Submitted:
16 July 2024
Posted:
16 July 2024
You are already at the latest version
Abstract
Keywords:
1. Introduction
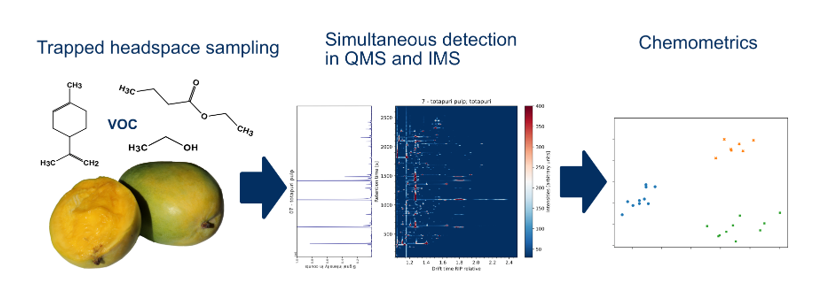
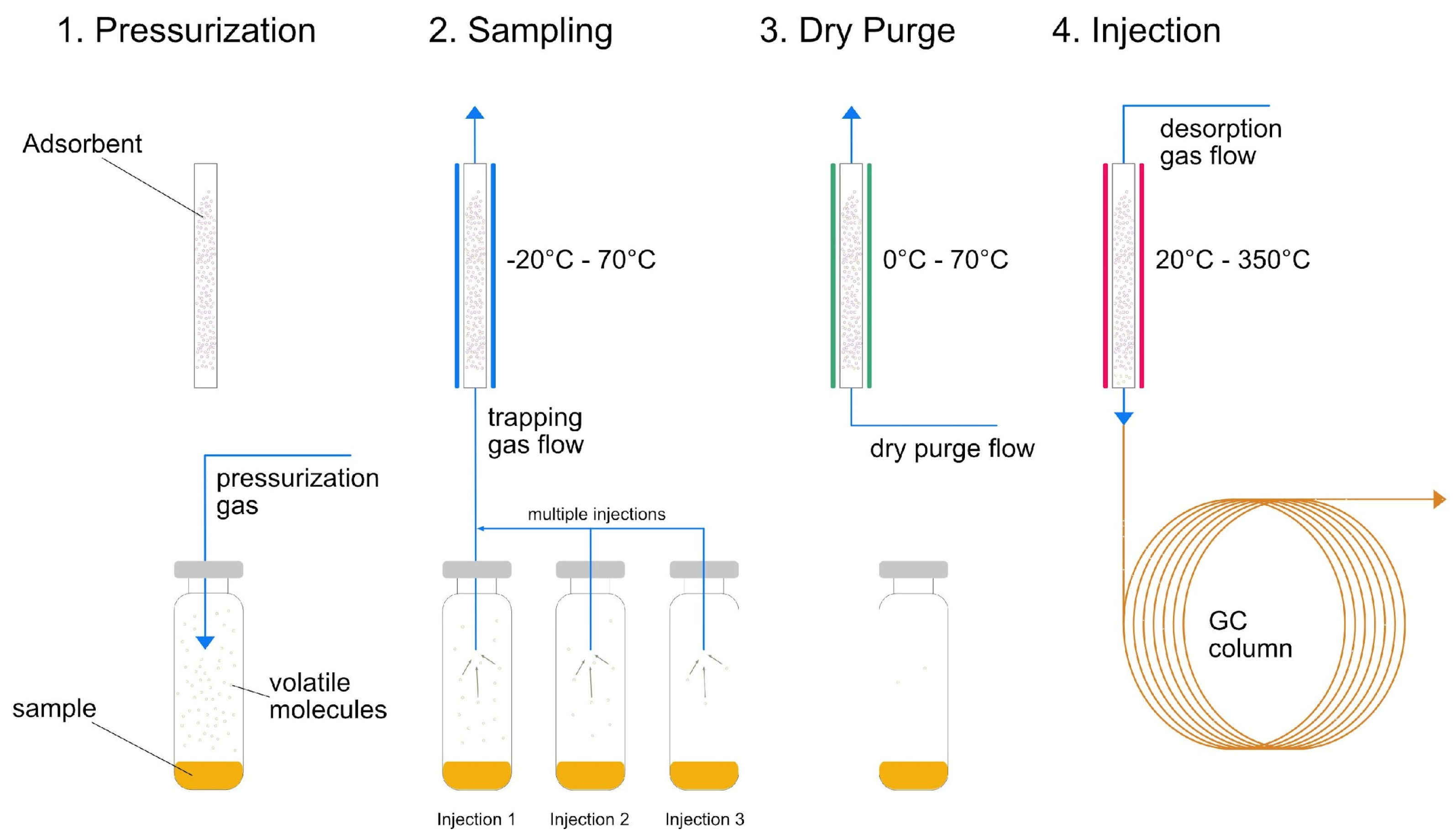
1.1. Trapped Headspace Sampling
- K = partition coefficient of a defined compounds
- CS = concentration of the compound in sample phase
- CG = concentration of the compound in vapor phase
1.2. Ion Mobility Spectrometry in VOC Analysis
1.3. VOC Analysis of Mangos
2. Materials and Methods
2.1. Reagents and Mango Samples
2.2. Instrumentation (HS-GC-MS-IMS)
2.3. Data Processing and Evaluation
3. Results & Discussion
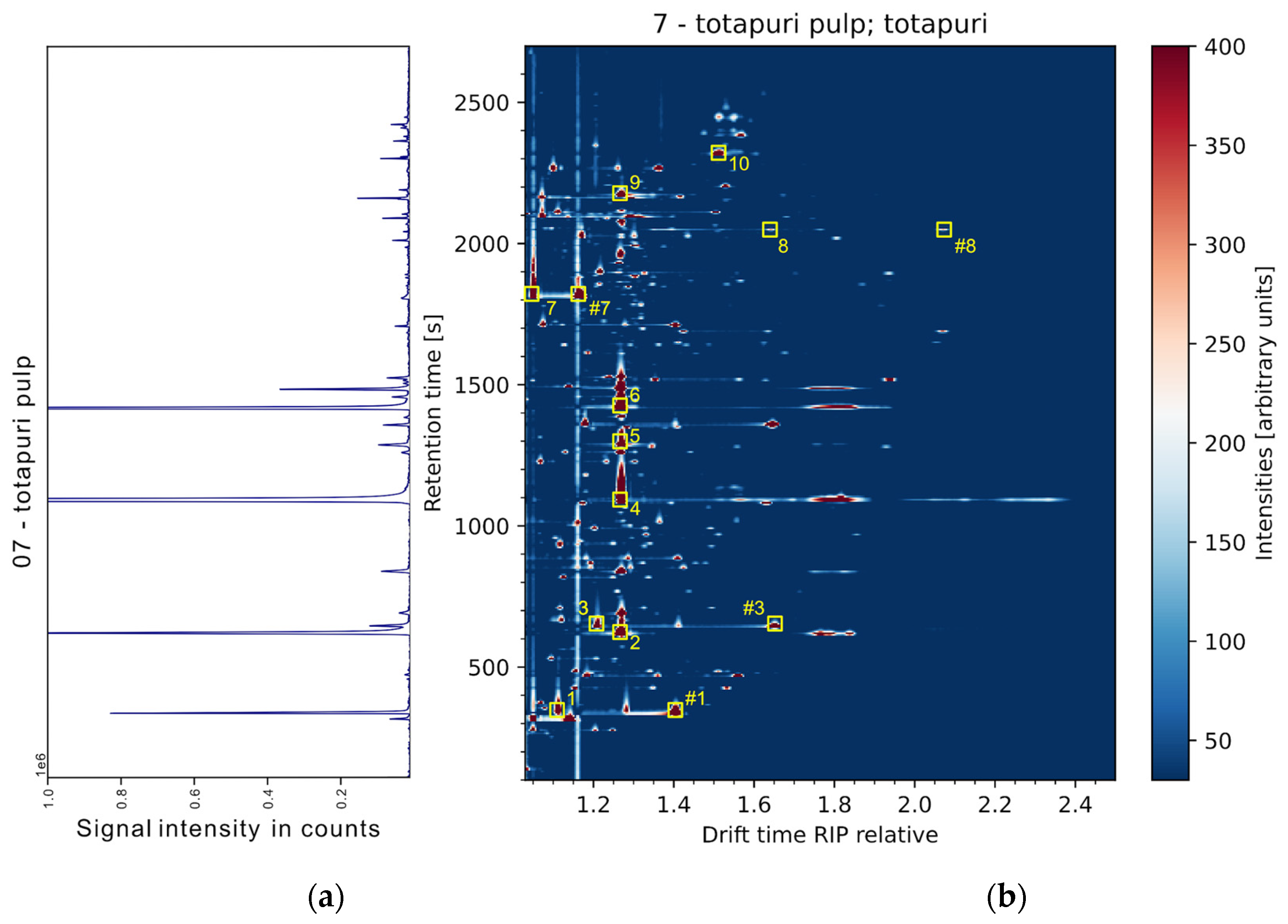
3.1. Performance of the THS-GC-MS-IMS System
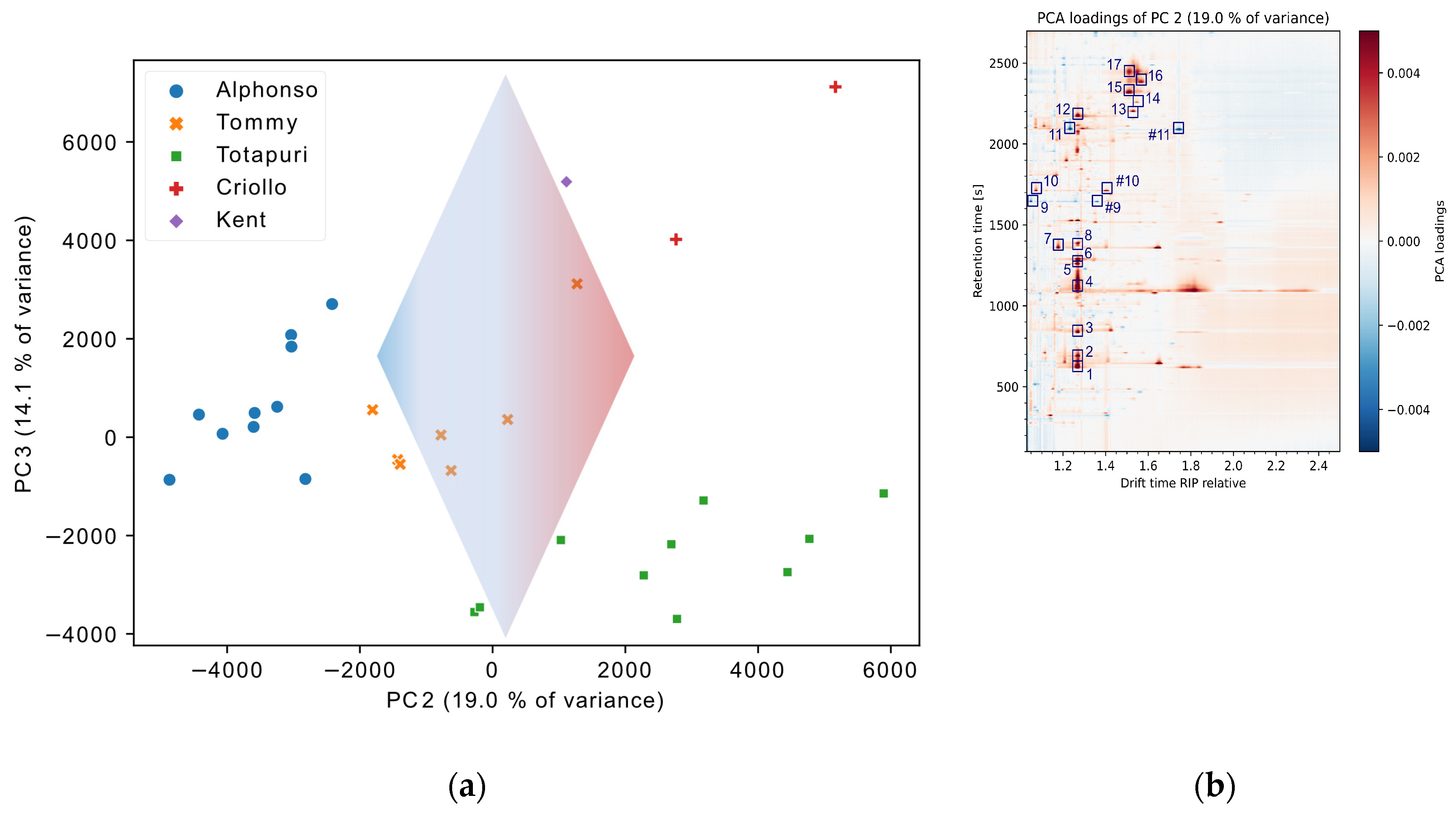
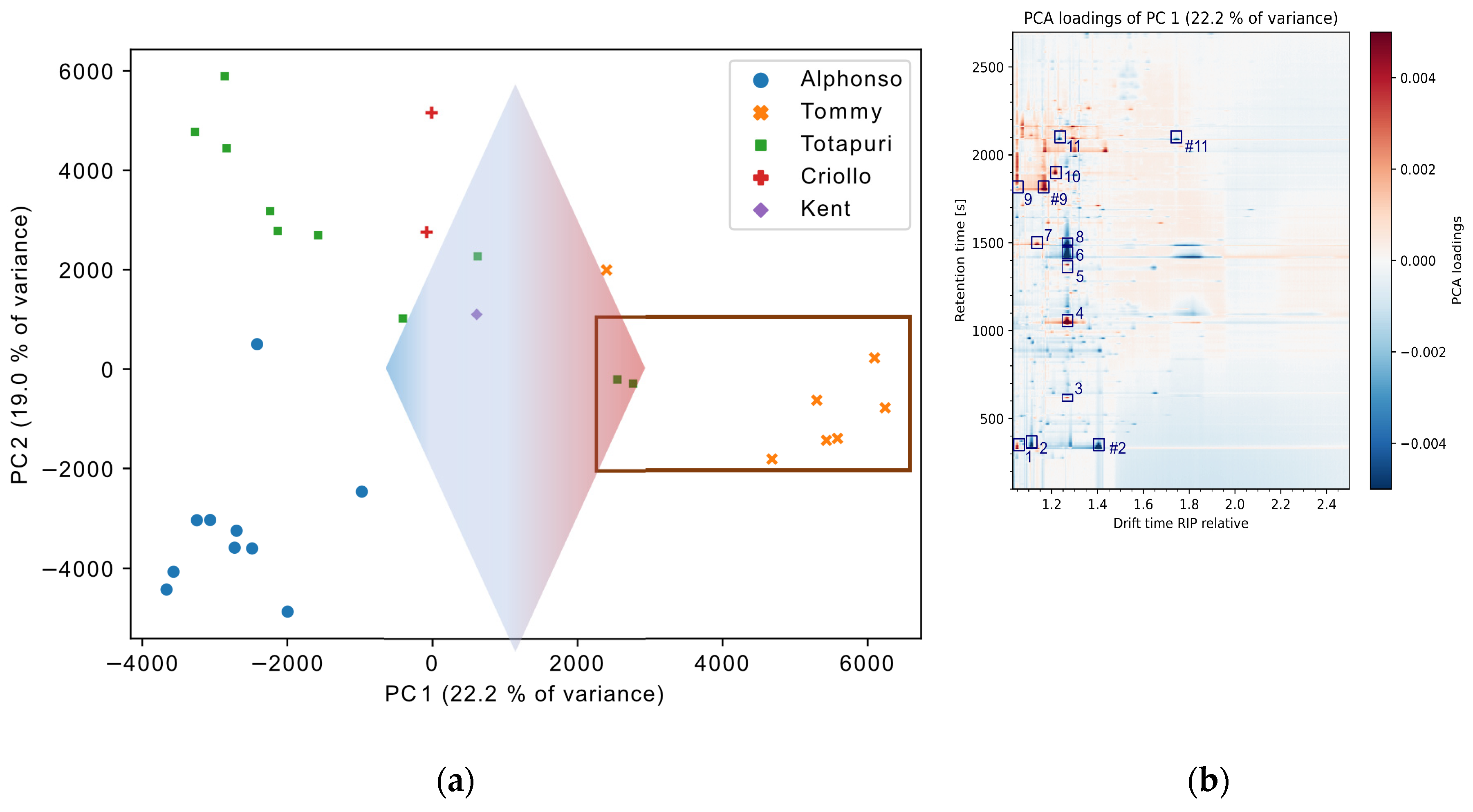
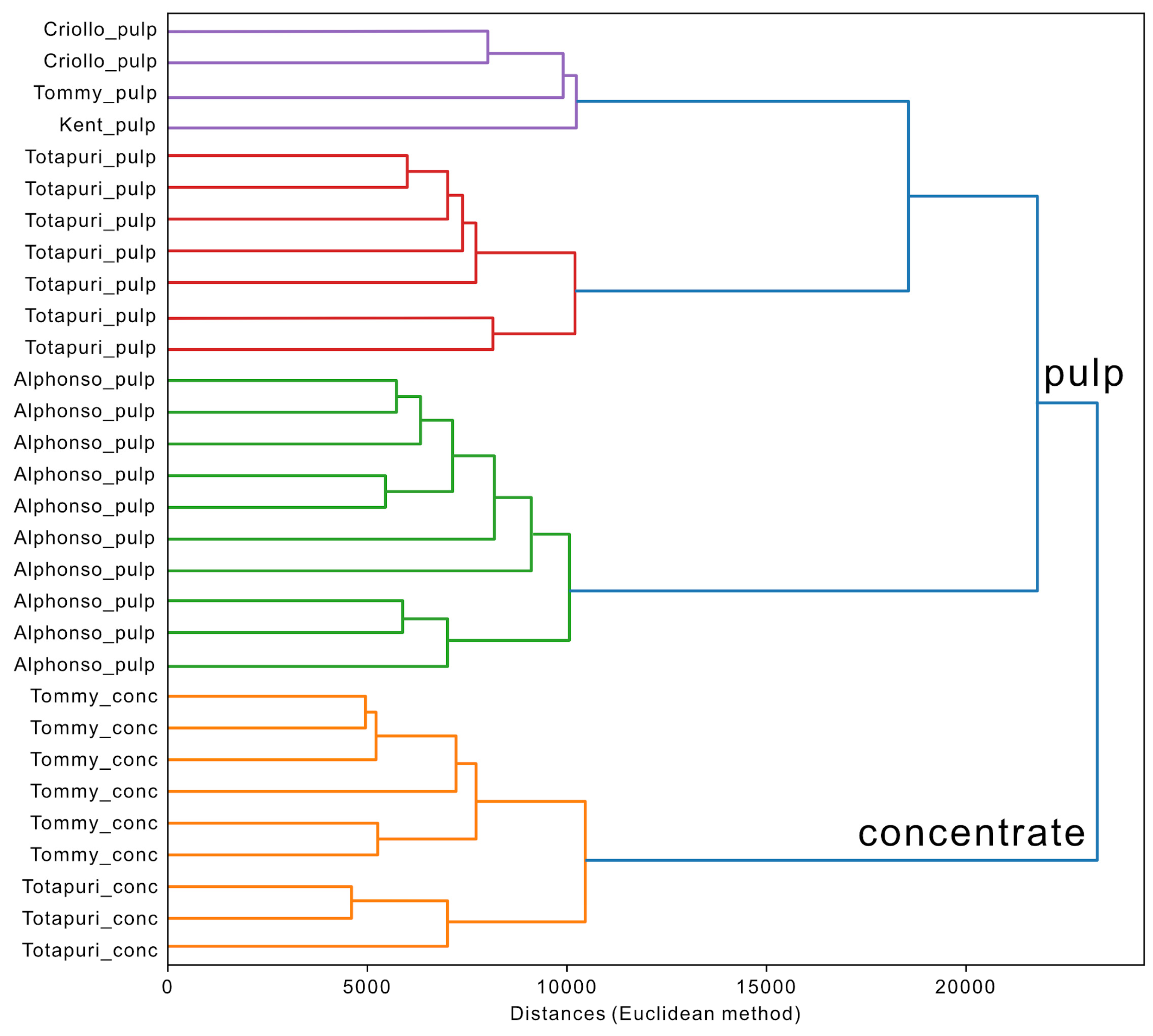
3.2. Exploratory Data Evaluation of Non-Targeted IMS Data
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Gerhardt, N.; Birkenmeier, M.; Schwolow, S.; Rohn, S.; Weller, P. Volatile-Compound Fingerprinting by Headspace-Gas-Chromatography Ion-Mobility Spectrometry (HS-GC-IMS) as a Benchtop Alternative to 1H NMR Profiling for Assessment of the Authenticity of Honey. Anal. Chem. 2018, 90, 1777–1785. [CrossRef]
- Brendel, R.; Schwolow, S.; Rohn, S.; Weller, P. Volatilomic Profiling of Citrus Juices by Dual-Detection HS-GC-MS-IMS and Machine Learning-An Alternative Authentication Approach. J. Agric. Food Chem. 2021, 69, 1727–1738. [CrossRef]
- Capitain, C.; Weller, P. Non-Targeted Screening Approaches for Profiling of Volatile Organic Compounds Based on Gas Chromatography-Ion Mobility Spectroscopy (GC-IMS) and Machine Learning. Molecules 2021, 26. [CrossRef]
- Karpas, Z. Applications of ion mobility spectrometry (IMS) in the field of foodomics. Food Research International 2013, 54, 1146–1151. [CrossRef]
- Parastar, H.; Weller, P. Towards greener volatilomics: Is GC-IMS the new Swiss army knife of gas phase analysis? TrAC Trends in Analytical Chemistry 2024, 170, 117438. [CrossRef]
- Borsdorf, H.; Eiceman, G.A. Ion Mobility Spectrometry: Principles and Applications. Applied Spectroscopy Reviews 2006, 41, 323–375. [CrossRef]
- Schanzmann, H.; Ruzsanyi, V.; Ahmad-Nejad, P.; Telgheder, U.; Sielemann, S. A novel coupling technique based on thermal desorption gas chromatography with mass spectrometry and ion mobility spectrometry for breath analysis. J. Breath Res. 2023, 18. [CrossRef]
- Kremser, A.; Jochmann, M.A.; Schmidt, T.C. Systematic comparison of static and dynamic headspace sampling techniques for gas chromatography. Anal. Bioanal. Chem. 2016, 408, 6567–6579. [CrossRef]
- Gas chromatography; Poole, C.F., Ed., 2nd ed.; Elsevier: Cambridge, 2021, ISBN 9780128206751.
- Soria, A.C.; García-Sarrió, M.J.; Sanz, M.L. Volatile sampling by headspace techniques. TrAC Trends in Analytical Chemistry 2015, 71, 85–99. [CrossRef]
- Costa Freitas, A.M.; Gomes da Silva, M.D.R.; Cabrita, M.J. Sampling Techniques for the Determination of Volatile Components in Grape Juice, Wine and Alcoholic Beverages. Comprehensive sampling and sample preparation: Analytical techniques for scientists; Academic Press: Amsterdam, Netherlands, 2012; pp 27–41, ISBN 9780123813749.
- Ikem, A. Measurement of volatile organic compounds in bottled and tap waters by purge and trap GC–MS: Are drinking water types different? Journal of Food Composition and Analysis 2010, 23, 70–77. [CrossRef]
- Schulz, K.; Dressler, J.; Sohnius, E.-M.; Lachenmeier, D.W. Determination of volatile constituents in spirits using headspace trap technology. Journal of Chromatography A 2007, 1145, 204–209. [CrossRef]
- Manzini, S.; Durante, C.; Baschieri, C.; Cocchi, M.; Sighinolfi, S.; Totaro, S.; Marchetti, A. Optimization of a Dynamic Headspace-Thermal Desorption-Gas Chromatography/Mass Spectrometry procedure for the determination of furfurals in vinegars. Talanta 2011, 85, 863–869. [CrossRef]
- Jeleń, H.; Gracka, A.; Myśków, B. Static Headspace Extraction with Compounds Trapping for the Analysis of Volatile Lipid Oxidation Products. Food Anal. Methods 2017, 10, 2729–2734. [CrossRef]
- Soria, A.C.; García-Sarrió, M.J.; Ruiz-Matute, A.I.; Sanz, M.L. Headspace Techniques for Volatile Sampling. Green Extraction Techniques - Principles, Advances and Applications; Elsevier, 2017; pp 255–278, ISBN 9780128110829.
- Christmann, J.; Weber, M.; Rohn, S.; Weller, P. Nontargeted Volatile Metabolite Screening and Microbial Contamination Detection in Fermentation Processes by Headspace GC-IMS. Anal. Chem. 2024. [CrossRef]
- Sammarco, G.; Bardin, D.; Quaini, F.; Dall'Asta, C.; Christmann, J.; Weller, P.; Suman, M. A geographical origin assessment of Italian hazelnuts: Gas chromatography-ion mobility spectrometry coupled with multivariate statistical analysis and data fusion approach. Food Res. Int. 2023, 171, 113085. [CrossRef]
- Babis, J.S.; Sperline, R.P.; Knight, A.K.; Jones, D.A.; Gresham, C.A.; Denton, M.B. Performance evaluation of a miniature ion mobility spectrometer drift cell for application in hand-held explosives detection ion mobility spectrometers. Anal. Bioanal. Chem. 2009, 395, 411–419. [CrossRef]
- Capitain, C.C.; Nejati, F.; Zischka, M.; Berzak, M.; Junne, S.; Neubauer, P.; Weller, P. Volatilomics-Based Microbiome Evaluation of Fermented Dairy by Prototypic Headspace-Gas Chromatography-High-Temperature Ion Mobility Spectrometry (HS-GC-HTIMS) and Non-Negative Matrix Factorization (NNMF). Metabolites 2022, 12. [CrossRef]
- Gerhardt, N.; Schwolow, S.; Rohn, S.; Pérez-Cacho, P.R.; Galán-Soldevilla, H.; Arce, L.; Weller, P. Quality assessment of olive oils based on temperature-ramped HS-GC-IMS and sensory evaluation: Comparison of different processing approaches by LDA, kNN, and SVM. Food Chem. 2019, 278, 720–728. [CrossRef]
- Brendel, R.; Schwolow, S.; Rohn, S.; Weller, P. Gas-phase volatilomic approaches for quality control of brewing hops based on simultaneous GC-MS-IMS and machine learning. Anal. Bioanal. Chem. 2020, 412, 7085–7097. [CrossRef]
- Schanzmann, H.; Augustini, A.L.R.M.; Sanders, D.; Dahlheimer, M.; Wigger, M.; Zech, P.-M.; Sielemann, S. Differentiation of Monofloral Honey Using Volatile Organic Compounds by HS-GCxIMS. Molecules 2022, 27. [CrossRef]
- Lauricella, M.; Emanuele, S.; Calvaruso, G.; Giuliano, M.; D'Anneo, A. Multifaceted Health Benefits of Mangifera indica L. (Mango): The Inestimable Value of Orchards Recently Planted in Sicilian Rural Areas. Nutrients 2017, 9. [CrossRef]
- Tharanathan, R.N.; Yashoda, H.M.; Prabha, T.N. Mango (Mangifera indica L.), “The King of Fruits”—An Overview. Food Reviews International 2006, 22, 95–123. [CrossRef]
- Shimizu, K.; Matsukawa, T.; Kanematsu, R.; Itoh, K.; Kanzaki, S.; Shigeoka, S.; Kajiyama, S.'i. Volatile profiling of fruits of 17 mango cultivars by HS-SPME-GC/MS combined with principal component analysis. Biosci. Biotechnol. Biochem. 2021, 85, 1789–1797. [CrossRef]
- Tandel, J.; Tandel, Y.; Kapadia, C.; Singh, S.; Gandhi, K.; Datta, R.; Singh, S.; Yirgu, A. Nontargeted Metabolite Profiling of the Most Prominent Indian Mango (Mangifera indica L.) Cultivars Using Different Extraction Methods. ACS Omega 2023, 8, 40184–40205. [CrossRef]
- Musharraf, S.G.; Uddin, J.; Siddiqui, A.J.; Akram, M.I. Quantification of aroma constituents of mango sap from different Pakistan mango cultivars using gas chromatography triple quadrupole mass spectrometry. Food Chem. 2016, 196, 1355–1360. [CrossRef]
- Pino, J.A.; Mesa, J.; Muñoz, Y.; Martí, M.P.; Marbot, R. Volatile components from mango (Mangifera indica L.) cultivars. J. Agric. Food Chem. 2005, 53, 2213–2223. [CrossRef]
- Mahattanatawee, K.; Goodner, K.; Baldwin, E.A. Volatile constituents and character impact compounds of selected Florida's tropical fruit. Proc Fla State Hort Soc 2005, 118.
- Pandit, S.S.; Chidley, H.G.; Kulkarni, R.S.; Pujari, K.H.; Giri, A.P.; Gupta, V.S. Cultivar relationships in mango based on fruit volatile profiles. Food Chem. 2009, 114, 363–372. [CrossRef]
- Ziegler, H. Flavourings: Production, composition, applications, regulations, 2nd, completely rev ed.; Wiley: Chichester, Weinheim, 2007, ISBN 3527611452.
- Farag, M.A.; Dokalahy, E.U.; Eissa, T.F.; Kamal, I.M.; Zayed, A. Chemometrics-Based Aroma Discrimination of 14 Egyptian Mango Fruits of Different Cultivars and Origins, and Their Response to Probiotics Analyzed via SPME Coupled to GC-MS. ACS Omega 2022, 7, 2377–2390. [CrossRef]
- Indrati, N.; Sumpavapol, P.; Samakradhamrongthai, R.S.; Phonsatta, N.; Poungsombat, P.; Khoomrung, S.; Panya, A. Volatile and non-volatile compound profiles of commercial sweet pickled mango and its correlation with consumer preference. Int J of Food Sci Tech 2022, 57, 3760–3770. [CrossRef]
- Bonneau, A.; Boulanger, R.; Lebrun, M.; Maraval, I.; Gunata, Z. Aroma compounds in fresh and dried mango fruit (M angifera indica L . cv. K ent): impact of drying on volatile composition. Int J of Food Sci Tech 2016, 51, 789–800. [CrossRef]
- Li, L.; Yi, P.; Sun, J.; Tang, J.; Liu, G.; Bi, J.; Teng, J.; Hu, M.; Yuan, F.; He, X.; et al. Genome-wide transcriptome analysis uncovers gene networks regulating fruit quality and volatile compounds in mango cultivar 'Tainong' during postharvest. Food Res. Int. 2023, 165, 112531. [CrossRef]
- Xie, H.; Meng, L.; Guo, Y.; Xiao, H.; Jiang, L.; Zhang, Z.; Song, H.; Shi, X. Effects of Volatile Flavour Compound Variations on the Varying Aroma of Mangoes 'Tainong' and 'Hongyu' during Storage. Molecules 2023, 28. [CrossRef]
- Christmann, J.; Rohn, S.; Weller, P. gc-ims-tools – A new Python package for chemometric analysis of GC–IMS data. Food Chem. 2022, 224, 133476. [CrossRef]
- Parastar, H.; Christmann, J.; Weller, P. Automated 2D peak detection in gas chromatography-ion mobility spectrometry through persistent homology. Analytica Chimica Acta 2024, 1289, 342204. [CrossRef]
- Capitain, C.C.; Zischka, M.; Sirkeci, C.; Weller, P. Evaluation of IMS drift tube temperature on the peak shape of high boiling fragrance compounds towards allergen detection in complex cosmetic products and essential oils. Talanta 2023, 257, 124397.
- D'Orazio, G.; Fanali, C.; Asensio-Ramos, M.; Fanali, S. Chiral separations in food analysis. TrAC Trends in Analytical Chemistry 2017, 96, 151–171. [CrossRef]
- Christmann, J.; Rohn, S.; Weller, P. Finding features - variable extraction strategies for dimensionality reduction and marker compounds identification in GC-IMS data. Food Research International 2022, 161, 111779. [CrossRef]
- Kulkarni, R.; Chidley, H.; Deshpande, A.; Schmidt, A.; Pujari, K.; Giri, A.; Gershenzon, J.; Gupta, V. An oxidoreductase from 'Alphonso' mango catalyzing biosynthesis of furaneol and reduction of reactive carbonyls. Springerplus 2013, 2, 494. [CrossRef]
- Kallio, H.P. Historical Review on the Identification of Mesifurane, 2,5-Dimethyl-4-methoxy-3(2 H)-furanone, and Its Occurrence in Berries and Fruits. J. Agric. Food Chem. 2018, 66, 2553–2560. [CrossRef]
- Zhang, W.; Zhu, G.; Zhu, G. The imitation and creation of a mango flavor. Food Sci. Technol 2022, 42. [CrossRef]
- Surburg, H.; Panten, J. Common fragrance and flavor materials: Preparation, properties and uses, 6 completely revised and updated edition; Wiley-VCH: Weinheim, 2016, ISBN 978-3-527-69317-7.
- Burdock, G.A.; Fenaroli, G. Fenaroli's handbook of flavor ingredients, 6th ed.; CRC Press/Taylor & Francis Group: Boca Raton Fla., 2010, ISBN 9780429150838.

| Volatile compound | Retention time IMS [min] |
Retention time MS [min] | RT Difference MS-IMS [min] |
MS match quality [%] |
|---|---|---|---|---|
| ethyl acetate | 5.53 ± 0.02 | 5.50 ± 0.04 | 0.03 | 97 |
| α-pinene | 10.28 ± 0.01 | 10.23 ± 0.01 | 0.05 | 96 |
| ethyl butyrate | 10.70 ± 0.02 | 10.66 ± 0.02 | 0.04 | 95 |
| β-myrcene | 18.15 ± 0.02 | 18.12 ± 0.03 | 0.03 | 95 |
| D-limonene | 21.42 ± 0.01 | 21.38 ± 0.01 | 0.04 | 95 |
| trans-β-ocimene | 23.60 ± 0.03 | 23.56 ± 0.01 | 0.04 | 95 |
| acetic acid | 30.10 ± 0.04 | 30.05 ± 0.05 | 0.05 | 95 |
| nonanal | 34.11 ± 0.02 | 34.02 ± 0.01 | 0.07 | 95 |
| α-terpineol | 36.06 ± 0.03 | 36.01 ± 0.01 | 0.05 | 95 |
| β-caryophyllene | 38.51 ± 0.01 | 38.36 ± 0.01 | 0.15 | 96 |
|
Mango cultivar |
||||||
| Nr. | Volatile compound | Alphonso | Totapuri | Criollo | Tommy | Kent |
| 1 | ethanol | x | x | x | x | x |
| 2 | ethyl acetate | x | x | x | x | x |
| 3 | unidentified | IMS only | IMS only | IMS only | IMS only | IMS only |
| 4 | ethylcyclohexane | x | x | x | x. | x |
| 5 | ethyl propanoate | x | x (pulp only) |
x | x (pulp only) |
x |
| 6 | propyl acetate | x | x (pulp only) |
IMS only | n.d. | IMS only |
| 7 | methyl butyrate | IMS only | x (pulp only) |
x | x (pulp only) |
x |
| 8 | unidentified | IMS only | IMS only | IMS only | IMS only | IMS only |
| 9 | unidentified | IMS only | IMS only | IMS only | IMS only | IMS only |
| 10 | α-pinene | x | x | x | x | x |
| 11 | ethyl butyrate | x | x (pulp only) |
x | x | x |
| 12 | unidentified | IMS only | IMS only | IMS only | IMS only | IMS only |
| 13 | camphene | x | x | x | x | x |
| 14 | 2-butenoic acid, methyl ester, (z)- | IMS only | x (pulp only) |
x | x (pulp only) |
x |
| 15 | butyl acetate | IMS only | x | x | x | x |
| 16 | unidentified | IMS only | IMS only | IMS only | IMS only | IMS only |
| 17 | β-pinene | x | x | x | x | n.d. |
| 18 | 1-butanol | x | x (pulp only) |
x | x | x |
| 19 | isovaleraldehyd | x | x | x | x | x |
| 20 | isobutanol | x | x (pulp only) |
x | x | IMS only |
| 21 | isobutyraldehyde | x | x | x | x | x |
| unidentified | IMS only | n.d. | n.d. | n.d. | IMS only | |
| 22 | pentanal | x | x | x | x | x |
| 23 | isopentyl alcohol | x | x (pulp only) |
x | x | x |
| 24 | 3-carene | n.d. | n.d. | x | x | x |
| 25 | ethyl cyclopropancarboxylate | n.d. | x (pulp only) |
x | x | x |
| 26 | β-myrcene | x | x | x | x | x |
| 27 | α-phellandrene | IMS only | x | x | x | x |
| 28 | 2-butanone | x | x | x | x | x |
| 29 | α-terpinene | IMS only | x | x | x | x |
| 30 | isobutyl butyrate | IMS only | x (pulp only) |
n.d. | n.d. | n.d. |
| 31 | d-limonene | x | x | x | x | x |
| 32 | unidentified | IMS only | IMS only | IMS only | IMS only | IMS only |
| 33 | 2-butenoic acid, ethyl ester, (e) | n.d. | x (pulp only) |
x | x | x |
| 34 | β-phellandrene | n.d. | x | x | x | x |
| 35 | trans-β-ocimene | x | x | x | n.d. | n.d. |
| 36 | 4-carene | n.d. | n.d. | x | x | x |
| 37 | 2,3-butanedion | x | x (conc. only) |
x | x | x |
| 38 | cis- β-ocimene | x | x | x | n.d. | n.d. |
| 39 | butyl butyrate | IMS only | x (pulp only) |
x | x | x |
| 40 | α-terpinolene | n.d. | x | x | x | x |
| 41 | ethyl hexanoate | n.d. | n.d. | x | x | n.d. |
| 42 | 2-pronanone,1-methoxy | x | n.d. | x | n.d. | IMS only. |
| 43 | 2-methylbutyl butyrate | n.d. | x | x | n.d. | n.d. |
| 44 | isoamyl butyrate | x | x (pulp only) |
x | x | x |
| 45 | 3-penten-2-one | x | x | x | x | x |
| 46 | unidentified | IMS only | IMS only | IMS only | IMS only | IMS only |
| 47 | unidentified | IMS only | IMS only | IMS only | IMS only | IMS only |
| 48 | acetic acid | x | x | x | n.d. | x |
| 49 | alloocimene | x | x | x | n.d. | n.d. |
| 50 | ethyl-3-hydroxbutyrate | x | x | x | x | x |
| 51 | p-1,3,8-menthatriene | x | x | n.d. | n.d. | n.d. |
| 52 | neo-alloocimene | x | x | x | n.d. | n.d. |
| 53 | nonanal | x | x | x | x | x |
| 54 | furfural | x | x | x | x | x |
| 55 | ethyl octanoate | n.d. | x (pulp only) |
x | x | x |
| 56 | trans-sabinene hydrate | n.d. | x | n.d. | n.d. | n.d. |
| 57 | 2,5-dimethyl-4-methoxy-3(2h)-furanone | x | n.d. | n.d. | n.d. | n.d. |
| 58 | acetoin | n.d. | x | x | x | x |
| 59 | β-terpineol | n.d. | x | n.d | x | n.d. |
| 60 | unidentified | IMS only | IMS only | IMS only | IMS only | IMS only |
| 61 | α-terpineol | n.d. | x | n.d. | x | n.d. |
| 62 | α-copaene | n.d. | x | n.d. | x | n.d. |
| 63 | α-gurjujene | n.d. | x | x | x | x |
| 64 | unidentified | IMS only | IMS only | IMS only | IMS only | IMS only |
| 65 | β-caryophyllene | x | x | x | x | x |
| 66 | α-guaiene | n.d. | x | n.d. | n.d. | n.d. |
| 67 | unidentified | n.d. | IMS only | IMS only | IMS only | IMS only |
| 68 | ethyl decanoate | n.d. | n.d. | x | n.d. | n.d. |
| 69 | 1,4,7,-cycloundecatriene, 1,5,9,9- tetramethyl-, z,z,z |
x | x | x | x | x |
| 70 | 4,5-di-epi-aristolochene | n.d. | x | x | x | n.d |
| 71 | γ-gurjujene | n.d. | x | x | n.d. | n.d |
| eremophilene | n.d. | n.d. | QMS only | n.d. | n.d | |
| 72 | β-selinene | n.d. | x | x | x (pulp only) |
n.d. |
| 73 | α-selinene | n.d. | x | x | x (pulp only) |
n.d. |
| 74 | α-bulnesene | n.d. | x | x | n.d. | n.d. |
| 75 | δ-cadinene | n.d. | x | x | n.d. | n.d. |
| 76 | β-cadinene | n.d. | QMS only | n.d. | n.d. | n.d. |
| 77 | γ-butyrolactone | x | n.d. | x | n.d. | n.d. |
| 78 | γ-hexalactone | x | n.d. | x | n.d. | IMS only |
| 79 | cis-calamenene | n.d. | n.d. | QMS only | n.d. | n.d. |
| 80 | γ-octalactone | x | n.d. | x | n.d. | n.d. |
| 81 | ethyl dodecanoate | x | n.d. | x | n.d. | n.d. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





