1. Introduction
Cutaneous metastatic tumors are rare, developing in 0.7% to 9% of patients with visceral malignancies (1). Despite this relative rarity, the diagnosis of secondary involvement of the skin by a malignant tumor raises several challenges among clinicians and pathologists. From a pathological point of view, the differential diagnosis between primary and metastatic cutaneous neoplasms is often difficult, and occasionally impossible. In the second scenario, the identification of the primary origin of the neoplasm can also be challenging, especially in the case of an unknown primary, but is obviously important for the optimal management of the patient (2, 3). Immunohistochemical studies are a very important ancillary technique helping the pathologist to reach a precise diagnosis, based on the expression of specific antigenic markers by tumor cells. TRPS1 (Tricho-rhino-phalangeal syndrome 1), also known as “Transcriptional Repressor GATA binding 1” gene, is a GATA transcriptional activator (4) that has recently emerged as a useful markerTRPS1 was first described in the context of Tricho-rhino-phalangeal syndrome (TRPS, OMIM 190350), a genetic malformative disease characterized by craniofacial and skeletal abnormalities (5). TRPS1 controls the proliferation and differentiation of normal mammary epithelial cells. (6) Although it was initially reported as a highly sensitive and specific marker of epithelial and mesenchymal neoplasms of the breast (4), more recent data have shown that TRPS1 is expressed by various other non-breast neoplasms, including benign and malignant cutaneous ones (7, 8). However, very limited data exist so far regarding the expression of TRPS1 in metastatic cutaneous tumors. The aim of this study was to investigate the expression of TRPS1 in metastatic cutaneous malignances, in order namely to assess the value of this marker in diagnosing the origin of the corresponding primaries.
2. Materials and Methods
This study was a retrospective analysis on archival tissues. We retrieved cases of cutaneous metastatic carcinomas from the archives of the Pathology Department, AHEPA University Hospital, School of Medicine, Aristotle University of Thessaloniki (Greece), and the Pathology Laboratory of the Lyon-Sud University Hospital, Hospices Civils de Lyon (France). The cases were randomly selected using the electronic registry of each department. They included biopsy or excision specimens of metastases of known primary origin, for which sufficient formalin-fixed, paraffin-embedded archival material to perform an additional immunohistochemical study was available. The diagnosis had been made by examination of hematoxylin-eosin-stained sections and appropriate immunostainings, and after integration of clinical data retrieved from the patient’s medical files concerning a known history of malignancy. Routinely-stained sections from each case were reviewed and the most representative paraffin blocks were selected.
The immunohistochemical study (IHC) was performed in an automated Ventana machine on 3-micron thick tissue sections cut from the paraffin blocks. Briefly, the sections were deparaffinized and dehydrated. Ethylene-diamine tetra-acetic acid (EDTA) was used for antigen retrieval for 64 minutes. The anti-TRPS1 antibody (clone ZR382, Zeta Corporation, dilution 1/100) was incubated for 32 minutes. The antigen–antibody complex was visualized using diaminobenzidine (DAB) as chromogen. The slides were counterstained with Mayer’s hematoxylin for 10 min, washed in water, dehydrated and mounted. For each case, the percentage of positive tumor cells was assessed and scored semi-quantitatively (0: negative, 1: <25% positive cells, 2: 26-49% positive cells, 3: 50-74% positive cells, 4: >75% positive cells). The staining intensity was also evaluated semi-quantitatively (0: negative, 1: mild, 2: moderate, 3: strong), considering as 3 the highest intensity observed in mammary carcinomas. A Histoscore was calculated for each case by multiplying the two scores (percentage of positive cells and staining intensity, ranging from 0 to 12). Adnexal (pilosebaceous or sweat gland) structures served as internal positive controls in each slide.
3. Results
3.1. Cases Studied
A total of 72 biopsy specimens with metastatic skin carcinomas, obtained from 40 men (55,6%) and 32 women (44,4%) aged 23-94 (mean 65.6) years, were studied (
Table 1). Nineteen metastases (26.4%) were of mammary origin (3 from invasive lobular carcinomas/ILC and 16 from invasive breast carcinomas of no special type/IBC, NST). Fifty-three metastatic cases (73.6%) were from other (non-breast) origin, including: 22 from the lung, 21 from the digestive system, 5 and 4 from the male and the female genito-urinary tract, respectively, and one mesothelioma derived from peritoneum. The 22 lung tumors included 3 neuroedocrine neoplasms, 14 adenocarcinomas, 1 squamous cell carcinoma and 4 undifferentiated carcinomas (these were included in the broad category of non-small cell carcinomas). The tumors of the digestive system included 12 colorectal adenocarcinomas, 3 adenocarcinomas of the stomach (one of which was of the diffuse type), 2 cholangiocarcinomas of the extrahepatic bile ducts, and one case each of squamous cell carcinoma (SCC) of the tongue, pancreatic adenocarcinoma, appendiceal adenocarcinoma and intestinal neuroendocrine neoplasm. The 10 remaining cases included 4 renal carcinomas, 2 uterine cervix carcinomas, and one case each of prostatic adenocarcinoma, high-grade serous ovarian carcinoma, undifferentiated carcinoma (of the uterus or the ovary) and mesothelioma.
3.2. Immunohistochemical Expression of TRPS1
3.2.1. Normal Skin
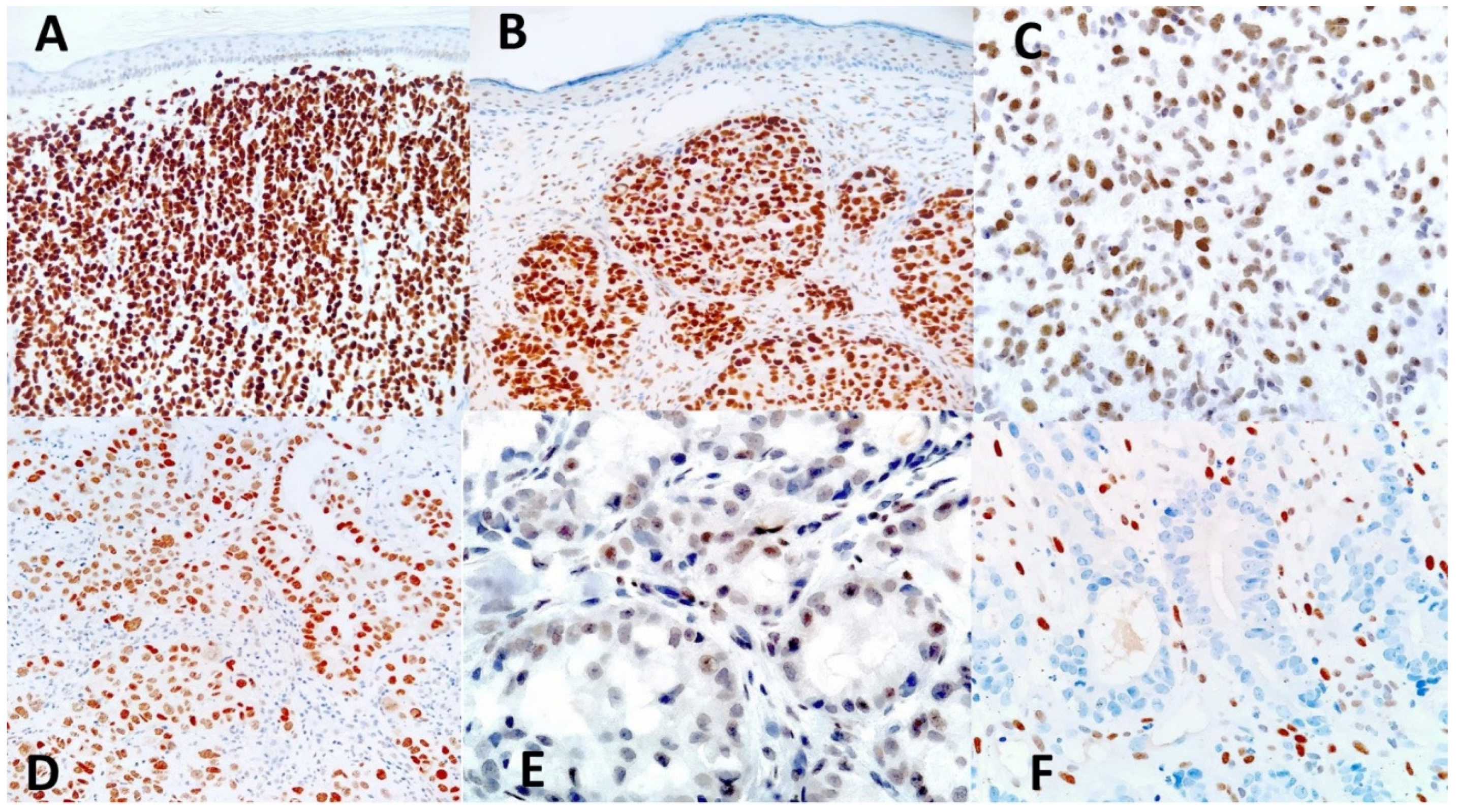
In normal skin adjacent to the tumors studied, nuclear expression of TRPS1 was mainly observed in cells of adnexal structures of the epidermis, i.e., pilosebaceous follicles and sweat glands, and served as internal positive controls. Strong TRPS1 expression was found in cells of both the secretory and the excretory part of eccrine sweat glands (
Figure 1A) and in cells of the hair follicle, including the fibroblasts of the dermal follicle papilla that showed strong expression (
Figure 1B,D). TRPS1 was also expressed within the sebaceous glands, more intensely by the less differentiated, peripheral cells (
Figure 1C). Occasionally, TRPS1 was expressed, although faintly, by some interstitial dermal cells and by epidermal keratinocytes (
Figure 2B). TRPS1 expression allowed to visualize the intraepidermal parts of the sweat ducts (acrosyringia –
Figure 1E) and hair-follicles (acrotrichia -
Figure 1F).
3.2.2. Cutaneous Metastatic Tumors (Table 1)
In metastatic tumors, TRPS1 expression varied significantly among the cases studied, both in terms of percentage of labelled cells and of staining intensity. In detail, all but one (18/19, 94.7%) metastatic breast carcinomas showed diffuse and strong TRPS1 positivity (histoscore 12), regardless of histological subtype (
Figure 2A). The TRPS1-negative case was from a female invasive breast carcinoma of no special type (grade 3 according to Nottingham histologic score). Half of the metastatic lung carcinoma cases showed some degree of TRPS1 expression, with one case (of a small-cell lung carcinoma) showing diffuse and strong positivity (histoscore 12) (
Figure 2B,E). The remaining positive metastatic lung carcinoma cases had histoscores ranging from 2 to 8. The majority (90.9%) of metastatic carcinomas of the digestive system (from gastric and colorectal adenocarcinomas, adenocarcinoma of the appendix, SCC of the tongue and cholangiocarcinomas) were TRPS1-negative (
Figure 2F). The remaining 2 (positive) cases included a case of intestinal neuroendocrine neoplasm with intermediate TRPS1 positivity (histoscore 6) and one case of pancreatic duct origin with weak TRPS1 expression (histoscore 1). Concerning the urogenital malignancies, 2/4 (50%) of renal clear cell carcinomas showed intermediate TRPS1 expression (histoscore 8-
Figure 2C), whereas the 2 other cases were negative, as was the case of prostatic adenocarcinoma. In tumors of the female genital tract, strong positivity (histoscore 12) was observed in one case of cervical carcinoma, whereas the second case was negative. Both the serous carcinomas of the ovary and the undifferentiated carcinoma of ovary/uterus were TRPS1-positive (histoscore 8-
Figure 2D). One case morphologically and immunohistochemically consistent with metastatic mesothelioma showed weak and patchy (<10% of neoplastic cells) TRPS1 positivity (histoscore 1).
4. Discussion
The microscopic diagnosis of cutaneous metastases is often fraught with difficulty. Indeed, although these tumors can often be broadly categorized into adenocarcinomas or squamous-cell carcinomas, they seldom display specific histological features that allow recognition of the primary site of origin. This situation is especially challenging when the metastasis is the first manifestation of an underlying, hitherto unknown, malignancy (2, 9). In this scenario, the search for specific and sensitive immunohistochemical markers provides useful, often invaluable, diagnostic information to the pathologist. In the setting of metastatic tumors, knowledge of the expression of a given marker by the primary tumors is certainly of paramount importance. TRPS1 is an emerging immunohistochemical marker that has been used as a sensitive and specific marker of breast carcinoma (6) and of mammary Paget’s disease (10). More recent studies have shown that TRPS1 is also expressed, although less frequently, by other, non-breast, malignancies (6, 11).
Although the expression of TRPS1 has been studied in primary tumors of the skin (7, 8, 10, 12-14), very limited data exist on cutaneous metastatic tumors, as only one study has investigated this topic in a limited number of cases (7). In the present study, we aimed to assess the usefulness of TRPS1 in the diagnosis of cutaneous metastatic tumors by studying a larger case cohort. We found that the vast majority (18/19, i.e., 94.7%) of cutaneous metastases of mammary origin (both from ILC and IBC-NST primary types) showed strong TRPS1 expression (histoscore 12). This result is in keeping with results of the literature showing very frequent and strong TRPS1 expression by primary breast tumors (6, 15, 16) and with the study by Taniguchi et al. (7), that found all 8 cases to be TRPS1-positive. Remarkably, in our study we found one case of TRPS1-negative metastatic breast carcinoma. This is not totally surprising, as 9% of primary breast carcinomas are TRPS1-negative (6), and indicates that, even though strong TRPS1 positivity is in favor of the mammary origin of a metastatic tumor, negativity of TRPS1 cannot completely rule out this origin of a cutaneous metastasis.
Regarding the remaining metastatic tumors of non-breast origin, we found TRPS1 expression in about one third of cases, including metastases from the lung, pancreas, kidney and the gynecologic tract. This result is consistent with the results of previous studies showing expression of TRPS1 by the corresponding primary malignancies, including those of the prostate and bladder (17), the endometrium and ovary (18, 19), salivary glands (20, 21) and the gastrointestinal tract (22). Of note, however, contrasting with the mammary metastases, metastases of non-mammary origin expressed TRPS1 more weakly and focally, with the exception of a case of small-cell lung carcinoma that presented diffuse and strong TRPS1 positivity. Remarkably, we found all three cases of gastric adenocarcinoma to be TRPS1-negative. Considering that gastric poorly-cohesive or Lauren diffuse-type carcinomas often share common morphological and immunohistochemical features with ILC (23, 24), we believe that TRPS1 may serve as an interesting marker in the differential diagnosis between these two tumor types.
The rather high percentage of TRPS1-positive metastatic non-mammary tumors we found (36.5%) is at some variance with the study of Taniguchi et al. (7), who found only 1/11 of cutaneous metastasis of a lung carcinoma to express weakly TRPS1, and with the results of Ai et al. (6) who found a rather low percentage of visceral (non-mammary) tumors to be TRPS1-positive. These discrepancies could be due to several reasons: a) the study of Ai et al. (6) used tissue microarrays, and may therefore have missed the TRPS1-positive parts in tumors with focal TRPS1 positivity; b) differences in the immunohistochemical protocol used (different antibody); c) finally, it cannot be excluded that metastatic cells show an upregulated TRPS1 expression compared with the primary tumors of origin, as some studies have suggested a possible prognostic significance of TRPS1 in specific tumors (4, 18, 19). In this respect, it will be interesting to compare metastases to the skin (or other site) with their corresponding primaries.
Another important diagnostic problem when facing a malignant skin tumor is the recognition of its primary (cutaneous) vs secondary (metastatic) nature. Several markers have been investigated to this end (such as hormone receptors, p63, p40, several keratin polypeptides), but it seems that none is absolutely specific so as to reliably distinguish metastatic tumors from primary ones (25, 26), especially when in the case of adenocarcinomas, which can be metastatic (namely from the breast) or originate from the sweat gland apparatus of the skin. Data from the literature have shown that both benign and malignant adnexal skin tumors express TRPS1 to various degrees (7, 8, 12-14). It seems therefore that TRPS1 cannot reliably distinguish cutaneous primary (adnexal) from metastatic (adeno)carcinomas, even though metastatic breast tumors show a more consistent and strong TRPS1 immunoreactivirty than primary cutaneous adenocarcinomas.
5. Conclusions
We present here the largest cohort of metastatic carcinomas to the skin studied for the expression of TRPS1. Our findings suggest that strong expression of TRPS1 favors a mammary origin of a cutaneous metastasis, although it cannot exclude a different primary origin, namely from the lung, the kidney or the female genital tract. Conversely, negativity for TRPS1 cannot formally exclude the mammary origin of a cutaneous metastasis. The usefulness of TRPS1 in the distinction between primary and metastatic skin adenocarcinomas seems limited. With these limitations in mind, we consider that TRPS1 is a useful marker, to be included in a panel of other immunohistochemical markers for the diagnosis of cutaneous metastatic tumors, in the context of adequate clinical information. Further studies, comparing namely the expression of metastatic tumors with their primaries of origin, will shed more light on the significance of TRPS1 expression in metastatic skin tumors and its value as a diagnostic and/or prognostic marker.
Author Contributions
Conceptualization, Jean Kanitakis and Georgia Karayannopoulou; Methodology, Kassiani Boulogeorgou, Christos Topalidis and Triantafyllia Koletsa; Validation, Kassiani Boulogeorgou, Triantafyllia Koletsa, Jean KANITAKIS and Georgia Karayannopoulou; Formal analysis, Kassiani Boulogeorgou, Triantafyllia Koletsa and Georgia Karayannopoulou; Investigation, Kassiani Boulogeorgou; Resources, Christos Topalidis; Data curation, Christos Topalidis and Jean KANITAKIS; Writing – original draft, Kassiani Boulogeorgou and Triantafyllia Koletsa; Writing – review & editing, Triantafyllia Koletsa, Jean Kanitakis and Georgia Karayannopoulou; Supervision, Jean KANITAKIS. All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
The study was approved by the Ethical and Research Committee of the Aristotle University of Thessaloniki (Approval No. 200/16-04-2024).
Data Availability Statement
The original contributions presented in the study are included in the article/supplementary material, further inquiries can be directed to the corresponding author.
References
- Betlloch-Mas, I.; Soriano-García, T.; Boira, I.; et al. Cutaneous Metastases of Solid Tumors: Demographic, Clinical, and Survival Characteristics. Cureus. 2021, 13, e19970. [Google Scholar] [CrossRef] [PubMed]
- Krämer, A.; Pauli, C.; John Prall, O.W. Cancer of unknown primary site. In: WHO Classification of Tumors online 2023 (5th ed.). Available online: https://tumourclassification.iarc.who.int/chaptercontent/64/367.
- Prieto, V.G.; Requena, L.; Zalaudek, I.; Ko, J.S. Metastases to skin. In: WHO Classification of Tumors online 2023 (5th ed.). Available online: https://tumourclassification.iarc.who.int/chaptercontent/64/217.
- Yang, L.; Gong, X.; Wang, J.; Fan, Q.; Yuan, J.; Yang, X.; Sun, X.; Li, Y.; Wang, Y. Functional mechanisms of TRPS1 in disease progression and its potential role in personalized medicine. Pathol Res Pract. 2022, 237, 154022. [Google Scholar] [CrossRef] [PubMed]
- Momeni, P.; Glöckner, G.; Schmidt, O.; von Holtum, D.; Albrecht, B.; Gillessen-Kaesbach, G.; Hennekam, R.; Meinecke, P.; Zabel, B.; Rosenthal, A.; Horsthemke, B.; Lüdecke, H.J. Mutations in a new gene, encoding a zinc-finger protein, cause tricho-rhino-phalangeal syndrome type I. Nat Genet. 2000, 24, 71–74. [Google Scholar] [CrossRef] [PubMed]
- Ai, D.; Yao, J.; Yang, F.; Huo, L.; Chen, H.; Lu, W.; Soto, L.M.S.; Jiang, M.; Raso, M.G.; Wang, S.; Bell, D.; Liu, J.; Wang, H.; Tan, D.; Torres-Cabala, C.; Gan, Q.; Wu, Y.; Albarracin, C.; Hung, M.C.; Meric-Bernstam, F.; Wistuba, I.I.; Prieto, V.G.; Sahin, A.A.; Ding, Q. TRPS1: a highly sensitive and specific marker for breast carcinoma, especially for triple-negative breast cancer. Mod Pathol. 2021, 34, 710–719. [Google Scholar] [CrossRef] [PubMed]
- Taniguchi, K.; Goto, K.; Yabushita, H.; Yamasaki, R.; Ichimura, K. Transcriptional repressor GATA binding 1 (TRPS1) immunoexpression in normal skin tissues and various cutaneous tumors. J Cutan Pathol. 2023, 50, 1006–1013. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.A.; Cho, W.C. TRPS1 Expression in Endocrine Mucin-Producing Sweat Gland Carcinoma: Diagnostic Utility and Pitfalls. Am J Dermatopathol. 2024, 46, 133–135. [Google Scholar] [CrossRef] [PubMed]
- Habermehl, G.; Ko, J. Cutaneous metastases: A review and diagnostic approach to tumors of unknown origin. Arch Pathol Lab Med. 2019, 143, 943–957. [Google Scholar] [CrossRef] [PubMed]
- Cho, W.C.; Ding, Q.; Wang, W.L.; Nagarajan, P.; Curry, J.L.; Torres-Cabala, C.A.; Ivan, D.; Albarracin, C.T.; Sahin, A.; Prieto, V.G.; Aung, P.P. Immunohistochemical expression of TRPS1 in mammary Paget disease, extramammary Paget disease, and their close histopathologic mimics. J Cutan Pathol. 2023, 50, 434–440. [Google Scholar] [CrossRef] [PubMed]
- Cloutier, J.M.; Ingram, D.R.; Wani, K.; Lazar, A.J.; Wang, W.L. Frequent TRPS1 expression in synovial sarcoma is associated with SS18-SSX fusion oncoprotein activity. Hum Pathol. 2022, 130, 88–94. [Google Scholar] [CrossRef] [PubMed]
- Zengin, H.B.; Bui, C.M.; Rybski, K.; Pukhalskaya, T.; Yildiz, B.; Smoller, B.R. TRPS1 is differentially expressed in a variety of malignant and benign cutaneous sweat gland neoplasms. Dermatopathology (Basel). 2023;10(1):75-85. [CrossRef]
- Liu, Y.A.; Aung, P.P.; Wang, Y.; Ning, J.; Nagarajan, P.; Curry, J.L.; Torres-Cabala, C.A.; Ivan, D.; Prieto, V.G.; Ding, Q.; Cho, W.C. TRPS1 expression in non-melanocytic cutaneous neoplasms: an immunohistochemical analysis of 200 cases. J Pathol Transl Med. 2024, 58, 72–80. [Google Scholar] [CrossRef] [PubMed]
- Rybski KJ, Zengin HB, Smoller BR. TRPS1: A marker of follicular differentiation. Dermatopathology (Basel). 2023, 10, 173–183. [CrossRef] [PubMed]
- Wang, J.; Wang, W.L.; Sun, H.; Huo, L.; Wu, Y.; Chen, H.; Gan, Q.; Meis, J.M.; Maloney, N.; Lazar, A.J.; Yoon, E.C.; Albarracin, C.T.; Krishnamurthy, S.; Middleton, L.P.; Resetkova, E.; Yu, W.; Tan, D.; Lu, W.; Solis Soto, L.M.; Wang, S.; Wistuba, I.I.; Parwani, A.V.; Prieto, V.G.; Sahin, A.A.; Li, Z.; Ding, Q. Expression of TRPS1 in phyllodes tumor and sarcoma of the breast. Hum Pathol. 2022, 121, 73–80. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Peng, Y.; Sun, H.; Aung, P.P.; Resetkova, E.; Yam, C.; Sahin, A.A.; Huo, L.; Ding, Q. TRPS1 and GATA3 Expression in Invasive Breast Carcinoma With Apocrine Differentiation. Arch Pathol Lab Med. 2024, 148, 200–205. [Google Scholar] [CrossRef] [PubMed]
- Bachert, S.E.; Di, J.; Zhang, S.; Short, H.E.; Piecoro, D.W.; McDonald, R.J.; Myint, Z.W.; Hensley, P.J.; Allison, D.B. TRPS1 expression in primary and metastatic prostatic adenocarcinoma, muscle invasive bladder urothelial carcinoma, and breast carcinoma: Is TRPS1 truly specific and sensitive for a breast primary? Hum Pathol. 2024, 143: 42-49. [CrossRef]
- Wang, X.; Sun, J.; Liu, Y.; Lin, Z.; Jiang, X.; Ye, Y.; Lv, C.; Lian, X.; Xu, W.; Luo, S.; Liao, S.; Chen, Z.; Wang, S. Trps1 predicts poor prognosis in advanced high grade serous ovarian carcinoma. Int J Cancer. 2024, 154, 1639–1651. [Google Scholar] [CrossRef] [PubMed]
- Ruiz, F.; Tjendra, Y.; Millan, N.; Gomez-Fernandez, C.; Pinto, A. Trichorhinophalangeal Syndrome Type 1 Immunohistochemical Expression in Carcinomas of Gynecologic Origin. Am J Surg Pathol. 2024, 48, 546–550. [Google Scholar] [CrossRef] [PubMed]
- Tjendra, Y.; Kerr, D.A.; Gomez-Fernandez, C.; Velez Torres, J.M. TRPS1 immunohistochemical expression in salivary gland tumors: A pilot study. Am J Clin Pathol. 2023, 160, 633–639. [Google Scholar] [CrossRef] [PubMed]
- Salem, A.; Wu, Y.; Albarracin, C.T.; Middleton, L.P.; Kalhor, N.; Peng, Y.; Huang, X.; Aung, P.P.; Chen, H.; Sahin, A.A.; Ding, Q. A Comparative Evaluation of TRPS1 and GATA3 in adenoid cystic, secretory, and acinic cell carcinomas of the breast and salivary gland. Hum Pathol. 2024, 145, 42–47. [Google Scholar] [CrossRef] [PubMed]
- Hong, J.; Sun, J.; Huang, T. Increased expression of TRPS1 affects tumor progression and correlates with patients’ prognosis of colon cancer. Biomed Res Int. 2013, 2013, 454085. [Google Scholar] [CrossRef] [PubMed]
- Carneiro, F.; Fukayama, M.; Yasui, W.; Grabsch, H.I. Gastric adenocarcinoma. In: WHO classification of tumours online. Digestive system tumours 2023 (5th edition). Available online: https://tumourclassification.iarc.who.int/chaptercontent/31/29.
- Zarrilli, G.; Angerilli, V.; Cappellesso, R.; Galuppini, F.; Pennelli, G.; Farinati, F.; Nicolè, L.; Savarino, E.; Realdon, S.; Griguolo, G.; Bottosso, M.; Dieci, M.V.; Guarneri, V.; Dei Tos, A.P.; Lo Mele, M.; Fassan, M. Gastric metastases of breast cancer: Histopathological and molecular characterization of a single Institution case series. Pathol Res Pract. 2022, 233, 153872. [Google Scholar] [CrossRef]
- Chung, J.; Rico-Castillo, J.; Sebastiano, C.; Lee, J.B. Expression of p40 in Primary Cutaneous Mucinous Carcinoma Versus Primary Mucinous Carcinomas of the Breast and Colon. Am J Dermatopathol. 2021, 43, e175–e180. [Google Scholar] [CrossRef] [PubMed]
- Kanitakis, J.; Chouvet, B. Expression of p63 in cutaneous metastases. Am J Clin Pathol. 2007, 128, 753–758. [Google Scholar] [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).