1. Introduction
Thymic epithelial tumors are the most prevalent type of anterior mediastinal masses in adults and are a rare form of thoracic tumor, with an incidence rate of 0.23–0.30 per 100,000 [
1,
2]. Thymectomy is the standard treatment for these tumors [
1,
3]. decades, median sternotomy has been the gold-standard approach for thymectomy, providing excellent exposure of the thymic tissue; however, it is associated with high postoperative morbidity. Minimally invasive thymectomy, such as thymectomy via video-assisted thoracic surgery (VATS) and robotic-assisted thoracic surgery (RATS), has recently become a popular alternative procedure. Three recent meta-analyses have showed that minimally invasive thymectomy achieves better cosmetic outcomes, lower postoperative complication rates, lower blood loss, and shorter hospital stays, with comparable oncologic outcomes to those of open thymectomy [
4,
5,
6].
Robotic surgery provides many advantages, including improved three-dimensional imaging, hand-eye coordination, freedom of movement, and tremor filtration [
7]. Therefore, RATS thymectomy may enable meticulous dissection and safe handling of thymic tissue and vessels [
8,
9]. Since its first report in 2001 [
10], RATS thymectomy has gradually increased in popularity, with the lateral transthoracic approach initially serving as the standard approach for this technique.
Subxiphoid RATS thymectomy is emerging as an alternative approach with several advantages, including easier specimen removal, reduced postoperative pain, and a better surgical view of the upper thymic pole and bilateral phrenic nerves [
11,
12]. The procedure was initially performed using a subxiphoid port with two bilateral transthoracic ports [
13]. Many surgeons have attempted to reduce the number of ports in subxiphoid RATS thymectomy to achieve a more minimally invasive surgery. We also initially attempted two-port subxiphoid RATS thymectomy using the da Vinci Xi robotic surgical system (Xi) (Intuitive Surgical Inc., Sunnyvale, CA, USA) with a subxiphoid port and an additional unilateral transthoracic port, depending on the tumor location [
14]. As robotic technology developed, we attempted to perform a subxiphoid single-port RATS(SRATS) thymectomy using the da Vinci Single-Site
TM platform (DVSSP) (Intuitive Surgical Inc.)[
15]. However, DVSSP has several limitations, including the lack of articulating instruments and collisions between instruments.
The da Vinci SP robotic surgical system (SPS) (Intuitive Surgical Inc.) has recently been developed for single-port (SP) surgery. This system was first approved for general thoracic surgery in South Korea by the Korean Food and Drug Administration in 2020. The use of the SPS in general thoracic surgery may provide improved cosmetic results, reduced postoperative pain, and early recovery. Our previous reports have demonstrated its feasibility in general thoracic surgery, including mediastinal mass excision and thymectomy [
16,
17]. We have also gained experience in performing subxiphoid single-port VATS (SVATS) thymectomy for a decade. Although we believe that subxiphoid single-port RATS (SRATS) thymectomy has potential benefits over subxiphoid SVATS thymectomy, no study has compared the two procedures. Therefore, this study examined multi-institutional experience with subxiphoid SRATS thymectomy using the SPS and compared perioperative outcomes of this technique with those of subxiphoid SVATS thymectomy.
2. Materials & Methods
2.1. Study Design and Patient Selection
We examined the data of patients who underwent subxiphoid SRATS and SVATS thymectomy performed by three thoracic surgeons (JHC, JWH, and HKK) at three institutions in South Korea between September 2018 and March 2024. All patients in the SRATS group underwent subxiphoid SRATS thymectomy using SPS, and all patients in the SVATS group underwent subxiphoid SVATS thymectomy. The procedure selection depended on tumor characteristics, patient preference, and surgeon preference.
The indications and contraindications for both procedures were similar to those applied with conventional minimally invasive thymectomy. Height and obesity were not considered contraindications. However, creating a subxiphoid tunnel in patients with obesity may require additional time, potentially prolonging the total operative time. Furthermore, from an anatomical perspective, an elongated xiphoid process, inwardly curved xiphoid process, or pectus excavatum may constitute challenges for the subxiphoid approach. Tumor invasion of the great vessels was an absolute contraindication for SRATS thymectomy. Relative contraindications included a history of radiotherapy, tumor size of >8 cm, and tumors invading other organs or structures [
12,
15,
17].
Preoperative clinical variables, including age, sex, comorbidities, American Society of Anesthesiologists score, body mass index, neurological examination, blood tests, and chest computed tomography (CT) findings, were collected. Chest magnetic resonance imaging and fluorodeoxyglucose-positron emission tomography/CT were preferred in specific clinical situations, such as identifying anatomical structures, assessing surgery feasibility, and distinguishing thymic malignancy from thymic cyst and hyperplasia. However, CT-guided percutaneous biopsy was not usually performed in such cases. Preoperative neurological assessments with diagnosis of myasthenia gravis (MG) were conducted by a neurologist.
Intraoperative outcomes (including total operative time, docking time, estimated blood loss, and any intraoperative complications) and postoperative outcomes (such as postoperative pain, chest tube duration, postoperative hospital stay, pathological data, and postoperative complications) were recorded. Extended thymectomy was defined as the complete resection of the whole thymus with the surrounding adipose tissue. The pathological results were classified according to the World Health Organization classification with the tumor–node–metastasis staging system. Postoperative complications were categorized according to the Clavien–Dindo classification[
18].
2.2. Creation of The Subxiphoid Tunnel
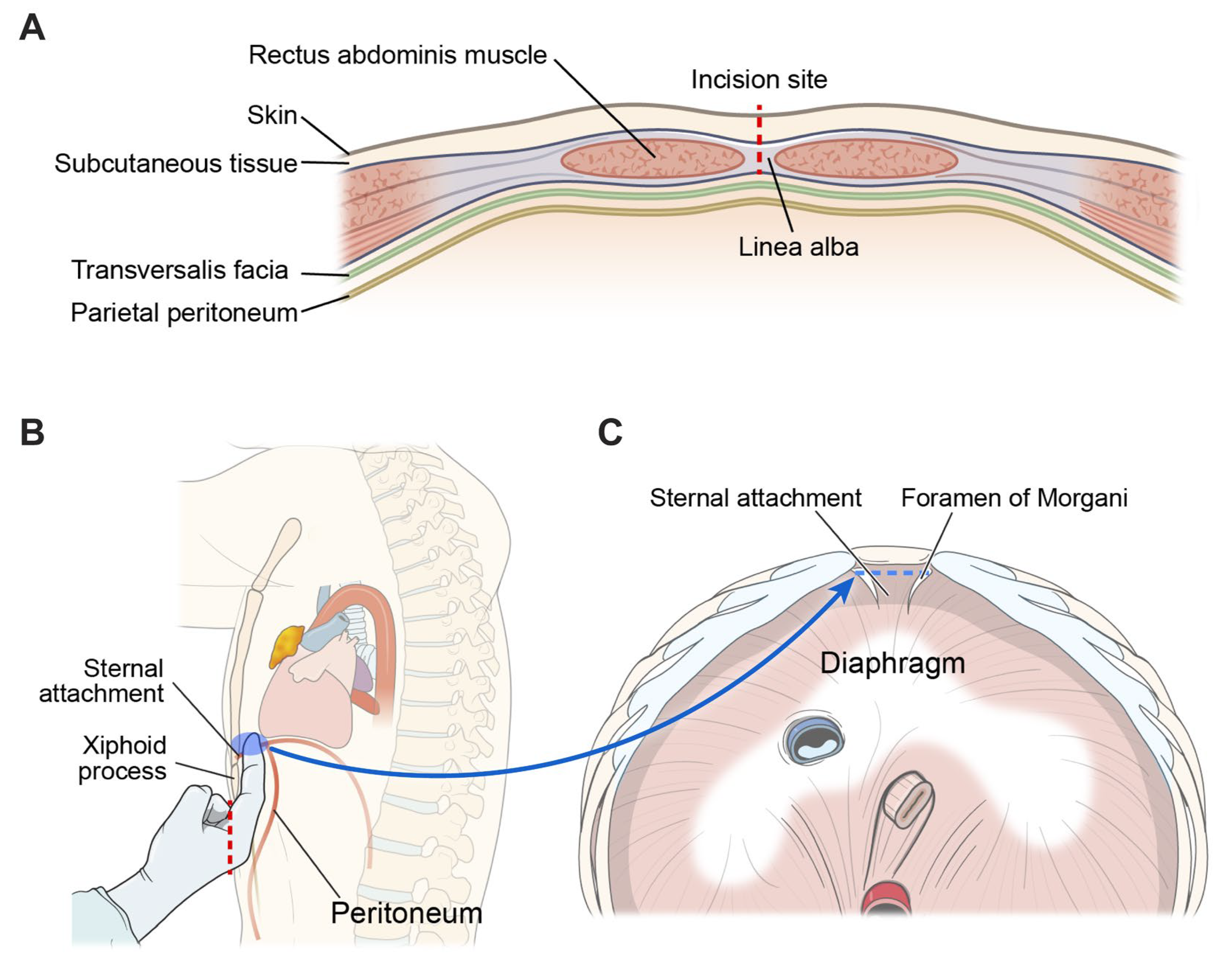
The patient was placed in the open-legged supine position under general anesthesia. Two-lung ventilation with a low tidal volume was the routine ventilation strategy, whereas one-lung ventilation was used for patients with tumors invading adjacent structures. A vertical incision measuring 2.5–4 cm was made 1–2 cm below the xiphoid process without its removal. The skin and subcutaneous fat and linea alba were carefully dissected to avoid damaging the peritoneum (
Figure 1a). The preperitoneal space and sternal attachment of diaphragm were bluntly dissected using a finger (
Figure 1b and 1c). A multi-channel port, such as the Lapsingle Vision (Sejong Medical, Gyeonggi, South Korea) or SP Access Port (Intuitive Surgical Inc.), was installed through a subxiphoid incision. Carbon dioxide (CO
2) gas was insufflated through a multi-channel port at a pressure of 6–10 mmHg.
2.3. Surgical Technique: Subxiphoid SRATS Thymectomy
In the early stages, the pericardial fat and tissue in the lower mediastinal area were dissected using VATS due to technical limitations of the SPS, requiring a minimum distance of 10 cm between the cannula and the target lesion. Details of the robotic-VATS hybrid approach were described in our previous study [
15,
16]. However, we currently perform pure robotic surgery, after utilizing the SP Access Port with the floating dock technique [
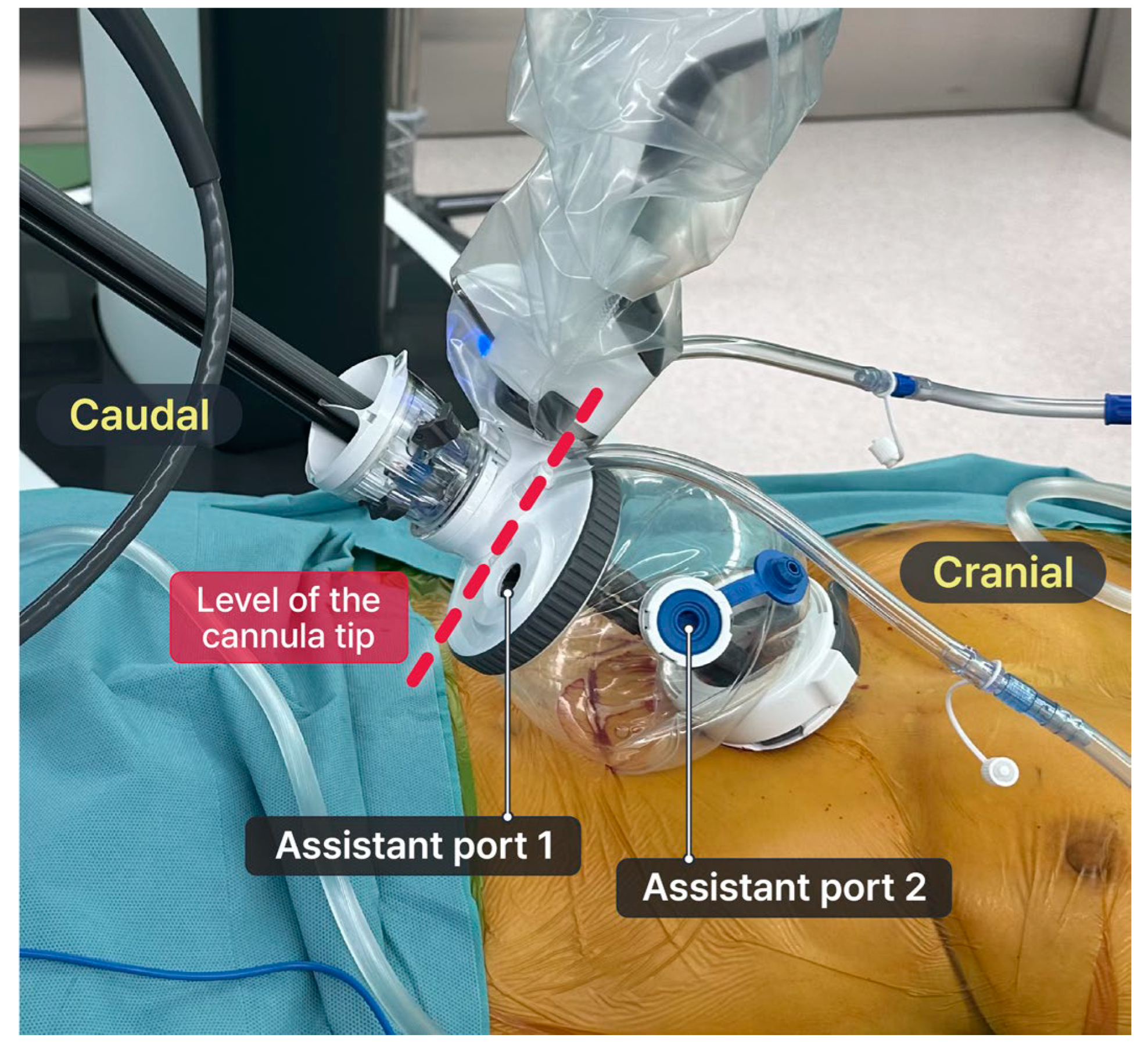
17]. The floating dock technique was necessary for pure robotic surgery in shallow spaces, specifically opening the mediastinum during thymectomy. The cannula tip was docked onto the tip of the multi-channel port and floated above the incision [
17,
19]. The port of entry floats above the incision, enabling the achievement of sufficient space from the canula tip to the target lesion and thereby allowing for the best range of motion within a confined space (
Figure 2). The bedside assistant can insert their instrument including a suction device and an endoscopic stapler Signia™ stapling system (Medtronic, Minneapolis, MN, USA) through the assistant port in the SP access port without the need for an additional port.
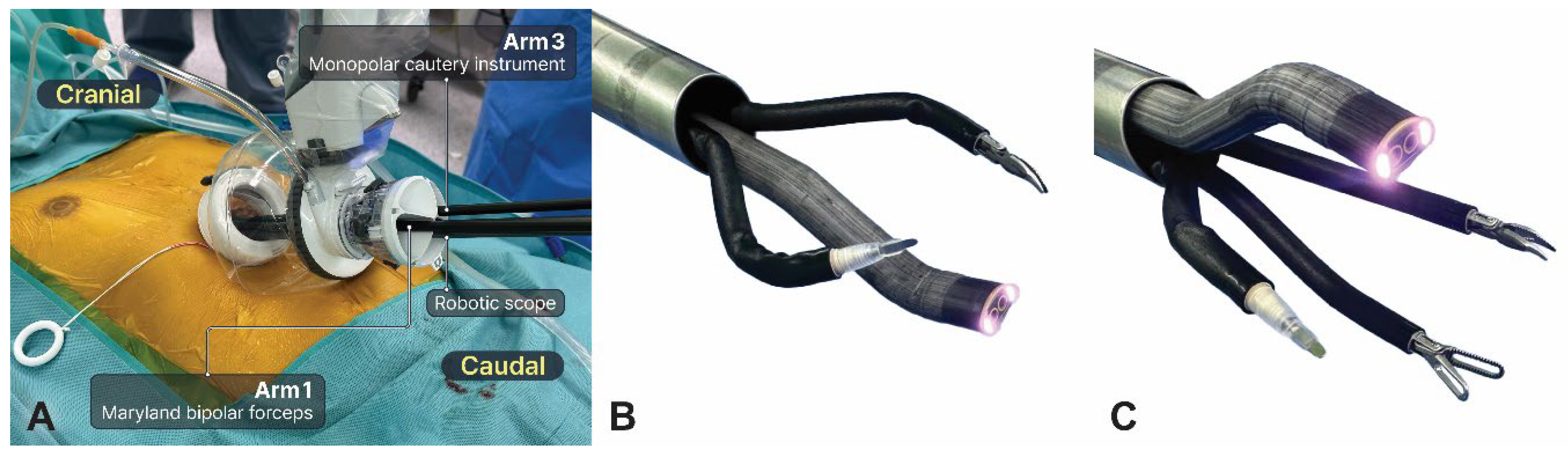
After positioning the da Vinci SP patient cart on the right side, a robotic scope was introduced into the lower middle hole (arm 2) to obtain the above view (
Figure 3a). Dissection was usually performed using Cadiere forceps, Maryland bipolar forceps, and monopolar cautery instruments. The procedure initially involved the use of two arms, with Maryland bipolar forceps and a monopolar cautery instrument placed at the left and right holes, respectively (
Figure 3b). The bilateral mediastinal pleura was opened after the connective tissue beneath the xiphoid process was dissected. Next, the robotic scope was repositioned at the upper middle hole to achieve the below view, whereas the Cadiere forceps were added at the lower middle hole (arm 2) (
Figure 3c). The surgical procedure is similar to that of the conventional subxiphoid RATS thymectomy. Thymus dissection started cranially from the diaphragm to the lower cervical region. The adipose tissue at the bilateral cardiophrenic angles was completely removed. After identifying the phrenic nerve, the first landmark for the lateral margin of dissection, the entire thymus gland was dissected cranially until the innominate vein was identified as the second landmark. Care was taken to avoid damage to the phrenic nerve and innominate vein. Several thymic vessels were ligated using the robotic Hem-o-Lok. Transferring the tumor to the contralateral pleural cavity can improve the exposure of the remaining thymus, particularly for large tumors. The upper pole of the thymus was carefully dissected, and the right upper pole was pulled downward and dissected freely, followed by dissection of the left upper pole. After the upper poles of the thymus and surrounding fatty tissue were removed, the resected specimen was extracted through the same incision using a retrieval bag. Finally, two chest drains (Evacuator Barovac, 100 mL, Sewoon Medical Co., Korea) were inserted into the bilateral pleural cavity through a subxiphoid incision once sufficient hemostasis was achieved. A video of the surgical procedure is provided (Video S1).
In emergency situations, such as intraoperative bleeding, conversion to sternotomy is needed. Preoperative skin preparation and draping, including that of the sternum, are essential for rapid conversion to median sternotomy. A vertical incision for subxiphoid tunnel facilitates conversion to sternotomy.
Stapling can be performed using an endoscopic stapler through an assistant port in the SP access port. If stapling is difficult or the surgical view is insufficient, an additional port is created. An additional 12-mm port can be placed in the 5th–7th intercostal space in the midclavicular line according to the target lesion if necessary.
2.4. Surgical Technique: Subxiphoid SVATS Thymectomy
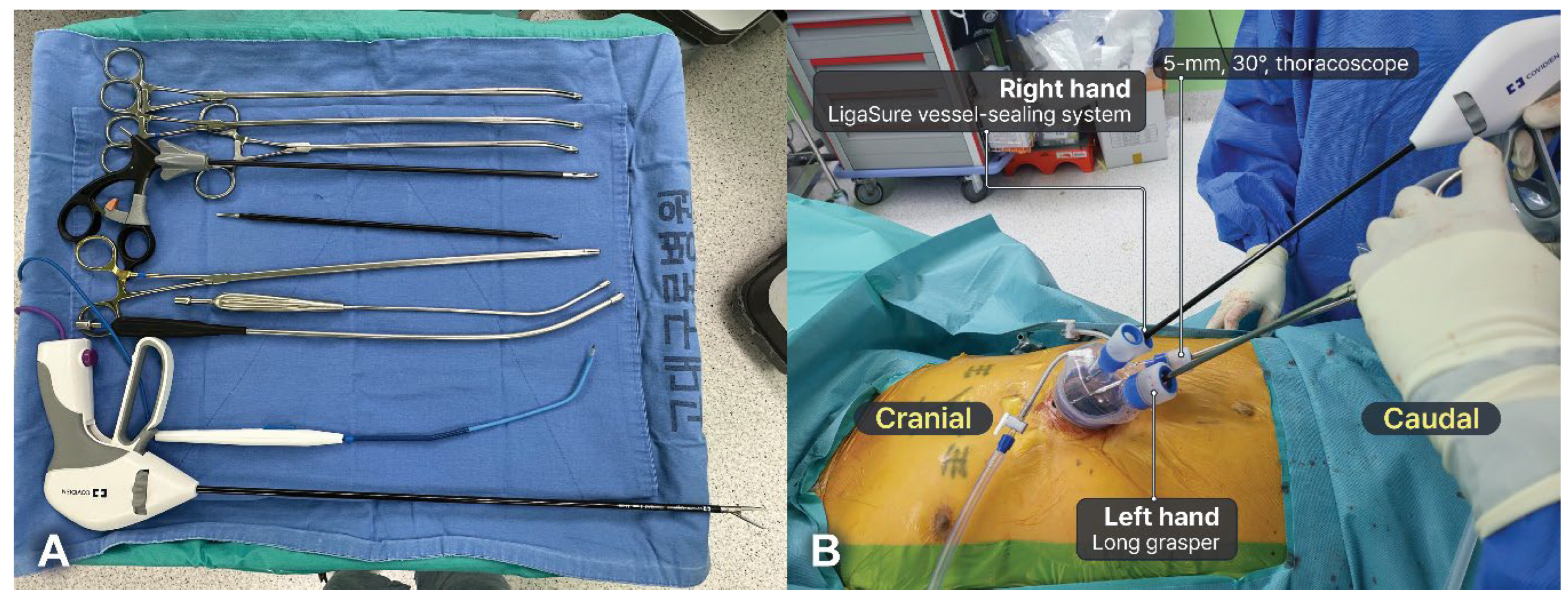
The surgeon stood between the patient’s legs or on their right side, depending on preference, whereas the assistants stood on the left side. A multi-channel port (Lapsingle Vision) was commonly used, and a 5-mm, 30° scope was typically used to minimize collisions between the scope and instruments. Conventional thoracoscopic instruments designed for SVATS were also used, and long-curved thoracoscopic instruments further reduced collisions between instruments (Figure 5a). A 5-mm camera scope was inserted through the most inferior part of the multi-channel port entrance, with the thoracoscopic instruments subsequently introduced through another multi-channel port entrance. Surgeons use thoracoscopic grasping forceps and a long bipolar energy device (44 cm, LigaSure, Covidien, Boulder, CO, USA) in their left and right hands, respectively (Figure 5b). A bipolar energy device was used for dissection, exposure, sealing, and cutting. Bipolar energy devices cause less thermal injury to the surrounding tissues and nerves, enabling safe dissection along the phrenic nerve and vessels[
20]. The position and type of the instrument can be adjusted, as necessary. In some cases, crossing hands to reduce interference between instruments may be necessary. The steps performed in SVATS were similar to those in SRATS.
Figure 4.
Subxiphoid single-port video-assisted thoracic surgery thymectomy. (a) Instruments for subxiphoid single-port video-assisted thoracic surgery thymectomy. (b) A total of four devices can be inserted through a multi-channel port. Surgeons commonly use a long-curved grasper in their left hand and a long bipolar energy device in their right hand. However, in some cases, the surgeon can cross the hands.
Figure 4.
Subxiphoid single-port video-assisted thoracic surgery thymectomy. (a) Instruments for subxiphoid single-port video-assisted thoracic surgery thymectomy. (b) A total of four devices can be inserted through a multi-channel port. Surgeons commonly use a long-curved grasper in their left hand and a long bipolar energy device in their right hand. However, in some cases, the surgeon can cross the hands.
2.5. Conversion to Sternotomy or Multi-Port Surgery
In emergency situations, such as intraoperative bleeding, conversion to sternotomy is needed. Preoperative skin preparation and draping, including that of the sternum, are essential for rapid conversion to median sternotomy. A vertical incision for subxiphoid tunnel facilitates conversion to sternotomy.
Stapling can be performed using an endoscopic stapler through an assistant port in the SP access port. If stapling is difficult or the surgical view is insufficient, an additional port is created. An additional 12-mm port can be placed in the 5th–7th intercostal space in the midclavicular line according to the target lesion if necessary.
2.6. Postoperative Management
Unless contraindicated, all patients received intravenous patient-controlled analgesia, along with oral analgesics including acetaminophen/tramadol combination tablets and nonsteroidal anti-inflammatory drugs. Pain scores were assessed daily every 8 h during hospitalization using the visual analog scale (range, 0–10). Drains were removed if the drain output was less than three times the patient’s body weight, and if no pleural effusion was observed on lateral decubitus chest radiography. Discharge occurred the day after drain removal.
2.7. Statistical Analyses
All statistical analyses were performed using IBM SPSS Statistics for Windows, version 27 (IBM Corp., Armonk, NY, USA). Categorical variables were compared using the chi-square or Fisher’s exact tests, and continuous variables were compared using Student’s t-test or Mann–Whitney U test. Categoical variables were expressed as counts (percentages), and continuous variables were presented as mean ± standard deviation. A two-sided p-value <0.05 was considered statistically significant.
2.8. Ethical Statement
This study was approved by the Institutional Review Board of the three institutions involved: Korea University Guro Hospital, Korea University Anam Hospital, and Korea University Ansan Hospital. The requirement to obtain informed consent was waived owing to the retrospective study design and given its minimal-risk nature, in accordance with institutional guidelines. This study was conducted in accordance with the principles of the Declaration of Helsinki (revised in 2013).
3. Results
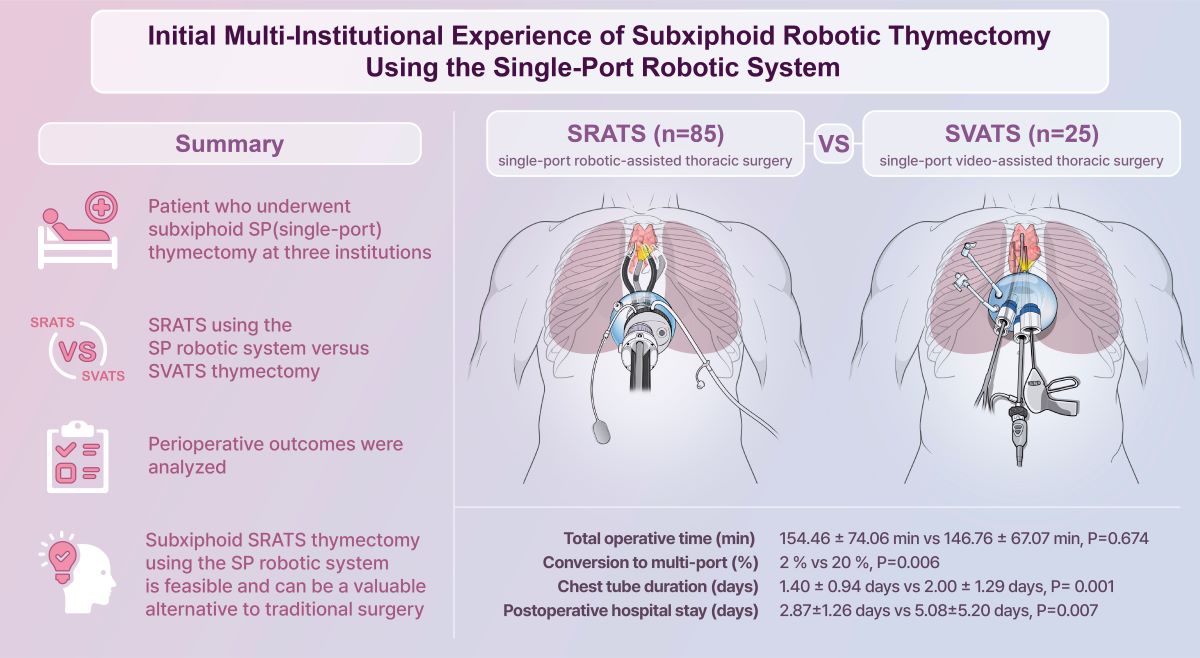
This study included 110 patients, of which 85 and 25 patients underwent SRATS and SVATS, respectively.
Table 1 summarizes patient characteristics in the two groups. The mean mass size was 3.29±1.61 and 3.84±2.01 cm in the SRATS and SVATS groups, respectively (P=0.216). No significant between-group differences were found in patient characteristics (P>0.05).
Table 2 shows the intraoperative outcomes of the two groups. All patients underwent successful R0 resection. There were no significant differences in the mean total operative time (154.46±74.06 vs. 146.76±67.07 min, P=0.674). Resection of adjacent structures was performed in four (4%) patients in the SRATS group and four (16%) patients in the SVATS group. In the SRATS group, two patients required innominate vein resection using an endoscopic stapler, with one requiring conversion to multi-port surgery (requiring an additional port). Additionally, one patient underwent pericardial resection and reconstruction without conversion (Video S2). Two patients in the SVATS group required resection of the invaded lung using an endoscopic stapler, and one of them required conversion to multi-port surgery. One patient underwent phrenic nerve resection and innominate vein repair with conversion to multi-port surgery. One patient underwent pericardial resection and reconstruction with conversion to median sternotomy. No cases of conversion to sternotomy occurred in the SRATS group. However, one patient required conversion to sternotomy in the SVATS group. Notably, a significantly lower conversion rate to multi-port surgery was observed in the SRATS group (
Table S1).
Table 3 summarizes postoperative outcomes and pathology results of the two groups. Notably, the chest tube drainage duration was significantly shorter in the SRATS group than in the SVATS group (1.40±0.94 vs. 2.00±1.29 days, P=0.001). Additionally, the postoperative hospital stay was significantly shorter in the SRATS group than in the SVATS group (2.87±1.26 vs. 5.08±5.20 days, P=0.007). No significant differences were found in peak pain score, pain score on POD 0–2, and postoperative complications rate between groups (P>0.05). Pathology results in the SRATS group included thymoma (n=36, 42%), thymic carcinoma (n=7, 8%), benign cystic lesions (n=32, 38%), and others (n=10, 12%). In the SVATS group, pathological outcomes were thymoma (n=10, 40%), thymic carcinoma (n=1, 4%), benign cystic lesions (n=10, 40%), and others (n=4, 16%) (P=0.882).
4. Discussion
We investigated the experience of three Korea institutions of subxiphoid SRATS thymectomy using the SPS and compared this technique with subxiphoid SVATS thymectomy. Subxiphoid SRATS thymectomy using the SPS is not widely performed. This study aimed to establish the feasibility and benefits of subxiphoid SRATS thymectomy using the SPS. To the best of our knowledge, this study is the first to compare perioperative outcomes of subxiphoid SRATS using the SPS with those of subxiphoid SVATS thymectomy and is the largest study to report on this novel technique. We demonstrated favorable outcomes in terms of the conversion rate to multi-port, chest tube drainage duration, and postoperative hospital stay. No significant difference in operative time between the SRATS and SVATS groups was found, although the total operative time was generally longer because an extended thymectomy was performed in almost all patients.
The SRATS group had a significantly lower conversion rate to multi-port surgery than the SVATS group, and there were no cases of conversion to median sternotomy within the SRATS group. Previous studies have reported that minimally invasive thymectomy had a lower conversion rate to median sternotomy, and our conversion rate was comparable to that reported in the literature (range, 0-14%) [
9,
21,
22,
23,
24,
25]. A potential reason for these positive outcomes is the improved accuracy of the procedure. Specifically, the enhanced 3-D surgical view of the robotic system using the articulating robot scope and precise movements of the robotic instruments are useful for upper thymic pole dissection or identification of the phrenic nerve.
Our study also demonstrated that subxiphoid SRATS thymectomy using the SPS is associated with a shorter chest tube drainage duration and postoperative hospital stay. There are several possible reasons for these findings. First, the articulating robotic arm and robot scope provide improved surgical view, allowing meticulous dissection. This facilitates delicate and complex procedures, allowing the complete removal of thymic tissue and the surrounding adipose tissue with better hemostasis and reduced damage to surrounding tissues. Therefore, chest drainage is reduced with shorter chest tube duration and postoperative hospital stay. Second, there may have been differences in the management protocols for these procedures as SRATS was introduced in the participating institutions in November 2020 while SVATS was introduced in September 2018.
The optimal approach for thymectomy remains controversial. The lateral transthoracic approach, such as the right, left, or bilateral approach, is the most commonly used approach for minimally invasive thymectomy. However, lateral transthoracic approach has limitations, such as the risk of intercostal nerve damage and difficulty in identifying the phrenic nerve and upper thymic pole. The unilateral transthoracic approach may not provide sufficient exposure of the contralateral phrenic nerve and pleural cavity. Therefore, the bilateral transthoracic approach should be considered for patients with MG to ensure complete removal of all thymic tissues. However, this approach may result in increased total operative time, postoperative pain, and number of ports, as well as poor cosmetic outcomes [
26].
In contrast, the subxiphoid approach for thymectomy provides an improved view of the bilateral phrenic nerves and the upper thymic pole above the innominate vein while minimizing postoperative pain. Chronic pain may be prevented using a subxiphoid incision because only cutaneous branches of intercostal nerves are present in this area. The subxiphoid approach can be applied in various fields of thoracic surgery, such as bullectomy, lung biopsy, bilateral metastasectomy, and major pulmonary resection [
27,
28,
29,
30]. Recently, three meta-analyses showed that subxiphoid VATS thymectomy is associated with less blood loss and postoperative pain, shorter chest tube duration, and shorter hospital stays than transthoracic VATS thymectomy [
31,
32,
33]. The subxiphoid approach for thymectomy was initially attempted using a subxiphoid port with two transthoracic or two subcostal ports. In particular, the combination of a subxiphoid port with two transthoracic ports may cause intercostal nerve damage due to port placement in the intercostal space. Although combining subxiphoid and subcostal ports can minimize intercostal nerve damage, it may be technically challenging when making subcostal ports.
SP surgery in the field of general thoracic surgery has potential benefits, including reduced postoperative pain, fewer postoperative complications, faster recovery, and improved cosmetic outcomes [
34,
35,
36]. Many surgeons who prefer SVATS have attempted subxiphoid SVATS thymectomy since this technique was first reported in 2012[
37]. Subxiphoid SVATS thymectomy is feasible but technically demanding, particularly for dissecting the upper thymic pole and cardiac-diaphragmatic angle. Inexperienced surgeons may experience poor surgical manipulability in SVATS due to collisions between thoracoscopic instruments and the scope. Therefore, surgeons must maintain an unnatural posture while standing beside the operating table. These limitations worsen surgeon ergonomics during the procedure.
Compared with subxiphoid SVATS thymectomy, subxiphoid SRATS thymectomy using the SPS improved surgeon ergonomics, surgical manipulability, and surgical view, particularly for dissecting the upper thymic pole. The SPS has several new features compared with the previous robotic system, featuring an articulating robot scope and three wristed arms that can be inserted through a single incision, allowing free movement in confined spaces, whereas the DVSSP includes only two non-flexible arms [
15]. The robot scope can also be adjusted to the cobra mode, enabling it to move above or below the instruments to reduce collisions and improve the surgical view. Furthermore, the features of the SPS provide several advantages over DVSSP and VATS, including more complex movements, meticulous dissection, and fewer collisions. Therefore, the SPS is more suitable for subxiphoid thymectomy. In particular, the articulating instrument of the SPS enables a more secure and comfortable dissection around the upper mediastinum, innominate vein, and upper thymic pole in patients with a tall stature or a long chest than VATS. Furthermore, the experience of the scopist, rather than solely that of the surgeon, within confined spaces where instrument collisions are common can be crucial to ensure clear visibility and safe dissection during SVATS, thereby introducing a certain degree of dependency. However, SRATS using the SPS enables surgeons to control instruments independently, including the articulating scope, thereby minimizing collisions and reducing dependency on an assistant.
The SPS has some technical limitations. First, this system does not include a robotic stapler or vessel-sealing device. Small vessels are ligated using robotic Hem-o-Lok, which can increase the total operative time. Although large vessels can be excised using an endoscopic stapler, this can be technically challenging and potentially time-consuming. Therefore, development of more dedicated and advanced robotic instruments for the SPS are required. Second, this system requires a minimum distance of 10 cm between the cannula and the target lesion. The floating dock technique is essential for pure robotic surgery and requires CO2 insufflation. Notably, CO2 insufflation provides an enlarged retrosternal space without a sternal retractor. Elevating the sternum may lead to injury. One-lung ventilation is not usually performed because CO2 insufflation displaces the lungs and pericardium downward. However, caution should be exercised when using CO2 insufflation in patients with compromised cardiac output because it can limit venous return and cardiac output. In such cases, reducing CO2 insufflation pressure or intermittent CO2 insufflation can be safely performed.
Contraindications for subxiphoid SRATS thymectomy using the SPS are unknown. Previously, contraindications for robotic thymectomy included large tumors (>8 cm) and tumors that invaded the pericardium or great vessels. However, some experienced robotic surgeons have attempted robotic thymectomy in complex cases, such as resecting and reconstructing the pericardium or vessels. Pericardial reconstruction using a Gore-Tex membrane can be safely performed using the SPS. Large tumors and those invading the lungs and innominate vein are not absolute contraindications for subxiphoid SRATS thymectomy using the SPS. However, tumor invasion of the great vessels is an absolute contraindication for SRATS thymectomy using the SPS. Nonetheless, advances in robotic technology may enable more complex thymectomy procedures in the future.
To date, the SPS has not been widely used in general thoracic surgery, and there is a paucity of clinical data on subxiphoid SRATS thymectomy using the SPS [
16,
17]. However, we believe that the true value of the SPS lies in its potential to be ideal for thymectomy in the field of thoracic surgery. Additionally, we believe that subxiphoid SRATS thymectomy using the SPS provides potential benefits by combining the advantages of robotic surgery, SP surgery, and the subxiphoid approach. Applying a new technique always involves challenges, and our study may contribute to the widespread adoption of the SPS in the field of general thoracic surgery. With advances in SPS technologies, including the dedicated robotic stapler and vessel-sealing device, we anticipate that this system will gain popularity for thymectomy.
This study has some limitations. First, this was a non-randomized, retrospective study with a small sample size. Second, indications for subxiphoid SRATS using the SPS may have differed among the three surgeons, and selection bias could not be excluded. Third, our study only included short-term outcomes; however, long-term follow-up data, including oncological results, are needed because of the indolent nature of the thymic epithelial tumor. Fourth, the control group did not include those who underwent RATS thymectomy using the conventional Xi robotic system or thymectomy via a transthoracic approach. Finally, SVATS thymectomy was initiated several years before the start of SRATS thymectomy, potentially introducing some bias.
Author Contributions
Conceptualization, J.H.L., J.H., J.H.C. and H.K.K.; methodology, J.H.L., S.Y.H. and H.K.K.; software, J.H.L. and H.K.K.; validation, J.H.L. and H.K.K.; formal analysis, J.H.L., E.Y. and H.K.K.; investigation, J.H.L. and S.L.; resources, J.H.L. and H.K.K.; data curation, J.H.L., J.H., Y.J., T.H.P., B.M.G. and J.H.C.; writing—original draft preparation, J.H.L., J.H., Y.J., J.H.C. and H.K.K.; writing—review and editing, J.H.L., J.H., Y.J., J.H.C. and H.K.K.; visualization, J.H.L., T.H.P. and B.M.G.; supervision, J.H.C. and H.K.K.; project administration, H.K.K.; funding acquisition, H.K.K. All authors have read and agreed to the published version of the manuscript.