Submitted:
11 July 2024
Posted:
12 July 2024
You are already at the latest version
Abstract
Keywords:
1. Introduction
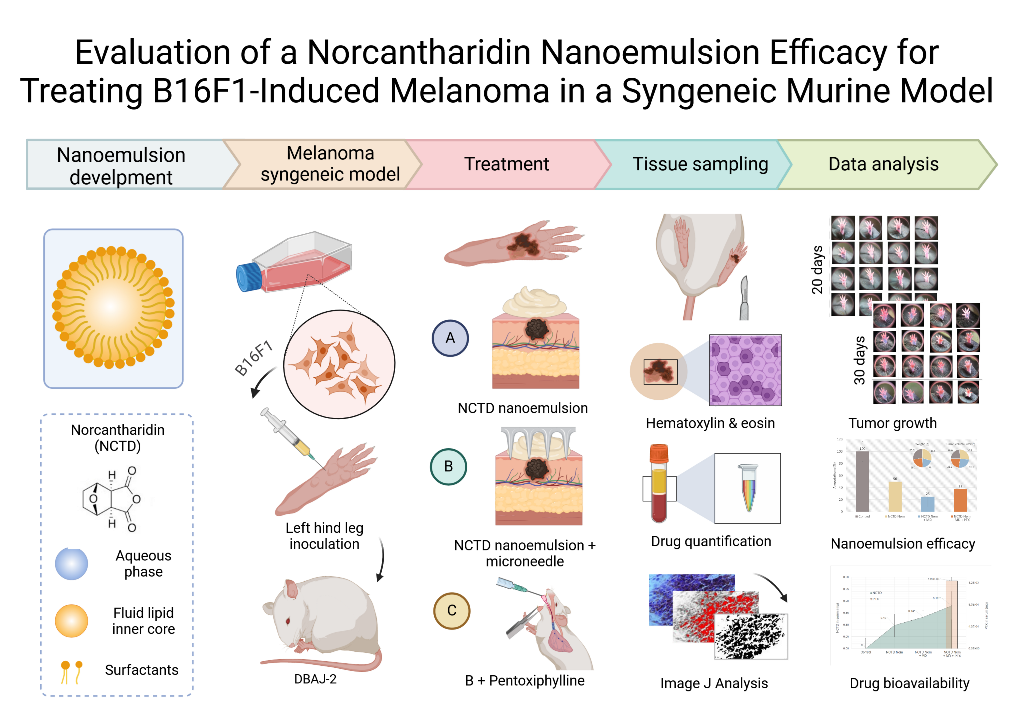
2. Results
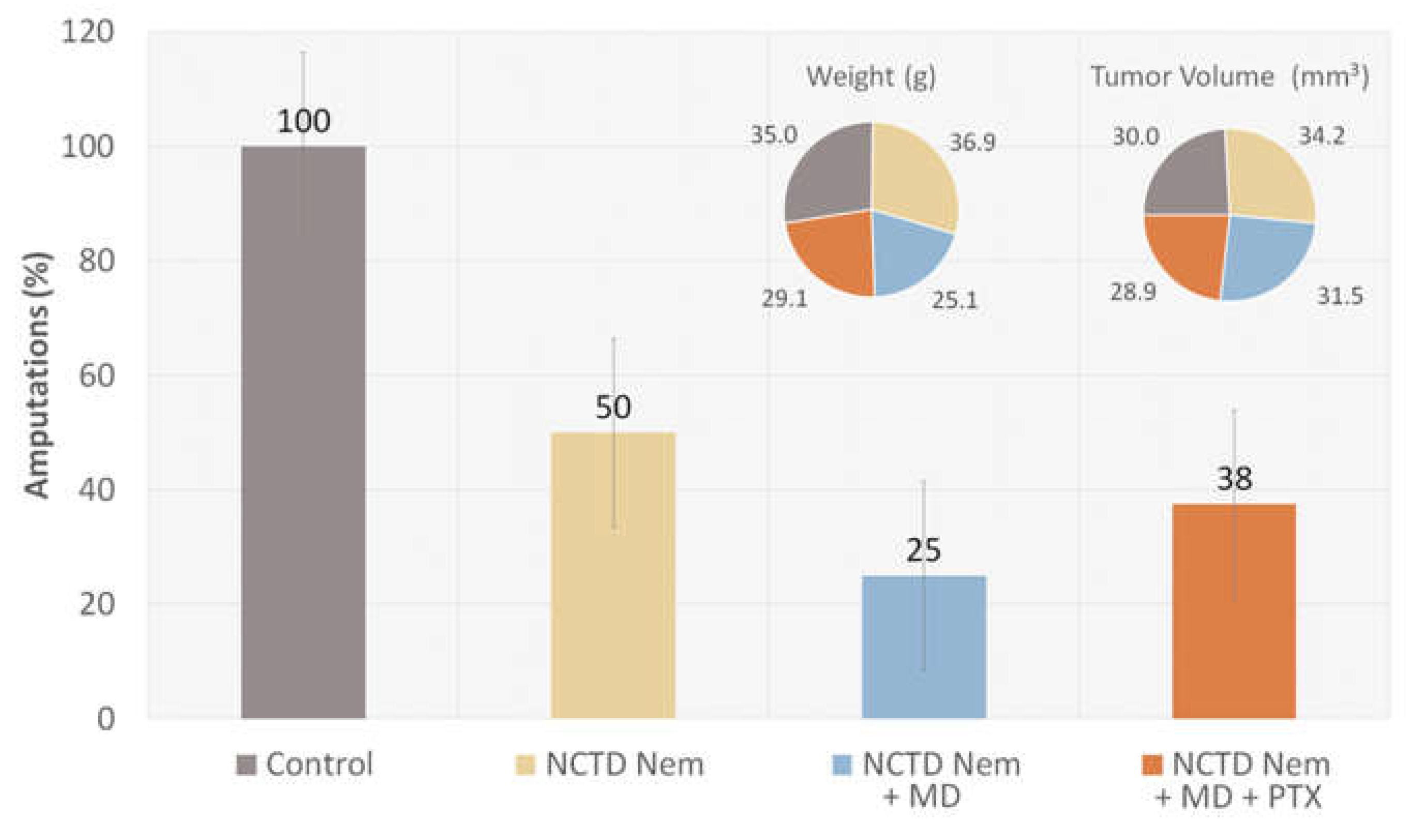
2.1. Tumor Induction and Application of treatments
2.2. Tumor Progression
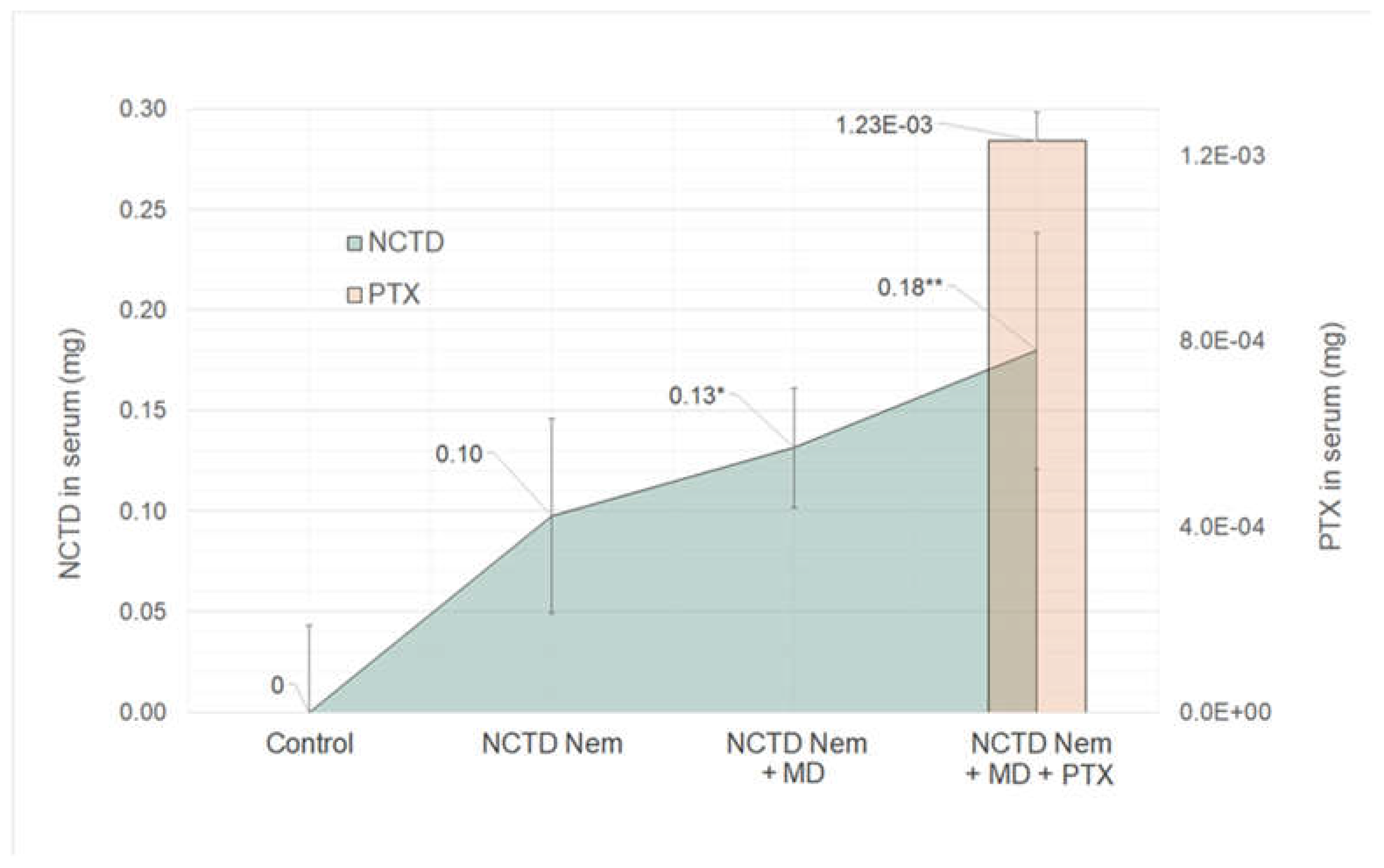
2.3. Drug Content in Serum Samples
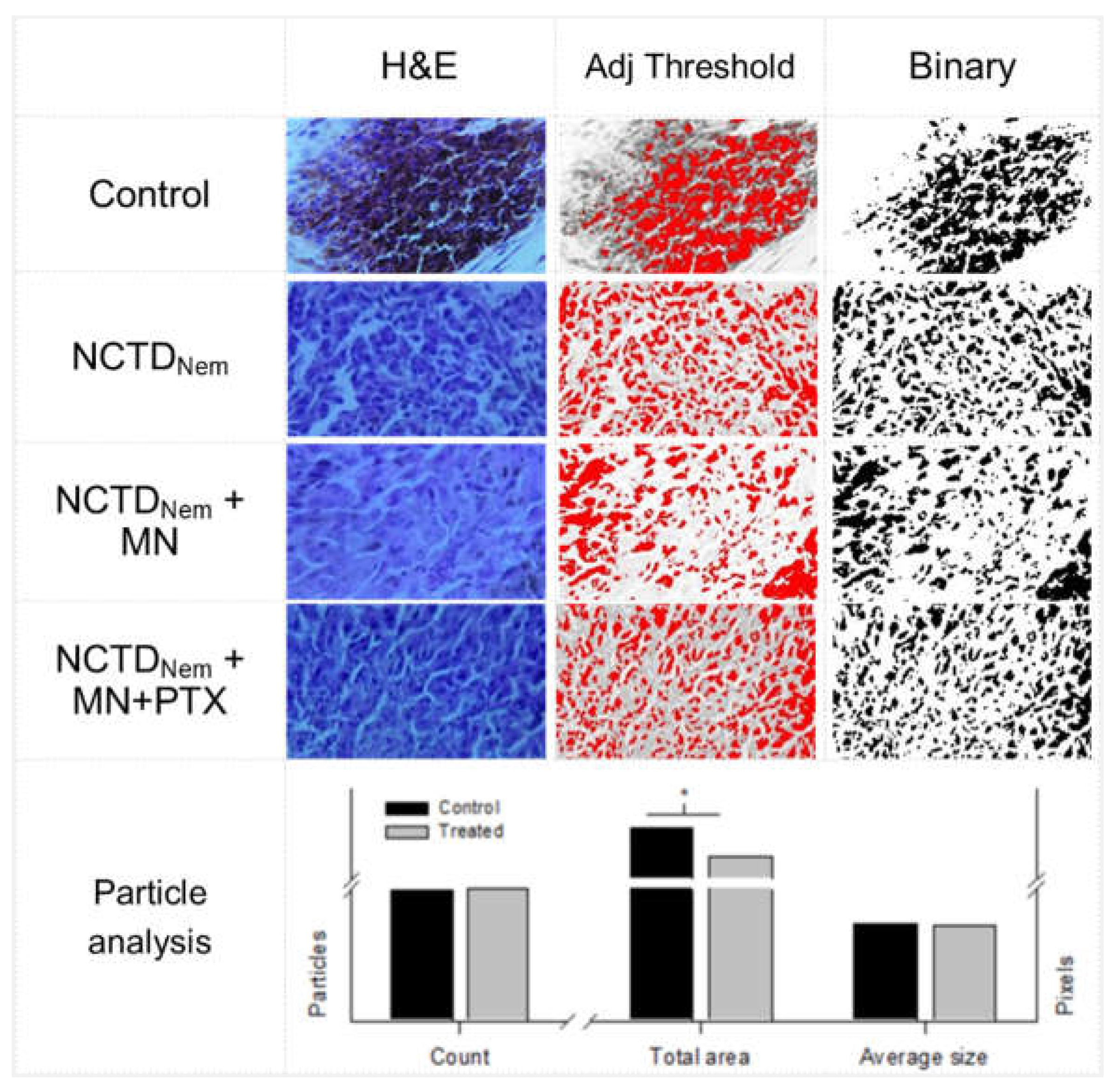
2.4. Histopathological Analysis
3. Discussion
4. Conclusions
5. Materials and Methods
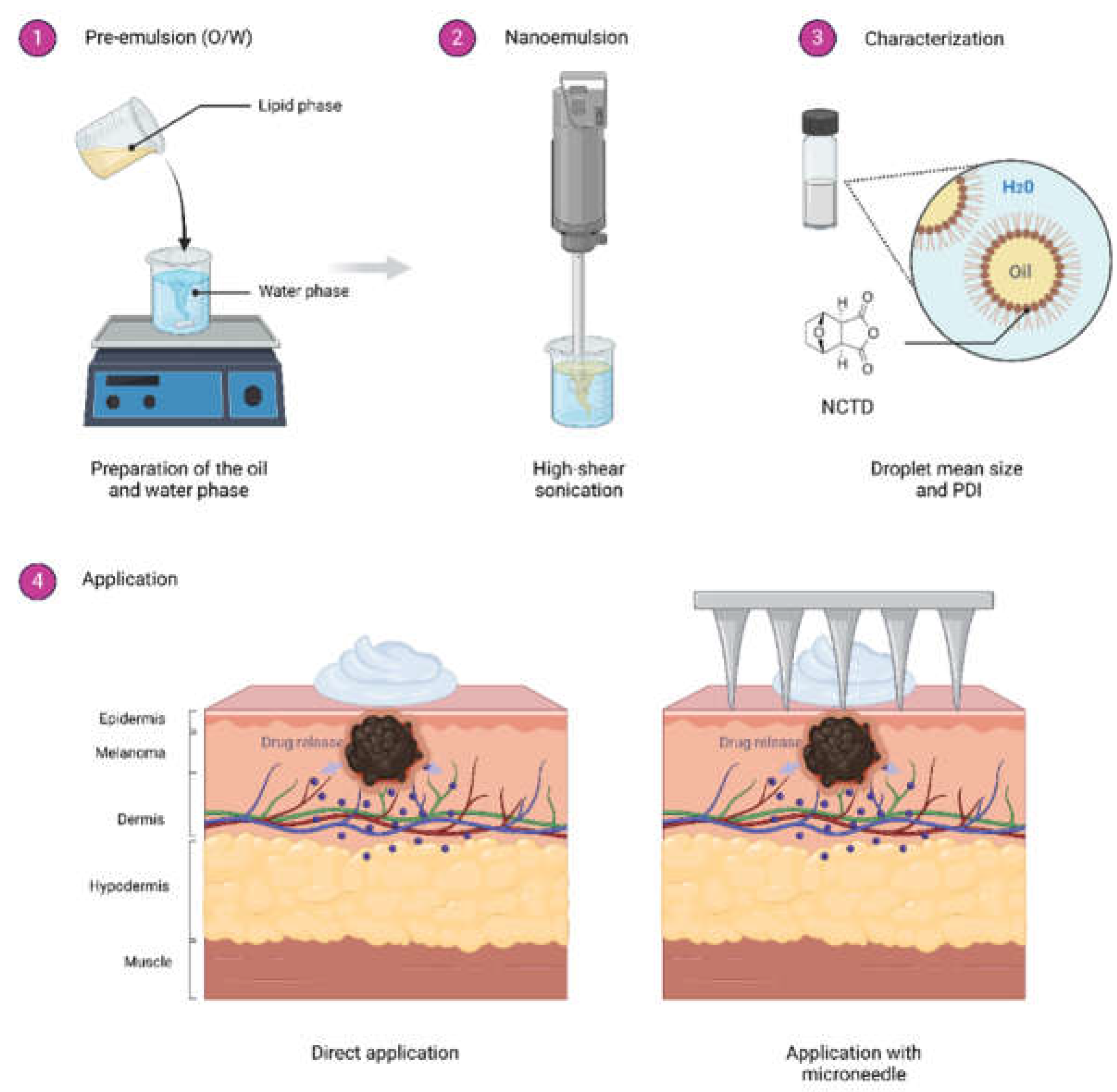
5.1. Nanoemulsion Development
5.2. Experimental Animals
5.3. Cell Culture
5.4. Syngeneic Graft Model
5.5. Surgical Intervention
5.6. Blood Samples
5.7. Drug Content Determination
5.8. Histological Analysis
5.9. Micrographs Image Analysis
5.10. Data Analysis
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| 5-FU | 5-Fluorouracil |
| BRAF | B-Raf Sarcoma Viral Oncogene B1 |
| CTLA-4 | Cytotoxic T-Lymphocyte Antigen 4 |
| LD50 | Lethal Dose 50 |
| NCTD | Norcantharidin |
| Nem | Nanoemulsion |
| MD | Microneedle |
| O/W | Oil/Water |
| PTX | Pentoxifylline |
| PBS | Phosphate-Buffered Saline |
| PDI | Polydispersity Index |
| PD-1 | Programmed Cell Death Protein-1 |
References
- Sladden, M.J.; Balch, C.; Barzilai, D.A.; Berg, D.; Freiman, A.; Handiside, T.; Hollis, S.; Lens, M.B.; Thompson, J.F. (2009). Surgical excision margins for primary cutaneous melanoma. The Cochrane Library. [CrossRef]
- Iarc.Fr. Retrieved June 28, 2024, from http://www.iarc.fr/en/publications/pdfs-online/pat-gen/bb6/.
- Eggermont, A.M.M.; Spatz, A.; Robert, C. Cutaneous melanoma. Lancet 2014, 383, 816–827. [Google Scholar] [CrossRef] [PubMed]
- Welch, H.G.; Woloshin, S.; Schwartz, L.M. Skin biopsy rates and incidence of melanoma: population based ecological study. BMJ 2005, 331, 481. [Google Scholar] [CrossRef]
- Tromme, I.; Legrand, C.; Devleesschauwer, B.; Leiter, U.; Suciu, S.; Eggermont, A.; Francart, J.; Calay, F.; Haagsma, J.A.; Baurain, J.-F.; Thomas, L.; Beutels, P.; Speybroeck, N. Melanoma burden by melanoma stage: Assessment through a disease transition model. Eur. J. Cancer 2016, 53, 33–41. [Google Scholar] [CrossRef]
- Friedman, R.J.; Rigel, D.S.; Kopf, A.W. Early detection of malignant melanoma: The role of physician examination and self-examination of the skin. CA: A Cancer Journal for Clinicians 1985, 35, 130–151. [Google Scholar] [CrossRef]
- Wang, S.Q.; Rabinovitz, H.; Kopf, A.W.; Oliviero, M. Current technologies for the in vivo diagnosis of cutaneous melanomas. Clinics in Dermatology 2004, 22, 217–222. [Google Scholar] [CrossRef] [PubMed]
- Read, T.; Lonne, M.; Sparks, D.S.; David, M.; Wagels, M.; Schaider, H.; Soyer, H.P.; Smithers, B.M. A systematic review and meta-analysis of locoregional treatments for in-transit melanoma. Journal of Surgical Oncology 2019, 119, 887–896. [Google Scholar] [CrossRef]
- Nieweg, O.E.; Gallegos-Hernández, J.F. La cirugía en melanoma cutáneo maligno y las nuevas drogas. Cirugia y cirujanos 2015, 83, 175–180. [Google Scholar] [CrossRef] [PubMed]
- Testori, A.A.E.; Ribero, S.; Indini, A.; Mandalà, M. Adjuvant treatment of melanoma: Recent developments and future perspectives. American Journal of Clinical Dermatology 2019, 20, 817–827. [Google Scholar] [CrossRef] [PubMed]
- Bastian, B.C. The molecular pathology of melanoma: An integrated taxonomy of melanocytic neoplasia. Annual Review of Pathology 2014, 9, 239–271. [Google Scholar] [CrossRef]
- Naguib, Y.W.; Kumar, A.; Cui, Z. The effect of microneedles on the skin permeability and antitumor activity of topical 5-fluorouracil. Acta Pharmaceutica Sinica. B 2014, 4, 94–99. [Google Scholar] [CrossRef]
- Giacone, D.V.; Dartora, V.F.M.C. , de Matos, J.K.R.; Passos, J.S.; Miranda, D.A.G., de Oliveira, E.A.; Silveira, E.R.; Costa-Lotufo, L.V.; Maria-Engler, S.S.; Lopes, L.B. Effect of nanoemulsion modification with chitosan and sodium alginate on the topical delivery and efficacy of the cytotoxic agent piplartine in 2D and 3D skin cancer models. International Journal of Biological Macromolecules 2020, 165, 1055–1065. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Wu, Q.; Zhang, Z.; Yuan, L.; Liu, X.; Zhou, L. Preparation of curcumin-loaded liposomes and evaluation of their skin permeation and pharmacodynamics. Molecules 2012, 17, 5972–5987. [Google Scholar] [CrossRef] [PubMed]
- Tagne, J.-B.; Kakumanu, S.; Nicolosi, R.J. Nanoemulsion preparations of the anticancer drug dacarbazine significantly increase its efficacy in a xenograft mouse melanoma model. Molecular Pharmaceutics 2008, 5, 1055–1063. [Google Scholar] [CrossRef] [PubMed]
- Tambunlertchai, S.; Geary, S.M.; Salem, A.K. Skin penetration enhancement strategies used in the development of melanoma topical treatments. The AAPS Journal 2021, 23. [Google Scholar] [CrossRef]
- McCluskey, A.; Ackland, S.P.; Bowyer, M.C.; Baldwin, M.L.; Garner, J.; Walkom, C.C.; Sakoff, J.A. Cantharidin analogues: synthesis and evaluation of growth inhibition in a panel of selected tumour cell lines. Bioorganic Chemistry 2003, 31, 68–79. [Google Scholar] [CrossRef] [PubMed]
- Pachuta-Stec, A.; Szuster-Ciesielska, A. New norcantharidin analogs: Synthesis and anticancer activity. Archiv Der Pharmazie 2015, 348, 897–907. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.; Ren, Y.; Tan, L.; Song, X.; Wang, M.; Li, Y.; Cao, Z.; Guo, C. Norcantharidin: research advances in pharmaceutical activities and derivatives in recent years. Biomed. Pharmacother. Biomed. Pharmacother. 2020, 131, 110755. [Google Scholar] [CrossRef] [PubMed]
- Dorn, D.C.; Kou, C.A.; Png, K.J.; Moore, M.A.S. The effect of cantharidins on leukemic stem cells. International Journal of Cancer. Journal International Du Cancer 2009, 124, 2186–2199. [Google Scholar] [CrossRef] [PubMed]
- Xie, M.-H.; Ge, M.; Peng, J.-B.; Jiang, X.-R.; Wang, D.-S.; Ji, L.-Q.; Ying, Y.; Wang, Z. In-vivo anti-tumor activity of a novel poloxamer-based thermosensitive in situ gel for sustained delivery of norcantharidin. Pharmaceutical Development and Technology 2019, 24, 623–629. [Google Scholar] [CrossRef]
- Lixin, W.; Haibing, H.; Xing, T.; Ruiying, S.; Dawei, C. A less irritant norcantharidin lipid microspheres: Formulation and drug distribution. International Journal of Pharmaceutics 2006, 323, 161–167. [Google Scholar] [CrossRef]
- Clinicaltrials.gov. (n.d.). Clinicaltrials.gov. Retrieved July 4, 2024, from https://clinicaltrials.gov/ct2/show/NCT04673396.
- Potez, M.; Trappetti, V.; Bouchet, A.; Fernandez-Palomo, C.; Güç, E.; Kilarski, W.W.; Hlushchuk, R.; Laissue, J.; Djonov, V. Characterization of a B16-F10 melanoma model locally implanted into the ear pinnae of C57BL/6 mice. PloS One 2018, 13, e0206693. [Google Scholar] [CrossRef] [PubMed]
- Martínez-Razo, G.; Pires, P.C.; Domínguez-López, M.L.; Veiga, F.; Vega-López, A.; Paiva-Santos, A.C. Norcantharidin nanoemulsion development, characterization, and in vitro antiproliferation effect on B16F1 melanoma cells. Pharmaceuticals 2023, 16, 501. [Google Scholar] [CrossRef] [PubMed]
- Gist.Pl. Retrieved June 28, 2024, from http://gist.pl/download/czerniak_esmo_rct_melanoma_guide_for_patients_2013.pdf.
- Herkenne, C.; Alberti, I.; Naik, A.; Kalia, Y.N.; Mathy, F.-X.; Préat, V.; Guy, R.H. In vivo methods for the assessment of topical drug bioavailability. Pharmaceutical Research 2008, 25. [Google Scholar] [CrossRef] [PubMed]
- Sharma, A.; Sharma, D.; Baldi, A.; Jyoti, K.; Chandra, R.; Madan, J. Imiquimod-oleic acid prodrug-loaded cream reduced drug crystallinity and induced indistinguishable cytotoxicity and apoptosis in mice melanoma tumour. Journal of Microencapsulation 2019, 36, 759–774. [Google Scholar] [CrossRef] [PubMed]
- Martínez-Razo, G.; Domínguez-López, M.L. , de la Rosa, J.M.; Fabila-Bustos, D.A.; Reyes-Maldonado, E.; Conde-Vázquez, E.; Vega-López, A. Norcantharidin toxicity profile: an in vivo murine study. Naunyn-Schmiedeberg’s Archives of Pharmacology 2023, 396, 99–108. [Google Scholar] [CrossRef] [PubMed]
- Pan, M.-S.; Cao, J.; Fan, Y.-Z. Insight into norcantharidin, a small-molecule synthetic compound with potential multi-target anticancer activities. Chinese Medicine 2020, 15. [Google Scholar] [CrossRef] [PubMed]
- Mei, L.; Sang, W.; Cui, K.; Zhang, Y.; Chen, F.; Li, X. Norcantharidin inhibits proliferation and promotes apoptosis via c-Met/Akt/mTOR pathway in human osteosarcoma cells. Cancer Science 2019, 110, 582–595. [Google Scholar] [CrossRef] [PubMed]
- Lu, K.; Cao, M.; Mao, W.; Sun, X.; Tang, J.; Shen, Y.; Sui, M. Targeted acid-labile conjugates of norcantharidin for cancer chemotherapy. Journal of Materials Chemistry 2012, 22, 15804. [Google Scholar] [CrossRef]
- Wang, Z.; You, D.; Lu, M.; He, Y.; Yan, S. Inhibitory effect of norcantharidin on melanoma tumor growth and vasculogenic mimicry by suppressing MMP-2 expression. Oncology Letters 2017, 13, 1660–1664. [Google Scholar] [CrossRef]
- Santos, A.C.; Morais, F.; Simões, A.; Pereira, I.; Sequeira, J.A.D.; Pereira-Silva, M.; Veiga, F.; Ribeiro, A. Nanotechnology for the development of new cosmetic formulations. Expert Opinion on Drug Delivery 2019, 16, 313–330. [Google Scholar] [CrossRef]
- Nayak, A.K.; Hasnain, M.S.; Aminabhavi, T.M.; Torchilin, V.P. (2022). Systems of Nanovesicular Drug Delivery. Academic Press.
- Correa-Lara, M.V.M.; Lara-Vega, I.; Nájera-Martínez, M.; Domínguez-López, M.L.; Reyes-Maldonado, E.; Vega-López, A. (2023). Tumor-infiltrating iNKT cells activated through c-kit/Sca-1 are induced by pentoxifylline, norcantharidin, and their mixtures for killing Murine melanoma cells. Pharmaceuticals, 16, 1472. [CrossRef]
- Hobohm, U. Fever therapy revisited. British Journal of Cancer 2005, 92, 421–425. [Google Scholar] [CrossRef] [PubMed]
- Zahl, P.-H.; Mæhlen, J.; Welch, H.G. The natural history of invasive breast cancers detected by screening mammography. Archives of Internal Medicine 2008, 168, 2311. [Google Scholar] [CrossRef] [PubMed]
- Güç, E.; Briquez, P.S.; Foretay, D.; Fankhauser, M.A.; Hubbell, J.A.; Kilarski, W.W.; Swartz, M.A. Local induction of lymphangiogenesis with engineered fibrin-binding VEGF-C promotes wound healing by increasing immune cell trafficking and matrix remodeling. Biomaterials 2017, 131, 160–175. [Google Scholar] [CrossRef] [PubMed]
- Bobek, V.; Kolostova, K.; Pinterova, D.; Kacprzak, G.; Adamiak, J.; Kolodziej, J.; Boubelik, M.; Kubecova, M.; Hoffman, R.M. A clinically relevant, syngeneic model of spontaneous, highly metastatic B16 mouse melanoma. Anticancer Research 2010, 30, 4799. [Google Scholar] [PubMed]
- Madera-Sandoval, R.L.; Tóvári, J.; Lövey, J.; Ranđelović, I.; Jiménez-Orozco, A.; Hernández-Chávez, V.G.; Reyes-Maldonado, E.; Vega-López, A. Combination of pentoxifylline and α-galactosylceramide with radiotherapy promotes necro-apoptosis and leukocyte infiltration and reduces the mitosis rate in murine melanoma. Acta Histochemica 2019, 121, 680–689. [Google Scholar] [CrossRef] [PubMed]
- Doornaert, B.; Leblond, V.; Galiacy, S.; Gras, G.; Planus, E.; Laurent, V.; Isabey, D.; Lafuma, C. Negative impact of DEP exposure on human airway epithelial cell adhesion, stiffness, and repair. American Journal of Physiology. Lung Cellular and Molecular Physiology 2003, 284, L119–L132. [Google Scholar] [CrossRef] [PubMed]
- Giavazzi, R.; Decio, A. (2014). Syngeneic Murine metastasis models: B16 melanoma. In Methods in Molecular Biology (pp. 131–140). Springer New York.
- Wei, C.-M.; Zhang, R.; Wang, B.-J.; Yuan, G.-Y.; Guo, R.-C. Determination and pharmacokinetic study of norcantharidin in human serum by HPLC-MS/MS method. Biomedical Chromatography: BMC 2008, 22, 44–49. [Google Scholar] [CrossRef]
- Lee, C.H.; Cho, J.; Lee, K. Tumour regression via integrative regulation of neurological, inflammatory, and hypoxic tumour microenvironment. Biomolecules & Therapeutics 2020, 28, 119–130. [Google Scholar] [CrossRef]
- Schneider, C.A.; Rasband, W.S.; Eliceiri, K.W. NIH Image to ImageJ: 25 years of image analysis. Nature Methods 2012, 9, 671–675. [Google Scholar] [CrossRef]
- Past: Paleontological statistics software package for education and data analysis. (n.d.). Palaeo-electronica.org. Retrieved June 28, 2024, from http://palaeo-electronica.org/2001_1/past/issue1_01.htm.
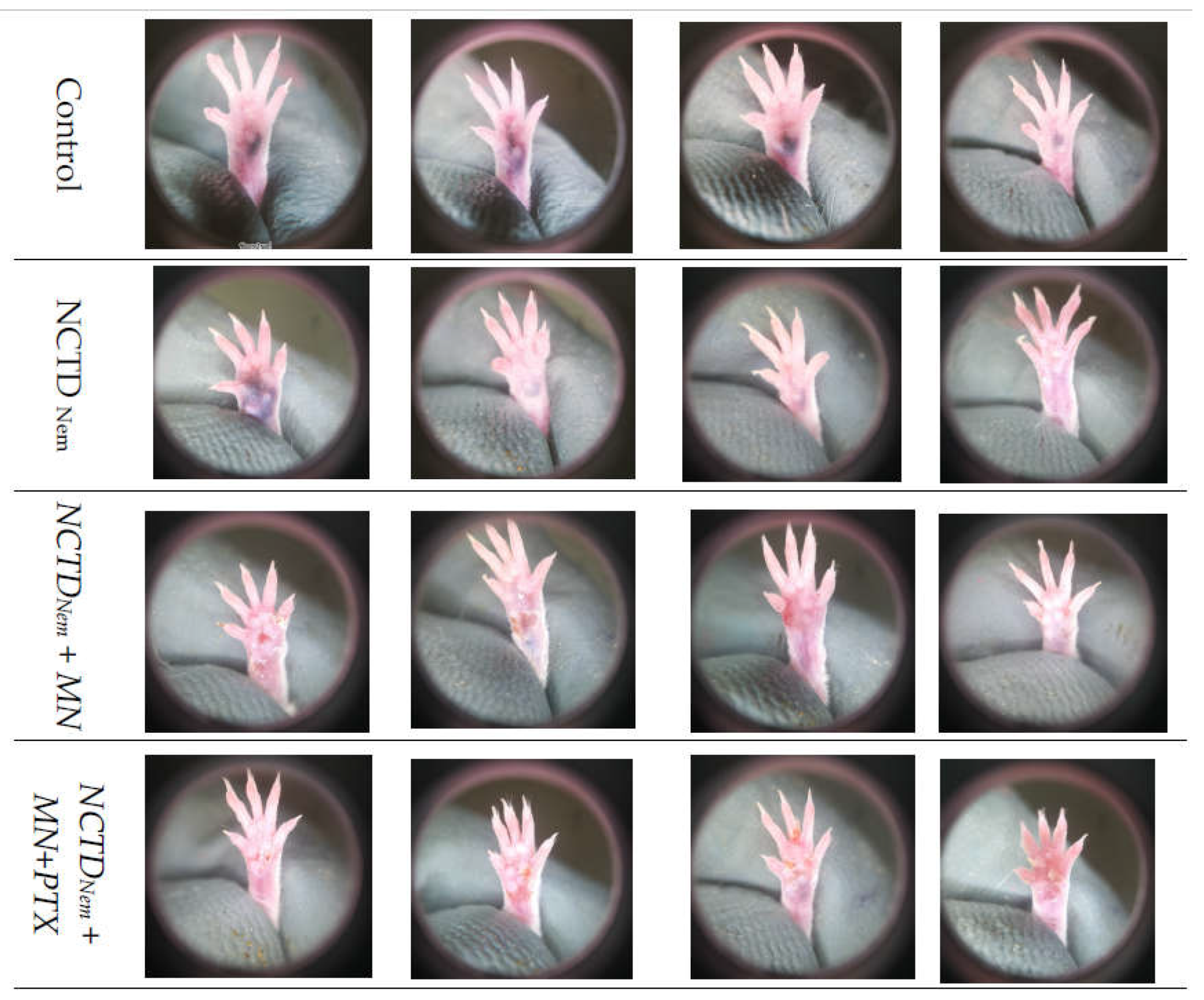
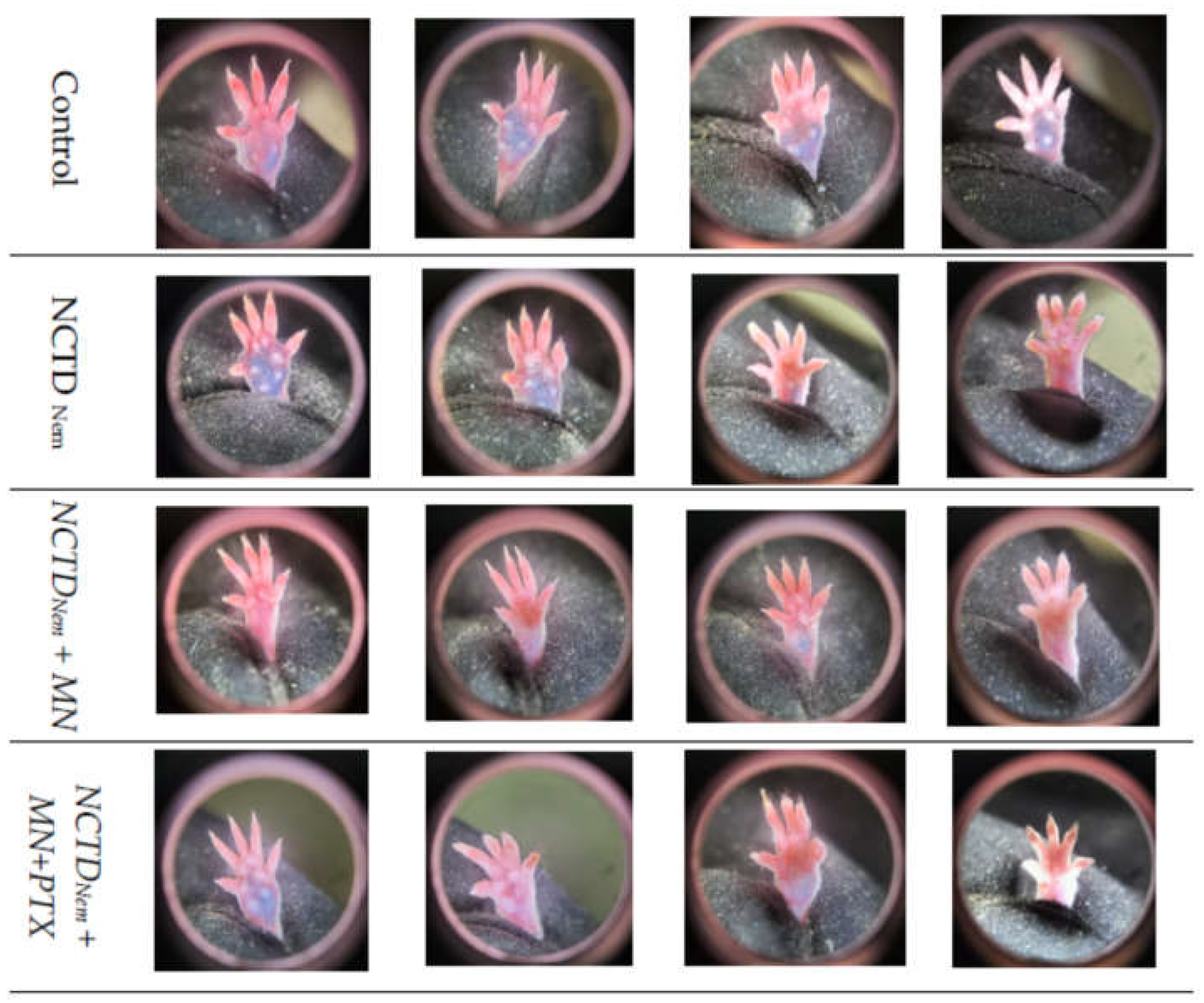
| Group | Size (cm) | +/-S.D | Mitosis | L.I. | Reg |
|---|---|---|---|---|---|
| Control | 0.6 | 0.09 | 1.6 | ** | - |
| NCTD Nem | 0.7 | 0.05 | 1.5 | ** | - |
| NCTD Nem + MD | 0.5 | 0.2 | 1 | *** | + |
| NCTD Nem | 0.6 | 0.2 | 2 | * | - |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





