Submitted:
24 July 2024
Posted:
26 July 2024
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Pulmonary Dendritic Cells (DCs)
3. Origin and Development of Lung DCs
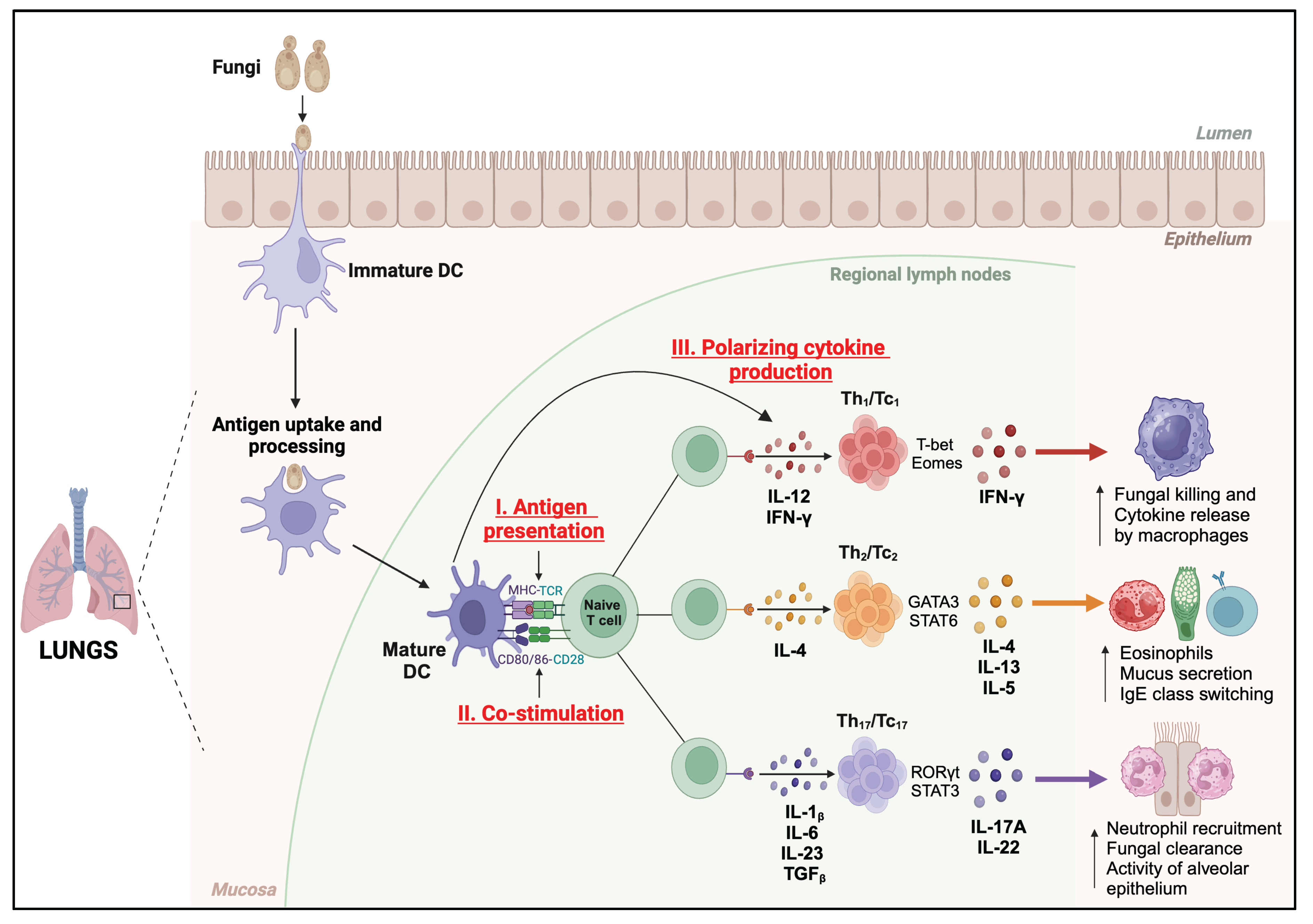
4. Functions of DC against Respiratory Fungal Pathogens
5. Dendritic Cell-Based Experimental Fungal Vaccines
4.1. Aspergillus spp.
4.2. Coccidiodes spp.
4.3. Paracoccidioides spp.
4.4. Cryptococcus spp.
6. Future Perspectives of Fungal DC Vaccines
7. Conclusions
| Fungi | Vaccine Type | Methodology | Major outcomes | References |
|---|---|---|---|---|
| Aspergillus spp. | RNA or live fungi/Crude antigen or subunit vaccine | Murine and human DC-pulsed (RNA complexed with DOTAP) | Enhanced DC’s MHC-II, co-stimulatory molecules expression and increased Th1/Th2 response | [93] |
| Heat-killed fungi/ Crude antigen vaccine | Human DC-pulsed | In vivo protective antigen-specific Th1 response (High IFN-g and IL-10 production) | [95] | |
| Heat inactivated fungi/viral transduction | Murine DC-pulsed and IL-12 gene therapy | Increased Th1 responses, improved survivability, and reduced fungal burden | [96] | |
| Cryptococcus spp. | Heat-killed Cryptococcus gattii mutant △cap60 | (DC-pulsed) | Protection and stimulation of tissue-resident memory Th17 cells in the lungs | [124,129] |
| Live or heat killed Cryptococcus neoformans mutant | - | Protective Th1-type adaptive immune response Induction of trained immunity of DCs |
[125] | |
| Coccidioides spp. | Ag2/Subunit vaccine (Ag2/PRA-cDNA transfected DC) |
DC-transfected | Reduced fungal burden and enhanced IFN-g production | [106,108] |
| Ag2/PRA primary DC | DC-pulsed | Reduced tissue injury in vaccinated mice, enhanced IgG levels, and increased IFN-g, IL-4, and IL-17 production |
[107,109] | |
| Paracoccidioides spp. |
Peptide vaccine (P10) P10 primary DC P10 primary monocyte derived-DC |
P10-primed DCs | Reduced fungal burden in both immunocompetent and immunosuppressed mice, protection against intratracheal challenge, protective Th1 responses, activation and upregulation of MHC-II, CD80, and CD86 on the DCs, and induction of CD4+ and CD8+ T cell proliferation. |
[117,118,119] |
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Denning, D.W. Global incidence and mortality of severe fungal disease. Lancet Infect Dis 2024, 24, e428–e438. [Google Scholar] [CrossRef] [PubMed]
- Bongomin, F.; Gago, S.; Oladele, R.O.; Denning, D.W. Global and Multi-National Prevalence of Fungal Diseases-Estimate Precision. J Fungi (Basel) 2017, 3. [Google Scholar] [CrossRef] [PubMed]
- Benedict, K.; Whitham, H.K.; Jackson, B.R. Economic Burden of Fungal Diseases in the United States. Open Forum Infect Dis 2022, 9, ofac097. [Google Scholar] [CrossRef] [PubMed]
- Steinman, R.M.; Cohn, Z.A. Identification of a novel cell type in peripheral lymphoid organs of mice. I. Morphology, quantitation, tissue distribution. J Exp Med 1973, 137, 1142–1162. [Google Scholar] [CrossRef] [PubMed]
- Steinman, R.M. Dendritic cells and the control of immunity: enhancing the efficiency of antigen presentation. Mt Sinai J Med 2001, 68, 160–166. [Google Scholar] [PubMed]
- Guilliams, M.; Lambrecht, B.N.; Hammad, H. Division of labor between lung dendritic cells and macrophages in the defense against pulmonary infections. Mucosal Immunol 2013, 6, 464–473. [Google Scholar] [CrossRef] [PubMed]
- Bosteels, C.; Neyt, K.; Vanheerswynghels, M.; van Helden, M.J.; Sichien, D.; Debeuf, N.; De Prijck, S.; Bosteels, V.; Vandamme, N.; Martens, L.; et al. Inflammatory Type 2 cDCs Acquire Features of cDC1s and Macrophages to Orchestrate Immunity to Respiratory Virus Infection. Immunity 2020, 52, 1039–1056. [Google Scholar] [CrossRef] [PubMed]
- Kawasaki, T.; Ikegawa, M.; Kawai, T. Antigen Presentation in the Lung. Front Immunol 2022, 13, 860915. [Google Scholar] [CrossRef] [PubMed]
- Kischkel, B.; Rossi, S.A.; Santos, S.R.; Nosanchuk, J.D.; Travassos, L.R.; Taborda, C.P. Therapies and Vaccines Based on Nanoparticles for the Treatment of Systemic Fungal Infections. Front Cell Infect Microbiol 2020, 10, 463. [Google Scholar] [CrossRef]
- Rizzo, J.; Rodrigues, M.L.; Janbon, G. Extracellular Vesicles in Fungi: Past, Present, and Future Perspectives. Front Cell Infect Microbiol 2020, 10, 346. [Google Scholar] [CrossRef]
- Guilliams, M.; Henri, S.; Tamoutounour, S.; Ardouin, L.; Schwartz-Cornil, I.; Dalod, M.; Malissen, B. From skin dendritic cells to a simplified classification of human and mouse dendritic cell subsets. Eur J Immunol 2010, 40, 2089–2094. [Google Scholar] [CrossRef] [PubMed]
- Condon, T.V.; Sawyer, R.T.; Fenton, M.J.; Riches, D.W. Lung dendritic cells at the innate-adaptive immune interface. J Leukoc Biol 2011, 90, 883–895. [Google Scholar] [CrossRef] [PubMed]
- Bachem, A.; Guttler, S.; Hartung, E.; Ebstein, F.; Schaefer, M.; Tannert, A.; Salama, A.; Movassaghi, K.; Opitz, C.; Mages, H.W.; et al. Superior antigen cross-presentation and XCR1 expression define human CD11c+CD141+ cells as homologues of mouse CD8+ dendritic cells. J Exp Med 2010, 207, 1273–1281. [Google Scholar] [CrossRef] [PubMed]
- Desch, A.N.; Randolph, G.J.; Murphy, K.; Gautier, E.L.; Kedl, R.M.; Lahoud, M.H.; Caminschi, I.; Shortman, K.; Henson, P.M.; Jakubzick, C.V. CD103+ pulmonary dendritic cells preferentially acquire and present apoptotic cell-associated antigen. J Exp Med 2011, 208, 1789–1797. [Google Scholar] [CrossRef]
- Huber, A.; Dammeijer, F.; Aerts, J.; Vroman, H. Current State of Dendritic Cell-Based Immunotherapy: Opportunities for in vitro Antigen Loading of Different DC Subsets? Front Immunol 2018, 9, 2804. [Google Scholar] [CrossRef]
- Cruz, F.M.; Chan, A.; Rock, K.L. Pathways of MHC I cross-presentation of exogenous antigens. Semin Immunol 2023, 66, 101729. [Google Scholar] [CrossRef]
- Raymond, M.; Rubio, M.; Fortin, G.; Shalaby, K.H.; Hammad, H.; Lambrecht, B.N.; Sarfati, M. Selective control of SIRP-alpha-positive airway dendritic cell trafficking through CD47 is critical for the development of T(H)2-mediated allergic inflammation. J Allergy Clin Immunol 2009, 124, 1333–1342. [Google Scholar] [CrossRef]
- Hammad, H.; Lambrecht, B.N. Dendritic cells and epithelial cells: linking innate and adaptive immunity in asthma. Nat Rev Immunol 2008, 8, 193–204. [Google Scholar] [CrossRef]
- Roy, R.M.; Klein, B.S. Dendritic cells in antifungal immunity and vaccine design. Cell Host Microbe 2012, 11, 436–446. [Google Scholar] [CrossRef]
- von Garnier, C.; Filgueira, L.; Wikstrom, M.; Smith, M.; Thomas, J.A.; Strickland, D.H.; Holt, P.G.; Stumbles, P.A. Anatomical location determines the distribution and function of dendritic cells and other APCs in the respiratory tract. J Immunol 2005, 175, 1609–1618. [Google Scholar] [CrossRef]
- Gardner, A.; Ruffell, B. moDCs, Less Problems. Immunity 2018, 48, 6–8. [Google Scholar] [CrossRef] [PubMed]
- Banki, Z.; Werner, R.; Riepler, L.; Rossler, A.; Mullauer, B.; Hegen, V.; Bayer, W.; Verbeek, J.S.; Dittmer, U.; Stoiber, H. Fcgamma Receptor Type I (CD64)-Mediated Impairment of the Capacity of Dendritic Cells to Activate Specific CD8 T Cells by IgG-opsonized Friend Virus. Viruses 2019, 11. [Google Scholar] [CrossRef] [PubMed]
- Tamoutounour, S.; Henri, S.; Lelouard, H.; de Bovis, B.; de Haar, C.; van der Woude, C.J.; Woltman, A.M.; Reyal, Y.; Bonnet, D.; Sichien, D.; et al. CD64 distinguishes macrophages from dendritic cells in the gut and reveals the Th1-inducing role of mesenteric lymph node macrophages during colitis. Eur J Immunol 2012, 42, 3150–3166. [Google Scholar] [CrossRef] [PubMed]
- Langlet, C.; Tamoutounour, S.; Henri, S.; Luche, H.; Ardouin, L.; Gregoire, C.; Malissen, B.; Guilliams, M. CD64 expression distinguishes monocyte-derived and conventional dendritic cells and reveals their distinct role during intramuscular immunization. J Immunol 2012, 188, 1751–1760. [Google Scholar] [CrossRef] [PubMed]
- Cheong, C.; Matos, I.; Choi, J.H.; Dandamudi, D.B.; Shrestha, E.; Longhi, M.P.; Jeffrey, K.L.; Anthony, R.M.; Kluger, C.; Nchinda, G.; et al. Microbial stimulation fully differentiates monocytes to DC-SIGN/CD209(+) dendritic cells for immune T cell areas. Cell 2010, 143, 416–429. [Google Scholar] [CrossRef]
- Leon, B.; Lopez-Bravo, M.; Ardavin, C. Monocyte-derived dendritic cells formed at the infection site control the induction of protective T helper 1 responses against Leishmania. Immunity 2007, 26, 519–531. [Google Scholar] [CrossRef] [PubMed]
- Bosteels, C.; Neyt, K.; Vanheerswynghels, M.; van Helden, M.J.; Sichien, D.; Debeuf, N.; De Prijck, S.; Bosteels, V.; Vandamme, N.; Martens, L.; et al. Inflammatory Type 2 cDCs Acquire Features of cDC1s and Macrophages to Orchestrate Immunity to Respiratory Virus Infection. Immunity 2020, 52, 1039–1056. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.; Briseno, C.G.; Durai, V.; Albring, J.C.; Haldar, M.; Bagadia, P.; Kim, K.W.; Randolph, G.J.; Murphy, T.L.; Murphy, K.M. Mafb lineage tracing to distinguish macrophages from other immune lineages reveals dual identity of Langerhans cells. J Exp Med 2016, 213, 2553–2565. [Google Scholar] [CrossRef]
- Backer, R.A.; Probst, H.C.; Clausen, B.E. Classical DC2 subsets and monocyte-derived DC: Delineating the developmental and functional relationship. Eur J Immunol 2023, 53, e2149548. [Google Scholar] [CrossRef]
- Gautier, E.L.; Shay, T.; Miller, J.; Greter, M.; Jakubzick, C.; Ivanov, S.; Helft, J.; Chow, A.; Elpek, K.G.; Gordonov, S.; et al. Gene-expression profiles and transcriptional regulatory pathways that underlie the identity and diversity of mouse tissue macrophages. Nat Immunol 2012, 13, 1118–1128. [Google Scholar] [CrossRef]
- Liu, K.; Victora, G.D.; Schwickert, T.A.; Guermonprez, P.; Meredith, M.M.; Yao, K.; Chu, F.F.; Randolph, G.J.; Rudensky, A.Y.; Nussenzweig, M. In vivo analysis of dendritic cell development and homeostasis. Science 2009, 324, 392–397. [Google Scholar] [CrossRef] [PubMed]
- Kopf, M.; Schneider, C.; Nobs, S.P. The development and function of lung-resident macrophages and dendritic cells. Nat Immunol 2015, 16, 36–44. [Google Scholar] [CrossRef]
- Patel, V.I.; Booth, J.L.; Duggan, E.S.; Cate, S.; White, V.L.; Hutchings, D.; Kovats, S.; Burian, D.M.; Dozmorov, M.; Metcalf, J.P. Transcriptional Classification and Functional Characterization of Human Airway Macrophage and Dendritic Cell Subsets. J Immunol 2017, 198, 1183–1201. [Google Scholar] [CrossRef] [PubMed]
- Anderson, D.A., 3rd; Dutertre, C.A.; Ginhoux, F.; Murphy, K.M. Genetic models of human and mouse dendritic cell development and function. Nat Rev Immunol 2021, 21, 101–115. [Google Scholar] [CrossRef] [PubMed]
- Diao, J.; Winter, E.; Cantin, C.; Chen, W.; Xu, L.; Kelvin, D.; Phillips, J.; Cattral, M.S. In situ replication of immediate dendritic cell (DC) precursors contributes to conventional DC homeostasis in lymphoid tissue. J Immunol 2006, 176, 7196–7206. [Google Scholar] [CrossRef] [PubMed]
- Maraskovsky, E.; Brasel, K.; Teepe, M.; Roux, E.R.; Lyman, S.D.; Shortman, K.; McKenna, H.J. Dramatic increase in the numbers of functionally mature dendritic cells in Flt3 ligand-treated mice: multiple dendritic cell subpopulations identified. J Exp Med 1996, 184, 1953–1962. [Google Scholar] [CrossRef] [PubMed]
- Eisenbarth, S.C. Dendritic cell subsets in T cell programming: location dictates function. Nat Rev Immunol 2019, 19, 89–103. [Google Scholar] [CrossRef] [PubMed]
- Schraml, B.U.; van Blijswijk, J.; Zelenay, S.; Whitney, P.G.; Filby, A.; Acton, S.E.; Rogers, N.C.; Moncaut, N.; Carvajal, J.J.; Reis e Sousa, C. Genetic tracing via DNGR-1 expression history defines dendritic cells as a hematopoietic lineage. Cell 2013, 154, 843–858. [Google Scholar] [CrossRef]
- Gilliet, M.; Boonstra, A.; Paturel, C.; Antonenko, S.; Xu, X.L.; Trinchieri, G.; O'Garra, A.; Liu, Y.J. The development of murine plasmacytoid dendritic cell precursors is differentially regulated by FLT3-ligand and granulocyte/macrophage colony-stimulating factor. J Exp Med 2002, 195, 953–958. [Google Scholar] [CrossRef] [PubMed]
- Adams, N.M.; Das, A.; Yun, T.J.; Reizis, B. Ontogeny and Function of Plasmacytoid Dendritic Cells. Annu Rev Immunol 2024, 42, 347–373. [Google Scholar] [CrossRef]
- Reynolds, G.; Tirard, A.; Villani, A.C. Plasmacytoid dendritic cells: Welcome back to the DC fold. Immunity 2022, 55, 380–382. [Google Scholar] [CrossRef] [PubMed]
- Ziegler-Heitbrock, L.; Ohteki, T.; Ginhoux, F.; Shortman, K.; Spits, H. Reclassification of plasmacytoid dendritic cells as innate lymphocytes is premature. Nature Reviews Immunology 2023, 23, 338–339. [Google Scholar] [CrossRef] [PubMed]
- Nagasawa, M.; Schmidlin, H.; Hazekamp, M.G.; Schotte, R.; Blom, B. Development of human plasmacytoid dendritic cells depends on the combined action of the basic helix-loop-helix factor E2-2 and the Ets factor Spi-B. Eur J Immunol 2008, 38, 2389–2400. [Google Scholar] [CrossRef] [PubMed]
- Cisse, B.; Caton, M.L.; Lehner, M.; Maeda, T.; Scheu, S.; Locksley, R.; Holmberg, D.; Zweier, C.; den Hollander, N.S.; Kant, S.G.; et al. Transcription factor E2-2 is an essential and specific regulator of plasmacytoid dendritic cell development. Cell 2008, 135, 37–48. [Google Scholar] [CrossRef] [PubMed]
- Merad, M.; Sathe, P.; Helft, J.; Miller, J.; Mortha, A. The dendritic cell lineage: ontogeny and function of dendritic cells and their subsets in the steady state and the inflamed setting. Annu Rev Immunol 2013, 31, 563–604. [Google Scholar] [CrossRef] [PubMed]
- Holt, P.G.; Haining, S.; Nelson, D.J.; Sedgwick, J.D. Origin and steady-state turnover of class II MHC-bearing dendritic cells in the epithelium of the conducting airways. J Immunol 1994, 153, 256–261. [Google Scholar] [CrossRef]
- Ginhoux, F.; Liu, K.; Helft, J.; Bogunovic, M.; Greter, M.; Hashimoto, D.; Price, J.; Yin, N.; Bromberg, J.; Lira, S.A.; et al. The origin and development of nonlymphoid tissue CD103+ DCs. J Exp Med 2009, 206, 3115–3130. [Google Scholar] [CrossRef] [PubMed]
- Cabeza-Cabrerizo, M.; Cardoso, A.; Minutti, C.M.; Pereira da Costa, M.; Reis e Sousa, C. Dendritic Cells Revisited. Annu Rev Immunol 2021, 39, 131–166. [Google Scholar] [CrossRef] [PubMed]
- Ersland, K.; Wuthrich, M.; Klein, B.S. Dynamic interplay among monocyte-derived, dermal, and resident lymph node dendritic cells during the generation of vaccine immunity to fungi. Cell Host Microbe 2010, 7, 474–487. [Google Scholar] [CrossRef]
- Trautwein-Weidner, K.; Gladiator, A.; Kirchner, F.R.; Becattini, S.; Rulicke, T.; Sallusto, F.; LeibundGut-Landmann, S. Antigen-Specific Th17 Cells Are Primed by Distinct and Complementary Dendritic Cell Subsets in Oropharyngeal Candidiasis. PLoS Pathog 2015, 11, e1005164. [Google Scholar] [CrossRef]
- Bacci, A.; Montagnoli, C.; Perruccio, K.; Bozza, S.; Gaziano, R.; Pitzurra, L.; Velardi, A.; d'Ostiani, C.F.; Cutler, J.E.; Romani, L. Dendritic cells pulsed with fungal RNA induce protective immunity to Candida albicans in hematopoietic transplantation. J Immunol 2002, 168, 2904–2913. [Google Scholar] [CrossRef]
- Montagnoli, C.; Bacci, A.; Bozza, S.; Gaziano, R.; Fiorucci, S.; Spreca, A.; Romani, L. The plasticity of dendritic cells at the host/fungal interface. Immunobiology 2001, 204, 582–589. [Google Scholar] [CrossRef] [PubMed]
- d'Ostiani, C.F.; Del Sero, G.; Bacci, A.; Montagnoli, C.; Spreca, A.; Mencacci, A.; Ricciardi-Castagnoli, P.; Romani, L.
- :: Implications for initiation of T helper cell immunity in vitro and in vivo. Journal of Experimental Medicine 2000, 191, 1661–1673. [CrossRef]
- Fei, M.; Bhatia, S.; Oriss, T.B.; Yarlagadda, M.; Khare, A.; Akira, S.; Saijo, S.; Iwakura, Y.; Fallert Junecko, B.A.; Reinhart, T.A.; et al. TNF-alpha from inflammatory dendritic cells (DCs) regulates lung IL-17A/IL-5 levels and neutrophilia versus eosinophilia during persistent fungal infection. Proc Natl Acad Sci U S A 2011, 108, 5360–5365. [Google Scholar] [CrossRef] [PubMed]
- Mezger, M.; Kneitz, S.; Wozniok, I.; Kurzai, O.; Einsele, H.; Loeffler, J. Proinflammatory response of immature human dendritic cells is mediated by dectin-1 after exposure to Aspergillus fumigatus germ tubes. J Infect Dis 2008, 197, 924–931. [Google Scholar] [CrossRef]
- Wiesner, D.L.; Specht, C.A.; Lee, C.K.; Smith, K.D.; Mukaremera, L.; Lee, S.T.; Lee, C.G.; Elias, J.A.; Nielsen, J.N.; Boulware, D.R.; et al. Chitin recognition via chitotriosidase promotes pathologic type-2 helper T cell responses to cryptococcal infection. PLoS Pathog 2015, 11, e1004701. [Google Scholar] [CrossRef]
- Guilliams, M.; Ginhoux, F.; Jakubzick, C.; Naik, S.H.; Onai, N.; Schraml, B.U.; Segura, E.; Tussiwand, R.; Yona, S. Dendritic cells, monocytes and macrophages: a unified nomenclature based on ontogeny. Nat Rev Immunol 2014, 14, 571–578. [Google Scholar] [CrossRef]
- Plantinga, M.; Guilliams, M.; Vanheerswynghels, M.; Deswarte, K.; Branco-Madeira, F.; Toussaint, W.; Vanhoutte, L.; Neyt, K.; Killeen, N.; Malissen, B.; et al. Conventional and monocyte-derived CD11b(+) dendritic cells initiate and maintain T helper 2 cell-mediated immunity to house dust mite allergen. Immunity 2013, 38, 322–335. [Google Scholar] [CrossRef]
- Osterholzer, J.J.; Curtis, J.L.; Polak, T.; Ames, T.; Chen, G.H.; McDonald, R.; Huffnagle, G.B.; Toews, G.B. CCR2 mediates conventional dendritic cell recruitment and the formation of bronchovascular mononuclear cell infiltrates in the lungs of mice infected with Cryptococcus neoformans. J Immunol 2008, 181, 610–620. [Google Scholar] [CrossRef]
- Goughenour, K.D.; Nair, A.S.; Xu, J.; Olszewski, M.A.; Wozniak, K.L. Dendritic Cells: Multifunctional Roles in Host Defenses to Cryptococcus Infections. J Fungi (Basel) 2023, 9. [Google Scholar] [CrossRef] [PubMed]
- Salazar, F.; Brown, G.D. Antifungal Innate Immunity: A Perspective from the Last 10 Years. J Innate Immun 2018, 10, 373–397. [Google Scholar] [CrossRef]
- Ramirez-Ortiz, Z.G.; Lee, C.K.; Wang, J.P.; Boon, L.; Specht, C.A.; Levitz, S.M. A nonredundant role for plasmacytoid dendritic cells in host defense against the human fungal pathogen Aspergillus fumigatus. Cell Host Microbe 2011, 9, 415–424. [Google Scholar] [CrossRef] [PubMed]
- Guo, Y.; Kasahara, S.; Jhingran, A.; Tosini, N.L.; Zhai, B.; Aufiero, M.A.; Mills, K.A.M.; Gjonbalaj, M.; Espinosa, V.; Rivera, A.; et al. During Aspergillus Infection, Monocyte-Derived DCs, Neutrophils, and Plasmacytoid DCs Enhance Innate Immune Defense through CXCR3-Dependent Crosstalk. Cell Host Microbe 2020, 28, 104–116. [Google Scholar] [CrossRef] [PubMed]
- Araujo, E.F.; Medeiros, D.H.; Galdino, N.A.; Condino-Neto, A.; Calich, V.L.; Loures, F.V. Tolerogenic Plasmacytoid Dendritic Cells Control Paracoccidioides brasiliensis Infection by Inducting Regulatory T Cells in an IDO-Dependent Manner. PLoS Pathog 2016, 12, e1006115. [Google Scholar] [CrossRef] [PubMed]
- Joo, H.; Upchurch, K.; Zhang, W.; Ni, L.; Li, D.; Xue, Y.; Li, X.H.; Hori, T.; Zurawski, S.; Liu, Y.J.; et al. Opposing Roles of Dectin-1 Expressed on Human Plasmacytoid Dendritic Cells and Myeloid Dendritic Cells in Th2 Polarization. J Immunol 2015, 195, 1723–1731. [Google Scholar] [CrossRef] [PubMed]
- Loures, F.V.; Rohm, M.; Lee, C.K.; Santos, E.; Wang, J.P.; Specht, C.A.; Calich, V.L.; Urban, C.F.; Levitz, S.M. Recognition of Aspergillus fumigatus hyphae by human plasmacytoid dendritic cells is mediated by dectin-2 and results in formation of extracellular traps. PLoS Pathog 2015, 11, e1004643. [Google Scholar] [CrossRef] [PubMed]
- Segura, E.; Albiston, A.L.; Wicks, I.P.; Chai, S.Y.; Villadangos, J.A. Different cross-presentation pathways in steady-state and inflammatory dendritic cells. Proc Natl Acad Sci U S A 2009, 106, 20377–20381. [Google Scholar] [CrossRef] [PubMed]
- Embgenbroich, M.; Burgdorf, S. Current Concepts of Antigen Cross-Presentation. Front Immunol 2018, 9, 1643. [Google Scholar] [CrossRef] [PubMed]
- Bevan, M.J. Cross-priming for a secondary cytotoxic response to minor H antigens with H-2 congenic cells which do not cross-react in the cytotoxic assay. J Exp Med 1976, 143, 1283–1288. [Google Scholar] [CrossRef]
- Segura, E.; Villadangos, J.A. Antigen presentation by dendritic cells in vivo. Curr Opin Immunol 2009, 21, 105–110. [Google Scholar] [CrossRef]
- Lin, J.S.; Yang, C.W.; Wang, D.W.; Wu-Hsieh, B.A. Dendritic cells cross-present exogenous fungal antigens to stimulate a protective CD8 T cell response in infection by Histoplasma capsulatum. J Immunol 2005, 174, 6282–6291. [Google Scholar] [CrossRef] [PubMed]
- Carvalho, A.; De Luca, A.; Bozza, S.; Cunha, C.; D'Angelo, C.; Moretti, S.; Perruccio, K.; Iannitti, R.G.; Fallarino, F.; Pierini, A.; et al. TLR3 essentially promotes protective class I-restricted memory CD8(+) T-cell responses to Aspergillus fumigatus in hematopoietic transplanted patients. Blood 2012, 119, 967–977. [Google Scholar] [CrossRef] [PubMed]
- Backer, R.; van Leeuwen, F.; Kraal, G.; den Haan, J.M. CD8- dendritic cells preferentially cross-present Saccharomyces cerevisiae antigens. Eur J Immunol 2008, 38, 370–380. [Google Scholar] [CrossRef] [PubMed]
- Gardner, A.; de Mingo Pulido, A.; Ruffell, B. Dendritic Cells and Their Role in Immunotherapy. Front Immunol 2020, 11, 924. [Google Scholar] [CrossRef] [PubMed]
- Ogasawara, M.; Miyashita, M.; Yamagishi, Y.; Ota, S. Phase I/II Pilot Study of Wilms' Tumor 1 Peptide-Pulsed Dendritic Cell Vaccination Combined With Conventional Chemotherapy in Patients With Head and Neck Cancer. Ther Apher Dial 2019, 23, 279–288. [Google Scholar] [CrossRef] [PubMed]
- Tanyi, J.L.; George, E. Personalized vaccination against ovarian cancer: what are the possibilities? Expert Rev Vaccines 2018, 17, 955–958. [Google Scholar] [CrossRef] [PubMed]
- Rosenblatt, J.; Avivi, I.; Vasir, B.; Uhl, L.; Munshi, N.C.; Katz, T.; Dey, B.R.; Somaiya, P.; Mills, H.; Campigotto, F.; et al. Vaccination with dendritic cell/tumor fusions following autologous stem cell transplant induces immunologic and clinical responses in multiple myeloma patients. Clin Cancer Res 2013, 19, 3640–3648. [Google Scholar] [CrossRef] [PubMed]
- Van Tendeloo, V.F.; Van de Velde, A.; Van Driessche, A.; Cools, N.; Anguille, S.; Ladell, K.; Gostick, E.; Vermeulen, K.; Pieters, K.; Nijs, G.; et al. Induction of complete and molecular remissions in acute myeloid leukemia by Wilms' tumor 1 antigen-targeted dendritic cell vaccination. Proc Natl Acad Sci U S A 2010, 107, 13824–13829. [Google Scholar] [CrossRef] [PubMed]
- Tel, J.; Aarntzen, E.H.; Baba, T.; Schreibelt, G.; Schulte, B.M.; Benitez-Ribas, D.; Boerman, O.C.; Croockewit, S.; Oyen, W.J.; van Rossum, M.; et al. Natural human plasmacytoid dendritic cells induce antigen-specific T-cell responses in melanoma patients. Cancer Res 2013, 73, 1063–1075. [Google Scholar] [CrossRef]
- Cheever, M.A.; Higano, C.S. PROVENGE (Sipuleucel-T) in prostate cancer: the first FDA-approved therapeutic cancer vaccine. Clin Cancer Res 2011, 17, 3520–3526. [Google Scholar] [CrossRef]
- Inacio, M.M.; Moreira, A.L.E.; Cruz-Leite, V.R.M.; Mattos, K.; Silva, L.O.S.; Venturini, J.; Ruiz, O.H.; Ribeiro-Dias, F.; Weber, S.S.; Soares, C.M.A.; Borges, C.L. Fungal Vaccine Development: State of the Art and Perspectives Using Immunoinformatics. J Fungi (Basel) 2023, 9. [Google Scholar] [CrossRef] [PubMed]
- Tacken, P.J.; Zeelenberg, I.S.; Cruz, L.J.; van Hout-Kuijer, M.A.; van de Glind, G.; Fokkink, R.G.; Lambeck, A.J.; Figdor, C.G. Targeted delivery of TLR ligands to human and mouse dendritic cells strongly enhances adjuvanticity. Blood 2011, 118, 6836–6844. [Google Scholar] [CrossRef] [PubMed]
- Lam, J.S.; Huang, H.; Levitz, S.M. Effect of differential N-linked and O-linked mannosylation on recognition of fungal antigens by dendritic cells. PLoS One 2007, 2, e1009. [Google Scholar] [CrossRef] [PubMed]
- Huang, H.; Ostroff, G.R.; Lee, C.K.; Specht, C.A.; Levitz, S.M. Robust stimulation of humoral and cellular immune responses following vaccination with antigen-loaded beta-glucan particles. mBio 2010, 1. [Google Scholar] [CrossRef] [PubMed]
- Carter, R.W.; Thompson, C.; Reid, D.M.; Wong, S.Y.; Tough, D.F. Preferential induction of CD4+ T cell responses through in vivo targeting of antigen to dendritic cell-associated C-type lectin-1. J Immunol 2006, 177, 2276–2284. [Google Scholar] [CrossRef]
- Dutta, O.; Masso-Silva, J.A.; Wang, K.; Rivera, A. Host response to pulmonary fungal infections: A highlight on cell-driven immunity to Cryptococcus species and Aspergillus fumigatus. Curr Pharmacol Rep 2017, 3, 335–345. [Google Scholar] [CrossRef] [PubMed]
- Aimanianda, V.; Bayry, J.; Bozza, S.; Kniemeyer, O.; Perruccio, K.; Elluru, S.R.; Clavaud, C.; Paris, S.; Brakhage, A.A.; Kaveri, S.V.; et al. Surface hydrophobin prevents immune recognition of airborne fungal spores. Nature 2009, 460, 1117–1121. [Google Scholar] [CrossRef] [PubMed]
- Hoenigl, M.; Seidel, D.; Sprute, R.; Cunha, C.; Oliverio, M.; Goldman, G.H.; Ibrahim, A.S.; Carvalho, A. COVID-19-associated fungal infections. Nat Microbiol 2022, 7, 1127–1140. [Google Scholar] [CrossRef] [PubMed]
- Pfaller, M.A.; Diekema, D.J. Rare and emerging opportunistic fungal pathogens: concern for resistance beyond Candida albicans and Aspergillus fumigatus. J Clin Microbiol 2004, 42, 4419–4431. [Google Scholar] [CrossRef]
- Rivera, A.; Hohl, T.M.; Collins, N.; Leiner, I.; Gallegos, A.; Saijo, S.; Coward, J.W.; Iwakura, Y.; Pamer, E.G. Dectin-1 diversifies Aspergillus fumigatus-specific T cell responses by inhibiting T helper type 1 CD4 T cell differentiation. J Exp Med 2011, 208, 369–381. [Google Scholar] [CrossRef]
- Rivera, A.; Lodge, J.; Xue, C. Harnessing the Immune Response to Fungal Pathogens for Vaccine Development. Annu Rev Microbiol 2022, 76, 703–726. [Google Scholar] [CrossRef]
- Oliveira, L.V.N.; Wang, R.; Specht, C.A.; Levitz, S.M. Vaccines for human fungal diseases: close but still a long way to go. NPJ Vaccines 2021, 6, 33. [Google Scholar] [CrossRef] [PubMed]
- Bozza, S.; Gaziano, R.; Lipford, G.B.; Montagnoli, C.; Bacci, A.; Di Francesco, P.; Kurup, V.P.; Wagner, H.; Romani, L. Vaccination of mice against invasive aspergillosis with recombinant Aspergillus proteins and CpG oligodeoxynucleotides as adjuvants. Microbes Infect 2002, 4, 1281–1290. [Google Scholar] [CrossRef] [PubMed]
- Bozza, S.; Gaziano, R.; Spreca, A.; Bacci, A.; Montagnoli, C.; di Francesco, P.; Romani, L. Dendritic cells transport conidia and hyphae of Aspergillus fumigatus from the airways to the draining lymph nodes and initiate disparate Th responses to the fungus. J Immunol 2002, 168, 1362–1371. [Google Scholar] [CrossRef] [PubMed]
- Bozza, S.; Perruccio, K.; Montagnoli, C.; Gaziano, R.; Bellocchio, S.; Burchielli, E.; Nkwanyuo, G.; Pitzurra, L.; Velardi, A.; Romani, L. A dendritic cell vaccine against invasive aspergillosis in allogeneic hematopoietic transplantation. Blood 2003, 102, 3807–3814. [Google Scholar] [CrossRef]
- Shao, C.; Qu, J.; He, L.; Zhang, Y.; Wang, J.; Zhou, H.; Wang, Y.; Liu, X. Dendritic cells transduced with an adenovirus vector encoding interleukin-12 are a potent vaccine for invasive pulmonary aspergillosis. Genes Immun 2005, 6, 103–114. [Google Scholar] [CrossRef]
- Perruccio, K.; Bozza, S.; Montagnoli, C.; Bellocchio, S.; Aversa, F.; Martelli, M.; Bistoni, F.; Velardi, A.; Romani, L. Prospects for dendritic cell vaccination against fungal infections in hematopoietic transplantation. Blood Cells Mol Dis 2004, 33, 248–255. [Google Scholar] [CrossRef]
- Awasthi, S. Dendritic cell-based vaccine against coccidioides infection. Ann N Y Acad Sci 2007, 1111, 269–274. [Google Scholar] [CrossRef]
- Chechi, J.L.; da Costa, F.A.C.; Figueiredo, J.M.; de Souza, C.M.; Valdez, A.F.; Zamith-Miranda, D.; Camara, A.C.; Taborda, C.P.; Nosanchuk, J.D. Vaccine development for pathogenic fungi: current status and future directions. Expert Rev Vaccines 2023, 22, 1136–1153. [Google Scholar] [CrossRef]
- Zou, G.; Wei, Y. World Health Organization's first-ever release of a fungal priority pathogens list: A reply action proposal for the prevention and treatment of fungal pathogens. Eco Environ Health 2023, 2, 43–44. [Google Scholar] [CrossRef]
- Liu, W.; Li, R.Y. [Enlightenment of World Health Organization fungal priority pathogens list]. Zhonghua Liu Xing Bing Xue Za Zhi 2023, 44, 1984–1987. [Google Scholar] [CrossRef]
- Sondermeyer, G.L.; Lee, L.A.; Gilliss, D.; Vugia, D.J. Coccidioidomycosis-Associated Deaths in California, 2000-2013. Public Health Rep 2016, 131, 531–535. [Google Scholar] [CrossRef] [PubMed]
- Loh, J.T.; Lam, K.P. Fungal infections: Immune defense, immunotherapies and vaccines. Adv Drug Deliv Rev 2023, 196, 114775. [Google Scholar] [CrossRef]
- Peyclit, L.; Yousfi, H.; Rolain, J.M.; Bittar, F. Drug Repurposing in Medical Mycology: Identification of Compounds as Potential Antifungals to Overcome the Emergence of Multidrug-Resistant Fungi. Pharmaceuticals (Basel) 2021, 14. [Google Scholar] [CrossRef]
- Nicola, A.M.; Albuquerque, P.; Paes, H.C.; Fernandes, L.; Costa, F.F.; Kioshima, E.S.; Abadio, A.K.R.; Bocca, A.L.; Felipe, M.S. Antifungal drugs: New insights in research & development. Pharmacol Ther 2019, 195, 21–38. [Google Scholar] [CrossRef] [PubMed]
- Awasthi, S.; Awasthi, V.; Magee, D.M.; Coalson, J.J. Efficacy of antigen 2/proline-rich antigen cDNA-transfected dendritic cells in immunization of mice against Coccidioides posadasii. J Immunol 2005, 175, 3900–3906. [Google Scholar] [CrossRef] [PubMed]
- Awasthi, S.; Vilekar, P.; Conkleton, A.; Rahman, N. Dendritic cell-based immunization induces Coccidioides Ag2/PRA-specific immune response. Vaccine 2019, 37, 1685–1691. [Google Scholar] [CrossRef]
- Awasthi, S. Intranasal Antifungal Vaccination Using DNA-Transfected Dendritic Cells. Methods Mol Biol 2017, 1625, 75–83. [Google Scholar] [CrossRef] [PubMed]
- Vilekar, P.; Awasthi, V.; Lagisetty, P.; King, C.; Shankar, N.; Awasthi, S. In vivo trafficking and immunostimulatory potential of an intranasally-administered primary dendritic cell-based vaccine. BMC Immunol 2010, 11, 60. [Google Scholar] [CrossRef]
- L, B.R.D.S.; C, P.T.; J, D.N. Advances in Fungal Peptide Vaccines. J Fungi (Basel) 2020, 6. [Google Scholar] [CrossRef]
- Prado, M.; Silva, M.B.; Laurenti, R.; Travassos, L.R.; Taborda, C.P. Mortality due to systemic mycoses as a primary cause of death or in association with AIDS in Brazil: a review from 1996 to 2006. Mem Inst Oswaldo Cruz 2009, 104, 513–521. [Google Scholar] [CrossRef]
- Ferreira, K.S.; Bastos, K.R.; Russo, M.; Almeida, S.R. Interaction between Paracoccidioides brasiliensis and pulmonary dendritic cells induces interleukin-10 production and toll-like receptor-2 expression: possible mechanisms of susceptibility. J Infect Dis 2007, 196, 1108–1115. [Google Scholar] [CrossRef] [PubMed]
- Silva, L.B.R.; Taira, C.L.; Cleare, L.G.; Martins, M.; Junqueira, M.; Nosanchuk, J.D.; Taborda, C.P. Identification of Potentially Therapeutic Immunogenic Peptides From Paracoccidioides lutzii Species. Front Immunol 2021, 12, 670992. [Google Scholar] [CrossRef]
- Travassos, L.R.; Taborda, C.P. Linear Epitopes of Paracoccidioides brasiliensis and Other Fungal Agents of Human Systemic Mycoses As Vaccine Candidates. Front Immunol 2017, 8, 224. [Google Scholar] [CrossRef]
- Jannuzzi, G.P.; de Almeida, J.R.F.; Dos Santos, S.S.; de Almeida, S.R.; Ferreira, K.S. Notch Signaling is Required for Dendritic Cell Maturation and T Cell Expansion in Paracoccidioidomycosis. Mycopathologia 2018, 183, 739–749. [Google Scholar] [CrossRef] [PubMed]
- Travassos, L.R.; Taborda, C.P. New advances in the development of a vaccine against paracoccidioidomycosis. Front Microbiol 2012, 3, 212. [Google Scholar] [CrossRef]
- Magalhaes, A.; Ferreira, K.S.; Almeida, S.R.; Nosanchuk, J.D.; Travassos, L.R.; Taborda, C.P. Prophylactic and therapeutic vaccination using dendritic cells primed with peptide 10 derived from the 43-kilodalton glycoprotein of Paracoccidioides brasiliensis. Clin Vaccine Immunol 2012, 19, 23–29. [Google Scholar] [CrossRef] [PubMed]
- Silva, L.B.R.; Taira, C.L.; Dias, L.S.; Souza, A.C.O.; Nosanchuk, J.D.; Travassos, L.R.; Taborda, C.P. Experimental Therapy of Paracoccidioidomycosis Using P10-Primed Monocyte-Derived Dendritic Cells Isolated From Infected Mice. Front Microbiol 2019, 10, 1727. [Google Scholar] [CrossRef] [PubMed]
- Silva, L.B.R.; Dias, L.S.; Rittner, G.M.G.; Munoz, J.E.; Souza, A.C.O.; Nosanchuk, J.D.; Travassos, L.R.; Taborda, C.P. Dendritic Cells Primed with Paracoccidioides brasiliensis Peptide P10 Are Therapeutic in Immunosuppressed Mice with Paracoccidioidomycosis. Front Microbiol 2017, 8, 1057. [Google Scholar] [CrossRef]
- Lizarazo, J.; Escandon, P.; Agudelo, C.I.; Firacative, C.; Meyer, W.; Castaneda, E. Retrospective study of the epidemiology and clinical manifestations of Cryptococcus gattii infections in Colombia from 1997-2011. PLoS Negl Trop Dis 2014, 8, e3272. [Google Scholar] [CrossRef]
- Centers for Disease, C.; Prevention. Emergence of Cryptococcus gattii-- Pacific Northwest, 2004-2010. MMWR Morb Mortal Wkly Rep 2010, 59, 865–868. [Google Scholar]
- Rajasingham, R.; Govender, N.P.; Jordan, A.; Loyse, A.; Shroufi, A.; Denning, D.W.; Meya, D.B.; Chiller, T.M.; Boulware, D.R. The global burden of HIV-associated cryptococcal infection in adults in 2020: a modelling analysis. Lancet Infect Dis 2022, 22, 1748–1755. [Google Scholar] [CrossRef] [PubMed]
- Smith, R.M.; Mba-Jonas, A.; Tourdjman, M.; Schimek, T.; DeBess, E.; Marsden-Haug, N.; Harris, J.R. Treatment and outcomes among patients with Cryptococcus gattii infections in the United States Pacific Northwest. PLoS One 2014, 9, e88875. [Google Scholar] [CrossRef] [PubMed]
- Ueno, K.; Kinjo, Y.; Okubo, Y.; Aki, K.; Urai, M.; Kaneko, Y.; Shimizu, K.; Wang, D.N.; Okawara, A.; Nara, T.; et al. Dendritic cell-based immunization ameliorates pulmonary infection with highly virulent Cryptococcus gattii. Infect Immun 2015, 83, 1577–1586. [Google Scholar] [CrossRef] [PubMed]
- Hole, C.R.; Wager, C.M.L.; Castro-Lopez, N.; Campuzano, A.; Cai, H.; Wozniak, K.L.; Wang, Y.; Wormley, F.L., Jr. Induction of memory-like dendritic cell responses in vivo. Nat Commun 2019, 10, 2955. [Google Scholar] [CrossRef]
- Nami, S.; Mohammadi, R.; Vakili, M.; Khezripour, K.; Mirzaei, H.; Morovati, H. Fungal vaccines, mechanism of actions and immunology: A comprehensive review. Biomed Pharmacother 2019, 109, 333–344. [Google Scholar] [CrossRef]
- Caballero Van Dyke, M.C.; Wormley, F.L., Jr. A Call to Arms: Quest for a Cryptococcal Vaccine. Trends Microbiol 2018, 26, 436–446. [Google Scholar] [CrossRef] [PubMed]
- Wozniak, K.L.; Ravi, S.; Macias, S.; Young, M.L.; Olszewski, M.A.; Steele, C.; Wormley, F.L. Insights into the mechanisms of protective immunity against Cryptococcus neoformans infection using a mouse model of pulmonary cryptococcosis. PLoS One 2009, 4, e6854. [Google Scholar] [CrossRef]
- Ueno, K.; Urai, M.; Sadamoto, S.; Shinozaki, M.; Takatsuka, S.; Abe, M.; Otani, Y.; Yanagihara, N.; Shimizu, K.; Iwakura, Y.; et al. A dendritic cell-based systemic vaccine induces long-lived lung-resident memory Th17 cells and ameliorates pulmonary mycosis. Mucosal Immunol 2019, 12, 265–276. [Google Scholar] [CrossRef]
- Rizzo, J.; Chaze, T.; Miranda, K.; Roberson, R.W.; Gorgette, O.; Nimrichter, L.; Matondo, M.; Latge, J.P.; Beauvais, A.; Rodrigues, M.L. Characterization of Extracellular Vesicles Produced by Aspergillus fumigatus Protoplasts. mSphere 2020, 5. [Google Scholar] [CrossRef]
- Marina, C.L.; Burgel, P.H.; Agostinho, D.P.; Zamith-Miranda, D.; Las-Casas, L.O.; Tavares, A.H.; Nosanchuk, J.D.; Bocca, A.L. Nutritional Conditions Modulate C. neoformans Extracellular Vesicles' Capacity to Elicit Host Immune Response. Microorganisms 2020, 8. [Google Scholar] [CrossRef] [PubMed]
- Joffe, L.S.; Nimrichter, L.; Rodrigues, M.L.; Del Poeta, M. Potential Roles of Fungal Extracellular Vesicles during Infection. mSphere 2016, 1. [Google Scholar] [CrossRef] [PubMed]
- Sandbrink, J.B.; Shattock, R.J. RNA Vaccines: A Suitable Platform for Tackling Emerging Pandemics? Front Immunol 2020, 11, 608460. [Google Scholar] [CrossRef]
- Oda, Y.; Kumagai, Y.; Kanai, M.; Iwama, Y.; Okura, I.; Minamida, T.; Yagi, Y.; Kurosawa, T.; Greener, B.; Zhang, Y.; Walson, J.L. Immunogenicity and safety of a booster dose of a self-amplifying RNA COVID-19 vaccine (ARCT-154) versus BNT162b2 mRNA COVID-19 vaccine: a double-blind, multicentre, randomised, controlled, phase 3, non-inferiority trial. Lancet Infect Dis 2024, 24, 351–360. [Google Scholar] [CrossRef] [PubMed]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





