Submitted:
28 July 2024
Posted:
30 July 2024
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Detailed Case Description
2.1. Materials and Methods
2.1.1. Clinical and Neuropsychological Workup:
2.1.2. Structural and Functional Neuroimaging Tests:
2.1.3. Laboratory Study (Including AD Biomarkers):
2.1.4. Genetic Tests:
2.2. Case A
2.2.1. Clinical Course:
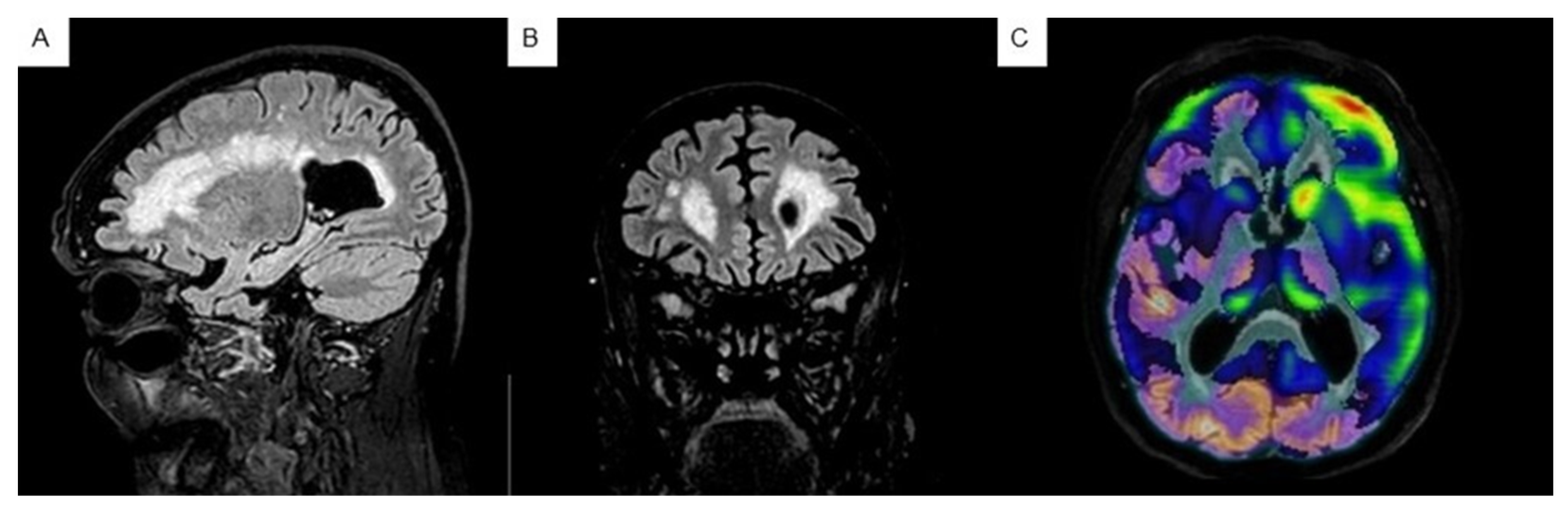
2.2.2. Neuroimaging Tests:
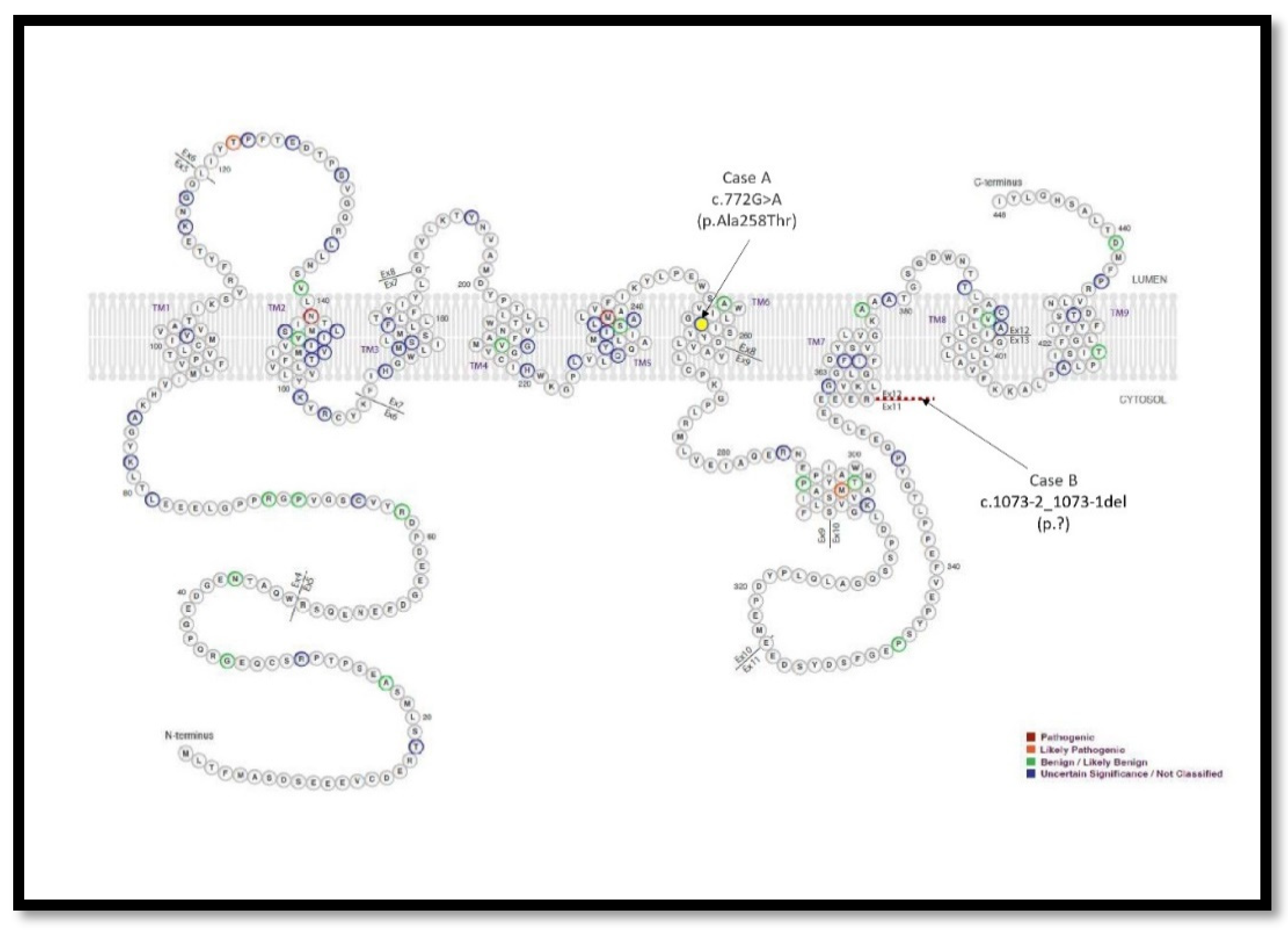
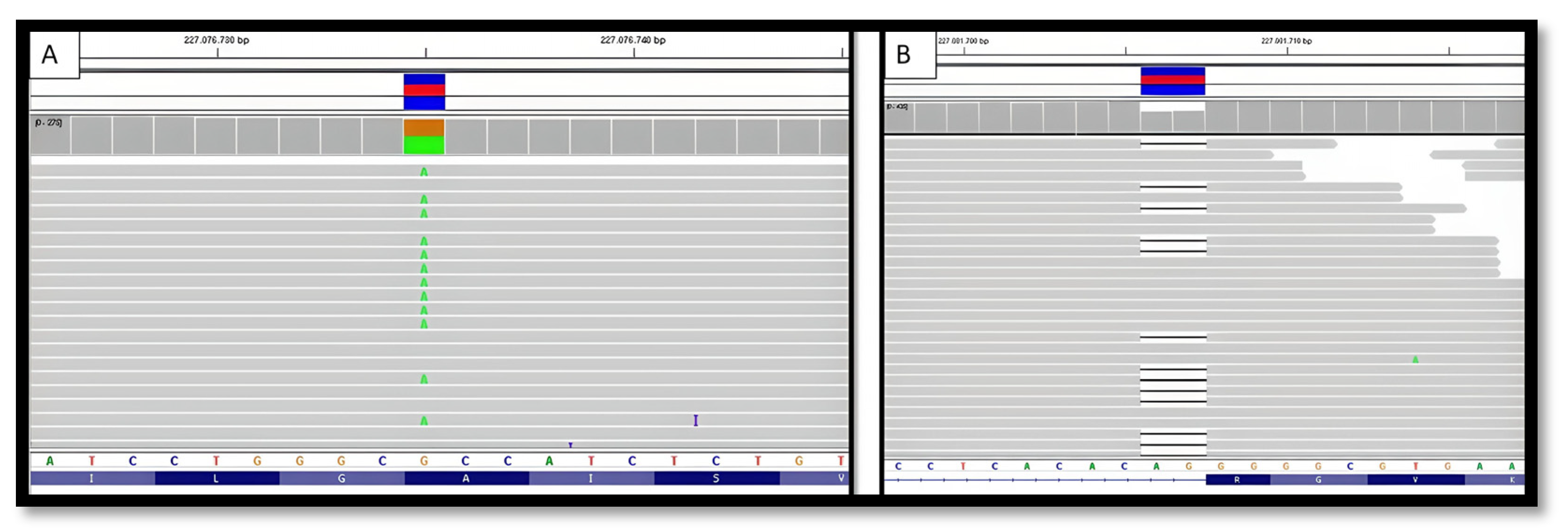
2.2.3. Genetic Testing:
2.3. Case B
2.3.1. Clinical Course:
2.3.2. Neuroimaging Tests:
2.3.2. Genetic Testing:
2.4. AD Blood and CSF Biomarkers:
3. Discussion
4. Conclusions
Limitations and Strengths of the Study
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Orphanet. Copyright, INSERM 1999. Available in http://www.orpha.net.
- Sociedad Española de Neurología. Guía oficial de práctica clínica en demencias. Madrid. 2018.
- Goate A, Chartier-Harlin M-C, Mullan M, Brown J, Crawford F, Fidani L, et al. Segregation of a missense mutation in the amyloid precursor protein gene with familial Alzheimer’s disease. Nature 1991;349: 704–6. [CrossRef]
- Sherrington R, Rogaev EI, Liang Y, Rogaev EA, Levesque G, Ikeda M, et al. Cloning of a gene bearing missense mutations in early-onset familial Alzheimer’s disease. Nature 1995;375: 754–60. [CrossRef]
- Levy-Lahad E, Wasco W, Poorkaj P, Romano DM, Oshima J, Pettingell WH, et al. Candidate gene for the chromosome 1 familial Alzheimer’ disease locus. Science 1995;269: 973–7. [CrossRef]
- Chen, M. The maze of APP processing in Alzheimer’s disease: Where did we go wrong in reasoning? Front Cell Neurosci. 2015;9.
- Cai Y, An SSA, Kim, S. Mutations in presenilin 2 and its implications in Alzheimer’s disease and other dementia-associated disorders. Clin Interv Aging. 2015;10: 1163-72. [CrossRef]
- Canevelli M, Piscopo P, Talarico G, Vanacore N, Blasimme A, Crestini A, et al. Familial Alzheimer’s disease sustained by presenilin 2 mutations: Systematic review of literature and genotype-phenotype correlation. Neurosci Biobehav Rev. 2014;42: 170-9. [CrossRef]
- Shea YF, Chu LW, Chan AOK, Ha J, Li Y, Song YQ. A systematic review of familial Alzheimer’s disease: Differences in presentation of clinical features among three mutated genes and potential ethnic differences. J Formos Med Assoc. 2016;115(2): 6775. [CrossRef]
- Shea YF, Chu LW, Chan AOK, Ha J, Li Y, Song YQ. A systematic review of familial Alzheimer’s disease: Differences in presentation of clinical features among three mutated genes and potential ethnic differences. J Formos Med Assoc. 2016; 115(2):67-75. [CrossRef]
- Folstein, M.F., Fostein, S.E., McHugh, P.R. 1975, «MiniMental State». A practical method for grading the cognitive state of patients for the clinician. J Psychiat Res; 12: 189-198. [CrossRef]
- Morris, J.C. The Clinical Dementia Rating (CDR): current version and scoring rules. Neurology, 1993;43: 2412-14.
- Aphasia severity subscale of the Boston Diagnostic Aphasia examination. H.; Goodglass, E. Kaplan and B Barresi. Spanish adaptation by J.E. García-Albea. 3rd edition. Buenos Aires -Madrid. Editorial Médica Panamericana 2005.
- Peña-Casanova, J., Gramunt, N.F., Gich, J.F. 2004, Neuropsychological tests. Fundamentals for evidence-based clinical psychology. Masson. Barcelona.
- Buschke, H., Kuslansky, G., Katz, M., et al. 1999, Screening for dementia with the Memory Impairment Screen. Neurology; 52: 231-8. [CrossRef]
- Cummings, J.L., Megra, M., Gray, K. et al. 1994, «The neuropsychiatric inventory. Comprehensive assessment of psychopathology in dementia», Neurology, 308-2314.
- Lawton, M.P., Brody, E.M. 1969, Assessment of older people: Self-Maintaining and Instrumental Activities of Daily Living. Gerontologist; 9: 179-186.
- Global Biomarker Standardization Consortium (GBSC) | Alzheimer’s Association [Internet]. [cited 2021 Dec 27]. Available from: https://www.alz.org/research/for_researchers/partnerships/gbsc.
- Carrillo MC, Blennow K, Soares H, Lewczuk P, Mattsson N, Oberoi P, et al. Global standardization measurement of cerebral spinal fluid for Alzheimer’s disease: an update from the Alzheimer’s Association Global Biomarkers Consortium. Alzheimers Dement. 2013;9:137–140. [CrossRef]
- Delaby C, Muñoz L, Torres S, Nadal A, Le Bastard N, Lehmann S, et al. Impact of CSF storage volume on the analysis of Alzheimer’s disease biomarkers on an automated platform. Clin Chim Acta Elsevier. 2019;490:98–101. [CrossRef]
- Greaves CV, Rohrer JD. An update on genetic frontotemporal dementia. J Neurol. 2019 Aug;266(8):2075-2086. [CrossRef]
- Perrone F, Cacace R, Van Mossevelde S, Van den Bossche T, De Deyn PP, Cras P, Engelborghs S, van der Zee J, Van Broeckhoven, C. Genetic screening in early-onset dementia patients with unclear phenotype: relevance for clinical diagnosis. Neurobiol Aging. 2018;69: 292.
- Farrer LA, Cupples LA, Haines JL, Hyman B, Kukull WA, Mayeux R, Myers RH, Pericak-Vance MA, Risch N, van Duijn CM. Effects of age, sex, and ethnicity on the association between apolipoprotein E genotype and Alzheimer disease. A meta-analysis. APOE and Alzheimer Disease Meta Analysis Consortium. JAMA. 1997 Oct 22-29;278(16):1349-56.
- Mattsson N, Zetterberg H, Janelidze S, Insel PS, Andreasson U, Stomrud E, Palmqvist S, Baker D, Tan Hehir CA, Jeromin A, Hanlon D, Song L, Shaw LM, Trojanowski JQ, Weiner MW, Hansson O, Blennow K; A.DNI Investigators. Plasma tau in Alzheimer disease. Neurology. 2016 Oct 25;87(17):1827-1835. [CrossRef]
- Janelidze S, Berron D, Smith R, Strandberg O, Proctor NK, Dage JL, Stomrud E, Palmqvist S, Mattsson-Carlgren N, Hansson, O. Associations of Plasma Phospho-Tau217 Levels With Tau Positron Emission Tomography in Early Alzheimer Disease. JAMA Neurol. 2021 Feb 1;78(2):149-156. [CrossRef]
- Illán-Gala I, Lleo A, Karydas A, Staffaroni AM, Zetterberg H, Sivasankaran R, Grinberg LT, Spina S, Kramer JH, Ramos EM, Coppola G, La Joie R, Rabinovici GD, Perry DC, Gorno-Tempini ML, Seeley WW, Miller BL, Rosen HJ, Blennow K, Boxer AL, Rojas JC. Plasma Tau and Neurofilament Light in Frontotemporal Lobar Degeneration and Alzheimer Disease. Neurology. 2021 Feb 2;96(5):e671-e683. [CrossRef]
- Dumurgier J, Schraen S, Gabelle A, Vercruysse O, Bombois S, Laplanche JL, et al.Cerebrospinal fluid amyloid-beta 42/40 ratio in clinical setting of memory centers: a multicentric study. Alzheimers Res Ther. 2015; 7(1): 30.
- Lewczuk P, Lelental N, Spitzer P, Maler JM, Kornhuber, J. Amyloid-beta 42/40 cerebrospinal fluid concentration ratio in the diagnostics of Alzheimer’s disease: validation of two novel assays. J Alzheimers Dis. 2015; 43(1): 183-91.
- Scheuner D, Eckman C, Jensen M, Song X, Citron M, Suzuki N, et al. Secreted amyloid β-protein similar to that in the senile plaques of Alzheimer’s disease is increased in vivo by the presenilin 1 and 2 and APP mutations linked to familial Alzheimer’s disease. Nat Med. 1996;2(8): 864-70. [CrossRef]
- Shen J, Kelleher RJ. The presenilin hypothesis of Alzheimer’s disease: Evidence for a loss-of-function pathogenic mechanism. Proc Natl Acad Sci U S A. 2007;104: 403-9. [CrossRef]
- Jayadev S, Leverenz JB, Steinbart E, Stahl J, Klunk W, Yu CE, et al. Alzheimer’s disease phenotypes and genotypes associated with mutations in presenilin 2. Brain. 2010;133(4): 1143-54. [CrossRef]
- Zarea A, Charbonnier C, Rovelet-Lecrux A, Nicolas G, Rousseau S, Borden A, et al. Seizures in dominantly inherited Alzheimer disease. Neurology. 2016;87(9): 912-9. [CrossRef]
- Lleó A, Blesa R, Queralt R, Ezquerra M, Molinuevo JL, Peña-Casanova J, et al. Frequency of mutations in the presenilin and amyloid precursor protein genes in earlyonset Alzheimer disease in Spain. Arch Neurol. 2002;59(11): 1759-63. [CrossRef]
- Tabaee Damavandi P, Storti B, Fabin N, Bianchi E, Ferrarese C, DiFrancesco JC. Epilepsy in cerebral amyloid angiopathy: an observational retrospective study of a large population. Epilepsia. 2023 Feb;64(2):500-510. [CrossRef]
- Banerjee G, Collinge J, Fox NC, Lashley T, Mead S, Schott JM, Werring DJ, Ryan NS. Clinical considerations in early-onset cerebral amyloid angiopathy. Brain. 2023 Oct 3;146(10):3991-4014. [CrossRef]
- Moscoso A, Rey-Bretal D, Silva-Rodríguez J, Aldrey JM, Cortés J, Pías-Peleteiro J, Ruibal Á, Aguiar P; A.lzheimer’s Disease Neuroimaging Initiative. White matter hyperintensities are associated with subthreshold amyloid accumulation. Neuroimage. 2020 Sep;218:116944. [CrossRef]
- Moscoso A, Silva-Rodríguez J, Aldrey JM, Cortés J, Pías-Peleteiro JM, Ruibal Á, Aguiar P; A.lzheimer’s Disease Neuroimaging Initiative. 18F-florbetapir PET as a marker of myelin integrity across the Alzheimer's disease spectrum. Eur J Nucl Med Mol Imaging. 2022 Mar;49(4):1242-1253. [CrossRef]
- Kapasi A, DeCarli C, Schneider JA. Impact of multiple pathologies on the threshold for clinically overt dementia. Acta Neuropathol. 2017 Aug;134(2):171-186. [CrossRef]
- Chang Wong E, Chang Chui, H. Vascular Cognitive Impairment and Dementia. Continuum (Minneap Minn). 2022 Jun 1;28(3):750-780.
- Marcon G, Di Fede G, Giaccone G, Rossi G, Giovagnoli AR, MacCagnano E, et al. A novel italian presenilin 2 gene mutation with prevalent behavioral phenotype. J Alzheimer’s .Dis. 2009;16(3): 509-11. [CrossRef]
| CASE B | CASE A | |||||
|---|---|---|---|---|---|---|
| CDR_ | 26/04/2017 | 04/12/2018 | 04/06/2020 | 18/06/2014 | 22/06/2016 | 22/06/2018 |
| CDR_MEMORY | 0,5 | 2 | 3 | 1 | NV* | NV |
| CDR_ORIENTATION | 1 | 1 | 3 | 1 | 2 | 3 |
| CDR_REASONING AND PROBLEM SOLVING | 0 | 2 | 3 | 1 | NV | 3 |
| CDR_OUT-OF-HOME ACTIVITIES | 0,5 | 2 | 3 | 1 | 3 | 3 |
| CDR_DOMESTIC ACTIVITIES AND HOBBIES | 0 | 2 | 3 | 1 | 2 | 3 |
| CDR_SELF-CARE | 0 | 2 | 3 | 1 | 2 | 3 |
| CDR_TOTAL | 0,5 | 2 | 3 | 1 | 2 | 3 |
| MMSE | 21 | 8 | 0 | 8 | NV | NV |
| NPI_TOTAL | 25 | 34 | 46 | 12 | 16 | 25 |
| NPI_DELUSIONS (frequencyXgravity) | 1X1 | 0 | 0 | 0 | 0 | 0 |
| NPI_HALLUCINATIONS (frequencyXgravity) | 0 | 0 | 0 | 0 | 0 | 0 |
| NPI_AGITATION (frequencyXgravity) | 0 | 0 | 4X2 | 0 | 0 | 1X1 |
| NPI_DEPRESSION-DYSPHORIA (frequencyXgravity) | 4X2 | 4X1 | 0 | 4X1 | 4X2 | 4X2 |
| NPI_ANXIETY (frequencyXgravity) | 4X2 | 4X1 | 4x2 | 4X1 | 0 | 1X1 |
| NPI_EUPHORIA (frequencyXgravity) | 0 | 4X2 | 4x2 | 0 | 0 | 4X3 |
| NPI_APATHY (frequencyXgravity) | 4X2 | 4X2 | 4x2 | 0 | 4X2 | 1X1 |
| NPI_DISINHIBITION (frequencyXgravity) | 0 | 4X2 | 4x2 | 2X2 | 0 | 1X1 |
| NPI_IRRITABILITY (frequencyXgravity) | 0 | 2X2 | 3x2 | 2X2break(hygiene) | 0 | 1X1 |
| NPI_ABERRANT MOTOR BEHAVIOR (frequencyXgravity) | 0 | 0 | 0 | 0 | 0 | 0 |
| NPI_ CAREGIVER STRESS | 6 | 8 | 10 | 3 | 3 | 3 |
| APETITE | OK | HYPERPHAGIA | HYPERPHAGIA | HYPERPHAGIA | HYPERPHAGIA | OK |
| SLEEP | OK | OK with medication | OK with breakmedication | OK | Fragmentated | Fragmentated |
| BARTHEL | 100 | 90 | 40 | 100 | 50 | 20 |
| AIVD | 8 | 2 | 2 | 3 | 0 | 0 |
| MIS | 2/8 | 0/8 | 0 | 2/8 | NV | NV |
| SEMANTIC FLUENCY | -- | 2 | 0 | 2 | 0 | 0 |
| PHONETIC FLUENCY | -- | 0 | 0 | 0 | 0 | 0 |
| VISOCONSTRUCTIVE PX (pentagons) | 0 (JUST ONE) | 0 | 0 | |||
| ORIENTATION | 5/10 | 1/10 | 0/10 | 2/10 | NV | NV |
| Boston Aphasia Severity Scale | 4/5 | 3/5 | 1/,5 | 2/5 | 1/5 | 0/5 |
| Cut-off points | None proposed | None proposed | < 0.083 | > 2 pg/mL |
| ID | Aβ 1-42 | Aβ 1-40 | β-amyloid ratio (1-42/1-40) | pTau |
| Case A | 18.31 | 318.71 | 0.057 | 2.39 |
| Case B | 2.96 | 265.42 | 0.011 | 2.91 |
| Cut-off points | <638 pg/mL | None proposed | < 0.069 | >56.5 pg/mL | >404 pg/mL |
| ID | Aβ 1-42 | Aβ 1-40 | β-amyloid ratio (1-42/1-40) | pTau | t-tau |
| Case A | 988 | 10693 | 0.092 | 122.5 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





