Submitted:
02 August 2024
Posted:
06 August 2024
You are already at the latest version
Abstract
Keywords:
1. Introduction
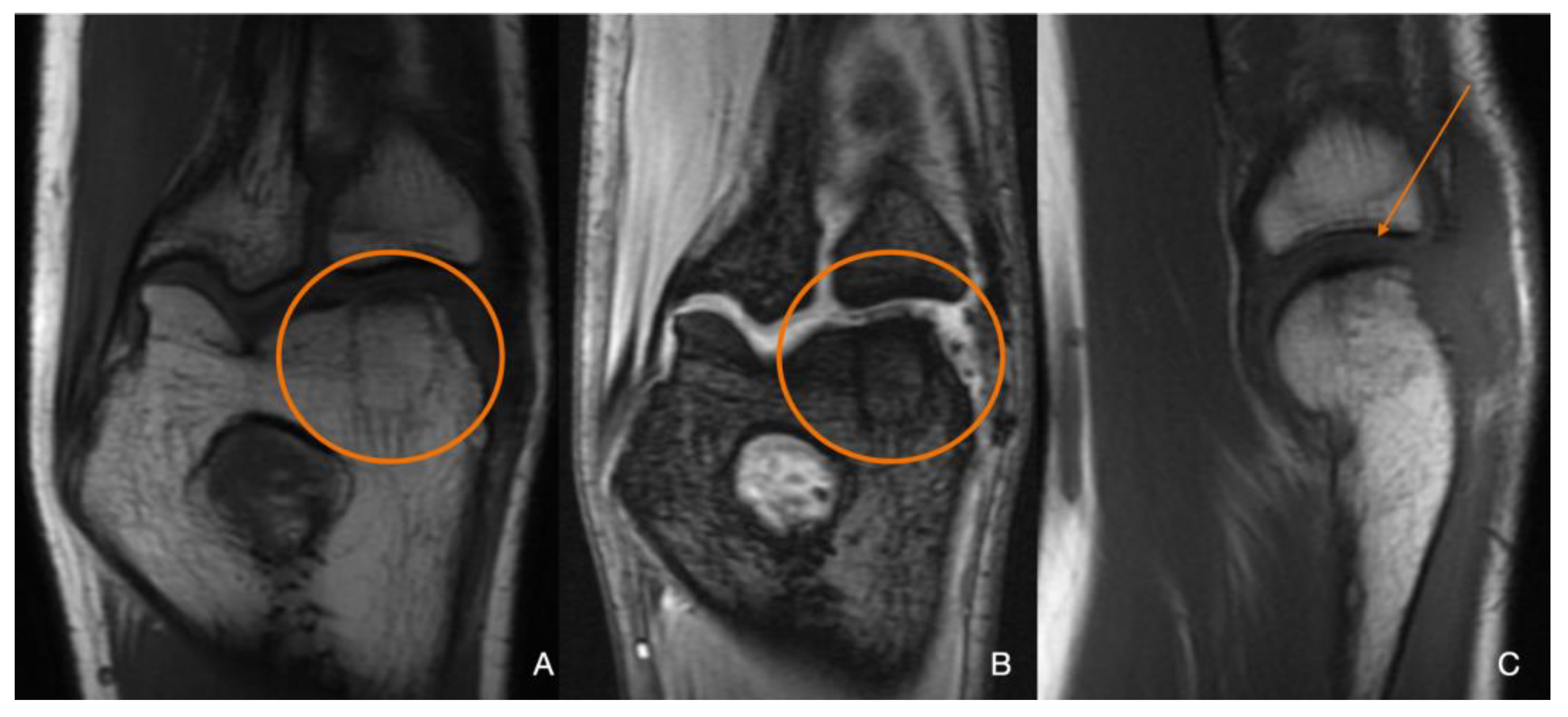
2. Materials and Methods
3. Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Bruns, J.; Werner, M.; Habermann, C. Osteochondritis Dissecans: Etiology, Pathology, and Imaging with a Special Focus on the Knee Joint. Cartilage 2018, 9, 346–362. [CrossRef]
- Tóth, F.; Nissi, M.J.; Ellermann, J.M.; Wang, L.; Shea, K.G.; Polousky, J.; Carlson, C.S. Novel Application of Magnetic Resonance Imaging Demonstrates Characteristic Differences in Vasculature at Predilection Sites of Osteochondritis Dissecans. Am. J. Sports Med. 2015, 43, 2522–2527. [CrossRef]
- Chau, M.M.; Klimstra, M.A.; Wise, K.L.; Ellermann, J.M.; Tóth, F.; Carlson, C.S.; Nelson, B.J.; Tompkins, M.A. Osteochondritis Dissecans. J. Bone Joint Surg. Am. 2021, 103, 1132–1151. [CrossRef]
- Hefti, F.; Beguiristain, J.; Krauspe, R.; Möller-Madsen, B.; Riccio, V.; Tschauner, C.; Wetzel, R.; Zeller, R. Osteochondritis Dissecans: A Multicenter Study of the European Pediatric Orthopedic Society. J. Pediatr. Orthop. Part B 1999, 8, 231–245.
- Tóth, F.; Nissi, M.J.; Wang, L.; Ellermann, J.M.; Carlson, C.S. Surgical Induction, Histological Evaluation, and MRI Identification of Cartilage Necrosis in the Distal Femur in Goats to Model Early Lesions of Osteochondrosis. Osteoarthr. Cartil. OARS Osteoarthr. Res. Soc. 2015, 23, 300–307. [CrossRef]
- Kamei, G.; Adachi, N.; Deie, M.; Nakamae, A.; Nakasa, T.; Shibuya, H.; Okuhara, A.; Niimoto, T.; Kazusa, H.; Ohkawa, S.; et al. Characteristic Shape of the Lateral Femoral Condyle in Patients with Osteochondritis Dissecans Accompanied by a Discoid Lateral Meniscus. J. Orthop. Sci. Off. J. Jpn. Orthop. Assoc. 2012, 17, 124–128. [CrossRef]
- Ishikawa, M.; Adachi, N.; Yoshikawa, M.; Nakamae, A.; Nakasa, T.; Ikuta, Y.; Hayashi, S.; Deie, M.; Ochi, M. Unique Anatomic Feature of the Posterior Cruciate Ligament in Knees Associated With Osteochondritis Dissecans. Orthop. J. Sports Med. 2016, 4, 2325967116648138. [CrossRef]
- Takigami, J.; Hashimoto, Y.; Tomihara, T.; Yamasaki, S.; Tamai, K.; Kondo, K.; Nakamura, H. Predictive Factors for Osteochondritis Dissecans of the Lateral Femoral Condyle Concurrent with a Discoid Lateral Meniscus. Knee Surg. Sports Traumatol. Arthrosc. Off. J. ESSKA 2018, 26, 799–805. [CrossRef]
- Gonzalez-Herranz, P.; Rodriguez, M.L.; de la Fuente, C. Femoral Osteochondritis of the Knee: Prognostic Value of the Mechanical Axis. J. Child. Orthop. 2017, 11, 1–5. [CrossRef]
- McElroy, M.J.; Riley, P.M.; Tepolt, F.A.; Nasreddine, A.Y.; Kocher, M.S. Catcher’s Knee: Posterior Femoral Condyle Juvenile Osteochondritis Dissecans in Children and Adolescents. J. Pediatr. Orthop. 2018, 38, 410–417. [CrossRef]
- Matching Osteochondritis Dissecans Lesions in Identical Twin Brothers - PubMed Available online: https://pubmed.ncbi.nlm.nih.gov/24025016/ (accessed on 11 July 2024).
- Oberti, V.; Sanchez Ortiz, M.; Allende, V.; Masquijo, J. Prevalencia de Hipovitaminosis D En Pacientes Con Osteocondritis Disecante Juvenil. Rev. Esp. Cir. Ortopédica Traumatol. 2021, 65, 132–137. [CrossRef]
- Bruns, J.; Werner, M.; Soyka, M. Is Vitamin D Insufficiency or Deficiency Related to the Development of Osteochondritis Dissecans? Knee Surg. Sports Traumatol. Arthrosc. Off. J. ESSKA 2016, 24, 1575–1579. [CrossRef]
- Maier, G.S.; Lazovic, D.; Maus, U.; Roth, K.E.; Horas, K.; Seeger, J.B. Vitamin D Deficiency: The Missing Etiological Factor in the Development of Juvenile Osteochondrosis Dissecans? J. Pediatr. Orthop. 2019, 39, 51–54. [CrossRef]
- Ellermann, J.M.; Ludwig, K.D.; Nissi, M.J.; Johnson, C.P.; Strupp, J.P.; Wang, L.; Zbýň, Š.; Tóth, F.; Arendt, E.; Tompkins, M.; et al. Three-Dimensional Quantitative Magnetic Resonance Imaging of Epiphyseal Cartilage Vascularity Using Vessel Image Features. JBJS Open Access 2019, 4, e0031. [CrossRef]
- Olstad, K.; Ekman, S.; Carlson, C.S. An Update on the Pathogenesis of Osteochondrosis. Vet. Pathol. 2015, 52, 785–802. [CrossRef]
- Tóth, F.; Tompkins, M.A.; Shea, K.G.; Ellermann, J.M.; Carlson, C.S. Identification of Areas of Epiphyseal Cartilage Necrosis at Predilection Sites of Juvenile Osteochondritis Dissecans in Pediatric Cadavers. J. Bone Joint Surg. Am. 2018, 100, 2132–2139. [CrossRef]
- McCoy, A.M.; Toth, F.; Dolvik, N.I.; Ekman, S.; Ellermann, J.; Olstad, K.; Ytrehus, B.; Carlson, C.S. Articular Osteochondrosis: A Comparison of Naturally-Occurring Human and Animal Disease. Osteoarthr. Cartil. OARS Osteoarthr. Res. Soc. 2013, 21, 10.1016/j.joca.2013.08.011. [CrossRef]
- Cabral, J.; Duart, J. Osteochondritis Dissecans of the Knee in Adolescents: How to Treat Them? J. Child. Orthop. 2023, 17, 54–62. [CrossRef]
- Takahara, M.; Ogino, T.; Tsuchida, H.; Takagi, M.; Kashiwa, H.; Nambu, T. Sonographic Assessment of Osteochondritis Dissecans of the Humeral Capitellum. AJR Am. J. Roentgenol. 2000, 174, 411–415. [CrossRef]
- Berndt, A.L.; Harty, M. Transchondral Fractures (Osteochondritis Dissecans) of the Talus. J. Bone Joint Surg. Am. 1959, 41-A, 988–1020.
- Guhl, J.F. Arthroscopic Treatment of Osteochondritis Dissecans. Clin. Orthop. 1982, 65–74.
- Brittberg, M.; Winalski, C.S. Evaluation of Cartilage Injuries and Repair. J. Bone Joint Surg. Am. 2003, 85-A Suppl 2, 58–69. [CrossRef]
- Dipaola, J.D.; Nelson, D.W.; Colville, M.R. Characterizing Osteochondral Lesions by Magnetic Resonance Imaging. Arthrosc. J. Arthrosc. Relat. Surg. Off. Publ. Arthrosc. Assoc. N. Am. Int. Arthrosc. Assoc. 1991, 7, 101–104. [CrossRef]
- Wall, E.J.; Vourazeris, J.; Myer, G.D.; Emery, K.H.; Divine, J.G.; Nick, T.G.; Hewett, T.E. The Healing Potential of Stable Juvenile Osteochondritis Dissecans Knee Lesions. J. Bone Joint Surg. Am. 2008, 90, 2655–2664. [CrossRef]
- Gentile, P.; Chiono, V.; Carmagnola, I.; Hatton, P.V. An Overview of Poly(Lactic-Co-Glycolic) Acid (PLGA)-Based Biomaterials for Bone Tissue Engineering. Int. J. Mol. Sci. 2014, 15, 3640–3659. [CrossRef]
- Sanders, T.L.; Pareek, A.; Obey, M.R.; Johnson, N.R.; Carey, J.L.; Stuart, M.J.; Krych, A.J. High Rate of Osteoarthritis After Osteochondritis Dissecans Fragment Excision Compared With Surgical Restoration at a Mean 16-Year Follow-Up. Am. J. Sports Med. 2017, 45, 1799–1805. [CrossRef]
- Dankelman, L.H.M.; Schilstra, S.; IJpma, F.F.A.; Doornberg, J.N.; Colaris, J.W.; Verhofstad, M.H.J.; Wijffels, M.M.E.; Prijs, J.; Machine Learning Consortium Artificial Intelligence Fracture Recognition on Computed Tomography: Review of Literature and Recommendations. Eur. J. Trauma Emerg. Surg. Off. Publ. Eur. Trauma Soc. 2023, 49, 681–691. [CrossRef]
- Adams, S.J.; Henderson, R.D.E.; Yi, X.; Babyn, P. Artificial Intelligence Solutions for Analysis of X-Ray Images. Can. Assoc. Radiol. J. J. Assoc. Can. Radiol. 2021, 72, 60–72. [CrossRef]
- Kraus, M.; Anteby, R.; Konen, E.; Eshed, I.; Klang, E. Artificial Intelligence for X-Ray Scaphoid Fracture Detection: A Systematic Review and Diagnostic Test Accuracy Meta-Analysis. Eur. Radiol. 2024, 34, 4341–4351. [CrossRef]
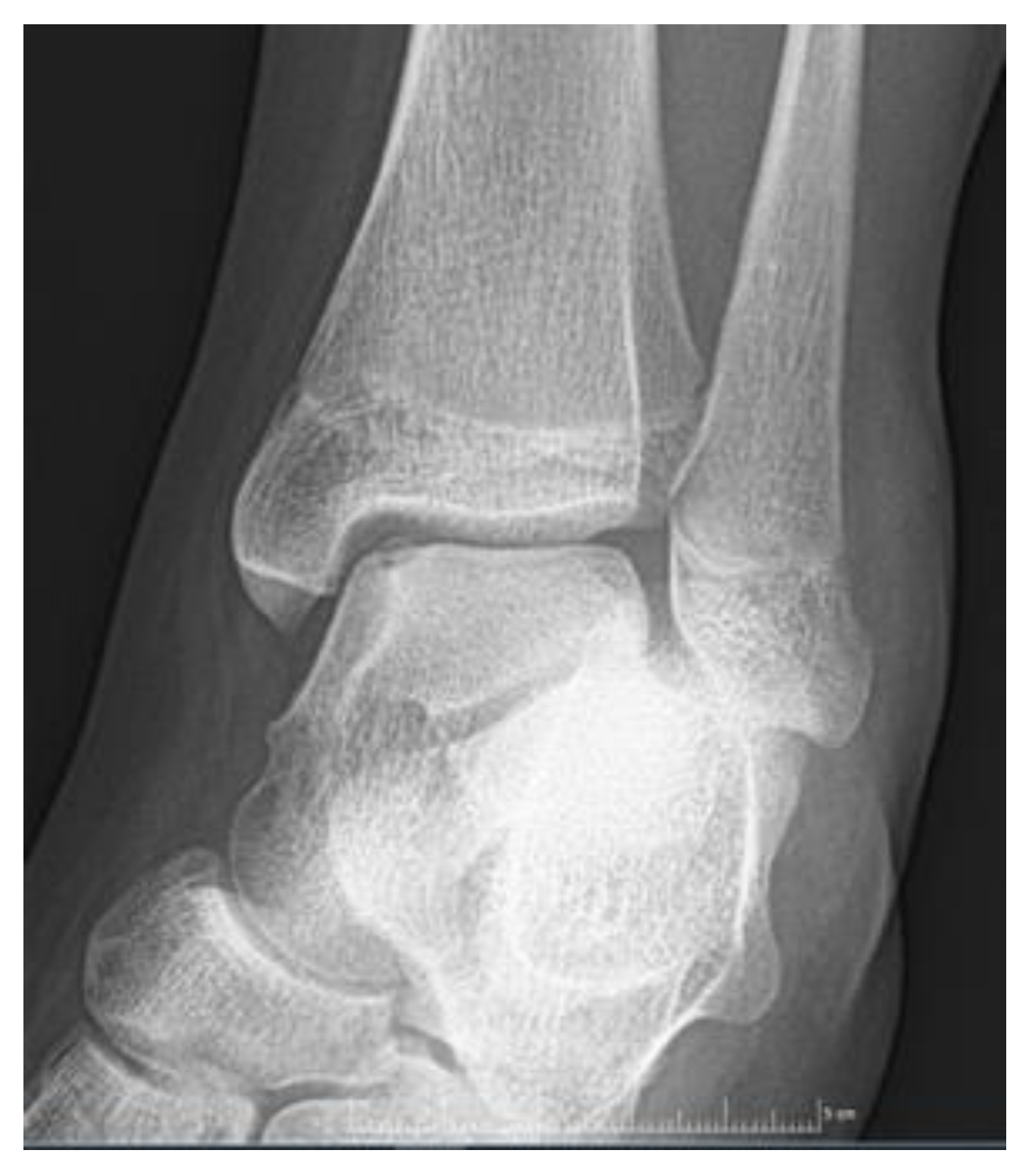

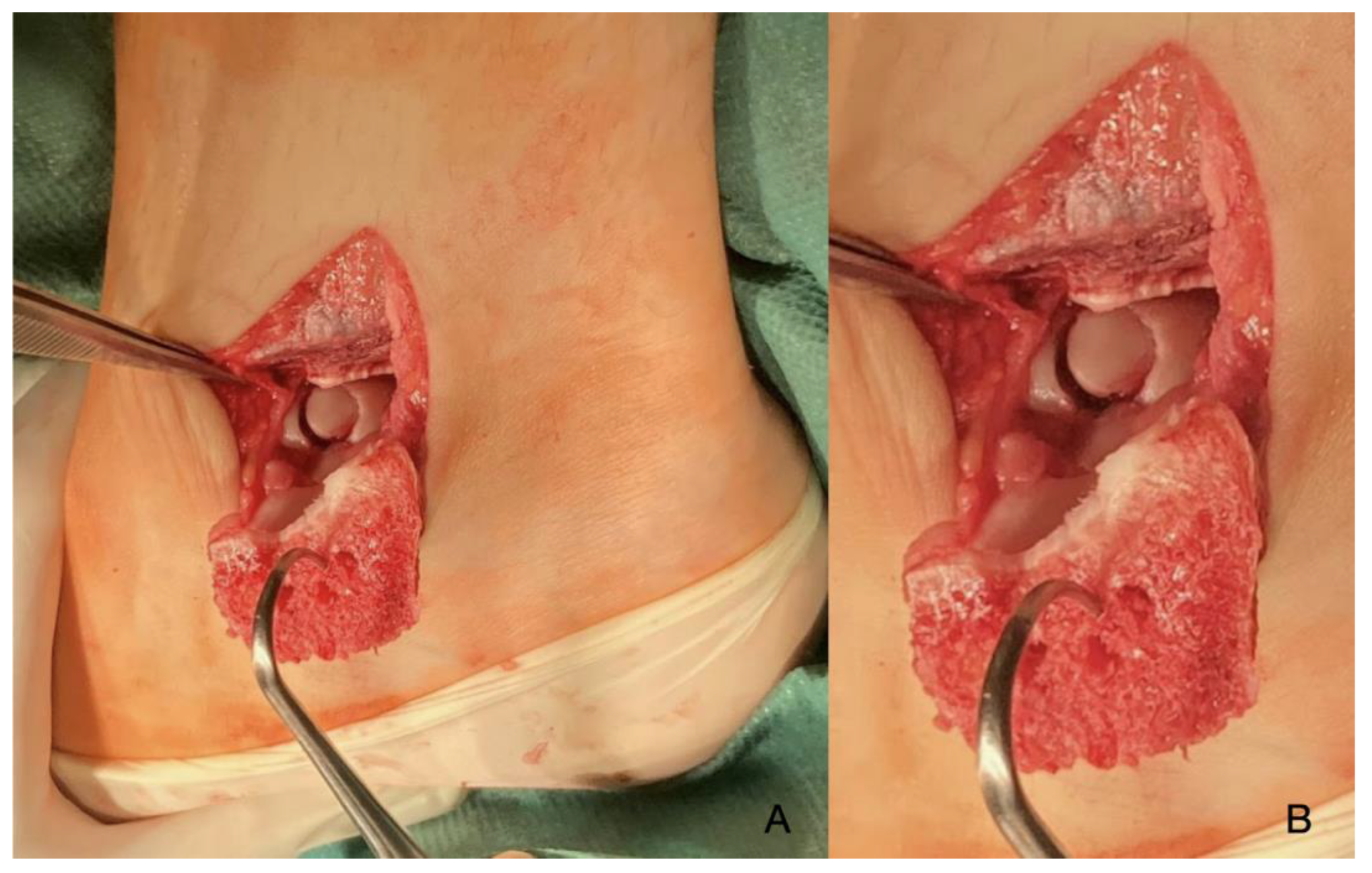
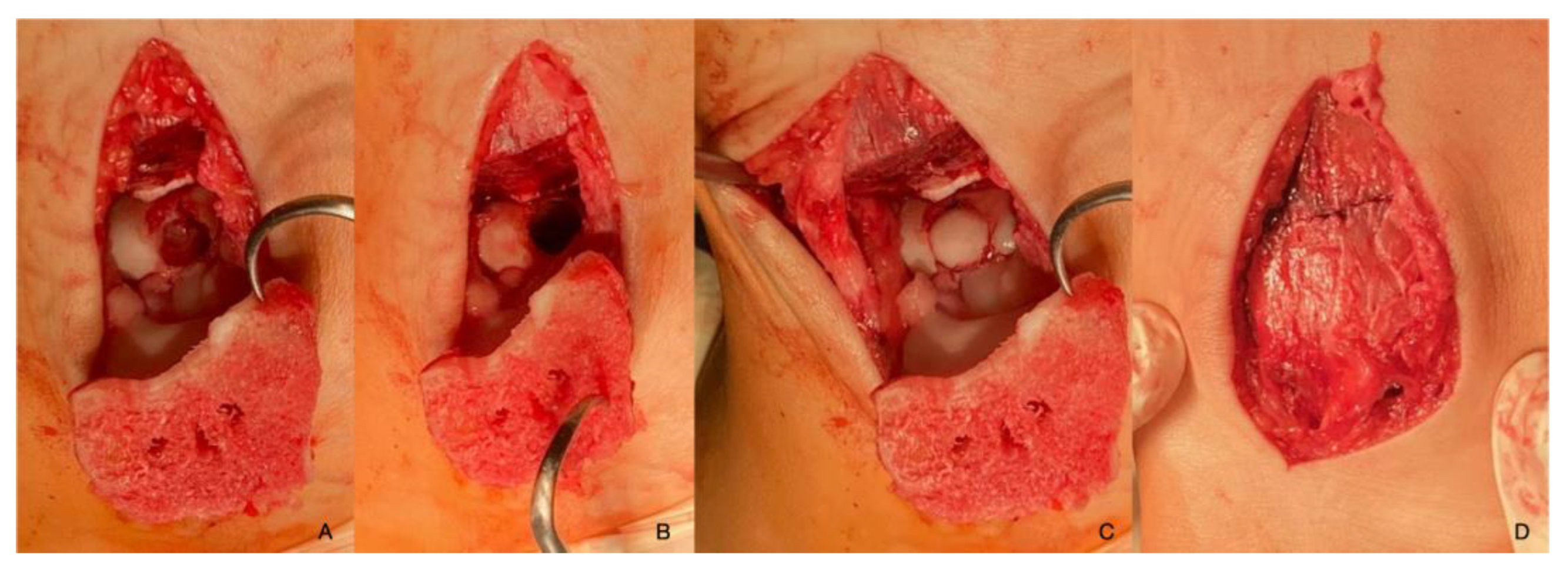
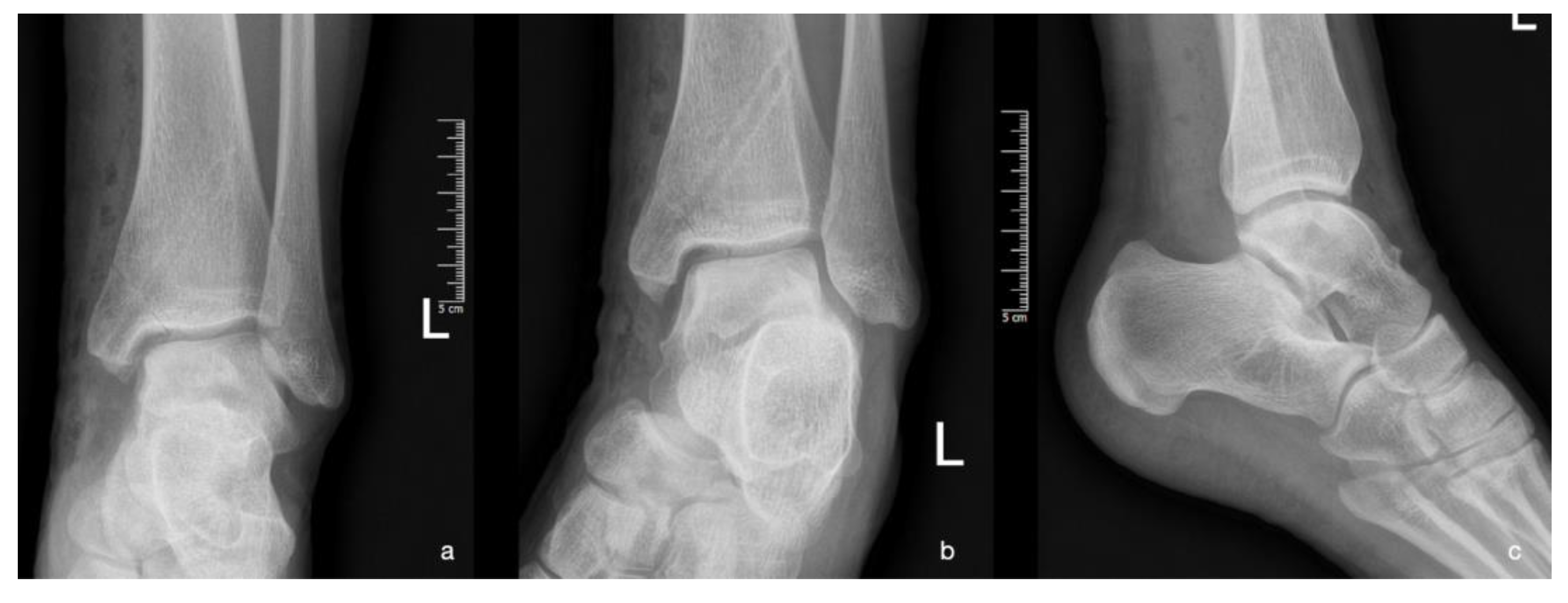
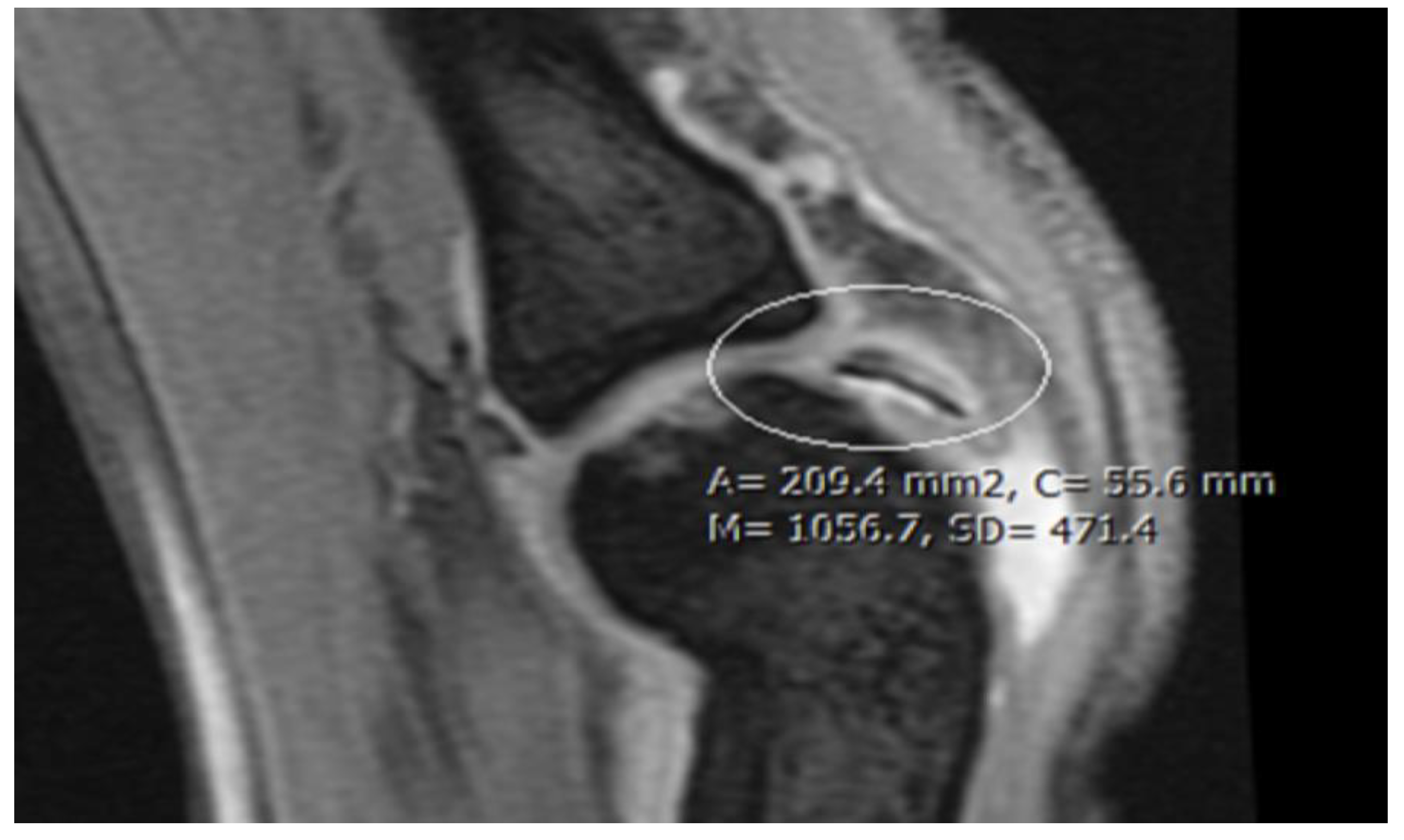
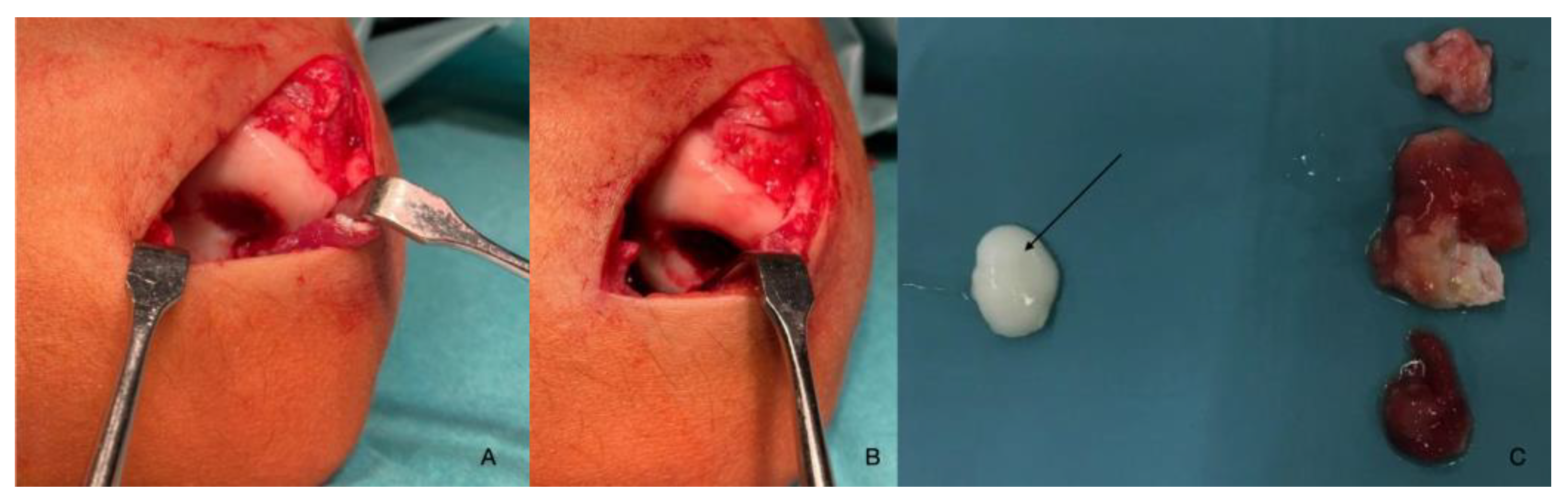
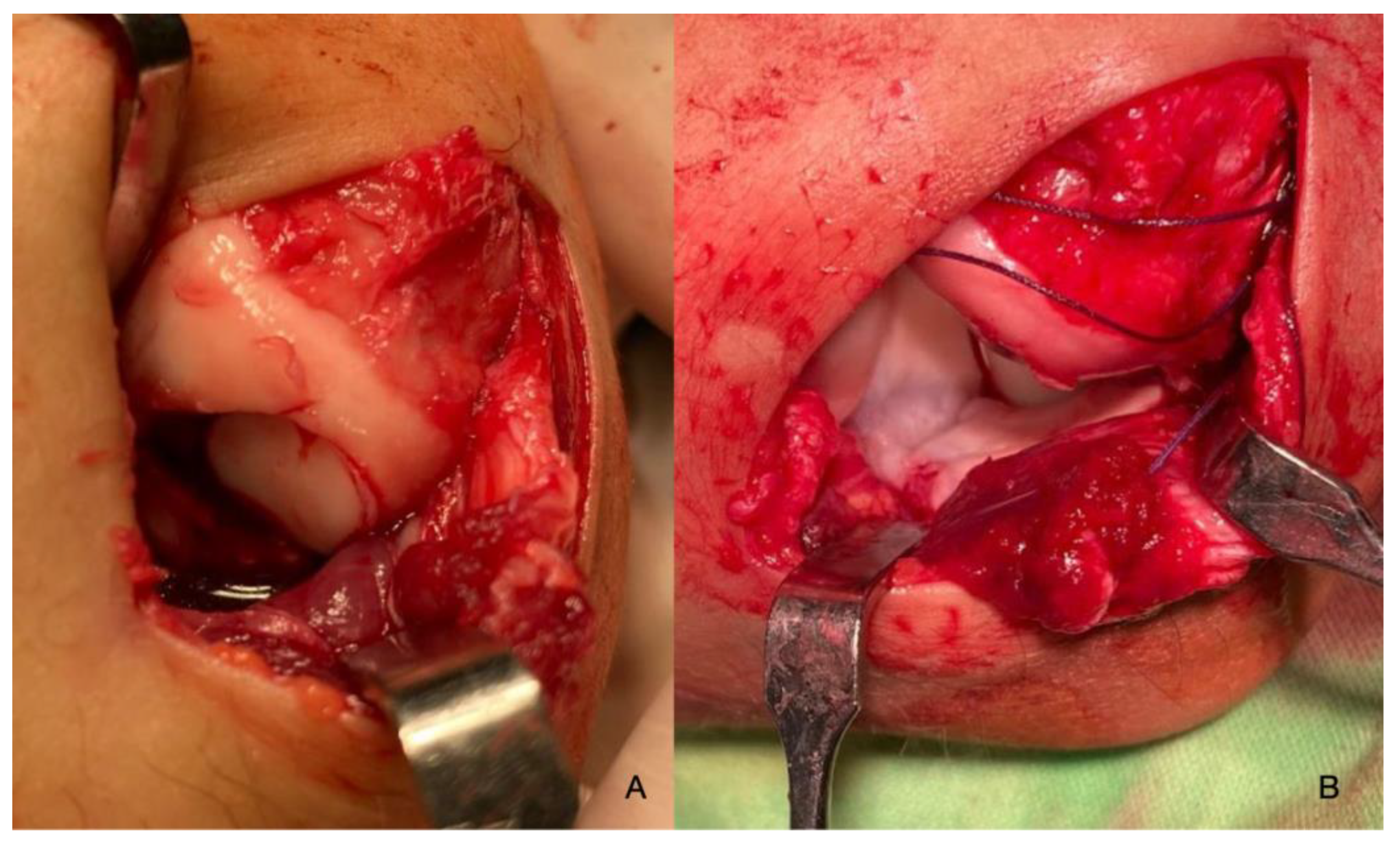
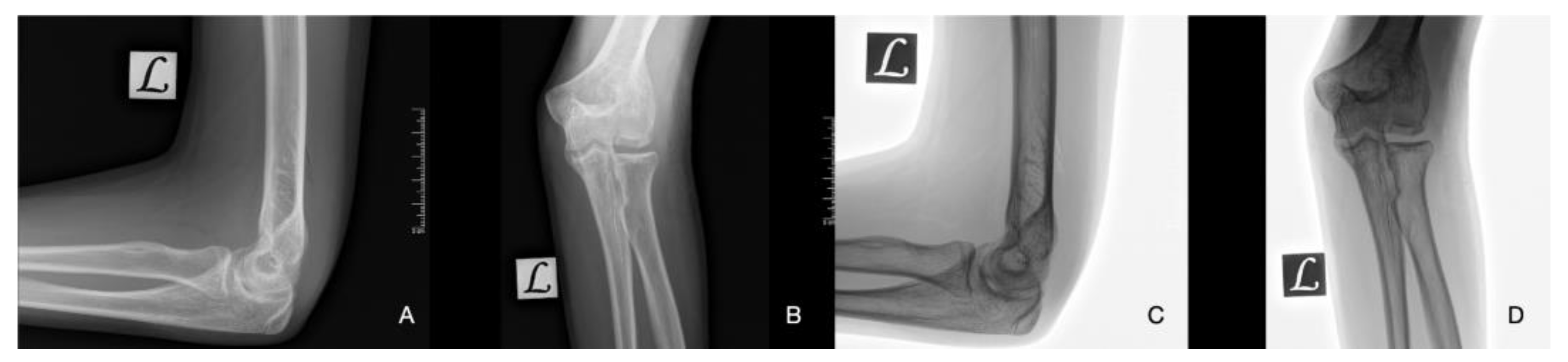

| Imaging: | X-Ray | Arthroscopy | MRI | |||
|---|---|---|---|---|---|---|
| Classification | Berndt and Harty [21] - 1959 | Guhl [22] - 1982 | ICRS by Brittberg and Winalsky [23]- 2003 | DiPaola [24] - 1991 | Hefti [4] - 1999 | Ellerman [15] - 2019 |
| Stage 1 | Small subchondral compression | Intact Lesion | A stable lesion of the softened area covered by intact cartilage. | Thickening of articular cartilage and low signal changes | Small change of signal, without clear fragment margins. | Epiphyseal cartilage lesion with necrotic center |
| Stage 2 | Partially detached osteochondral fragment | A lesion with signs of early separation | Lesions with partial discontinuity which are stable when probed | Articular cartilage has been breached with a low signal rim behind the fragment indicating fibrous attachment | OSteochondral fragment with clear margins, without fluid in between | Epiphyseal cartilage lesion with complete or incomplete rim calcification |
| Stage 3 | Completely detached, non-displaced | Partially detached lesion | Lesions with complete discontinuity which are not dislocated (Dead in situ) | High signal changes behind the fragment indicate synovial fluid between the fragment and the underlying subchondral bone | Fluid is visible partially between the fragment and bone | Partially or completely ossified lesion |
| Stage 4 | Completely detached and displaced - loose body | Craters with loose bodies (salvageable or non-salvageable) | Empty defect bed with loose or dislocated fragment | Loose body | Fluid surrounds the fragment but it is still in situ | A healed osseous lesion with scar |
| Stage 5 | Scranton and McDermott modification : Subchondral Cyst | - | - | - | The fragment is completely detached and displaced | Unhealed, detached osseous lesion (Sequestrum) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





