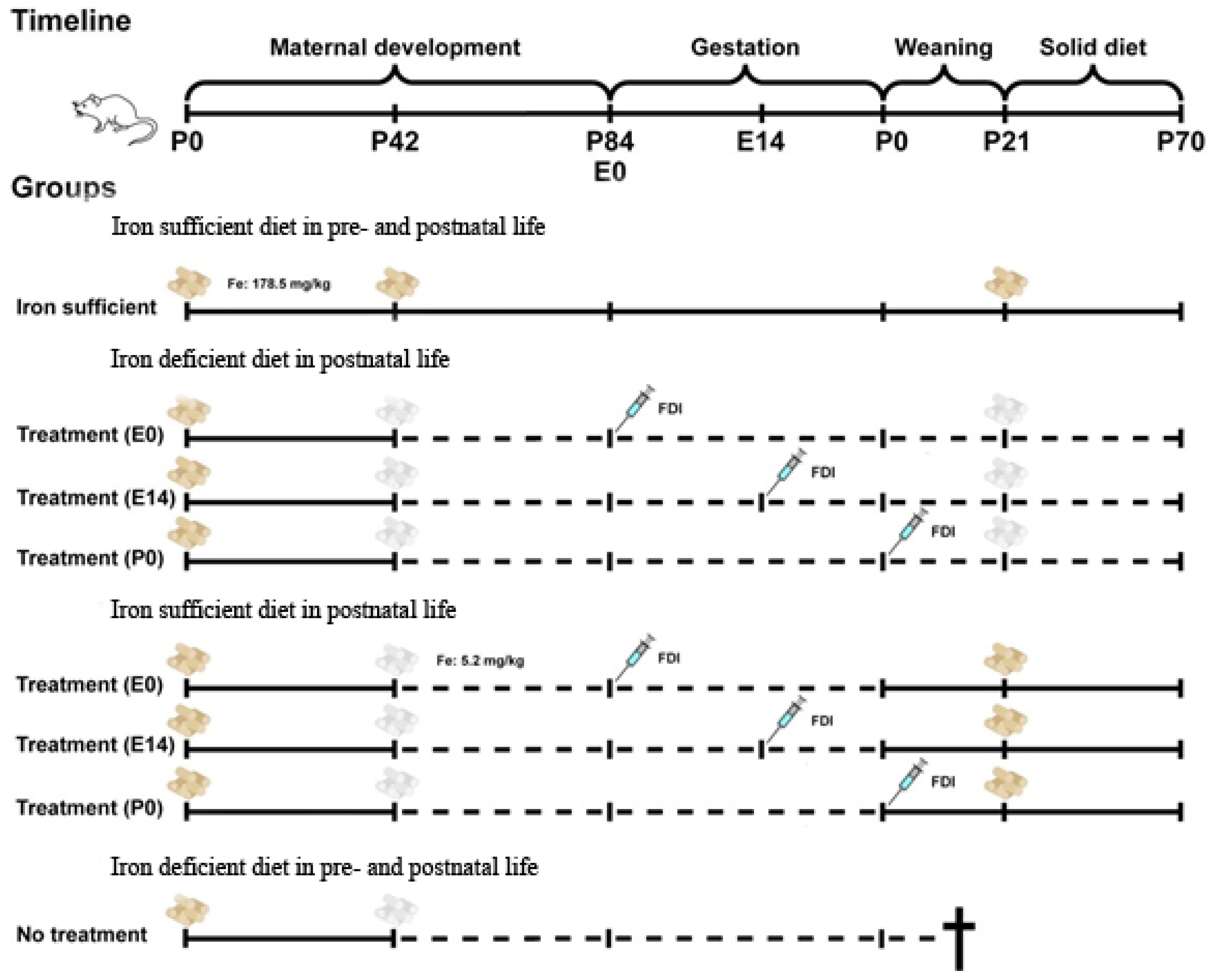
Materials and Methods
Gestational Fe Deficiency
Metal Analyses
Gene Expression Analyses
Statistics
Results
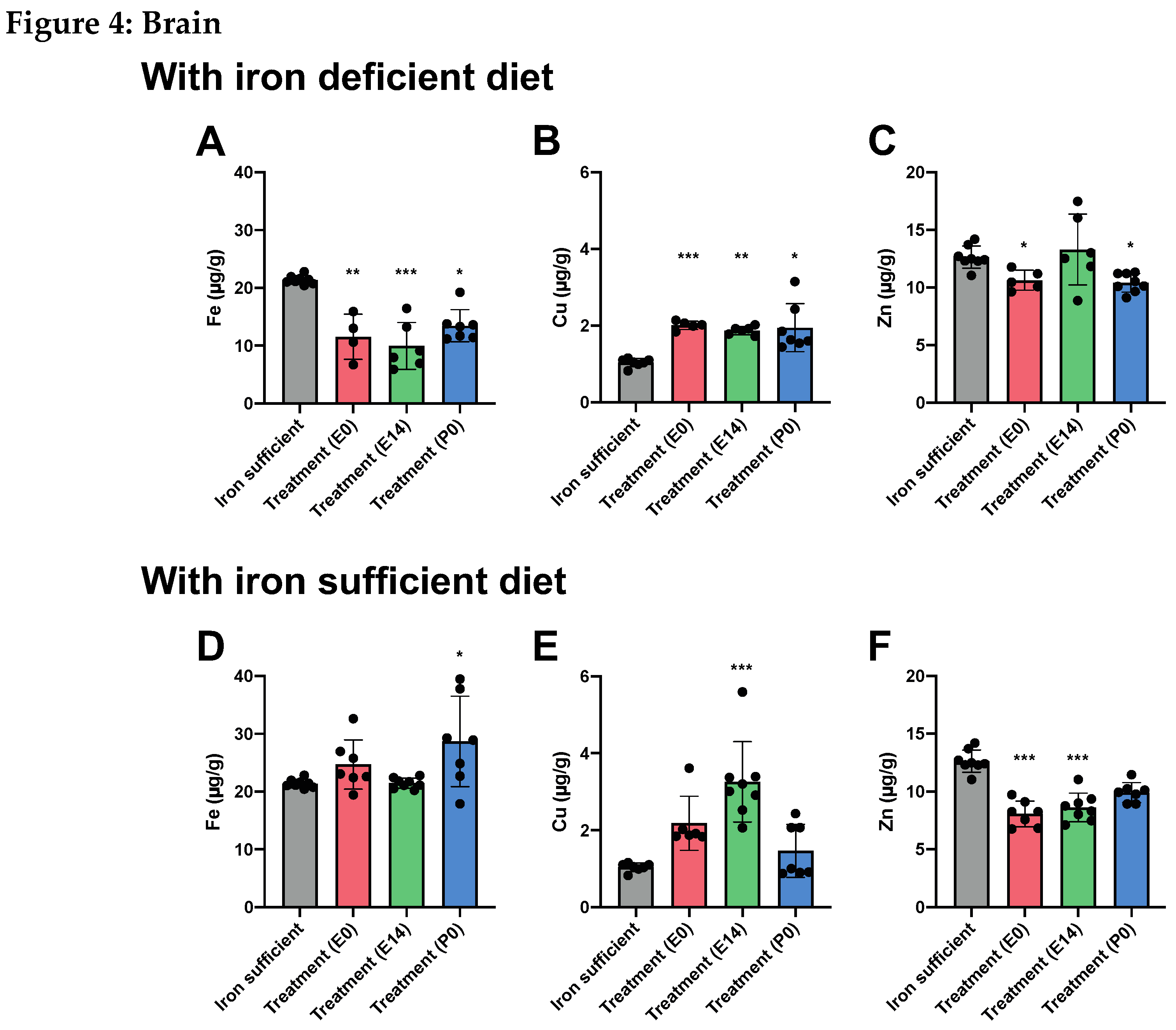
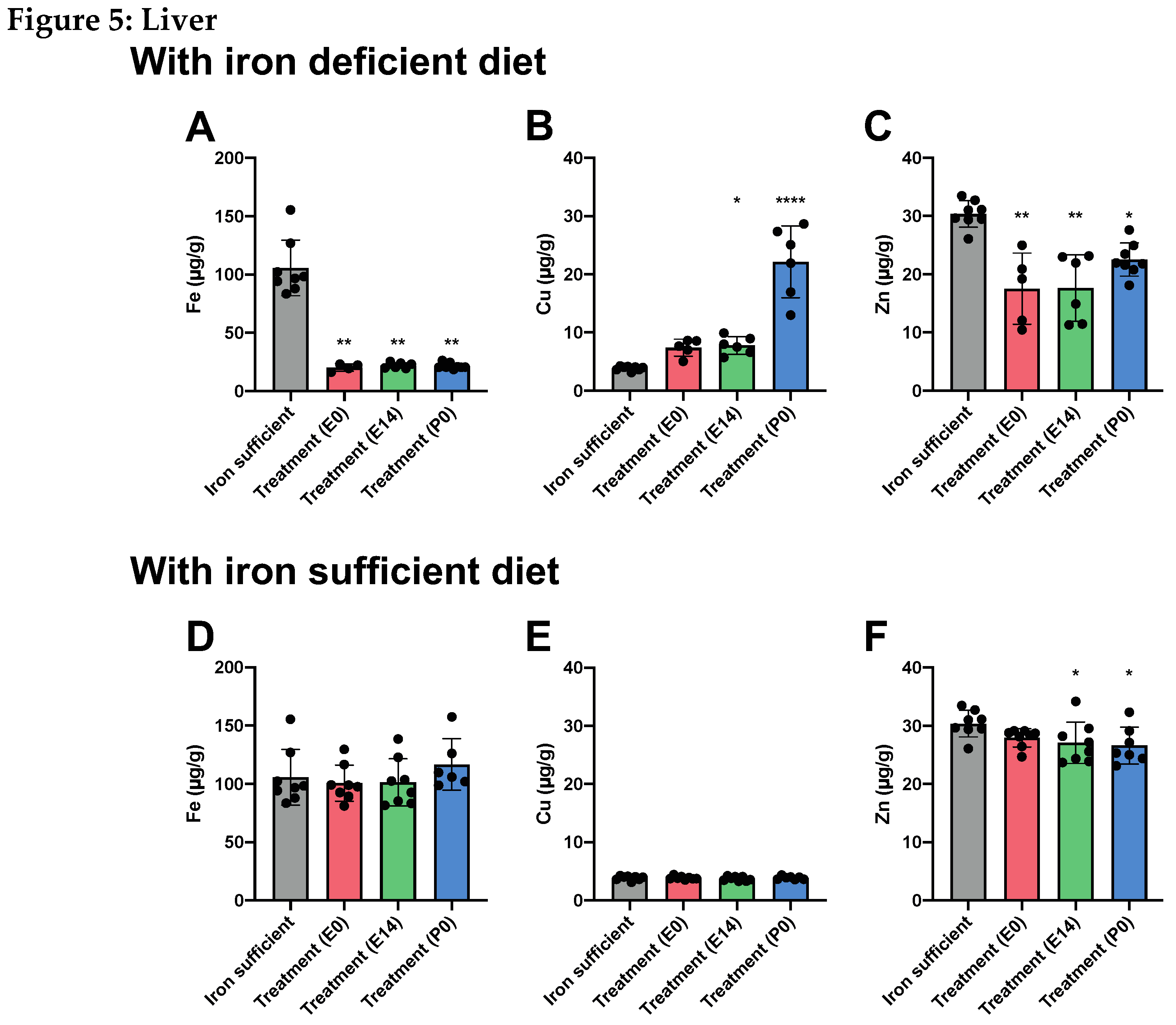
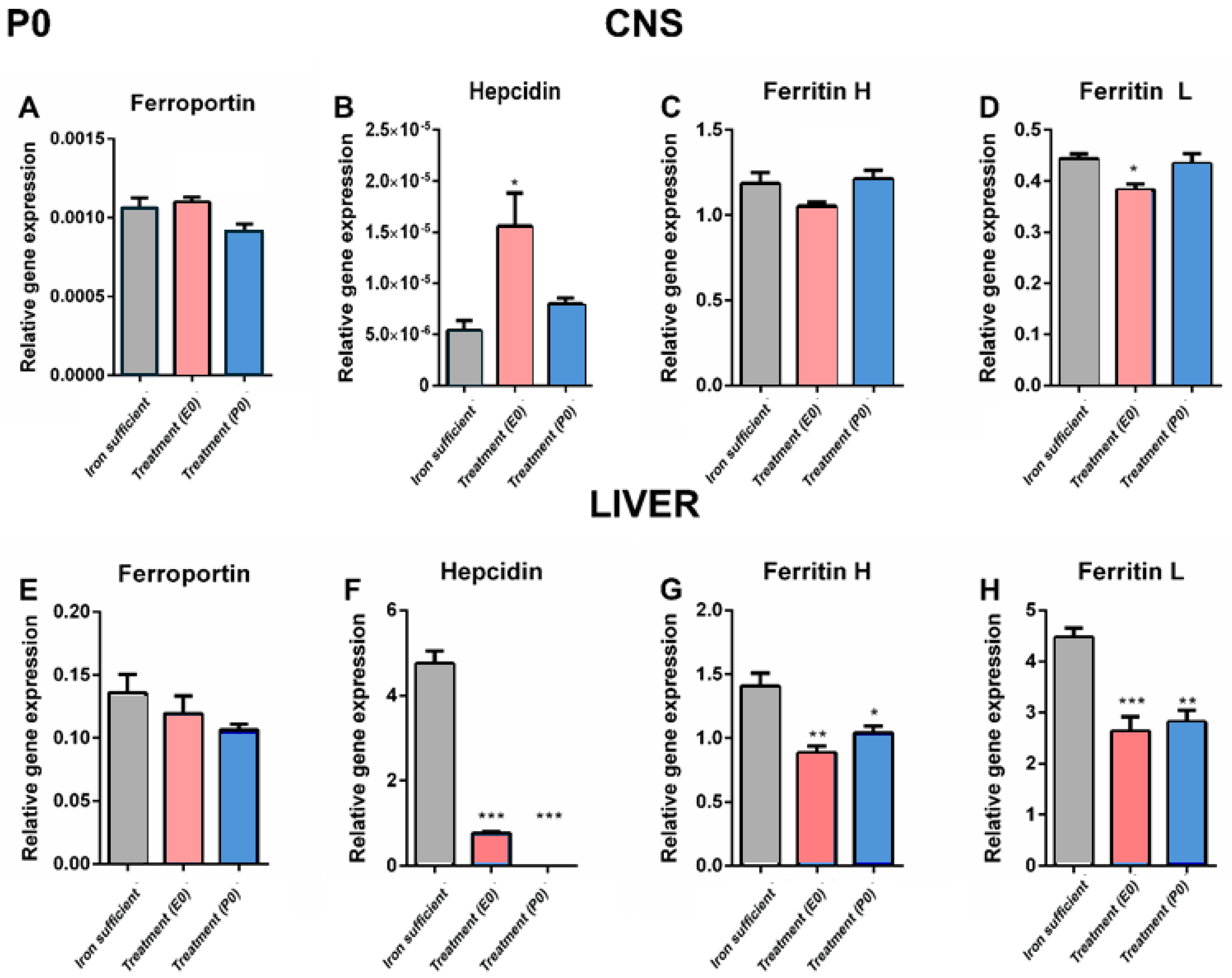
Discussion
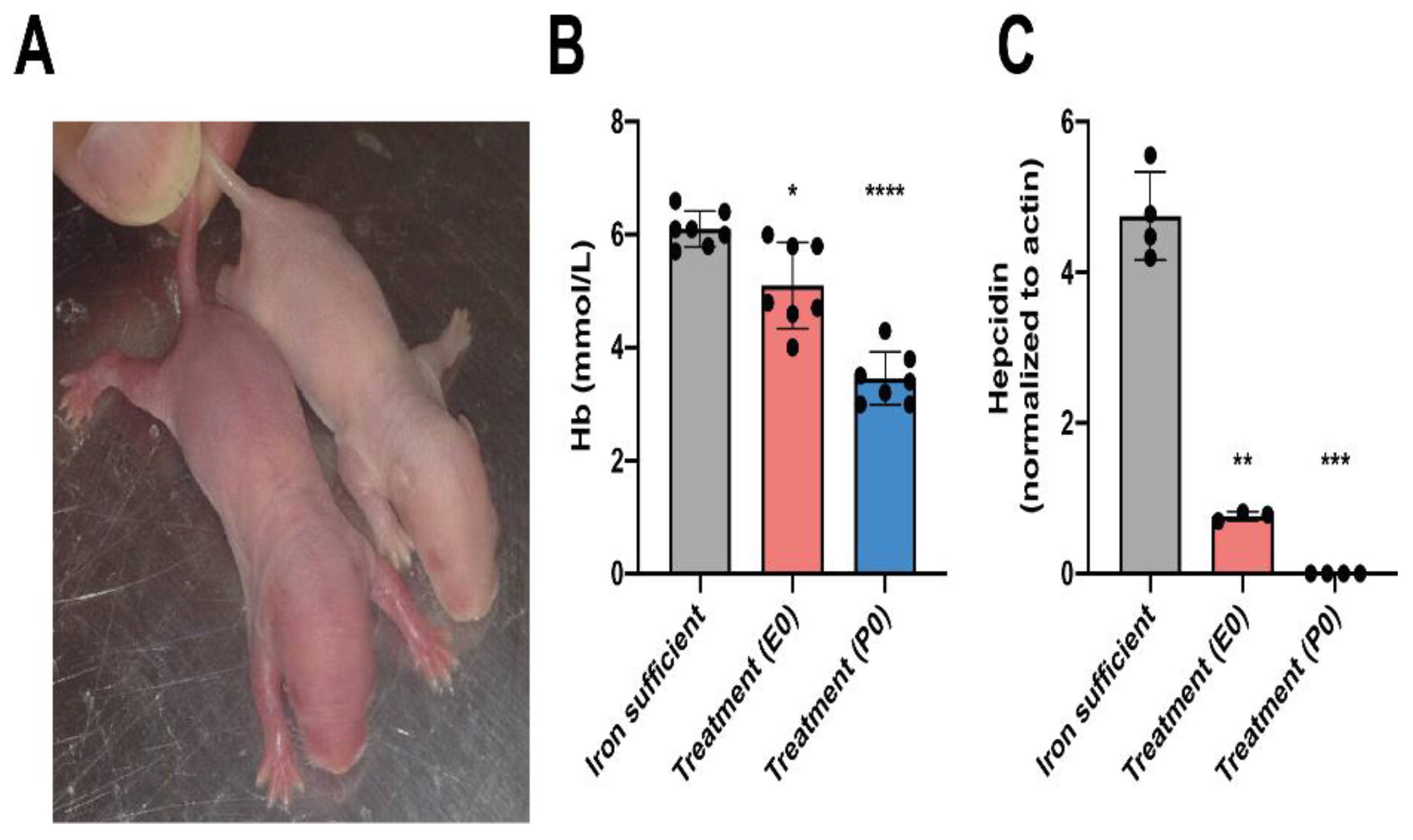
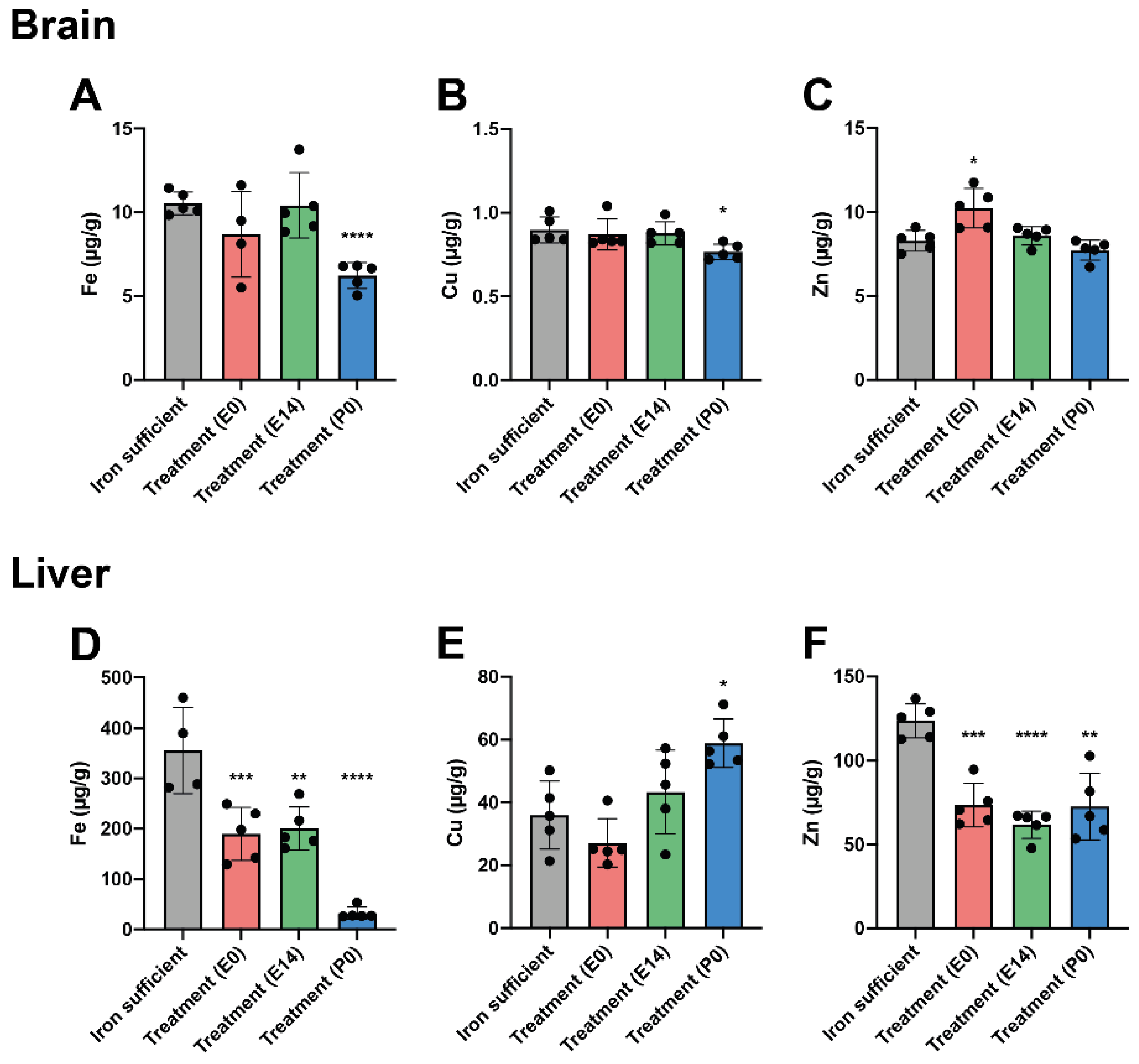
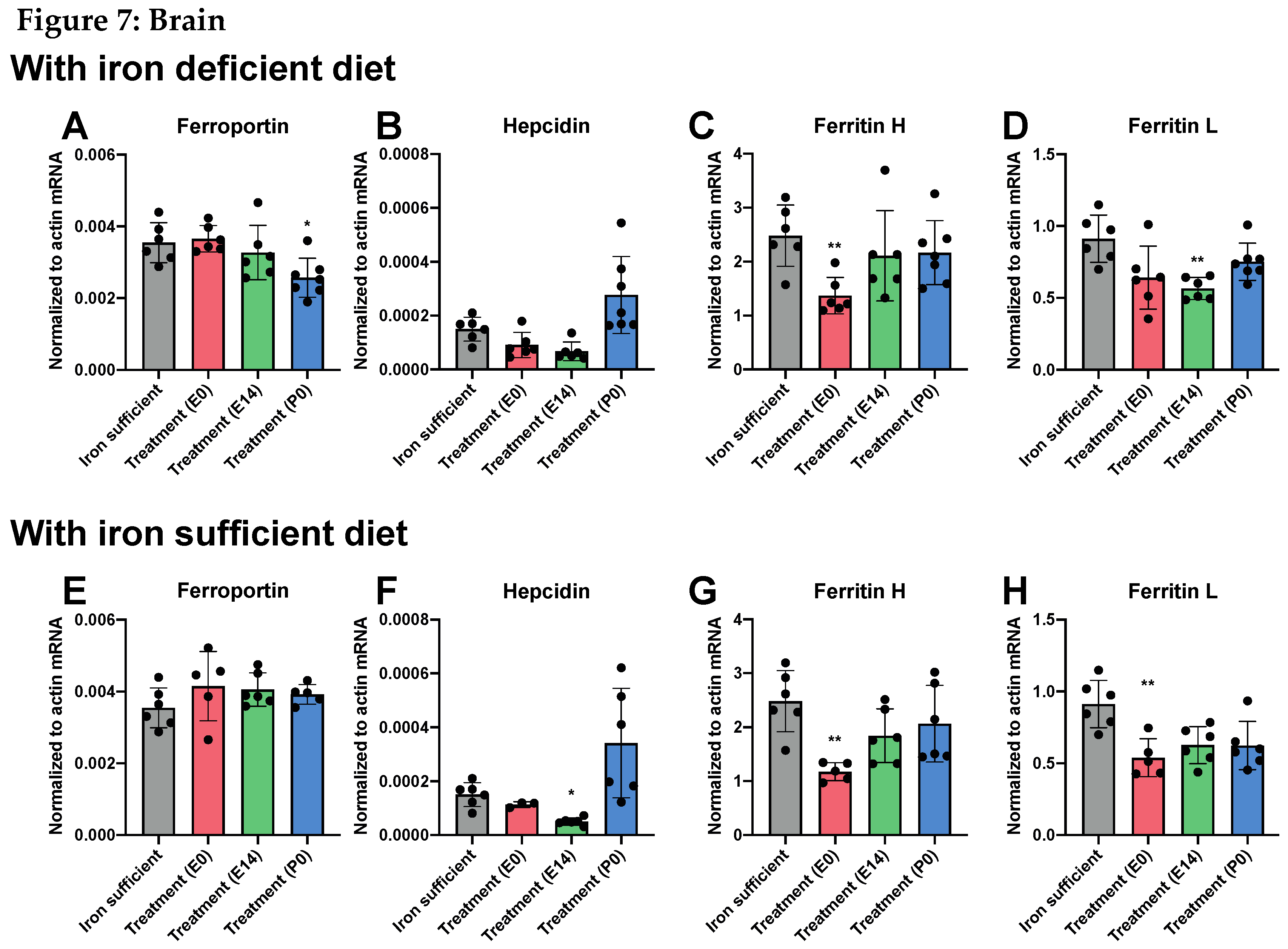
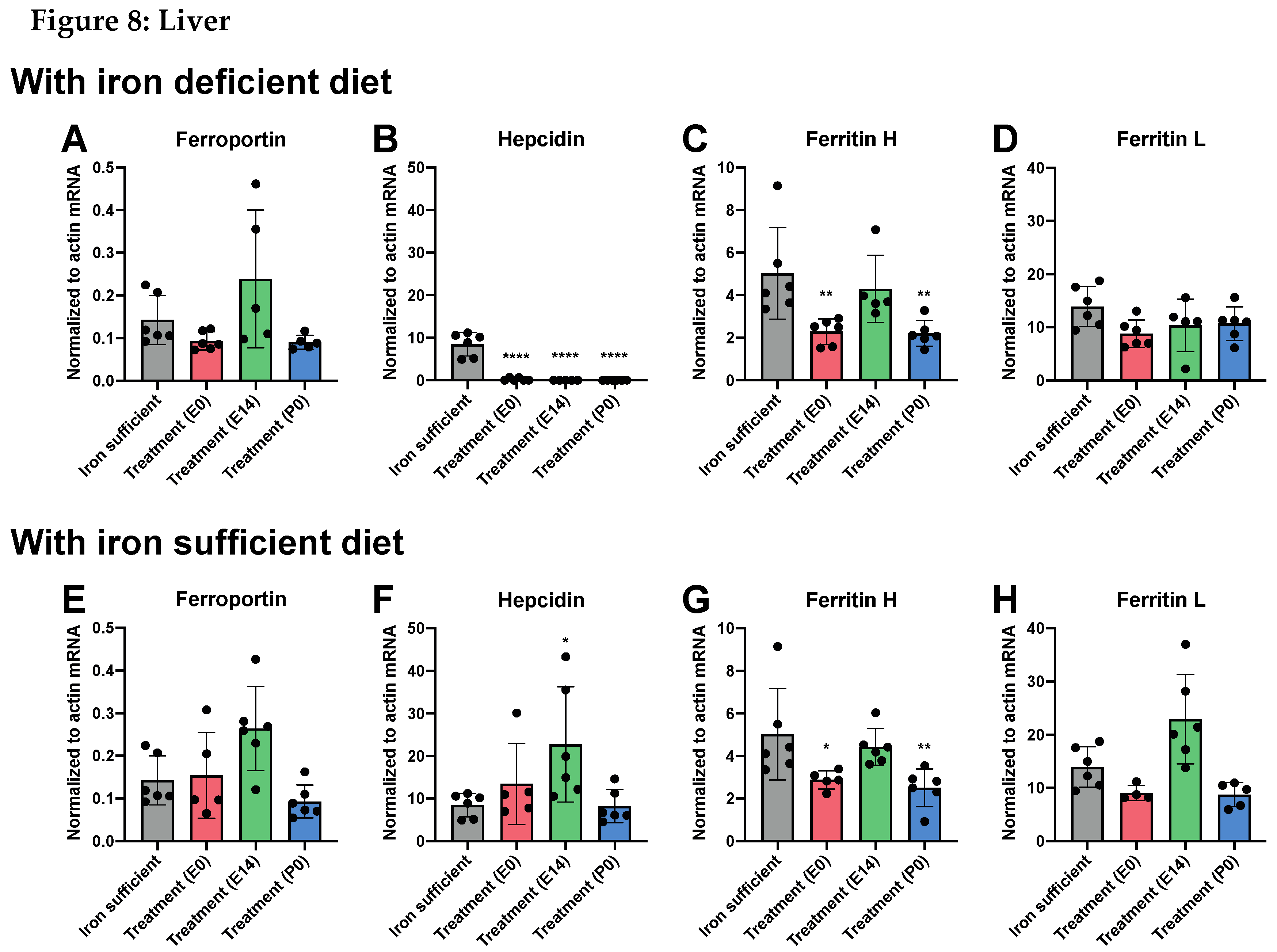
Fe and Fe-Related Genes in Offspring on P0
Fe and Fe-Related Genes in Offspring on P70
The Effect of ID and Fe Therapy on Cu Levels
The Effect of ID and Fe Therapy on Zn Levels
Conclusions
Author contributions
Funding
Acknowledgments
Conflicts of interest
References
- Zimmermann, M.B., Hurrell, R.F. Nutritional iron deficiency. Lancet 2007;370,511-520. [CrossRef]
- Heath, A.L., Fairweather-Tait, S.J. Clinical implications of changes in the modern diet: iron intake, absorption and status. Best Pract. Res. Clin. Haematol. 2002;15:225-41. [CrossRef]
- Scholl, T.O. Iron status during pregnancy: setting the stage for mother and infant. Am. J. Clin. Nutr. 2005;81:1218S-22S. [CrossRef]
- Zariwala, M.G., Somavarapu, S., Farnaud, S., Renshaw, D. Comparison study of oral iron preparations using a human intestinal model. Sci. Pharm. 2013;81:1123-39. [CrossRef]
- Froessler, B., Collingwood, J., Hodyl, N.A., Dekker, G. Intravenous ferric carboxymaltose for anaemia in pregnancy. BMC Pregnancy Childbirth. 2014;14:115,2393-14-115. [CrossRef]
- Laskey, J., Webb, I., Schulman, H.M., Ponka, P. Evidence that transferrin supports cell proliferation by supplying iron for DNA synthesis. Exp. Cell Res. 1988;176:87-95. [CrossRef]
- Lozoff, B., Jimenez, E., Hagen, J., Mollen, E., Wolf, A.W. Poorer behavioral and developmental outcome more than 10 years after treatment for iron deficiency in infancy. Pediatrics. 2000;105:E51. [CrossRef]
- Levy, J.E., Jin, O., Fujiwara, Y., Kuo, F., Andrews, N.C. Transferrin receptor is necessary for development of erythrocytes and the nervous system. Nat. Genet. 1999;21:396-9. [CrossRef]
- Jefferies, W.A., Brandon, M.R., Hunt, S.V., Williams, A.F., Gatter, K.C., Mason, D.Y. Transferrin receptor on endothelium of brain capillaries. Nature. 1984;312:162-3. [CrossRef]
- Focht, S.J., Snyder, B.S., Beard, J.L., Van Gelder, W., Williams, L.R., Connor, J.R. Regional distribution of iron, transferrin, ferritin, and oxidatively-modified proteins in young and aged Fischer 344 rat brains. Neuroscience. 1997;79:255-61. [CrossRef]
- Zheng, W., Monnot, A.D. Regulation of brain iron and copper homeostasis by brain barrier systems: implication in neurodegenerative diseases. Pharmacol. Ther. 2012;133:177-88. [CrossRef]
- Skjorringe, T., Burkhart, A., Johnsen, K.B., Moos, T. Divalent metal transporter 1 (DMT1) in the brain: implications for a role in iron transport at the blood-brain barrier, and neuronal and glial pathology. Front. Mol. Neurosci. 2015;8:19. [CrossRef]
- Zhang, D.L., Hughes, R.M., Ollivierre-Wilson, H., Ghosh, M.C., Rouault, T.A. A ferroportin transcript that lacks an iron-responsive element enables duodenal and erythroid precursor cells to evade translational repression. Cell. Metab. 2009;9:461-73. [CrossRef]
- Darshan, D., Frazer, D.M., Wilkins, S.J., Anderson, G.J. Severe iron deficiency blunts the response of the iron regulatory gene Hamp and pro-inflammatory cytokines to lipopolysaccharide. Haematologica. 2010;95:1660-7. [CrossRef]
- Urrutia, P., Aguirre, P., Esparza, A., Tapia, V., Mena, N.P., Arredondo, M., Gonzalez-Billault, C., Nunez, M.T. Inflammation alters the expression of DMT1, FPN1 and hepcidin, and it causes iron accumulation in central nervous system cells. J. Neurochem. 2013;126:541-9. [CrossRef]
- Burkhart, A., Skjørringe, T., Johnsen, K.B., Siupka, P., Thomsen, L.B., Nielsen, M.S., Moos, T. Expression of iron-related proteins at the neurovascular unit supports reduction and reoxidation of iron for transport through the blood-brain barrier. Mol. Neurobiol. 2016;53:7237-7253. [CrossRef]
- Bastian, T.W., Prohaska, J.R., Georgieff, M.K., Anderson, G.W. Perinatal iron and copper deficiencies alter neonatal rat circulating and brain thyroid hormone concentrations. Endocrinology. 2010;151:4055-65. [CrossRef]
- Monnot, A.D., Behl, M., Ho, S., Zheng, W. Regulation of brain copper homeostasis by the brain barrier systems: effects of Fe-overload and Fe-deficiency. Toxicol. Appl. Pharmacol. 2011;256:249-57. [CrossRef]
- Oladiji, T. Tissue levels of iron, copper, zinc and magnesium in iron deficient rats. Biokemistri. 2003;14:1.
- Myers, S.A., Nield, A., Myers, M. Zinc transporters, mechanisms of action and therapeutic utility: implications for type 2 diabetes mellitus. J. Nutr. Metab. 2012;2012:173712. [CrossRef]
- Jahn, M.R., Andreasen, H.B., Futterer, S., Nawroth, T., Schunemann, V., Kolb, U., Hofmeister, W., Munoz, M., Bock, K., Meldal, M., Langguth, P. A comparative study of the physicochemical properties of iron isomaltoside 1000 (Monofer), a new intravenous iron preparation and its clinical implications. Eur. J. Pharm. Biopharm. 2011;78:480-91. [CrossRef]
- Auerbach, M., Henry, D., Derman, R. J., Achebe, M. M., Thomsen, L. L., Glaspy J. A prospective, multi-center, randomized comparison of iron isomaltoside 1000 versus iron sucrose in patients with iron deficiency anemia; the FERWON-IDA trial. Am. J. Hematol. 2019;94:1007-1014. [CrossRef]
- Wolf, M., Rubin, J., Achebe, M., Econs, M. J., Peacock, M., Imel, E.A., Thomsen L. L., et al. Effects of Iron Isomaltoside vs Ferric Carboxymaltose on Hypophosphatemia in Iron-Deficiency Anemia: Two Randomized Clinical Trials. JAMA 323;432-443. [CrossRef]
- Unger, E.L., Earley, C.J., Thomsen, L.L., Jones, B.C., Allen, R.P. Effects of IV iron isomaltoside-1000 treatment on regional brain iron status in an iron-deficient animal. Neuroscience. 2013;246:179-85. [CrossRef]
- Karakoc, Y., Buruk, M.S., Aktan, B., Kirvar, R., Erdogan, S., Sahbaz, M.A., Aksoy, S., Gulyasar T. Effects of chronic light/dark cycle on iron zinc and copper levels in different brain regions of rats. Biol Trace Elem Res 2011;144, 1003–1007. [CrossRef]
- Stephansen, D. A., Nielsen, A. H., Hvitved-Jacobsen, T., Arias, C. A., Brix, H., Vollertsen, J. Distribution of metals in fauna, flora and sediments of wet detention ponds and natural shallow lakes. Ecological Engineering 2014;66,43–51. [CrossRef]
- Bustin, S. A., Benes, V., Garson, J. A., Hellemans, J., Huggett, J., Kubista, M., Mueller, R., et al. The MIQE guidelines: minimum information for publication of quantitative real-time PCR experiments. Clin. Chem. 2009;55:611-22. [CrossRef]
- Pfaffl MW. A new mathematical model for relative quantification in real-time RT-PCR. Nucleic Acids Res. 2001;29:e45. [CrossRef]
- Piñero, D. J., Li, N. Q., Connor, J. R., & Beard, J. L. Variations in dietary iron alter brain iron metabolism in developing rats. The Journal of nutrition, 2000, 130, 254–263. [CrossRef]
- Lozoff, B., & Georgieff, M. K. Iron deficiency and brain development. Seminars in pediatric neurology, 2006; 13;158–165. [CrossRef]
- Felt, B. T., Beard, J. L., Schallert, T., Shao, J., Aldridge, J. W., Connor, J. R., Georgieff, M. K., & Lozoff, B. Persistent neurochemical and behavioral abnormalities in adulthood despite early iron supplementation for perinatal iron deficiency anemia in rats. Behavioural brain research 2006;171,261–270. [CrossRef]
- Rao, R., Tkac, I., Schmidt, A. T., & Georgieff, M. K. Fetal and neonatal iron deficiency causes volume loss and alters the neurochemical profile of the adult rat hippocampus. Nutritional neuroscience, 2011;14,59–65. [CrossRef]
- Mihaila, C., Schramm, J., Strathmann, F. G., Lee, D. L., Gelein, R. M., Luebke, A. E., & Mayer-Pröschel, M. Identifying a window of vulnerability during fetal development in a maternal iron restriction model. PloS one, 2011; 6, e17483. [CrossRef]
- Zhang, Q., Lu, X. M., Zhang, M., Yang, C. Y., Lv, S. Y., Li, S. F., Zhong, C. Y., & Geng, S. S. Adverse effects of iron deficiency anemia on pregnancy outcome and offspring development and intervention of three iron supplements. Scientific reports, 2021;11,1347. [CrossRef]
- Moos, T., Skjørringe, T., Thomsen, L.L. Iron deficiency and iron treatment in the fetal developing brain—a pilot study introducing an experimental rat model. Reprod Health 2018;15,93–120. [CrossRef]
- Ham, S. Y., Jun, J. H., Kim, H. B., Shim, J. K., Lee, G., & Kwak, Y. L. Regulators impeding erythropoiesis following iron supplementation in a clinically relevant rat model of iron deficiency anemia with inflammation. Life sciences, 2022;310,121124. [CrossRef]
- De Souza, L. V., Hoffmann, A., Fischer, C., Petzer, V., Asshoff, M., Theurl, I., Tymoszuk, P., Seifert, M., Brigo, N., Hilbe, R., Demetz, E., Von Raffay, L., Berger, S., Barros-Pinkelnig, M., & Weiss, G. Comparative analysis of oral and intravenous iron therapy in rat models of inflammatory anemia and iron deficiency. Haematologica, 2023; 108,135–149. [CrossRef]
- Camaschella, C., Pagani, A., Silvestri, L., & Nai, A. The mutual crosstalk between iron and erythropoiesis. International journal of hematology, 2022;116,182–191. [CrossRef]
- Evstatiev, R., Bukaty, A., Jimenez, K., Kulnigg-Dabsch, S., Surman, L., Schmid, W., Eferl, R., Lippert, K., Scheiber-Mojdehkar, B., Kvasnicka, H.M., Khare, V., Gasche, C. Iron deficiency alters megakaryopoiesis and platelet phenotype independent of thrombopoietin. Am. J. Hematol. 2014;89:524-9. [CrossRef]
- Gambling, L., Lang, C., McArdle, H.J. Fetal regulation of iron transport during pregnancy. Am. J. Clin. Nutr. 2011;94:1903S-7S. [CrossRef]
- Morgan, E.H., Moos, T. Mechanism and developmental changes in iron transport across the blood-brain barrier. Devl. Neurosci. 2002;24:106-13. [CrossRef]
- Moos, T., Morgan, E.H. A morphological study of the developmentally regulated transport of iron into the brain. Devl. Neurosci. 2002;24:99-105. [CrossRef]
- Wang, X., Wiesinger, J., Beard, J., Felt, B., Menzies, S., Earley, C., Allen, R., & Connor, J. Thy1 expression in the brain is affected by iron and is decreased in Restless Legs Syndrome. Journal of the neurological sciences, 2004; 220, 59–66. [CrossRef]
- Ozen, M., Kitase, Y., Vasan, V., Burkhardt, C., Ramachandra, S., Robinson, S., & Jantzie, L. L. Chorioamnionitis Precipitates Perinatal Alterations of Heme-Oxygenase-1 (HO-1) Homeostasis in the Developing Rat Brain. International journal of molecular sciences, 2021; 22, 5773. [CrossRef]
- Georgieff, M. K., Krebs, N. F., & Cusick, S. E. The Benefits and Risks of Iron Supplementation in Pregnancy and Childhood. Annual review of nutrition, 2019; 39, 121–146. [CrossRef]
- Clardy, S.L., Wang, X., Zhao W., Liu, W., Chase, G.A., Beard J.L., True Felt B., Connor J. R. Acute and chronic effects of developmental iron deficiency on mRNA expression patterns in the brain. J. Neural Transm. 2006;Suppl. 262:173–196. [CrossRef]
- Ortiz, E., Pasquini, J. M., Thompson, K., Felt, B., Butkus, G., Beard J., Connor J. R. Effect of manipulation of iron storage, transport, or availability on myelin composition and brain iron content in three different animal models. J. Neurosci. Res. 2004;77:681–689. [CrossRef]
- Markova, V., Holm, C., Pinborg, A.B., Thomsen, L.L., Moos, T. Impairment of the Developing Human Brain in Iron Deficiency: Correlations to Findings in Experimental Animals and Prospects for Early Intervention Therapy. Pharmaceuticals. 2019;12:120. [CrossRef]
- Lakhal-Littleton, S., Wolna, M., Carr, C.A., Miller, J.J., Christian, H.C., Ball, V., Santos. A., Diaz, R., Biggs, D., Stillion, R., Holdship, P., Larner, F., Tyler, D.J., Clarke, K., Davies, B., Robbins, P.A. Cardiac ferroportin regulates cellular iron homeostasis and is important for cardiac function. Proc. Natl. Acad. Sci. U. S. A. 2015;112:3164-9. [CrossRef]
- Vanlandewijck, M., He, L., Mäe, M. A., Andrae, J., Ando, K., Del Gaudio, F., et al. A molecular atlas of cell types and zonation in the brain vasculature. Nature, 2018;554(7693): 475–480. [CrossRef]
- Wilkinson, N., Pantopoulos, K. The IRP/IRE system in vivo: insights from mouse models. Front Pharmacol. 2014; 5:176. [CrossRef]
- Hansen, T.M., Nielsen, H., Bernth, N., Moos, T. Expression of ferritin protein and subunit mRNAs in normal and iron deficient rat brain. Brain Res Mol Brain Res. 1999; 65:186-97. [CrossRef]
- Han, J., Day, J.R., Connor, J.R., Beard, J.L. H and L ferritin subunit mRNA expression differs in brains of control and iron-deficient rats. J. Nutr. 2002; 132:2769-74. [CrossRef]
- Ma, J., Guo, Q., Shen, M. Q., Li, W., Zhong, Q. X., & Qian, Z. M. Apolipoprotein E is required for brain iron homeostasis in mice. Redox biology, 2023; 64, 102779. [CrossRef]
- Wu, Q., Hao, Q., Li, H., Wang, B., Wang, P., Jin, X., Yu, P., Gao, G., & Chang, Y. Z. Brain iron deficiency and affected contextual fear memory in mice with conditional Ferroportin1 ablation in the brain. FASEB journal : official publication of the Federation of American Societies for Experimental Biology, 2021; 35, e21174. [CrossRef]
- McMillen, S. A., Nonnecke, E. B., & Lönnerdal, B. Trace Element Interactions, Inflammatory Signaling, and Male Sex Implicated in Reduced Growth Following Excess Oral Iron Supplementation in Pre-Weanling Rats. Nutrients, 2022; 14, 3913. [CrossRef]
- Penkowa, M., Nielsen, H., Hidalgo, J., Bernth, N., Moos T. Distribution of metallothionein I + II and vesicular zinc in the developing central nervous system: correlative study in the rat. J. Comp. Neurol. 1999;412:303-18. [CrossRef]
- McCarthy, R. C., Kosman D. J. Glial cell ceruloplasmin and hepcidin differentially regulate iron efflux from brain microvascular endothelial cells. PLoS ONE 2014;9,e89003. [CrossRef]
- McCarthy, R. C., Kosman D. J. Mechanisms and regulation of iron trafficking across the capillary endothelial cells of the blood-brain barrier. Front Mol Neurosci 2015;8:31. [CrossRef]
- Sherman, A.R., Tissue, N.T. Tissue iron, copper and zinc levels in offspring of iron-sufficient and iron-deficient rats. J. Nutr. 1981;111,266–275. [CrossRef]
- Skjørringe, T., Møller, L.B., Moos T. Impairment of interrelated iron- and copper homeostatic mechanisms in brain contributes to the pathogenesis of neurodegenerative disorders. Front Pharmacol 2012;3, 169. [CrossRef]
- Gambling, L., Andersen, H.S., Czopek, A., Wojciak, R., Krejpcio, Z., McArdle, H.J. Effect of timing of iron supplementation on maternal and neonatal growth and iron status of iron-deficient pregnant rats. J. Physiol. 2004;561:195-203. [CrossRef]
- Carmona, A., Chen, S., Domart, F., Choquet, D., & Ortega, R. Imaging the structural organization of chemical elements in growth cones of developing hippocampal neurons. Metallomics : integrated biometal science, 2022; 14, mfab073. [CrossRef]
- Lenartowicz, M., Kennedy, C., Hayes, H., McArdle, H.J. Transcriptional regulation of copper metabolism genes in the liver of fetal and neonatal control and iron-deficient rats. Biometals. 2015;28:51-9. [CrossRef]
- Basha, M.R., Wei, W., Bakheet, S.A., Benitez, N., Siddiqi, H.K., Ge, Y.W., Lahiri, D.K., Zawia, N.H. The fetal basis of amyloidogenesis: exposure to lead and latent overexpression of amyloid precursor protein and beta-amyloid in the aging brain. J. Neurosci. 2005;25:823-9. [CrossRef]
- Nam, H., Knutson, M.D. Effect of dietary iron deficiency and overload on the expression of ZIP metal-ion transporters in rat liver. Biometals. 2012;25:115-24. [CrossRef]
- Solomons, N.W. Competitive interaction of iron and zinc in the diet: consequences for human nutrition. J. Nutr. 1986;116:927-35. [CrossRef]
| Pregnant females Weight Hepatic Fe Plasma Fe HgB Hepatic Cu Hepatic Zn |
| (g) (µg/g) (µg/g) (mmol/l) (µg/g) (µg/g) |
| Iron sufficient diet 221.3 ± 5.1 214.6 ± 22.5 460.3 ± 42.0 8.6 ± 0.4 2.7 ± 0.7 18.7 ± 5.5 |
| Iron deficient diet 237.4 ± 5.8 151.3 ± 24.2* 461.5 ± 14.2 8.3 ± 0.5 2.9 ± 0.9 17.8 ± 5.2 |
| Weight HGB MCV MCHC Reticulocytes Leucocytes Thrombocytes |
|
(g) (mmol/L) (fL) (mmol/L) (x109/L) (x109/L) (x109/L) (1) Iron sufficient: |
| 275 ± 5.4 8.9 ± 0.2 59.1 ± 0.8 20 ± 0.1 227.7 ± 6.8 4.8 ± 0.9 615.5 ± 51.6 |
| (II) Continuously iron deficient diet with a single intramuscular injection of ferric derisomaltoside given on: |
|
E0 235.3 ± 4.7** 3 ± 0.2*** 39.7 ± 0.7*** 11.3 ± 0.2*** 1773 ± 121**** 3.9 ± 0.8 891.4 ± 12.6*** E14 258.1 ± 7.6 3 ± 0.2*** 39.7 ± 0.3*** 11.8 ± 0.1*** 1380 ± 67.7*** 3.4 ± 0.5 912.2 ± 50.9*** |
|
P0 213.3 ± 9.7*** 3.7 ± 0.1*** 40.6 ± 0.4*** 12 ± 0.2*** 1591 ± 169.1**** 5.7 ± 0.7 911.3 ± 43.9*** |
| (III) Iron deficient diet + Iron-containing diet after weaning. Single intramuscular injection of ferric derisomaltoside given on: |
|
E0 272 ± 3.5 8.8 ± 0.1 59.8 ± 0.3 19.6 ± 0.2 256.2 ± 12.1 2.8 ± 1.1 588.3 ± 56.1 |
|
E14 264.6 ± 4.9 9.3 ± 0.3 59.8 ± 1.3 19.5 ± 0.3 275.8 ± 9.8 4 ± 0.4 586.7 ± 74.4 |
|
P0 256 ± 8.1 9 ± 0.2 62 ± 1.0* 19.3 ± 0.2 286.2 ± 19 4.8 ± 0.8 580 ± 35.6 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





