Submitted:
30 July 2024
Posted:
01 August 2024
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Materials and Methods
2.1. Plant Material
2.2. Phytochemical Analysis
2.3. In Vitro Bioaccessibility Assessment of Berberine and Protoberberine Derivatives
2.4. In Silico Pharmacokinetic Analysis and Targets Prediction of Berberine and Protoberberine Derivatives
2.5. In Vitro Cell Culture and Treatment
2.6. Cytotoxicity Assay of Extracts Containing Berberine and Protoberberine Derivatives
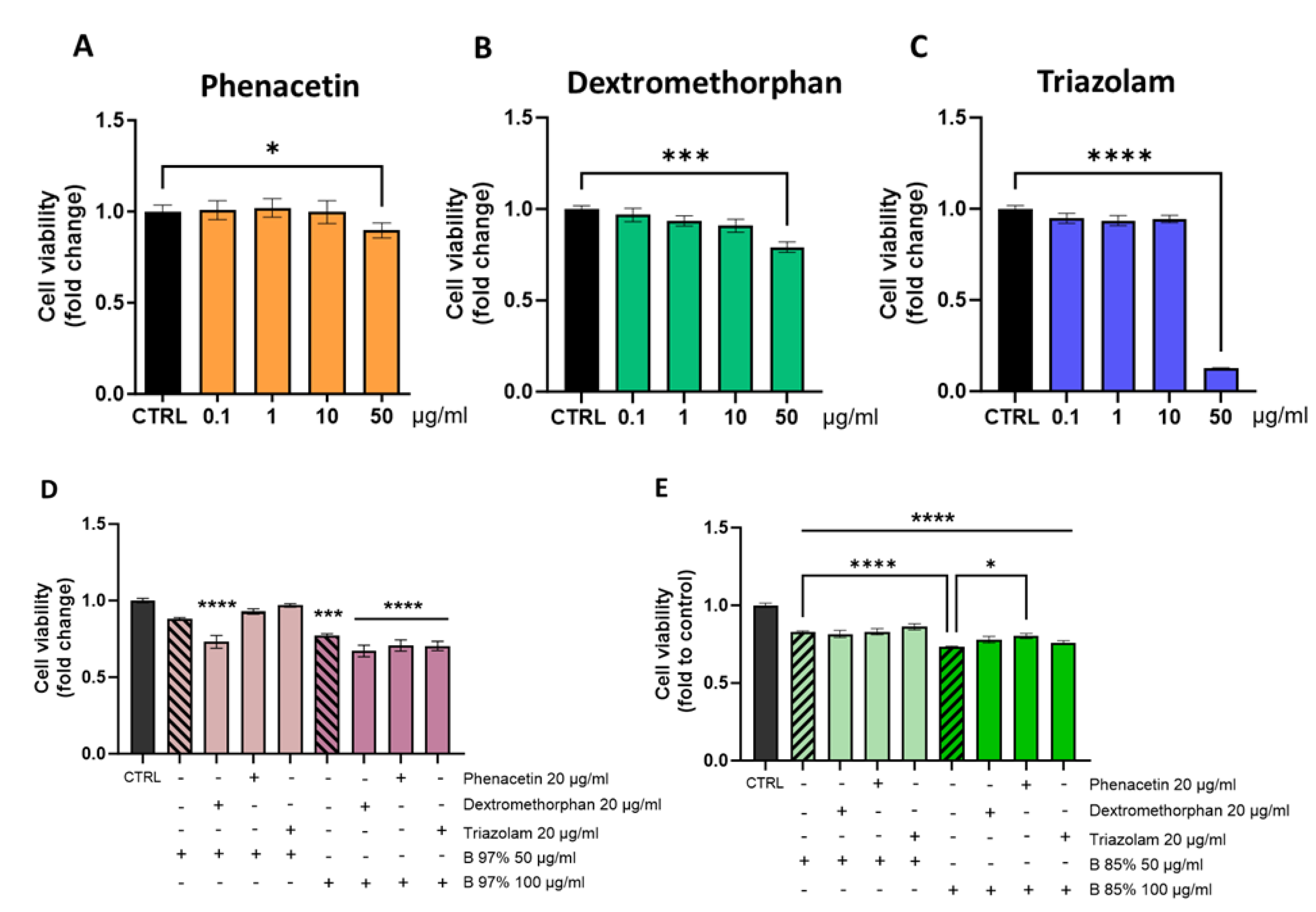
2.7. Cytotoxicity Assay of Extracts Containing Berberine and Protoberberine Derivatives in Presence of CYP450 Substrates
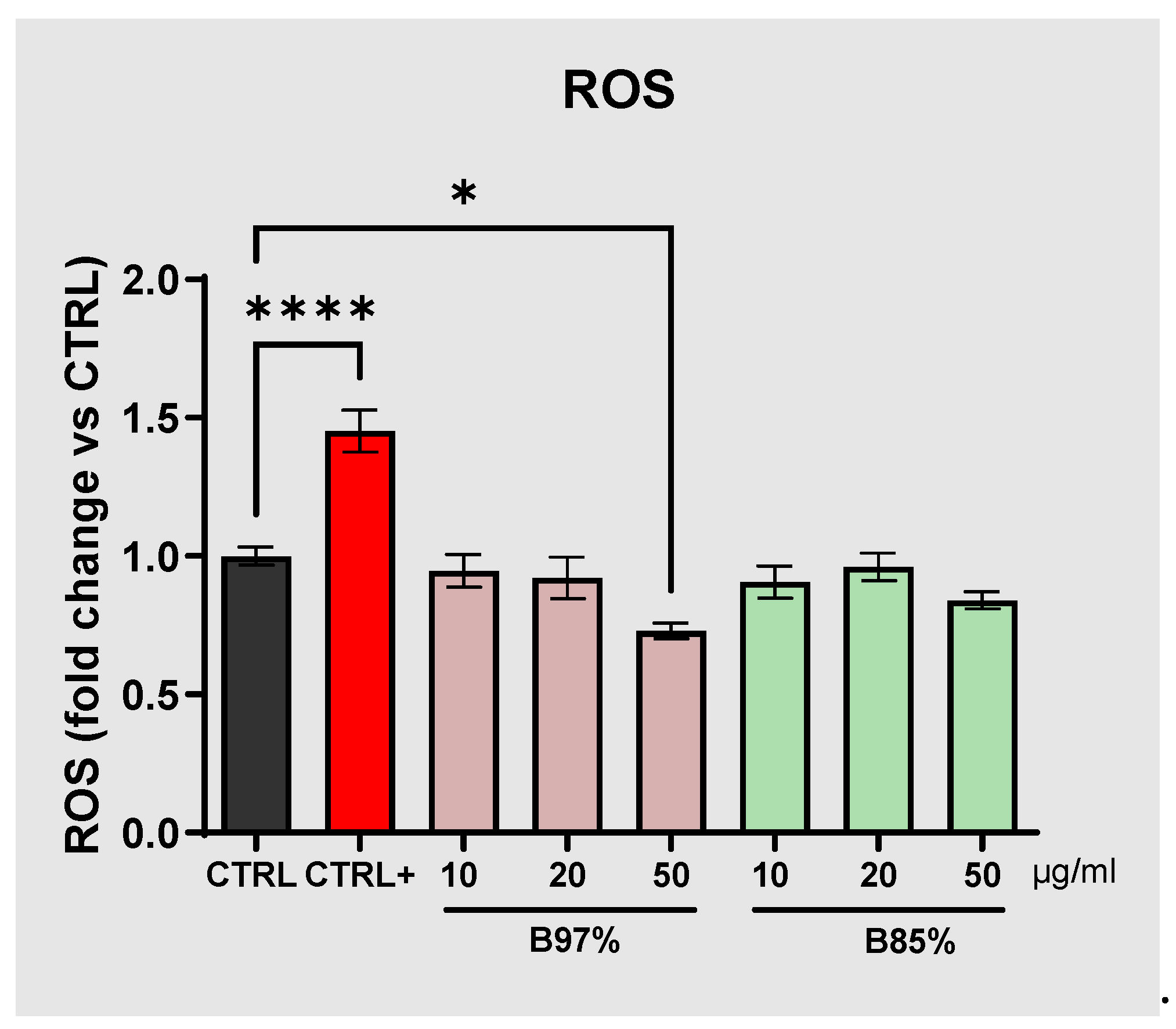
2.87. Dosage of Intracellular Reactive Oxygen Species (ROS) Level
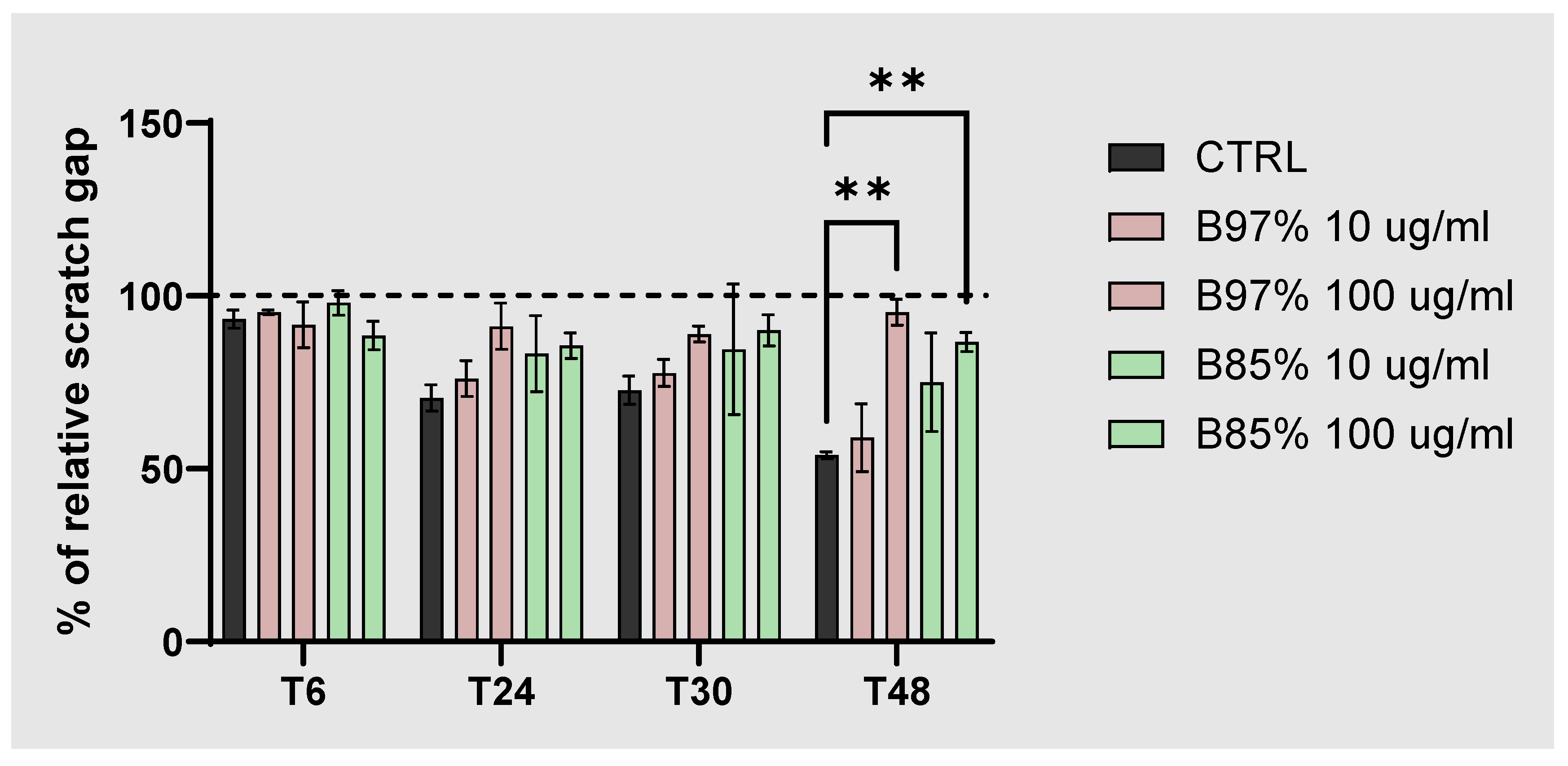
2.9. Migration Assay
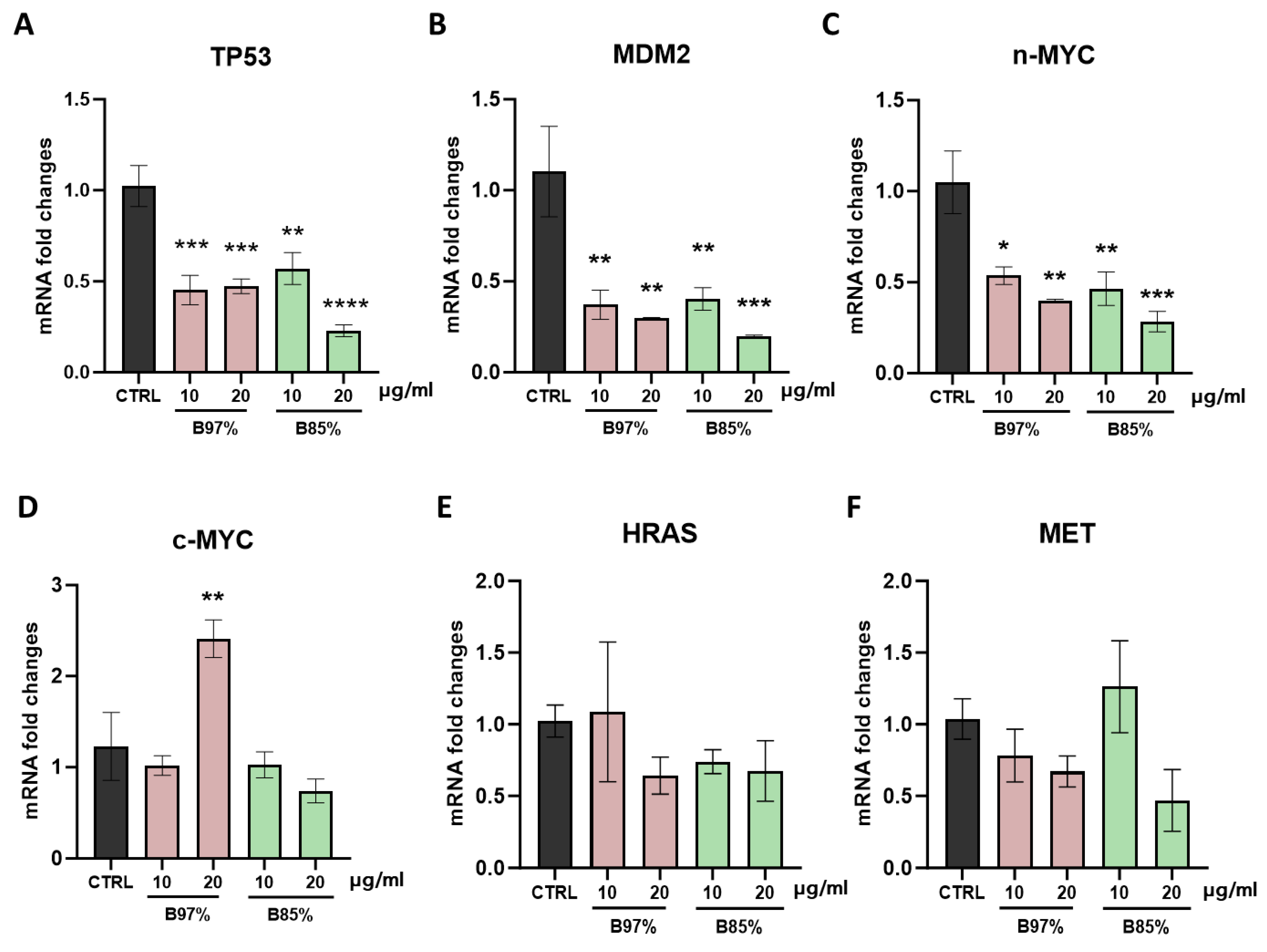
2.10. Total RNA Extraction, Reverse Transcription, and real-Time PCR
| Gene | NCBI GenBank | Sequence |
| Rplp13a | NM_012423.4 | fw: GTGCGTCTGAAGCCTACAAG rv: CGTTCTTCTCGGCCTGTTTC |
| Rps18 | NM_022551.3 | fw: TCTAGTGATCCCTGAAAAGT rv: AACACCACATGAGCATATC |
| Tp53 | NM_000546.6 | fw: AGGGATGTTTGGGAGATGTAAG rv: CCTGGTTAGTACGGTGAAGTG |
| c-Myc | NM_001354870.1 | fw: AAGCTGAGGCACACAAAGA rv: GCTTGGACAGGTTAGGAGTAAA |
| n-Myc | NM_005378.6 | fw: TCCAGCAGATGCCACATAAG rv: ACCTCTCATTACCCAGGATGTA |
| Met | NM_001127500.3 | fw: CCTGGGCACCGAAAGATAAA rv: CTCCTCTGCACCAAGGTAAAC |
| Mdm2 | NM_002392.6 | fw: AGGCTGATCTTGAACTCCTAAAC rv: CAGGTGCCTCACATCTGTAATC |
| Cdkn1a | NM_000389.5 | fw: CGGAACAAGGAGTCAGACATT rv: AGTGCCAGGAAAGACAACTAC |
| Snai1 | NM_005985.4 | fw: CAGATGAGGACAGTGGGAAAG rv: GAGACTGAAGTAGAGGAGAAGGA |
| Snai2 | NM_003068.5 | fw: AACTACAGCGAACTGGACAC rv: GAGGATCTCTGGTTGTGGTATG |
| Hras | NM_005343.4 | fw: AAGCAAGGAAGGAAGGAAGG rv: GTGGCATTTGGGATGTTCAAG |
| Cdk4 | NM_000075.4 | fw: GCTCTGCAGCACTCTTATCTAC rv: CTCAGTGTCCAGAAGGGAAATG |
| Bax | NM_004324 | fw: CTCCCCATCTTCAGATCATCAG rv: GGCAGAAGGCACTAATCAAGTC |
| Bcl2 | NM_000657 | fw: GACTGAGTACCTGAACCGGC rv: CTCAGCCCAGACTCACATCA |
2.11. Statistical Analysis
3. Results
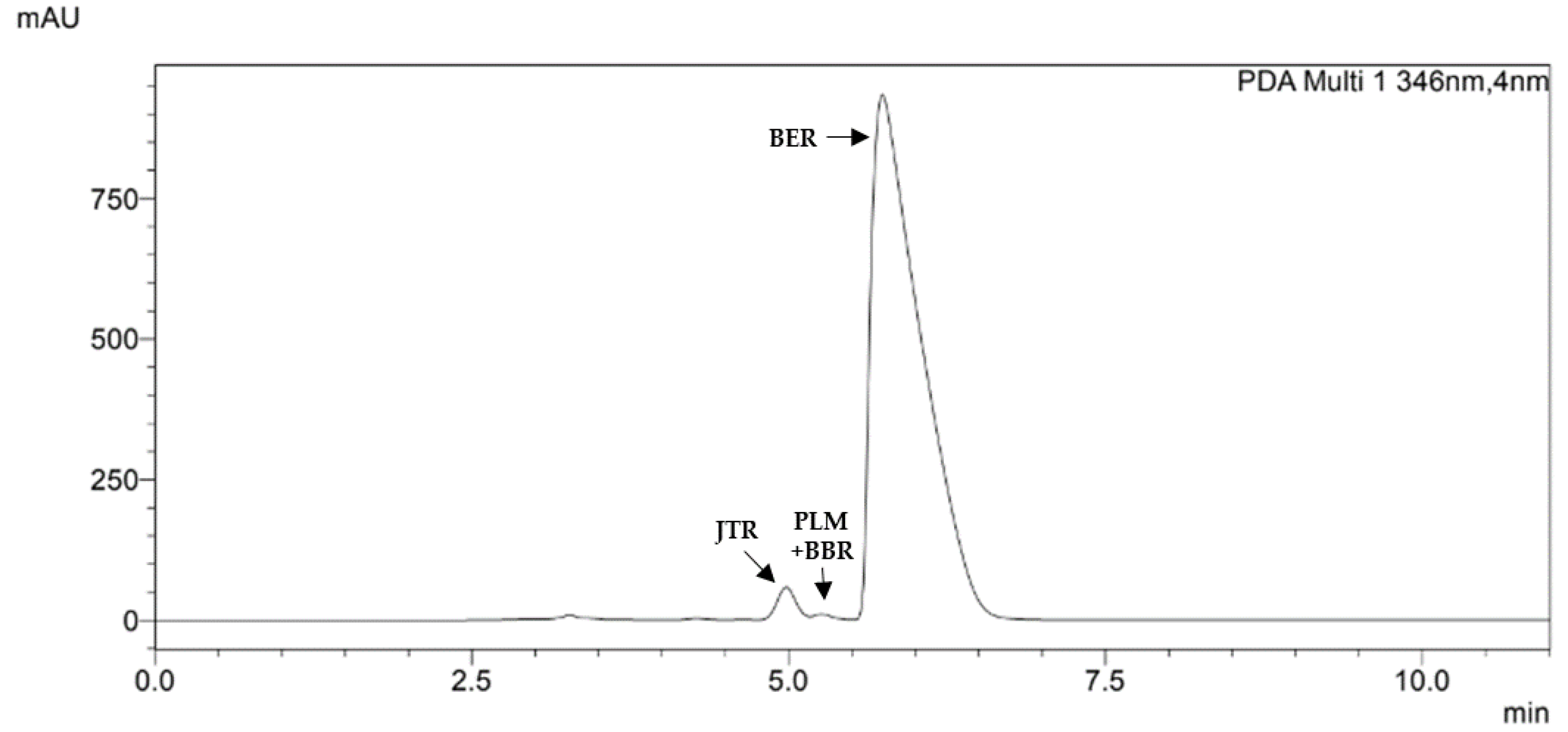
3.1. Chemical Analyses of Extracts Containing Berberine and Protoberberine Derivatives
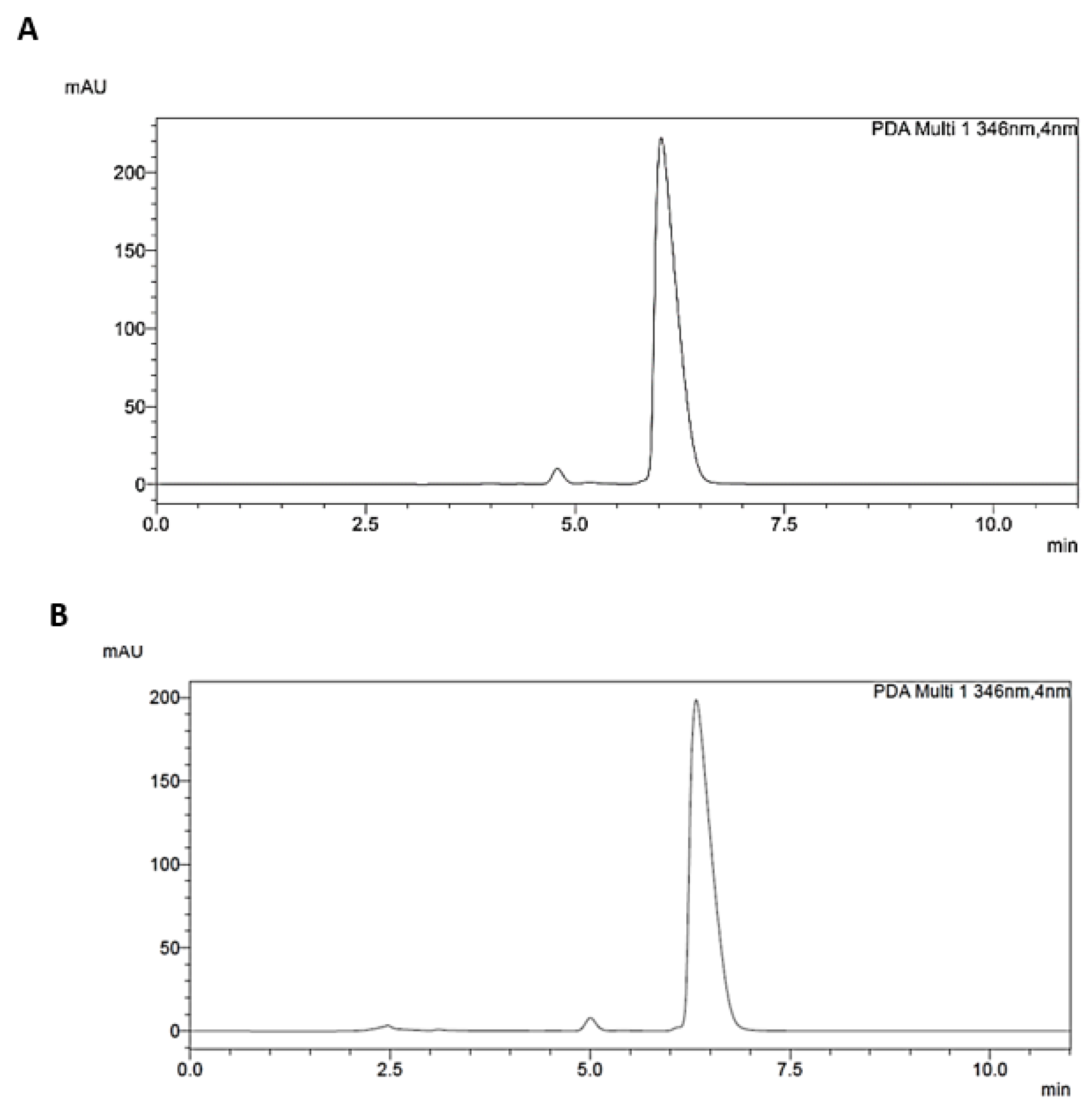
3.2. In Vitro Bioaccessibility Assessment of Berberine and Protoberberine Derivatives
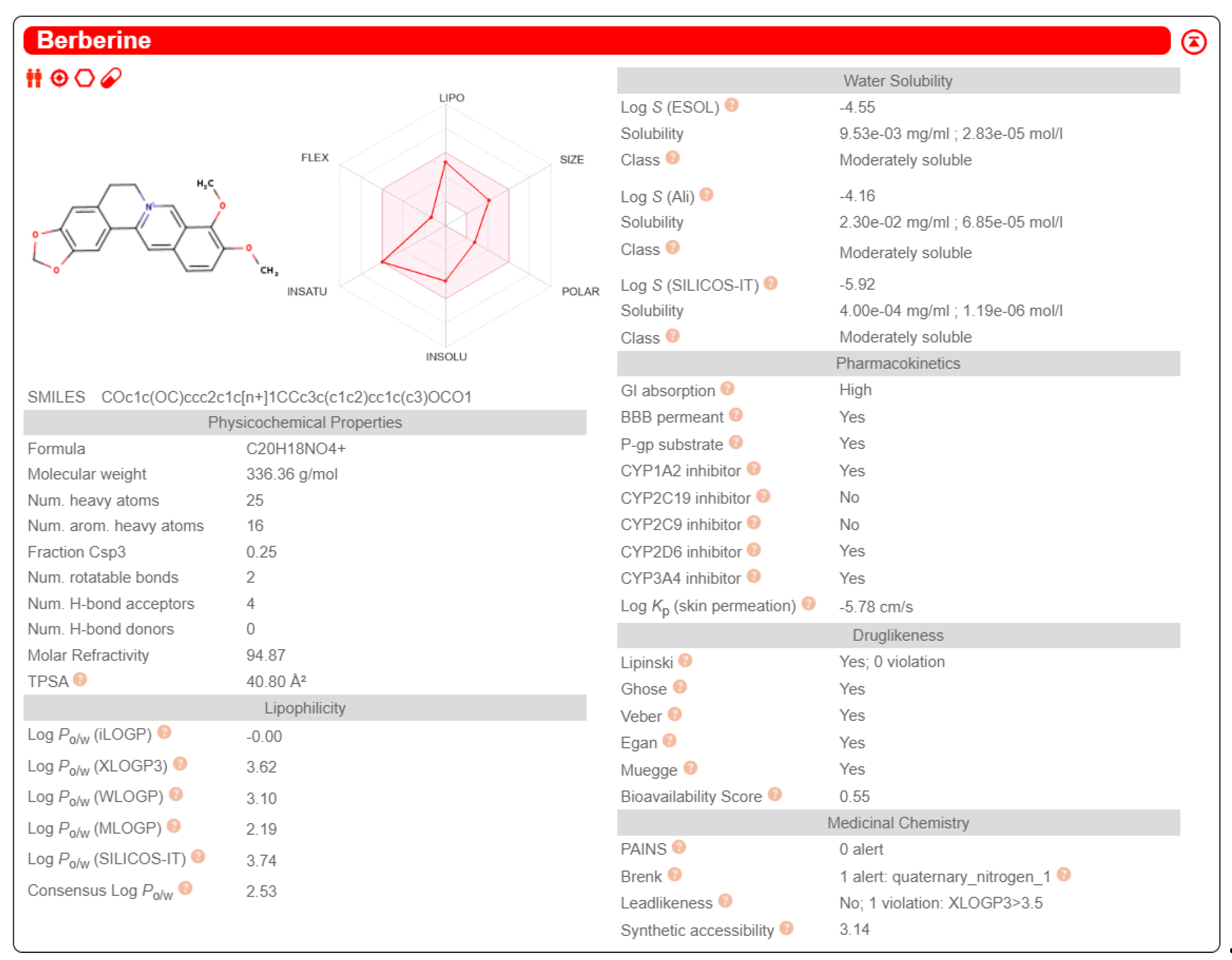
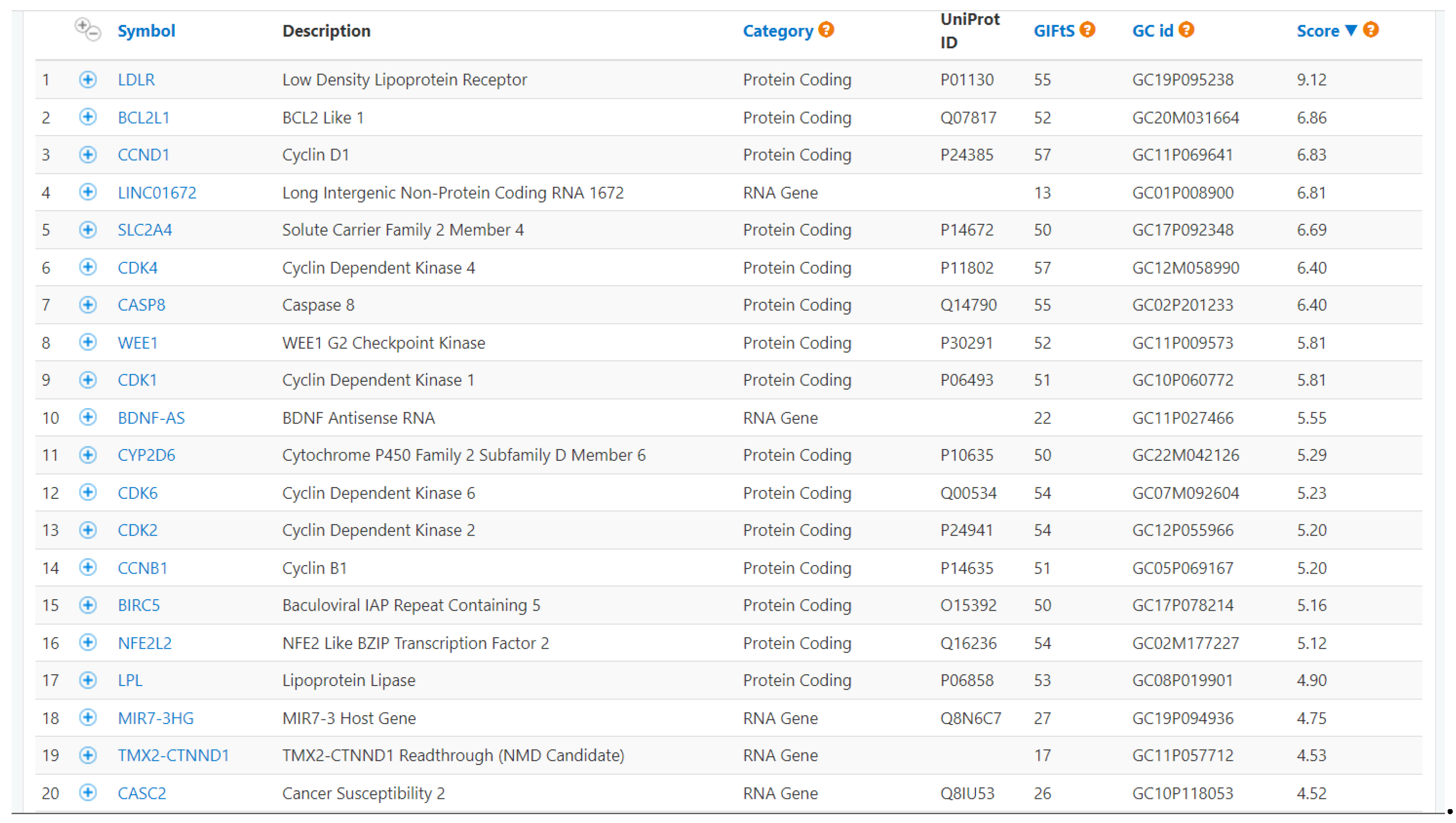
3.3 In Silico Pharmacokinetic Analysis and Targets Prediction of Berberine and Protoberberine Derivatives
3.4. In Vitro Cytotoxicity Evaluation of Berberis Aristata Bark Extracts
| IC50 (mg/ml) | |||||
| Sample | Treatment (h) | AGS | Caco-2 | HepG2 | HEK293 |
| B 97% | 4 | >200 | 166.93±8.44 | >200 | 142.65±12.01 |
| B 85% | 4 | >200 | 169.14±9.25 | >200 | 181.93±24.50 |
| B 97% | 24 | >200 | 105.59±11.21 | 198.85±8.50 | 127.16±25.18 |
| B 85% | 24 | >200 | 107.34±9.68 | 186.41±8.42 | 143.43±29.80 |
3.5. In Vitro Cytotoxicity Evaluation of Berberis Aristata Bark Extracts in Presence of CYP450 Substrates
3.6. Effect of Berberis Aristata Bark Extracts on Cell Migration
3.7. In Vitro Evaluation of ROS Production in Normal Kidney Cells Treated with Berberis Aristata Bark Extracts
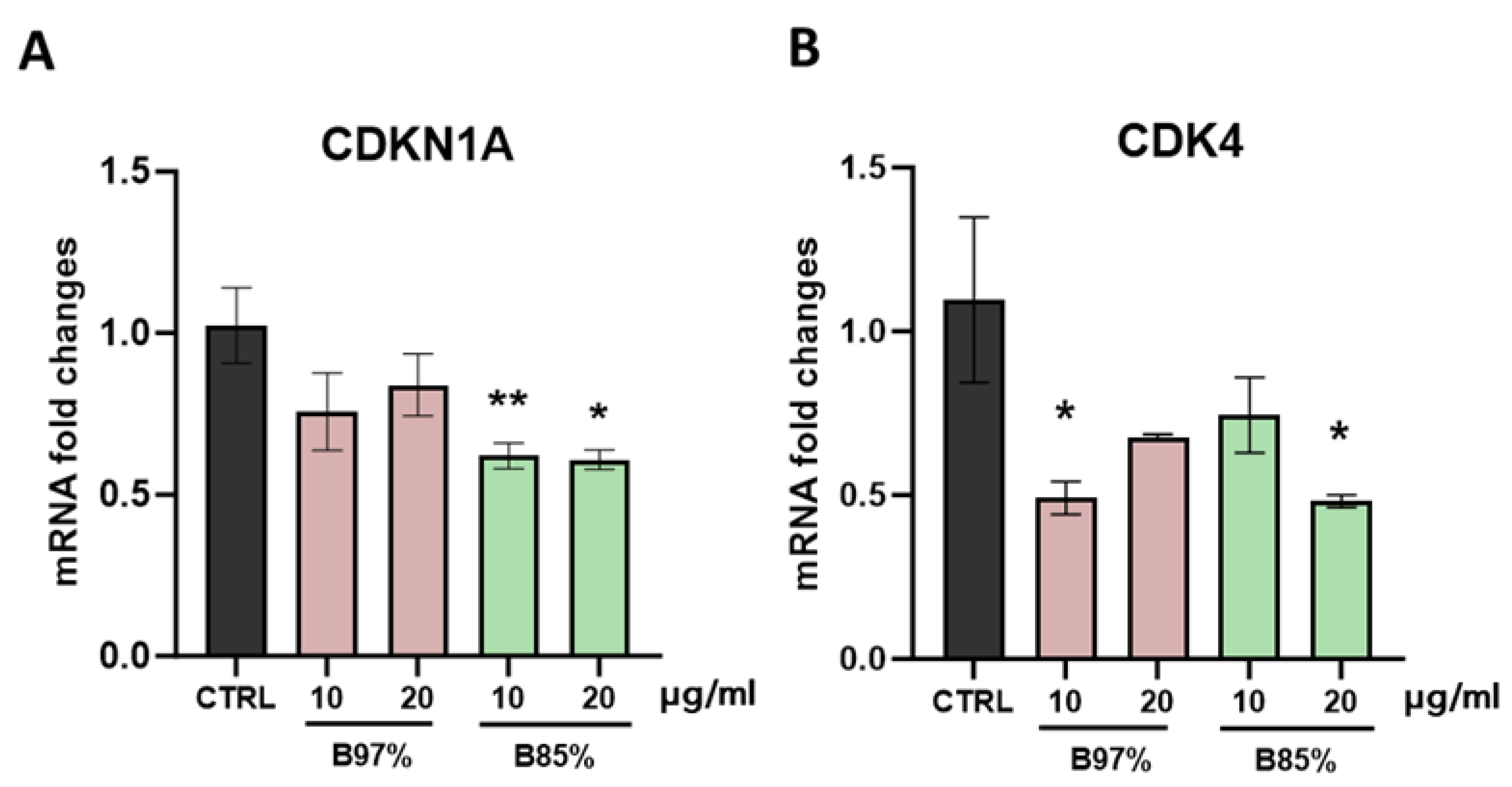
3.8. Transcriptional Effect of Berberis Aristata Bark Extracts on Target Genes Involved In Cell Cycle Control and Neoplastic Transformation
4. Discussion
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Berberine | C20H18NO4+ | CID 2353 - PubChem Available online:. Available online: https://pubchem.ncbi.nlm.nih.gov/compound/2353 (accessed on 2 January 2024).
- Neag, M.A.; Mocan, A.; Echeverría, J.; Pop, R.M.; Bocsan, C.I.; Crisan, G.; Buzoianu, A.D. Berberine: Botanical Occurrence, Traditional Uses, Extraction Methods, and Relevance in Cardiovascular, Metabolic, Hepatic, and Renal Disorders. Front Pharmacol 2018, 9, 343970. [Google Scholar] [CrossRef] [PubMed]
- Imenshahidi, M.; Hosseinzadeh, H. Berberine and Barberry (Berberis Vulgaris): A Clinical Review. Phytotherapy Research 2019, 33, 504–523. [Google Scholar] [CrossRef] [PubMed]
- European Pharmacopoeia Commission Berberis Aristata Stem (2851) Monograph in Ph. Eur. Post 2022. 2022.
- Xing, L.; Zhou, X.; Li, A.H.; Li, H.J.; He, C.X.; Qin, W.; Zhao, D.; Li, P.Q.; Zhu, L.; Cao, H.L. Atheroprotective Effects and Molecular Mechanism of Berberine. Front Mol Biosci 2021, 8, 762673. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Zhu, W.; Zhang, X.; Zhou, X.; Wu, W.; Shen, T. Efficacy and Safety of Berberine for Several Cardiovascular Diseases: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. Phytomedicine 2023, 112, 154716. [Google Scholar] [CrossRef] [PubMed]
- Cho, B.J.; Im, E.K.; Kwon, J.H.; Lee, K.H.; Shin, H.J.; Oh, J.; Kang, S.M.; Chung, J.H.; Jang, Y. Berberine Inhibits the Production of Lysophosphatidylcholine-Induced Reactive Oxygen Species and the ERK1/2 Pathway in Vascular Smooth Muscle Cells. Mol Cells 2005, 20, 429–434. [Google Scholar] [CrossRef] [PubMed]
- Ju, J.; Li, J.; Lin, Q.; Xu, H. Efficacy and Safety of Berberine for Dyslipidaemias: A Systematic Review and Meta-Analysis of Randomized Clinical Trials. Phytomedicine 2018, 50, 25–34. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Geng, Y.-N.; Jiang, J.-D.; Kong, W.-J. Antioxidant and Anti-Inflammatory Activities of Berberine in the Treatment of Diabetes Mellitus. 2014. [CrossRef]
- Chueh, W.H.; Lin, J.Y. Berberine, an Isoquinoline Alkaloid, Inhibits Streptozotocin-Induced Apoptosis in Mouse Pancreatic Islets through down-Regulating Bax/Bcl-2 Gene Expression Ratio. Food Chem 2012, 132, 252–260. [Google Scholar] [CrossRef]
- Liang, Y.; Xu, X.; Yin, M.; Zhang, Y.; Huang, L.; Chen, R.; Ni, J. Effects of Berberine on Blood Glucose in Patients with Type 2 Diabetes Mellitus: A Systematic Literature Review and a Meta-Analysis. Endocr J 2019, 66, 51–63. [Google Scholar] [CrossRef]
- Cao, C.; Su, M. Effects of Berberine on Glucose-Lipid Metabolism, Inflammatory Factors and Insulin Resistance in Patients with Metabolic Syndrome. Exp Ther Med 2019, 17, 3009. [Google Scholar] [CrossRef]
- Nazari, A.; Ghotbabadi, Z.R.; Kazemi, K.S.; Metghalchi, Y.; Tavakoli, R.; Rahimabadi, R.Z.; Ghaheri, M. The Effect of Berberine Supplementation on Glycemic Control and Inflammatory Biomarkers in Metabolic Disorders: An Umbrella Meta-Analysis of Randomized Controlled Trials. Clin Ther 2023. [Google Scholar] [CrossRef] [PubMed]
- Panigrahi, A.; Mohanty, S. Efficacy and Safety of HIMABERB® Berberine on Glycemic Control in Patients with Prediabetes: Double-Blind, Placebo-Controlled, and Randomized Pilot Trial. BMC Endocr Disord 2023, 23, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.; Yu, Z.; Li, Y.; Fichna, J.; Storr, M. Effects of Berberine in the Gastrointestinal Tract - a Review of Actions and Therapeutic Implications. Am J Chin Med (Gard City N Y) 2014, 42, 1053–1070. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Wu, X.; Yang, R.; Chen, F.; Liao, Y.; Zhu, Z.; Wu, Z.; Sun, X.; Wang, L. Effects of Berberine on the Gastrointestinal Microbiota. Front Cell Infect Microbiol 2020, 10. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Liu, Y.; Du, X.; Ma, H.; Yao, J. The Anti-Cancer Mechanisms of Berberine: A Review. Cancer Manag Res 2020, 12, 695. [Google Scholar] [CrossRef] [PubMed]
- Huang, C.; Tao, L.; Wang, X. lian; Pang, Z. Berberine Reversed the Epithelial-Mesenchymal Transition of Normal Colonic Epithelial Cells Induced by SW480 Cells through Regulating the Important Components in the TGF-β Pathway. J Cell Physiol 2019, 234, 11679–11691. [Google Scholar] [CrossRef] [PubMed]
- Gong, C.; Hu, X.; Xu, Y.; Yang, J.; Zong, L.; Wang, C.; Zhu, J.; Li, Z.; Lu, D. Berberine Inhibits Proliferation and Migration of Colorectal Cancer Cells by Downregulation of GRP78. Anticancer Drugs 2020, 31, 141–149. [Google Scholar] [CrossRef] [PubMed]
- Bertuccioli, A.; Cardinali, M.; Biagi, M.; Moricoli, S.; Morganti, I.; Zonzini, G.B.; Rigillo, G. Nutraceuticals and Herbal Food Supplements for Weight Loss: Is There a Prebiotic Role in the Mechanism of Action? Microorganisms 2021, 9. [Google Scholar] [CrossRef] [PubMed]
- Guo, P.; Cai, C.; Wu, X.; Fan, X.; Huang, W.; Zhou, J.; Wu, Q.; Huang, Y.; Zhao, W.; Zhang, F.; et al. An Insight into the Molecular Mechanism of Berberine towards Multiple Cancer Types through Systems Pharmacology. Front Pharmacol 2019, 10, 857. [Google Scholar] [CrossRef]
- Radzka, J.; Łapińska, Z.; Szwedowicz, U.; Gajewska-Naryniecka, A.; Gizak, A.; Kulbacka, J. Alternations of NF-ΚB Signaling by Natural Compounds in Muscle-Derived Cancers. Int J Mol Sci 2023, 24. [Google Scholar] [CrossRef]
- Zhang, Y.; Ma, J.; Zhang, W. Berberine for Bone Regeneration: Therapeutic Potential and Molecular Mechanisms. J Ethnopharmacol 2021, 277, 114249. [Google Scholar] [CrossRef] [PubMed]
- Noh, J.W.; Jun, M.S.; Yang, H.K.; Lee, B.C. Cellular and Molecular Mechanisms and Effects of Berberine on Obesity-Induced Inflammation. Biomedicines 2022, 10. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Chen, S.T.; Sun, Y.; Xu, Z.; Wang, Y.; Yao, S.Y.; Yao, W.B.; Gao, X.D. Fibroblast Growth Factor 21 Ameliorates Neurodegeneration in Rat and Cellular Models of Alzheimer’s Disease. Redox Biol 2019, 22. [Google Scholar] [CrossRef]
- Wong, S.K.; Chin, K.Y.; Ima-Nirwana, S. Berberine and Musculoskeletal Disorders: The Therapeutic Potential and Underlying Molecular Mechanisms. Phytomedicine 2020, 73, 152892. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.; Miao, Y.Q.; Fan, D.J.; Yang, S.S.; Lin, X.; Meng, L.K.; Tang, X. Bioavailability Study of Berberine and the Enhancing Effects of TPGS on Intestinal Absorption in Rats. AAPS PharmSciTech 2011, 12, 705–711. [Google Scholar] [CrossRef]
- He, C.Y.; Fu, J.; Shou, J.W.; Zhao, Z.X.; Ren, L.; Wang, Y.; Jiang, J.D. In Vitro Study of the Metabolic Characteristics of Eight Isoquinoline Alkaloids from Natural Plants in Rat Gut Microbiota. Molecules 2017, Vol. 22, Page 932 2017, 22, 932. [Google Scholar] [CrossRef]
- Zhong, F.; Chen, Y.; Chen, J.; Liao, H.; Li, Y.; Ma, Y. Jatrorrhizine: A Review of Sources, Pharmacology, Pharmacokinetics and Toxicity. Front Pharmacol 2022, 12, 783127. [Google Scholar] [CrossRef]
- Chen, N.; Yang, X.Y.; Guo, C.E.; Bi, X.N.; Chen, J.H.; Chen, H.Y.; Li, H.P.; Lin, H.Y.; Zhang, Y.J. The Oral Bioavailability, Excretion and Cytochrome P450 Inhibition Properties of Epiberberine: An in Vivo and in Vitro Evaluation. Drug Des Devel Ther 2018, 12, 57. [Google Scholar] [CrossRef]
- Basera, I.A.; Girmeorcid, A.; Bhatt, V.P.; Sasteorcid, G.; Pawarorcid, S.; Hingoraniorcid, L.; Shah, M.B. Development of Validated UHPLC–PDA with ESI–MS-MS Method for Concurrent Estimation of Magnoflorine, Berbamine, Columbamine, Jatrorrhizine, Palmatine and Berberine in Berberis Aristata. Acta Chromatogr 2021, 34, 412–421. [Google Scholar] [CrossRef]
- Yang, N.; Sun, R. Bin; Chen, X.L.; Zhen, L.; Ge, C.; Zhao, Y.Q.; He, J.; Geng, J.L.; Guo, J.H.; Yu, X.Y.; et al. In Vitro Assessment of the Glucose-Lowering Effects of Berberrubine-9-O-β-D-Glucuronide, an Active Metabolite of Berberrubine. Acta Pharmacol Sin 2017, 38, 351. [Google Scholar] [CrossRef]
- Du, W.; Jin, L.; Li, L.; Wang, W.; Zeng, S.; Jiang, H.; Zhou, H. Development and Validation of a HPLC-ESI-MS/MS Method for Simultaneous Quantification of Fourteen Alkaloids in Mouse Plasma after Oral Administration of the Extract of Corydalis Yanhusuo Tuber: Application to Pharmacokinetic Study. Molecules : A Journal of Synthetic Chemistry and Natural Product Chemistry 2018, 23. [Google Scholar] [CrossRef]
- Guo, Y.; Pope, C.; Cheng, X.; Zhou, H.; Klaassen, C.D. Dose-Response of Berberine on Hepatic Cytochromes P450 MRNA Expression and Activities in Mice. J Ethnopharmacol 2011, 138, 111. [Google Scholar] [CrossRef] [PubMed]
- Zhou, H.; Shi, R.; Ma, B.; Ma, Y.; Wang, C.; Wu, D.; Wang, X.; Cheng, N. CYP450 1A2 and Multiple UGT1A Isoforms Are Responsible for Jatrorrhizine Metabolism in Human Liver Microsomes. Biopharm Drug Dispos 2013, 34, 176–185. [Google Scholar] [CrossRef] [PubMed]
- Vrba, J.; Papouskova, B.; Pyszkova, M.; Zatloukalova, M.; Lemr, K.; Ulrichova, J.; Vacek, J. Metabolism of Palmatine by Human Hepatocytes and Recombinant Cytochromes P450. J Pharm Biomed Anal 2015, 102, 193–198. [Google Scholar] [CrossRef]
- Call for Data for the Scientific Opinion on the Evaluation of the Safety in Use of Plant Preparations Containing Berberine | EFSA Available online:. Available online: https://www.efsa.europa.eu/en/call/call-data-scientific-opinion-evaluation-safety-use-plant-preparations-containing-berberine (accessed on 2 January 2024).
- Governa, P.; Manetti, F.; Miraldi, E.; Biagi, M. Effects of in Vitro Simulated Digestion on the Antioxidant Activity of Different Camellia Sinensis (L.) Kuntze Leaves Extracts. European Food Research and Technology 2022, 248, 119–128. [Google Scholar] [CrossRef]
- Brodkorb, A.; Egger, L.; Alminger, M.; Alvito, P.; Assunção, R.; Ballance, S.; Bohn, T.; Bourlieu-Lacanal, C.; Boutrou, R.; Carrière, F.; et al. INFOGEST Static in Vitro Simulation of Gastrointestinal Food Digestion. Nature Protocols 2019 14:4 2019, 14, 991–1014. [Google Scholar] [CrossRef]
- Daina, A.; Michielin, O.; Zoete, V. SwissADME: A Free Web Tool to Evaluate Pharmacokinetics, Drug-Likeness and Medicinal Chemistry Friendliness of Small Molecules OPEN. 2017. [CrossRef]
- Safran, M.; Rosen, N.; Twik, M.; BarShir, R.; Stein, T.I.; Dahary, D.; Fishilevich, S.; Lancet, D. The GeneCards Suite. Practical Guide to Life Science Databases 2022, 27–56. [Google Scholar] [CrossRef]
- Yu, L.; Zhou, D.; Gao, L.; Zha, Y. Prediction of Drug Response in Multilayer Networks Based on Fusion of Multiomics Data. Methods 2021, 192, 85–92. [Google Scholar] [CrossRef]
- Pressi, G.; Rigillo, G.; Governa, P.; Borgonetti, V.; Baini, G.; Rizzi, R.; Guarnerio, C.; Bertaiola, O.; Frigo, M.; Merlin, M.; et al. A Novel Perilla Frutescens (L.) Britton Cell-Derived Phytocomplex Regulates Keratinocytes Inflammatory Cascade and Barrier Function and Preserves Vaginal Mucosal Integrity In Vivo. Pharmaceutics 2023, 15. [Google Scholar] [CrossRef]
- Borgonetti, V.; Benatti, C.; Governa, P.; Isoldi, G.; Pellati, F.; Alboni, S.; Tascedda, F.; Montopoli, M.; Galeotti, N.; Manetti, F.; et al. Non-Psychotropic Cannabis Sativa L. Phytocomplex Modulates Microglial Inflammatory Response through CB2 Receptors-, Endocannabinoids-, and NF-ΚB-Mediated Signaling. Phytotherapy Research 2022, 36, 2246–2263. [Google Scholar] [CrossRef]
- Pressi, G.; Bertaiola, O.; Guarnerio, C.; Barbieri, E.; Rigillo, G.; Governa, P.; Biagi, M.; Guzzo, F.; Semenzato, A. In Vitro Cell Culture of Rhus Coriaria L.: A Standardized Phytocomplex Rich of Gallic Acid Derivatives with Antioxidant and Skin Repair Activity. Cosmetics 2022, Vol. 9, Page 12 2022, 9, 12. [Google Scholar] [CrossRef]
- G. Rigillo, V. Basile, S.; Belluti, M. Ronzio, E. Sauta, A.; Ciarrocchi, L. Latella, S. Molinari, A.Vallarola, G. Messina, R.; Mantovani, D.D. and C.I. The Transcription Factor NF-Y Participates to Stem Cell Fate Decision and Regeneration in Adult Skeletal Muscle. Nat Commun, 2020.
- Basera, I.A.; Girmeorcid, A.; Bhatt, V.P.; Sasteorcid, G.; Pawarorcid, S.; Hingoraniorcid, L.; Shah, M.B. Development of Validated UHPLC–PDA with ESI–MS-MS Method for Concurrent Estimation of Magnoflorine, Berbamine, Columbamine, Jatrorrhizine, Palmatine and Berberine in Berberis Aristata. Acta Chromatogr 2021, 34, 412–421. [Google Scholar] [CrossRef]
- Ichim, M.C.; Booker, A. Chemical Authentication of Botanical Ingredients: A Review of Commercial Herbal Products. Front Pharmacol 2021, 12, 666850. [Google Scholar] [CrossRef] [PubMed]
- Danoun, S.; Balayssac, S.; Gilard, V.; Martino, R.; Malet-Martino, M. Quality Evaluation of Berberine Food Supplements with High-Field and Compact 1H NMR Spectrometers. J Pharm Biomed Anal 2023, 223. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.; Miao, Y.Q.; Fan, D.J.; Yang, S.S.; Lin, X.; Meng, L.K.; Tang, X. Bioavailability Study of Berberine and the Enhancing Effects of TPGS on Intestinal Absorption in Rats. AAPS PharmSciTech 2011, 12, 705–711. [Google Scholar] [CrossRef] [PubMed]
- Chen, N.; Yang, X.Y.; Guo, C.E.; Bi, X.N.; Chen, J.H.; Chen, H.Y.; Li, H.P.; Lin, H.Y.; Zhang, Y.J. The Oral Bioavailability, Excretion and Cytochrome P450 Inhibition Properties of Epiberberine: An in Vivo and in Vitro Evaluation. Drug Des Devel Ther 2018, 12, 57–65. [Google Scholar] [CrossRef]
- Maharjan, B.; Payne, D.T.; Ferrarese, I.; Giovanna Lupo, M.; Kumar Shrestha, L.; Hill, J.P.; Ariga, K.; Rossi, I.; Sharan Shrestha, S.; Panighel, G.; et al. Evaluation of the Effects of Natural Isoquinoline Alkaloids on Low Density Lipoprotein Receptor (LDLR) and Proprotein Convertase Subtilisin/Kexin Type 9 (PCSK9) in Hepatocytes, as New Potential Hypocholesterolemic Agents. Bioorg Chem 2022, 121. [Google Scholar] [CrossRef]
- Lee, S.; Lim, H.J.; Park, J.H.; Lee, K.S.; Jang, Y.; Park, H.Y. Berberine-Induced LDLR up-Regulation Involves JNK Pathway. Biochem Biophys Res Commun 2007, 362, 853–857. [Google Scholar] [CrossRef]
- Katiyar, S.K.; Meeran, S.M.; Katiyar, N.; Akhtar, S. P53 Cooperates Berberine-Induced Growth Inhibition and Apoptosis of Non-Small Cell Human Lung Cancer Cells in Vitro and Tumor Xenograft Growth in Vivo. Mol Carcinog 2009, 48, 24–37. [Google Scholar] [CrossRef]
- Hsu, W.H.; Hsieh, Y.S.; Kuo, H.C.; Teng, C.Y.; Huang, H.I.; Wang, C.J.; Yang, S.F.; Liou, Y.S.; Kuo, W.H. Berberine Induces Apoptosis in SW620 Human Colonic Carcinoma Cells through Generation of Reactive Oxygen Species and Activation of JNK/P38 MAPK and FasL. Arch Toxicol 2007, 81, 719–728. [Google Scholar] [CrossRef]
- Cheng, Z.; Kang, C.; Che, S.; Su, J.; Sun, Q.; Ge, T.; Guo, Y.; Lv, J.; Sun, Z.; Yang, W.; et al. Berberine: A Promising Treatment for Neurodegenerative Diseases. Front Pharmacol 2022, 13. [Google Scholar] [CrossRef] [PubMed]
- Chen, F.L.; Yang, Z.H.; Liu, Y.; Li, L.X.; Liang, W.C.; Wang, X.C.; Zhou, W.B.; Yang, Y.H.; Hu, R.M. Berberine Inhibits the Expression of TNFalpha, MCP-1, and IL-6 in AcLDL-Stimulated Macrophages through PPARgamma Pathway. Endocrine 2008, 33, 331–337. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Cao, B.; Liu, X.; Fu, X.; Xiong, Z.; Chen, L.; Sartor, O.; Dong, Y.; Zhang, H. Berberine Suppresses Androgen Receptor Signaling in Prostate Cancer. Mol Cancer Ther 2011, 10, 1346. [Google Scholar] [CrossRef] [PubMed]
- Jantová, S.; čipák, L.; čerňáková, M.; Košt‘álová, D. Effect of Berberine on Proliferation, Cell Cycle and Apoptosis in HeLa and L1210 Cells. Journal of Pharmacy and Pharmacology 2010, 55, 1143–1149. [Google Scholar] [CrossRef]
- Li, Z.; Chen, M.; Wang, Z.; Fan, Q.; Lin, Z.; Tao, X.; Wu, J.; Liu, Z.; Lin, R.; Zhao, C. Berberine Inhibits RA-FLS Cell Proliferation and Adhesion by Regulating RAS/MAPK/FOXO/HIF-1 Signal Pathway in the Treatment of Rheumatoid Arthritis. Bone Joint Res 2023, 12, 91–102. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Liu, F.; Jiang, S.; Liu, J.; Chen, X.; Zhang, S.; Zhao, H. Berberine Hydrochloride Inhibits Cell Proliferation and Promotes Apoptosis of Non-Small Cell Lung Cancer via the Suppression of the MMP2 and Bcl-2/Bax Signaling Pathways. Oncol Lett 2018, 15, 7409. [Google Scholar] [CrossRef] [PubMed]
- Lo, S.N.; Wang, C.W.; Chen, Y.S.; Huang, C.C.; Wu, T.S.; Li, L.A.; Lee, I.J.; Ueng, Y.F. Berberine Activates Aryl Hydrocarbon Receptor but Suppresses CYP1A1 Induction through MiR-21-3p Stimulation in MCF-7 Breast Cancer Cells. Molecules : A Journal of Synthetic Chemistry and Natural Product Chemistry 2017, 22. [Google Scholar] [CrossRef] [PubMed]
- Wei, W.; Yao, J.X.; Zhang, T.T.; Wen, J.Y.; Zhang, Z.; Luo, Y.M.; Cao, Y.; Li, H. Network Pharmacology Reveals That Berberine May Function against Alzheimer’s Disease via the AKT Signaling Pathway. Front Neurosci 2023, 17, 1059496. [Google Scholar] [CrossRef] [PubMed]
- Zhao, L.; Liu, S.; Wang, M.; Zhi, M.; Geng, X.; Hou, C.; Wang, W.; Zhao, D. Berberine Restored Nitrergic and Adrenergic Function in Mesenteric and Iliac Arteries from Streptozotocin-Induced Diabetic Rats. J Ethnopharmacol 2019, 244. [Google Scholar] [CrossRef]
- Mak, S.; Luk, W.W.K.; Cui, W.; Hu, S.; Tsim, K.W.K.; Han, Y. Synergistic Inhibition on Acetylcholinesterase by the Combination of Berberine and Palmatine Originally Isolated from Chinese Medicinal Herbs. J Mol Neurosci 2014, 53, 511–516. [Google Scholar] [CrossRef]
- Campbell, K.J.; Tait, S.W.G. Targeting BCL-2 Regulated Apoptosis in Cancer. Open Biol 2018, 8. [Google Scholar] [CrossRef]
- Qian, S.; Wei, Z.; Yang, W.; Huang, J.; Yang, Y.; Wang, J. The Role of BCL-2 Family Proteins in Regulating Apoptosis and Cancer Therapy. Front Oncol 2022, 12. [Google Scholar] [CrossRef]
- Singh, R.; Letai, A.; Sarosiek, K. Regulation of Apoptosis in Health and Disease: The Balancing Act of BCL-2 Family Proteins. Nat Rev Mol Cell Biol 2019, 20, 175–193. [Google Scholar] [CrossRef]
- Li, J.; Li, O.; Kan, M.; Zhang, M.; Shao, D.; Pan, Y.; Zheng, H.; Zhang, X.; Chen, L.; Liu, S. Berberine Induces Apoptosis by Suppressing the Arachidonic Acid Metabolic Pathway in Hepatocellular Carcinoma. Mol Med Rep 2015, 12, 4572–4577. [Google Scholar] [CrossRef]
- Palma, T.V.; Lenz, L.S.; Bottari, N.B.; Pereira, A.; Schetinger, M.R.C.; Morsch, V.M.; Ulrich, H.; Pillat, M.M.; de Andrade, C.M. Berberine Induces Apoptosis in Glioblastoma Multiforme U87MG Cells via Oxidative Stress and Independent of AMPK Activity. Mol Biol Rep 2020, 47, 4393–4400. [Google Scholar] [CrossRef]
- Kuo, C.L.; Chou, C.C.; Yung, B.Y.M. Berberine Complexes with DNA in the Berberine-Induced Apoptosis in Human Leukemic HL-60 Cells. Cancer Lett 1995, 93, 193–200. [Google Scholar] [CrossRef]
- Berberine Hydrochloride Inhibits Cell Proliferation and Promotes Apoptosis of Non-Small Cell Lung Cancer via the Suppression of the MMP2 and Bcl-2/Bax Signaling Pathways Available online:. Available online: https://www.spandidos-publications.com/10.3892/ol.2018.8249 (accessed on 14 December 2023).
- Liu, Z.; Liu, Q.; Xu, B.; Wu, J.; Guo, C.; Zhu, F.; Yang, Q.; Gao, G.; Gong, Y.; Shao, C. Berberine Induces P53-Dependent Cell Cycle Arrest and Apoptosis of Human Osteosarcoma Cells by Inflicting DNA Damage. Mutation Research - Fundamental and Molecular Mechanisms of Mutagenesis 2009, 662, 75–83. [Google Scholar] [CrossRef]
- Xie, J.; Xu, Y.; Huang, X.; Chen, Y.; Fu, J.; Xi, M.; Wang, L. Berberine-Induced Apoptosis in Human Breast Cancer Cells Is Mediated by Reactive Oxygen Species Generation and Mitochondrial-Related Apoptotic Pathway. Tumor Biology 2015, 36, 1279–1288. [Google Scholar] [CrossRef]
- Liu, L.; Fan, J.; Ai, G.; Liu, J.; Luo, N.; Li, C.; Cheng, Z. Berberine in Combination with Cisplatin Induces Necroptosis and Apoptosis in Ovarian Cancer Cells. Biol Res 2019, 52, 37. [Google Scholar] [CrossRef]
- Yao, Z.; Wan, Y.; Li, B.; Zhai, C.; Yao, F.; Kang, Y.; Liu, Q.; Lin, D. Berberine Induces Mitochondrial-Mediated Apoptosis and Protective Autophagy in Human Malignant Pleural Mesothelioma NCI-H2452 Cells. Oncol Rep 2018, 40, 3603–3610. [Google Scholar] [CrossRef]
- Cao, C.; Su, M. Effects of Berberine on Glucose-Lipid Metabolism, Inflammatory Factors and Insulin Resistance in Patients with Metabolic Syndrome. Exp Ther Med 2019, 17, 3009. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Li, D.; Kuang, H.; Feng, X.; Ai, W.; Wang, Y.; Shi, S.; Chen, J.; Fan, R. Berberine Increases Glucose Uptake and Intracellular ROS Levels by Promoting Sirtuin 3 Ubiquitination. Biomedicine and Pharmacotherapy 2020, 121. [Google Scholar] [CrossRef] [PubMed]
- Liang, Y.; Xu, X.; Yin, M.; Zhang, Y.; Huang, L.; Chen, R.; Ni, J. Effects of Berberine on Blood Glucose in Patients with Type 2 Diabetes Mellitus: A Systematic Literature Review and a Meta-Analysis. Endocr J 2019, 66, 51–63. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Liu, Y.; Du, X.; Ma, H.; Yao, J. The Anti-Cancer Mechanisms of Berberine: A Review. Cancer Manag Res 2020, 12, 695–702. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Wu, X.; Yang, R.; Chen, F.; Liao, Y.; Zhu, Z.; Wu, Z.; Sun, X.; Wang, L. Effects of Berberine on the Gastrointestinal Microbiota. Front Cell Infect Microbiol 2020, 10. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.; Yu, Z.; Li, Y.; Fichna, J.; Storr, M. Effects of Berberine in the Gastrointestinal Tract - a Review of Actions and Therapeutic Implications. Am J Chin Med (Gard City N Y) 2014, 42, 1053–1070. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.; Lim, H.J.; Park, J.H.; Lee, K.S.; Jang, Y.; Park, H.Y. Berberine-Induced LDLR up-Regulation Involves JNK Pathway. Biochem Biophys Res Commun 2007, 362, 853–857. [Google Scholar] [CrossRef] [PubMed]
- Tian, E.; Sharma, G.; Dai, C. Neuroprotective Properties of Berberine: Molecular Mechanisms and Clinical Implications. Antioxidants 2023, 12, 1883. [Google Scholar] [CrossRef] [PubMed]
- Cheng, Z.; Kang, C.; Che, S.; Su, J.; Sun, Q.; Ge, T.; Guo, Y.; Lv, J.; Sun, Z.; Yang, W.; et al. Berberine: A Promising Treatment for Neurodegenerative Diseases. Front Pharmacol 2022, 13. [Google Scholar] [CrossRef]
- Huang, Q.; Ji, D.; Tian, X.; Ma, L.; Sun, X. Berberine Inhibits Erastin-Induced Ferroptosis of Mouse Hippocampal Neuronal Cells Possibly by Activating the Nrf2-HO-1/GPX4 Pathway. Journal of Southern Medical University 2022, 42, 937–943. [Google Scholar] [CrossRef]
- Wei, W.; Yao, J.X.; Zhang, T.T.; Wen, J.Y.; Zhang, Z.; Luo, Y.M.; Cao, Y.; Li, H. Network Pharmacology Reveals That Berberine May Function against Alzheimer’s Disease via the AKT Signaling Pathway. Front Neurosci 2023, 17, 1059496. [Google Scholar] [CrossRef] [PubMed]
- Mak, S.; Luk, W.W.K.; Cui, W.; Hu, S.; Tsim, K.W.K.; Han, Y. Synergistic Inhibition on Acetylcholinesterase by the Combination of Berberine and Palmatine Originally Isolated from Chinese Medicinal Herbs. J Mol Neurosci 2014, 53, 511–516. [Google Scholar] [CrossRef]
- GU, Y.; ZHOU, Z. Berberine Inhibits the Proliferation, Invasion and Migration of Endometrial Stromal Cells by Downregulating MiR-429. Mol Med Rep 2021, 23. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Qing, L.; Xu, W.; Yang, Y.; You, C.; Lao, Y.; Dong, Z. Berberine Affects the Proliferation, Migration, Invasion, Cell Cycle, and Apoptosis of Bladder Cancer Cells T24 and 5637 by Down-Regulating the HER2/PI3K/AKT Signaling Pathway. Arch Esp Urol 2023, 76, 152–160. [Google Scholar] [CrossRef]
- Burkina, V.; Rasmussen, M.K.; Pilipenko, N.; Zamaratskaia, G. Comparison of Xenobiotic-Metabolising Human, Porcine, Rodent, and Piscine Cytochrome P450. Toxicology 2017, 375, 10–27. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Liu, Q.; Xu, B.; Wu, J.; Guo, C.; Zhu, F.; Yang, Q.; Gao, G.; Gong, Y.; Shao, C. Berberine Induces P53-Dependent Cell Cycle Arrest and Apoptosis of Human Osteosarcoma Cells by Inflicting DNA Damage. Mutation Research - Fundamental and Molecular Mechanisms of Mutagenesis 2009, 662, 75–83. [Google Scholar] [CrossRef]
- Khan, M.; Giessrigl, B.; Vonach, C.; Madlener, S.; Prinz, S.; Herbaceck, I.; Hölzl, C.; Bauer, S.; Viola, K.; Mikulits, W.; et al. Berberine and a Berberis Lycium Extract Inactivate Cdc25A and Induce α-Tubulin Acetylation That Correlate with HL-60 Cell Cycle Inhibition and Apoptosis. Mutation Research/Fundamental and Molecular Mechanisms of Mutagenesis 2010, 683, 123–130. [Google Scholar] [CrossRef]
- Liu, J.F.; Lai, K.C.; Peng, S.F.; Maraming, P.; Huang, Y.P.; Huang, A.C.; Chueh, F.S.; Huang, W.W.; Chung, J.G. Berberine Inhibits Human Melanoma A375.S2 Cell Migration and Invasion via Affecting the FAK, UPA, and NF-ΚB Signaling Pathways and Inhibits PLX4032 Resistant A375.S2 Cell Migration in Vitro. Molecules 2018, 23. [Google Scholar] [CrossRef]
- Yang, L.J.; He, J.B.; Jiang, Y.; Li, J.; Zhou, Z.W.; Zhang, C.; Tao, X.; Chen, A.F.; Peng, C.; Xie, H.H. Berberine Hydrochloride Inhibits Migration Ability via Increasing Inducible NO Synthase and Peroxynitrite in HTR-8/SVneo Cells. J Ethnopharmacol 2023, 305. [Google Scholar] [CrossRef]
- Tarawneh, N.; Hamadneh, L.; Abu-Irmaileh, B.; Shraideh, Z.; Bustanji, Y.; Abdalla, S. Berberine Inhibited Growth and Migration of Human Colon Cancer Cell Lines by Increasing Phosphatase and Tensin and Inhibiting Aquaporins 1, 3 and 5 Expressions. Molecules 2023, 28, 3823. [Google Scholar] [CrossRef]
- Srinivas, U.S.; Tan, B.W.Q.; Vellayappan, B.A.; Jeyasekharan, A.D. ROS and the DNA Damage Response in Cancer. Redox Biol 2019, 25. [Google Scholar] [CrossRef] [PubMed]
- Sarmiento-Salinas, F.L.; Perez-Gonzalez, A.; Acosta-Casique, A.; Ix-Ballote, A.; Diaz, A.; Treviño, S.; Rosas-Murrieta, N.H.; Millán-Perez-Peña, L.; Maycotte, P. Reactive Oxygen Species: Role in Carcinogenesis, Cancer Cell Signaling and Tumor Progression. Life Sci 2021, 284, 119942. [Google Scholar] [CrossRef] [PubMed]
- Kassab, R.B.; Vasicek, O.; Ciz, M.; Lojek, A.; Perecko, T. The Effects of Berberine on Reactive Oxygen Species Production in Human Neutrophils and in Cell-Free Assays. Interdiscip Toxicol 2017, 10, 61. [Google Scholar] [CrossRef] [PubMed]
- Lv, X.X.; Yu, X.H.; Wang, H.D.; Yan, Y.X.; Wang, Y.P.; Lu, D.X.; Qi, R. Bin; Hu, C.F.; Li, H.M. Berberine Inhibits Norepinephrine-Induced Apoptosis in Neonatal Rat Cardiomyocytes via Inhibiting ROS-TNF-α-Caspase Signaling Pathway. Chin J Integr Med 2013, 19, 424–431. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Yuan, X.; Zhang, F.; Han, Y.; Chang, X.; Xu, X.; Li, Y.; Gao, X. Berberine Ameliorates Fatty Acid-Induced Oxidative Stress in Human Hepatoma Cells. Scientific Reports 2017 7:1 2017, 7, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Dong, S.F.; Yasui, N.; Negishi, H.; Kishimoto, A.; Sun, J.N.; Ikeda, K. Increased Oxidative Stress in Cultured 3T3-L1 Cells Was Attenuated by Berberine Treatment. Nat Prod Commun 2015, 10. [Google Scholar] [CrossRef]
- Wen, L.; Han, Z.; Li, J.; Du, Y. C-MYC and HIF1α Promoter G-Quadruplexes Dependent Metabolic Regulation Mechanism of Berberine in Colon Cancer. J Gastrointest Oncol 2022, 13, 1152–1168. [Google Scholar] [CrossRef]
- Rauf, A.; Abu-Izneid, T.; Khalil, A.A.; Imran, M.; Shah, Z.A.; Bin Emran, T.; Mitra, S.; Khan, Z.; Alhumaydhi, F.A.; Aljohani, A.S.M.; et al. Berberine as a Potential Anticancer Agent: A Comprehensive Review. Molecules 2021, Vol. 26, Page 7368 2021, 26, 7368. [Google Scholar] [CrossRef]
- Almatroodi, S.A.; Alsahli, M.A.; Rahmani, A.H. Berberine: An Important Emphasis on Its Anticancer Effects through Modulation of Various Cell Signaling Pathways. Molecules 2022, 27. [Google Scholar] [CrossRef]
- Ho, Y.; Lu, C.-C.; Yang, J.-S.; Chiang, J.-H.; Li, T.-C.; Ip, S.; Hsia, T.; Liao, C.; Lin, J.-G.; Wood, W.; et al. Berberine Induced Apoptosis via Promoting the Expression of Caspase-8, -9 and -3, Apoptosis-Inducing Factor and Endonuclease G in SCC-4 Human Tongue Squamous Carcinoma Cancer Cells. Anticancer Res 2009. [Google Scholar]
- Li, J.; Li, O.; Kan, M.; Zhang, M.; Shao, D.; Pan, Y.; Zheng, H.; Zhang, X.; Chen, L.; Liu, S. Berberine Induces Apoptosis by Suppressing the Arachidonic Acid Metabolic Pathway in Hepatocellular Carcinoma. Mol Med Rep 2015, 12, 4572–4577. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Fan, J.; Ai, G.; Liu, J.; Luo, N.; Li, C.; Cheng, Z. Berberine in Combination with Cisplatin Induces Necroptosis and Apoptosis in Ovarian Cancer Cells. Biol Res 2019, 52, 37. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.-Y.; Kim, S.-H.; Cheong, H.-T.; Ra, C.-S.; Rhee, K.-J.; Jung, B.D. Berberine Induces P53-Dependent Apoptosis through Inhibition of DNA Methyltransferase3b in Hep3B Cells. Korean Journal of Clinical Laboratory Science 2020, 52, 69–77. [Google Scholar] [CrossRef]
- Palma, T.V.; Lenz, L.S.; Bottari, N.B.; Pereira, A.; Schetinger, M.R.C.; Morsch, V.M.; Ulrich, H.; Pillat, M.M.; de Andrade, C.M. Berberine Induces Apoptosis in Glioblastoma Multiforme U87MG Cells via Oxidative Stress and Independent of AMPK Activity. Mol Biol Rep 2020, 47, 4393–4400. [Google Scholar] [CrossRef]

| Parameter | Value |
| R2 | 0.99 |
| Linearity range | 0.15-7.5 mg in column |
| Equation | y=4317.80-98.57 |
| Intra- and inter-day variation | <3% |
| Sample |
Berberine % w/w db |
Sample |
Berberine % w/w db |
| A85 | 86.26 | A97 | 97.41 |
| B85 | 86.74 | B97 | 91.94 |
| C85 | 91.93 | C97 | 96.50 |
| D85 | 88.87 | D97 | 97.16 |
| E85 | 89.63 | E97 | 97.49 |
| F85 | 90.66 | F97 | 97.21 |
| G85 | 80.07 | G97 | 97.29 |
| J85 | 87.88 | J97 | 97.80 |
| mean± SD | 87.76 ± 3.64 | mean± SD | 96.60 ± 1.91 |
| Samples |
Jatrorrhizine % w/w db |
Berberrubine + palmatine % w/w db |
| A85 | 2.39 | 0.25 |
| B85 | 1.88 | 0.24 |
| C85 | 2.10 | 0.23 |
| D85 | 3.07 | 0.26 |
| E85 | 2.54 | 0.28 |
| F85 | 2.85 | 0.24 |
| GH | 2.59 | 0.20 |
| J85 | 3.12 | 0.25 |
| mean± SD | 2.57± 0.44 | 0.24± 0.02 |
| A97 | 2.25 | 0.24 |
| B97 | 1.49 | 0.08 |
| C97 | 2.98 | 0.11 |
| D97 | 2.57 | 0.23 |
| E97 | 2.04 | 0.13 |
| F97 | 2.04 | 0.14 |
| G97 | 2.05 | 0.28 |
| J97 | 1.71 | 0.13 |
| mean± SD | 2.14 ± 0.46 | 0.17 ± 0.07 |
| Samples |
Berberine % w/w db |
Jatrorrhizine % w/w db |
Berberrubine + palmatine % w/w db |
| B. aristata85% (B 85) | 87.57 ± 1.85 | 2.83 ± 0.48 | 0.26 ± 0.01 |
| B. aristata97% (B 97) | 97.35 ± 0.08 | 2.15 ± 0.14 | 0.26 ± 0.02 |
| Bioaccessibility rate % | ||
| Samples | Berberine | Jatrorrhizine |
| B. aristata 97% | > 95 | 83.57 ± 3.33 |
| B. aristata 85% | > 95 | 87.81 ± 3.69 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





