1. Introduction
According to the report of the European Environment Agency (EEA, 2017), air pollution is a major environmental and social problem, which leads to multiple challenges in terms of management and mitigation. Effective action to reduce the impacts of air pollution requires a good understanding of how pollutants are transported and transformed in the atmosphere, and how they affect humans, ecosystems, the climate and, subsequently, the society and economy [
1]. Suspended particulate matter (PM) is an important atmospheric pollutant with severe public health effects, particularly in urban areas which are heavily affected by emissions from vehicles, industry, and other sources of air pollution [
2]. The correlation between emissions and pollution levels differs depending on the city and even part of a city, where infrastructure and urban planning determine the emission pattern while meteorology and topography determine dispersion and transformation [
3].
Inhalable PM refers to particles with a diameter less than 10 μm. These particles have a complex composition, including organic constituents, inorganic salts, and trace elements [
4]. Different studies have associated PM10 with severe respiratory diseases, such as asthma [
5], lung cancer [
6], and chronic obstructive pulmonary disease [
7], as well as with cardiovascular diseases such as stroke, deep vein thrombosis, coronary events, myocardial infractions and atherosclerosis [
8]. Between 1990 and 2019, worldwide deaths due to air pollution enhanced by 2.62%. Furthermore, over 90% of the global population resides in areas that are not reach the air quality standards established by the WHO (2021) [
2].
Despite the impact of air quality on health, studies of air pollution are often restricted by the high costs of monitoring instruments and the too limited scale of sampling. Drawbacks of the conventional monitoring equipment is the high price, large size, heavy weight and energy consumption [
9]. For these reasons, bio-monitoring, that is the use of plants or lichens to estimate air quality, has been proposed as a cost-effective and environmentally friendly approach which can be an alternative for physical and chemical analytical methods of air pollution monitoring [
10]. In this way, plants got introduced as bio-monitors of trace elements accumulation due to their efficiency for trapping particulate matter [
11]. Air pollutants can be bounded in and on the cuticula and, eventually, taken up by plants via stomata, or indirectly by uptake via roots after deposition of the air pollutants on the soil [
12]. After penetration, the particles clog the stomata and decrease the foliar pH due to the presence of sulphate (SO
42-) and nitrate (NO
3-) ions in the dust [
13]. Atmospheric dust deposition on leaves is mainly influenced by the plant species (evergreen or deciduous, composition and thickness of wax layer) and the specific structure of their leaves (e.g. leaf size, shape, roughness, presence of trichomes), as well as the meteorological conditions (air humidity, rainfall, wind) and source-specific particle features (e.g. particle size distribution) [
14]. In this context, improvement of green spaces or shelter belts by planting suitable and tolerant species, selected for the specific area, can catch air pollutants and mitigate the pollution levels.
This study aimed to assess the levels of air pollution in sites with high, moderate and low traffic intensity, relating bio-monitors with automatic monitors data, as well as to assess the usefulness of destructive and non-destructive analytical techniques for the quantification of trace elements using outdoor exposed Hedera helix and Senecio cineraria plants.
3. Discussion
Meteorological conditions were recorded by Argentina´s National Meteorological service, Creative Commons 2.5 Argentina License (
Figure 1). According to the climate classification of Thornthwaite, the climate of the center and south of this agricultural zone (Santa Rosa) is dry subhumid, with little or no excess water, cold temperate mesothermal with a summer concentration of thermal efficiency less than 48 % [
15]. Temperature, precipitation, humidity and wind speed were related with concentrations recorded by automatic monitors (
Figure 2) by Spearman correlation test. It has been reported that a lower temperature leads to a higher humidity, which leads to an increase of both PM2.5 and PM10 deposition and absorptions by plants [
16]. The effect of air humidity on deposition is due to the fact that particles are mainly hygroscopic, and their size varies as a result of the absorption or discharge of water. In return, this leads to a change in their deposition properties as a function of diameter [
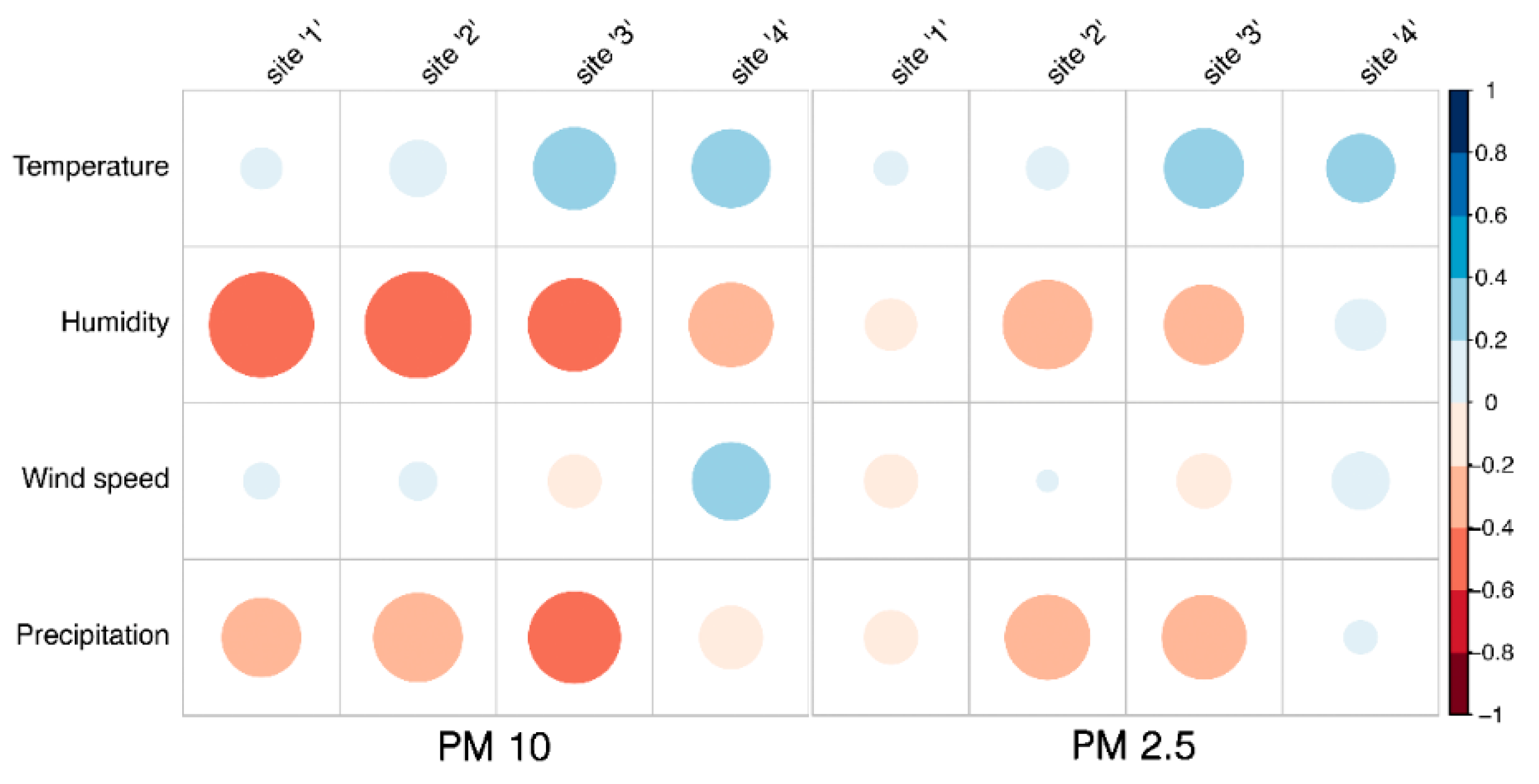
17]. Our results showed a positive correlation between temperature and PM10 and PM2.5 for sites 3 and 4 and a negative correlation between humidity and PM10 across all sites (
Figure 3). Regarding wind speed, Mei et al., [
18] reported that an increase in wind speed enhanced particle transport and reduced local particle concentrations, however, it did not affect the relative location of high particle concentration zones, which are more related to building height and design. Agreeing with Mei et al., we just found at site 4 (rural area) a positive correlation between wind speed and PM10 (
Figure 3). Additionally, Liu et al. [
19] reported that precipitation has a certain wet scavenging effect on PM2.5 and PM10, and the scavenging effect on PM10 is higher than that on PM2.5. They concluded that the scavenging effect of precipitation on PM10 is closely related to the initial concentration of PM10 before precipitation. The higher the initial concentration of PM10 is, the greater the removal by precipitation. A negative correlation was observed across all sites between precipitation and PM10, nonmatter the initial concentration of PM 10, whereas this correlation was evident only at sites 2 and 3 when considering PM2.5 (
Figure 3).
Gravimetric quantification of PM deposited on leaves (
Table 1) indicates a significant difference in the amounts of PM10 sequester by
Senecio cineraria in contrast with
Hedera helix. Deposition was already reported to be mainly affected by the shape of the plant and the structure of the leaves or needles [
20]. Also,
Senecio cineraria leaves are covered with fine matted hairs giving them a felted or woolly appearance [
21]. The accumulation of atmospheric PM reported by Castanheiro et al. [
22], was shown to be species specific (hedera accumulated more than strawberry) rather than influenced by the buildup of atmospheric dust. Urban, rural, and industrial areas were studied using the leaves of
Celtis occidentalis and
Trientalis europaea in the city of Debrecen (Hungary) [
11]. In contrast to our findings, an increasing amount of fine dust deposited on leaves was found in the urban area compared to the industrial and rural sites, suggesting that the higher vehicular traffic had a notable effect on dust emission and deposition on leaves at the urban site. Similarly, in the city of Gandhinagar, India, Chaudhary et al. [
23] found higher dust depositions on tree leaves in zones with intense traffic compared to commercial and residential zones. However, we did not find additional PM deposition at the urban site (site 1) compared to the peri-urban sites (sites 2 and 3) and the rural site (site 4). This might be due to the fact that in the urban site the diffusion of PM was not hindered by tall rows of buildings. There was not a correlation between the location where plants sequestered the highest amount of PM (site 3,
Table 1) and the location where the monitors reported the highest amount of PM (site 2,
Figure 1). Outcomes obtained with both techniques (automatic monitors and bio-monitors) are not associating, however they can be used as complementary tools to elucidate the complex, multifactorial process of PM diffusion and deposition. After characterizing the heavy metal concentrations in a total of 540 samples from four ecosystem compartments (plant leaves, foliar dust, surface soil, and subsoil), Li et al. [
24] concluded that foliar dust reflected pollution of atmospheric particulate matter in the most reliably ways among the four ecosystem compartments that were investigated.
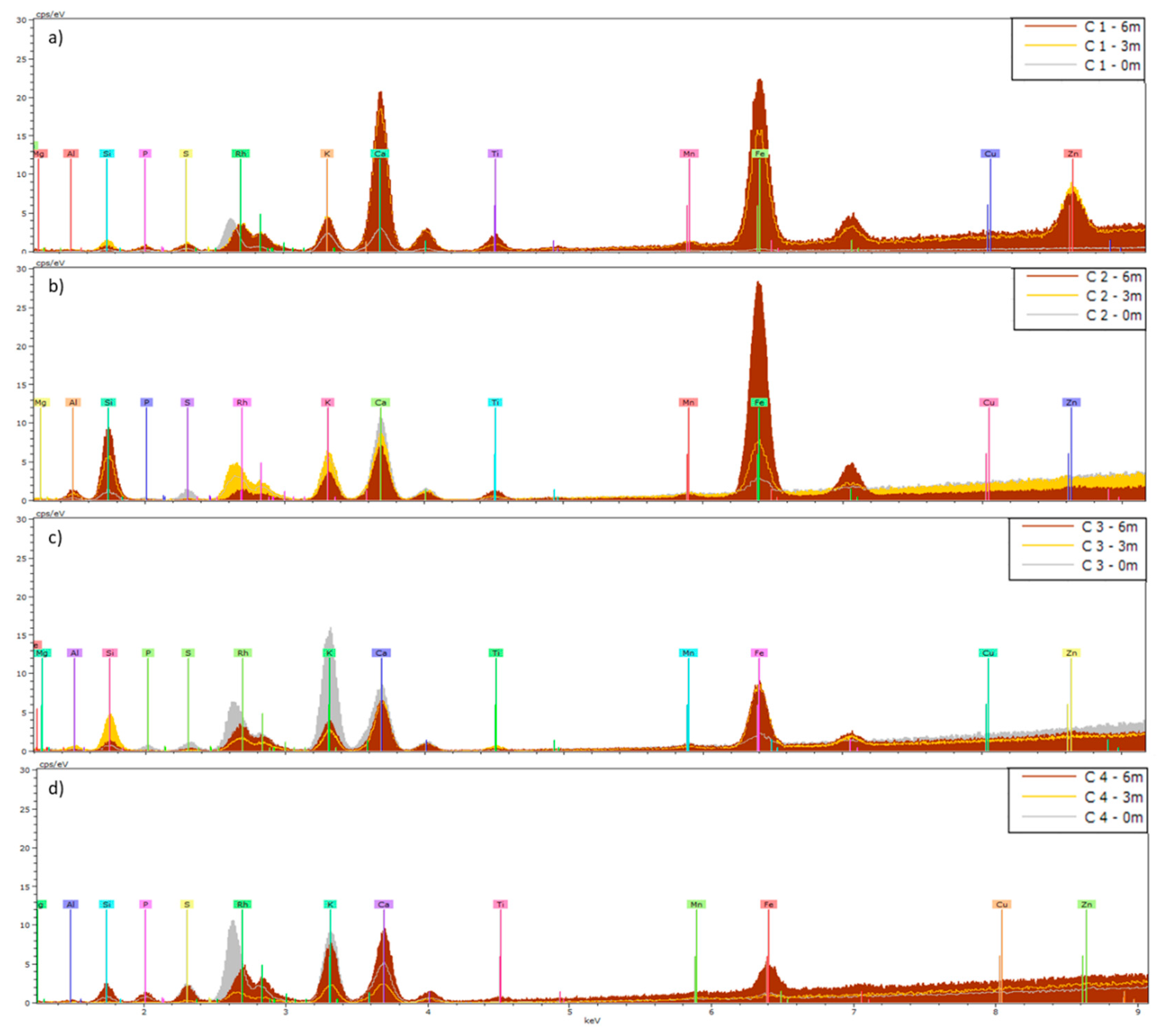
After 3 and 6 months of exposure, the predominant elements found enriching PM deposited on
Hedera an
Cineraria leaves included Ti, Zn and Fe, by XRF (
Figure 4 and
Figure 5). Some researchers have reported that Fe could be associated with soil resuspension since this element is a typical soil constituent [
25]. Zn and Fe can be derived from exhaust and non-exhaust road traffic [
26]. Zn is also associated to tires tread dust [
27]. While, Ti is widely used in paints as a UV filter as well as in plastics [
28] and has been detected before in outdoor air [
29,
30]. Similar results were reported by Castanheiro et al. [
22]. They studied accumulation of atmospheric dust on leaves of ivy and strawberry using XRF and concluded that XRF offers many advantages for multi-element, non-destructive analysis, which can be performed directly on the sample, at relatively low cost and with rapid output. However, disadvantages are due to the heterogeneity of plant material and matrix effects [
22]. Particularly when samples do not meet the condition of thin-film, self-absorption effects arise that complicate the process of matrix calibration required for quantitative analysis [
31]. In our study these effects were also observed as the variabilities between leaves from the same species and exposure time were larger than expected (
Table S1). Santos et al. [
25], used
Nerium oleander L. leaves as bio-monitor to evaluate levels of environmental pollutants in a sub-region in the Metropolitan Area of Rio de Janeiro City (Brazil) through XRF. In their study they highlighted the association between Fe, Cu, Zn, and Pb and vehicle and industrial emission sources and the usability of the XRF technique for environmental pollution analysis. While, according to Hulskotte et al. [
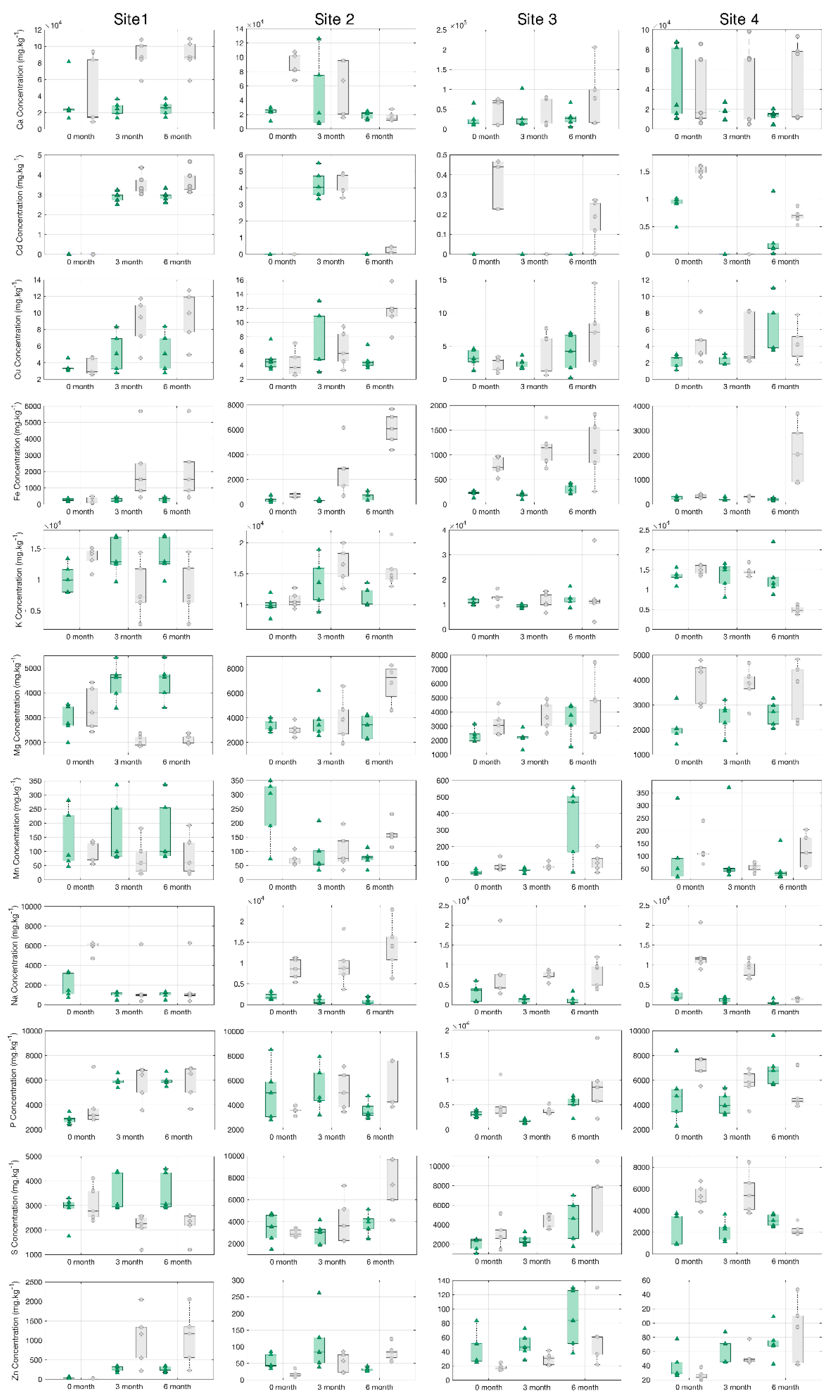
32] vehicle braking system is one of the most important sources of Cu particles. In our study, enrichment of Cd, Cu, Fe, Mn and Zn in leaves was found to result from accumulation of atmospheric dust by ICP (
Figure 6).
For the interpretation of field data and evaluation of trace element air pollution, reference values are needed. “Reference Plant” was proposed by Markert [
33] and it describes the average content of all the inorganic elements found in plants. The following values could be considered as ‘normal’ metal concentrations in leaves of plants from uncontaminated environments: 0.05 mg.kg
-1 Cd, 10 mg.kg
-1 Cu, 150 mg.kg
-1 Fe, 200 mg.kg
-1 Mn and 50 mg.kg
-1 Zn. These values of element concentration can be further used to establish the reference point of the “chemical fingerprint” [
34]. In our study, Cd, Fe and Zn exceed these thresholds (
Figure 6 and
Table S2). These elements may become a threat to human health and/or the environment. Chronic exposure to low levels of heavy metals can cause serious health effects in the long term [
35]. Gehring et al. [
36] described the adverse effects of PM constituents, in particular Si, K, Fe, Cu and Zn, on asthma, rhinitis, allergic sensitization, and lung function in schoolchildren.
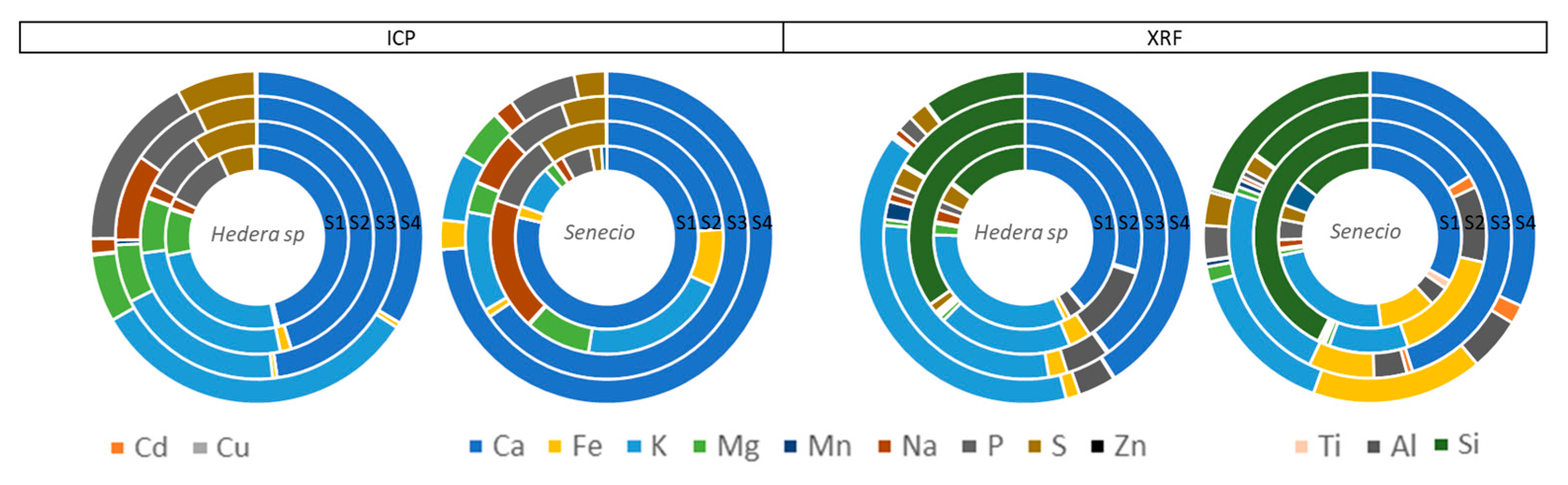
Finally, results obtained by XRF (not destructive) and ICP (destructive) analytical techniques were compared by Welch two sample t-test (
Figure 8). Castanheiro et al. [
22] also compared results obtained by XRF and HRICP-MS techniques of leaves of ivy and strawberry exposed to atmospheric dust. For a total of ten (common) elements (Si, K, Ca, Ti, Cr, Fe, Cu, Rb, Sr, Pb), they observed that concentrations were always higher when samples were investigated using XRF in comparison with ICP-MS. However, those ten elements were detected for most analysed leaves with ICP-MS, which was not the case for XRF. Therefore, they consider the accumulation of elements to be most accurate after quantification by HR-ICP-MS. In contrast, our study showed that XRF and ICP results were statistically similar for Ca, Fe, Na and Zn. While, Cd and Cu were just detected by ICP due to K peaks overlapping by XRF and Ti, Si and Al were just detected by XRF due to heterogeneity of plant material and low detection limits by ICP.
Although it is necessary to perform more research to elucidate whether these plants species can be used as bio-monitors in areas with higher pollution levels, Cineraria features make it a promising candidate to be adopted as a bio-monitor or even for PM mitigation when planted as green belts.
4. Materials and Methods
4.1. Selection of Plant Species
Hedera helix: is an evergreen climbing plant with alternating leaves, 50-100 mm long, with 15-20 mm long petioles. It possesses palmately five-lobed juvenile leaves on creeping, climbing stems and unlobed cordate adult leaves on fertile flowering stems exposed to full sun (Metcalf, 2005). This species was chosen for its known capacity to capture a wide spectrum of PM fractions (0.2-2.5 μm; 2.5-10 μm; 10-100 μm) [
37,
38].
Senecio cineraria: is a white-wooly, heat and drought tolerant evergreen subshrub. The leaves are pinnate or pinnatifid, 5-15 cm long and 3-7 cm broad, stiff, with oblong and obtuse segments, and like the stems, covered with long, thinly to thickly covered with grey-white to white hairs. The tomentum is thickest on the underside of the leaves, and can become worn off on the upper side, leaving the top surface glabrous with age [
39]. This species was chosen as its capacity to accumulate PM has not yet been evaluated and the characteristics of its leaves make it a good candidate to sequester PM.
4.2. Sites Selected, Air Quality Monitoring Stations and Daily Meteorological Conditions
Hedera helix and
Senecio cineraria plants were obtained from a plant nursery (Agropecuaria, Santa Rosa, Argentina). After the collection of non-exposed cleaned leaves, five plants of each species were placed at the final locations. Four locations in Santa Rosa, La Pampa, Argentina with different anthropogenic impacts were selected for the study. Site 1: University, urban area with intense car traffic based on Google Maps Traffic statistics (-36.625325 Lat., -64.293103 Long.); Site 2: Calo street, a suburban area with moderate car traffic and unpaved streets (-36.647888 Lat., -64.276939 Long.); Site 3: Felice street, located in a residential area with moderate car traffic (-36.627074 Lat., -64.323317 Long.); Site 4: Agronomic Campus, located 5 km from Santa Rosa city, rural area, with little car traffic (-36.548932 Lat., -64.299345 Long.) (
Figure S1). Plants were placed next to an automatic air quality measuring station, equipped with a laser scattering Sensor SDS-011 (Nova Fitness, Shandong, China) (Fig S2). Daily meteorological conditions (temperature, precipitation, humidity and wind speed) were recorded by Argentina´s National Meteorological service, Creative Commons 2.5 Argentina License. Plants were watered once a week to prevent drought stress. The watering was performed avoiding any physical contact with the leaves. Leaf sampling was conducted every three months during a period of six months, in the spring (December 15
th 2021) and in summer (March 16
th 2022). Leaves were sampled at 10 cm above the soil, to avoid direct soil contamination and to standardize any potential influence from resuspension of the soil in the pots. Three leaves per plant were collected per sampling location (n=60).
4.3. Sites Selected, Air Quality Monitoring Stations and Daily Meteorological Conditions
An Erlenmeyer was filled with 50 ml of ultrapure water and one leaf was added. The leaf was stirred in an orbital shaker for 60 min at 270 rpm as described previously [
40]. Subsequently, PM fractions were separated using Type 91 Whatman ashless filters with 10 µm retention and Type 42 with 2.5 µm retention. Before use, the filters were dried overnight in an oven at 60 °C, and their weight was determined to correct for air humidity. After filtration, filters were dried and post-weighed using a PIONER precision balance (OHAUS, Lindavista, Mexico) to calculate the weight of PM in each fraction of every sample. The leaf surface area was determined using Image J Analysis System [
41], which allowed to express the amount of PM as mg cm
-2 leaf area.
4.4. Leaf Surface Elemental Composition: XRF and ICP
Leaf samples were analyzed for their elemental composition using X-Ray Fluorescence (XRF) and Inductively Coupled Plasma-Atomic Emission Spectrometry (ICP). First, leaf samples were analyzed by XRF for the elements range Na–Ba. An XRF benchtop spectrometer (M4 Tornado, Bruker®, Germany), equipped with a Rh X-ray tube with polycapillary optics and XFlash® detector providing an energy resolution of better than 145 eV was employed. For the analyses of (i) Mg–Fe we used: tube voltage of 10 kV, current of 300 mA, live time of 500 s; and for (ii) Ti–Ba: 40 kV, 50 mA, 1000 s. The measured XRF spectra in each pixel of the XRF maps were deconvoluted using the software supplied with the M4 Tornado. Spectrum energy calibration was performed daily, before the analysis of each batch, by using a copper (Cu) and zirconium (Zr) Bruker® calibration standard block. Analytical quality control was ensured by analysis of steel certified reference material (SN0163). Data was normalized against a conservative element and results were informed in % weight normalized to 100 %.
Secondly, the same leaves were digested with 70% HNO3 in a heat block and dissolved in 5ml of 2% HCl using the USEPA 3050B Acid Digestion of Sediments, Sludges, and Soils (Environmental Protection Agency [EPA] 1996a). The concentrations of elements were determined by inductively coupled plasma-atomic emission spectrometry (HR-ICP-OES, Agilent Technologies, 700 series, Belgium). Blanks (only HNO3) were included. Also, certified reference materials, Cabbage-BCR and Spinach Leaves 1570a were included in each batch. The recoveries obtained were in the range of 40-67% for Cd, 77-90% for Cu, 79-90% for Mn, 61-70% for Pb and 79-91% Zn.
4.5. Statistical Analysis
Data normal distribution was verified with the Shapiro-Wilk test and the homogeneity of variances was confirmed by a Levene test. The differences between samples were tested using analysis of variance (ANOVA) for each variable. When group variances were unequal, the Games-Howell method was used for pairwise comparison between the groups. Two-sample t-test was applied to analyze months and species categories. Statistical analysis to compare possible significant differences between XRF and ICP techniques was carried out by the Welch T-test. Principal component analysis (PCA) was carried out to identify the potential contributions of Cd, Cu, Fe, Mn, and Zn and exposure time (0, 3 and 6 months) at each site. Additionally, Spearman correlation analysis was performed to assess correlations between meteorological variables (temperature, wind speed, humidity, and precipitation) and PM fractions (10 and 2.5) at each site. PCA was displayed by Matlab (The MathWorks Inc., Natick, MA), while the correlation matrix was generated using R (version 4.2.2).
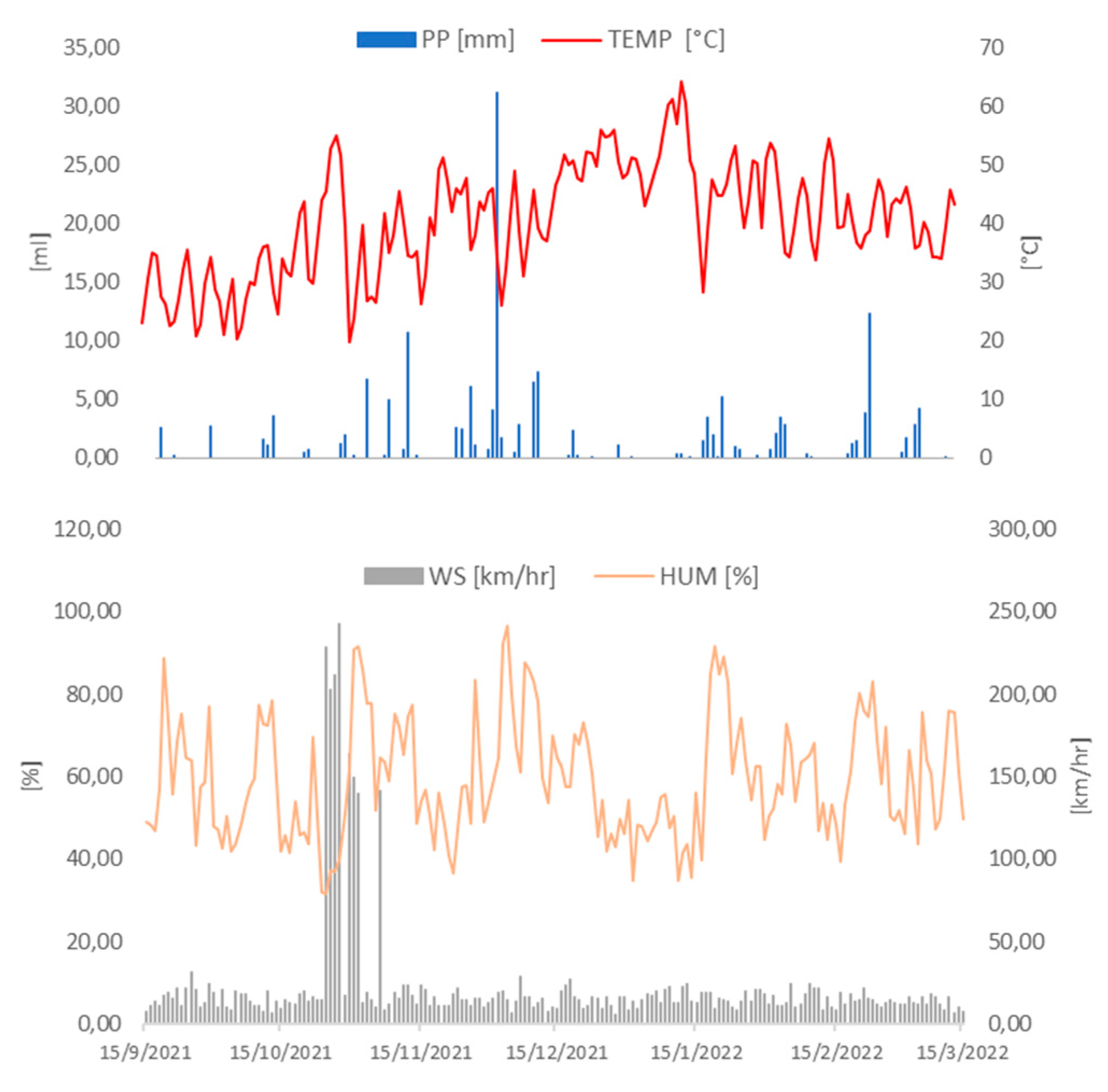
Figure 1.
Measurements from an automatic weather station from the National Weather Service of Argentina at Santa Rosa Aero station. Humidity (orange line), wind speed (grey bars), temperature (red line) and precipitation recorded (blue bars) between 15/09/2021 and 15/03/2022.
Figure 1.
Measurements from an automatic weather station from the National Weather Service of Argentina at Santa Rosa Aero station. Humidity (orange line), wind speed (grey bars), temperature (red line) and precipitation recorded (blue bars) between 15/09/2021 and 15/03/2022.
Figure 2.
Average concentrations of PM 10 and PM 2.5 recorded monthly at each site (n=5). Pink line represents the WHO annual limit recommended (PM 10= 15 µg m-3; PM2.5= 5 µg m-3).
Figure 2.
Average concentrations of PM 10 and PM 2.5 recorded monthly at each site (n=5). Pink line represents the WHO annual limit recommended (PM 10= 15 µg m-3; PM2.5= 5 µg m-3).
Figure 3.
Spearman correlation matrix, pairwise relationships between meteorological variables and PM concentrations recorded by monitors located at the four sites. Circle sizes dynamically adjust based on the magnitude of correlation and the color gradient indicate the strength and direction of correlations, from negative (red) to positive (blue).
Figure 3.
Spearman correlation matrix, pairwise relationships between meteorological variables and PM concentrations recorded by monitors located at the four sites. Circle sizes dynamically adjust based on the magnitude of correlation and the color gradient indicate the strength and direction of correlations, from negative (red) to positive (blue).
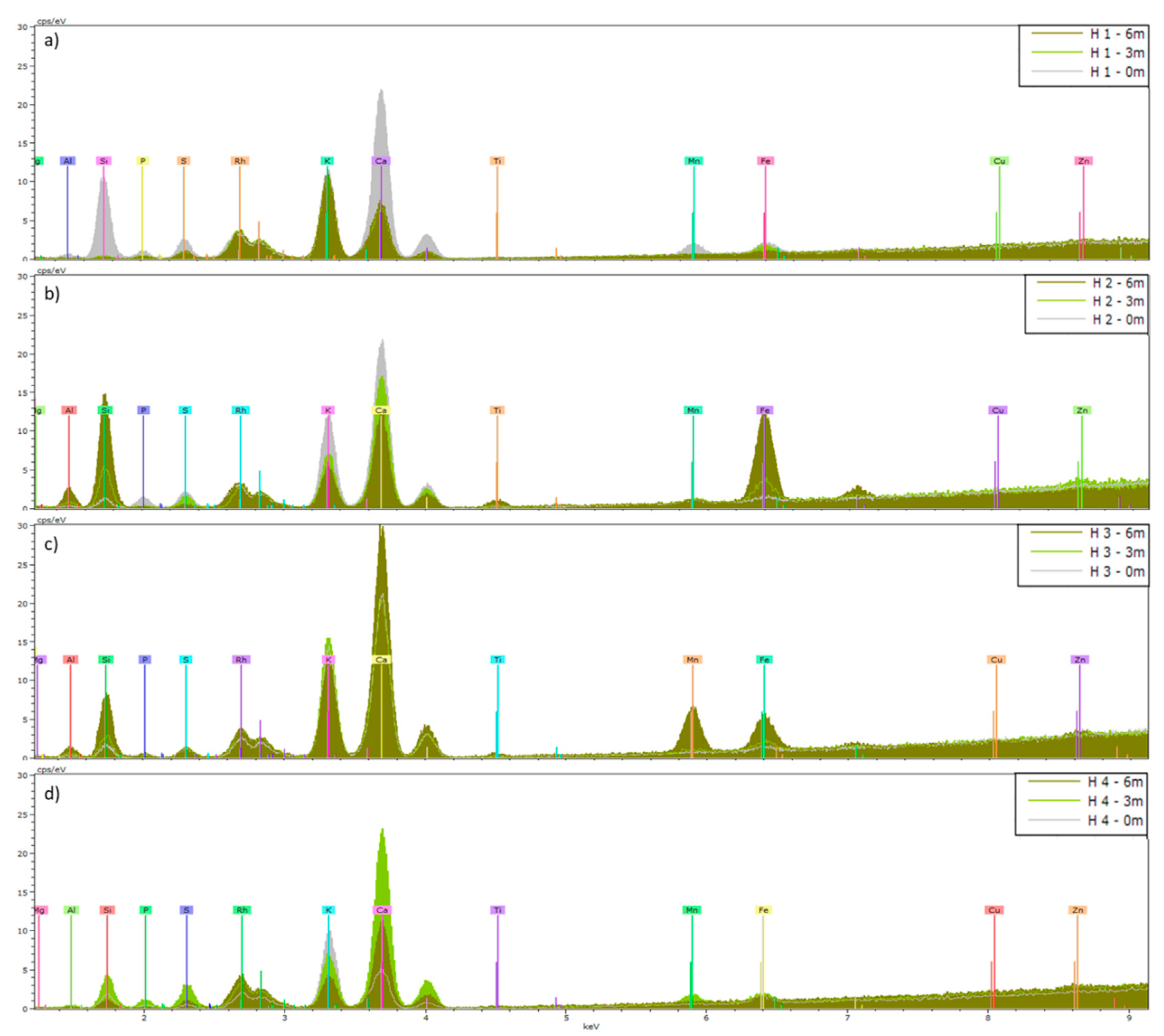
Figure 4.
Means (n=5) of XRF spectra of Hedera helix (H) leaves collected from a) site 1, b) site 2, c) site 3 and d) site 4. Leaves were analyzed before (0m) and after 3 and 6 months of exposure (3m and 6m). The KeV of the peaks shows which elements are present, and the height of a peak indicates the abundance of that element.
Figure 4.
Means (n=5) of XRF spectra of Hedera helix (H) leaves collected from a) site 1, b) site 2, c) site 3 and d) site 4. Leaves were analyzed before (0m) and after 3 and 6 months of exposure (3m and 6m). The KeV of the peaks shows which elements are present, and the height of a peak indicates the abundance of that element.
Figure 5.
Means (n=5) of XRF spectra of Senecio cineraria (C) leaves originating from a) site 1, b) site 2, c) site 3 and d) site 4. Leaves were analyzed before (0m) and after 3 and 6 months of exposure (3m and 6m). The KeV of the peaks shows which elements are present, and the height of a peak indicates the abundance of that element.
Figure 5.
Means (n=5) of XRF spectra of Senecio cineraria (C) leaves originating from a) site 1, b) site 2, c) site 3 and d) site 4. Leaves were analyzed before (0m) and after 3 and 6 months of exposure (3m and 6m). The KeV of the peaks shows which elements are present, and the height of a peak indicates the abundance of that element.
Figure 6.
Boxplot mean element concentrations (mg.kg-1) and standard error (n=5) in leaf tissue across sites and months for Hedera helix (green triangle) and Senecio cineraria (gray circle) by ICP.
Figure 6.
Boxplot mean element concentrations (mg.kg-1) and standard error (n=5) in leaf tissue across sites and months for Hedera helix (green triangle) and Senecio cineraria (gray circle) by ICP.
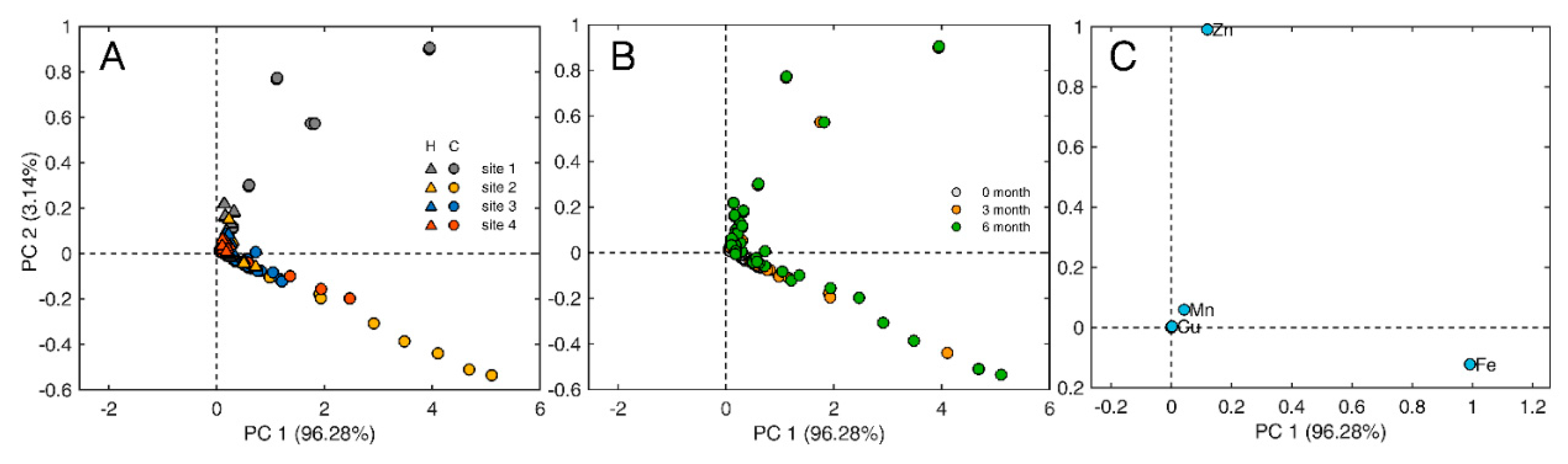
Figure 7.
Scores plot of the first two PCs obtained by PCA illustrating sample distributions based on (A) site and plant species ('C' for Senecio cineraria and ‘H’ for Hedera helix) and (B) exposure time in months. (C) Loading plot highlighting elements with main influence on the sample distribution.
Figure 7.
Scores plot of the first two PCs obtained by PCA illustrating sample distributions based on (A) site and plant species ('C' for Senecio cineraria and ‘H’ for Hedera helix) and (B) exposure time in months. (C) Loading plot highlighting elements with main influence on the sample distribution.
Figure 8.
Pie charts of average leaf surface elemental concentration measured by ICP and XRF for Hedera helix and Senecio cineraria plants after 6 months of exposure at sites 1, 2, 3 and 4. Cd and Cu (left side) were only detected by ICP. Al and Si (right side) were only detected using XRF.
Figure 8.
Pie charts of average leaf surface elemental concentration measured by ICP and XRF for Hedera helix and Senecio cineraria plants after 6 months of exposure at sites 1, 2, 3 and 4. Cd and Cu (left side) were only detected by ICP. Al and Si (right side) were only detected using XRF.
Table 1.
PM10 and PM2.5 (µg.cm-2) sequestered on leaf surface.
Table 1.
PM10 and PM2.5 (µg.cm-2) sequestered on leaf surface.
| Plant |
Time |
Site 1 |
Site 2 |
Site 3 |
Site 4 |
| PM10 |
PM2.5 |
PM10 |
PM2.5 |
PM10 |
PM2.5 |
PM10 |
PM2.5 |
| Hedera helix |
0m |
485±96a
|
681±125a
|
930±175a
|
115±44a
|
60±26a
|
30±23a
|
151±51a
|
109±90a
|
| 3m |
341±83a
|
552±588a
|
699±274ab
|
670±577ab
|
946±312b
|
965±343b
|
175±81a
|
215±90a
|
| 6m |
412±43a
|
505±465a
|
501±330ab
|
682±198b
|
697±92b
|
1016±682b
|
752±435b
|
601±359b
|
Senecio
cineraria
|
0m |
429±176a
|
691±235a |
260±153a
|
231±159a
|
55±26a
|
43±50a |
234±138a
|
152±48a
|
| 3m |
2893±3634ab
|
140±54b |
1686±2174ab
|
344±159ab
|
2450±264b
|
2450±343c |
158±83a
|
231±114a
|
| 6m |
2800±884b
|
132±44b |
649±242b
|
383±55ab
|
1056±538b
|
1047±582b |
623±329b
|
548±94b
|