Submitted:
10 July 2024
Posted:
11 July 2024
You are already at the latest version
Abstract
Keywords:
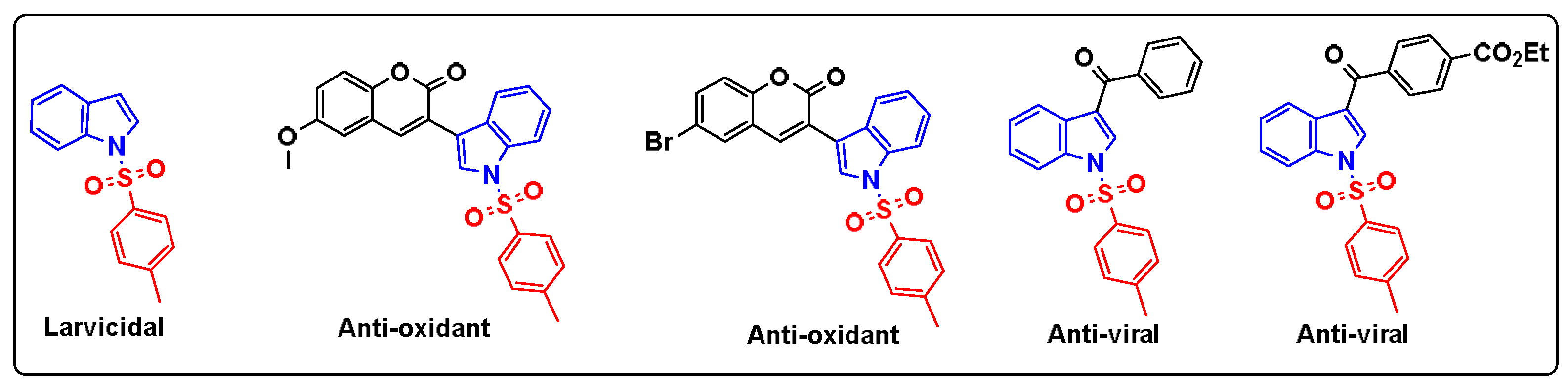
1. Introduction
2. Results and Discussion
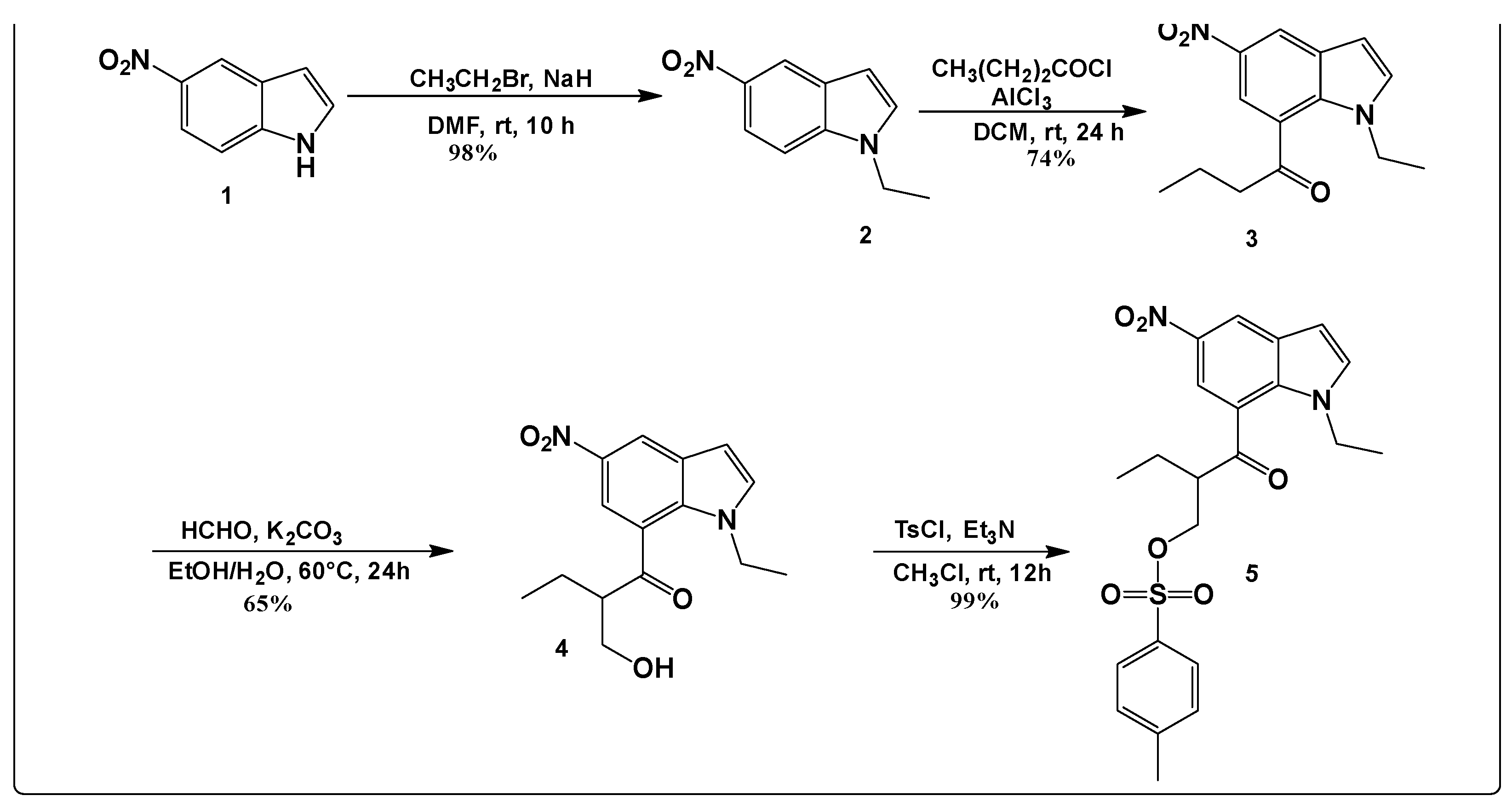
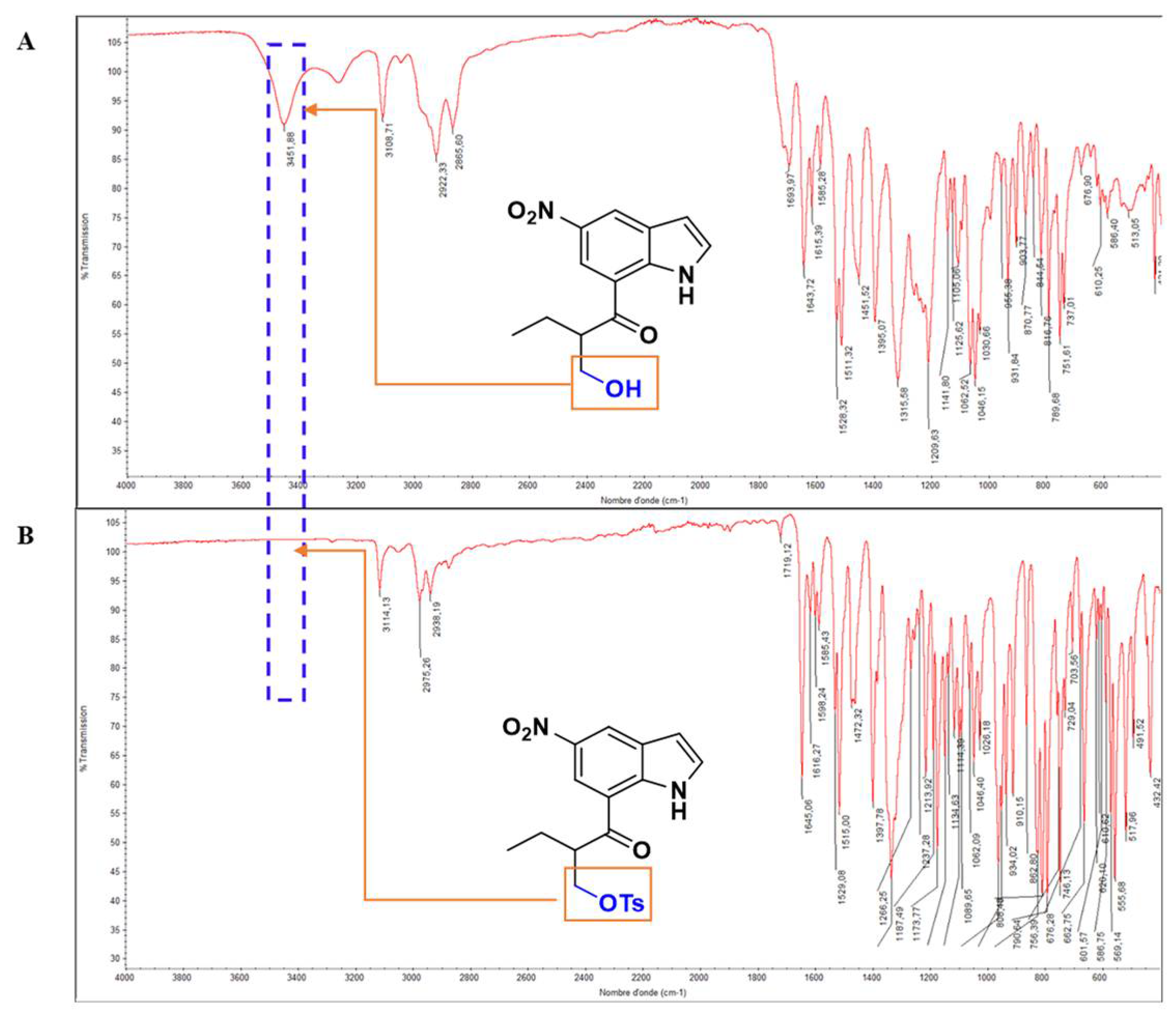
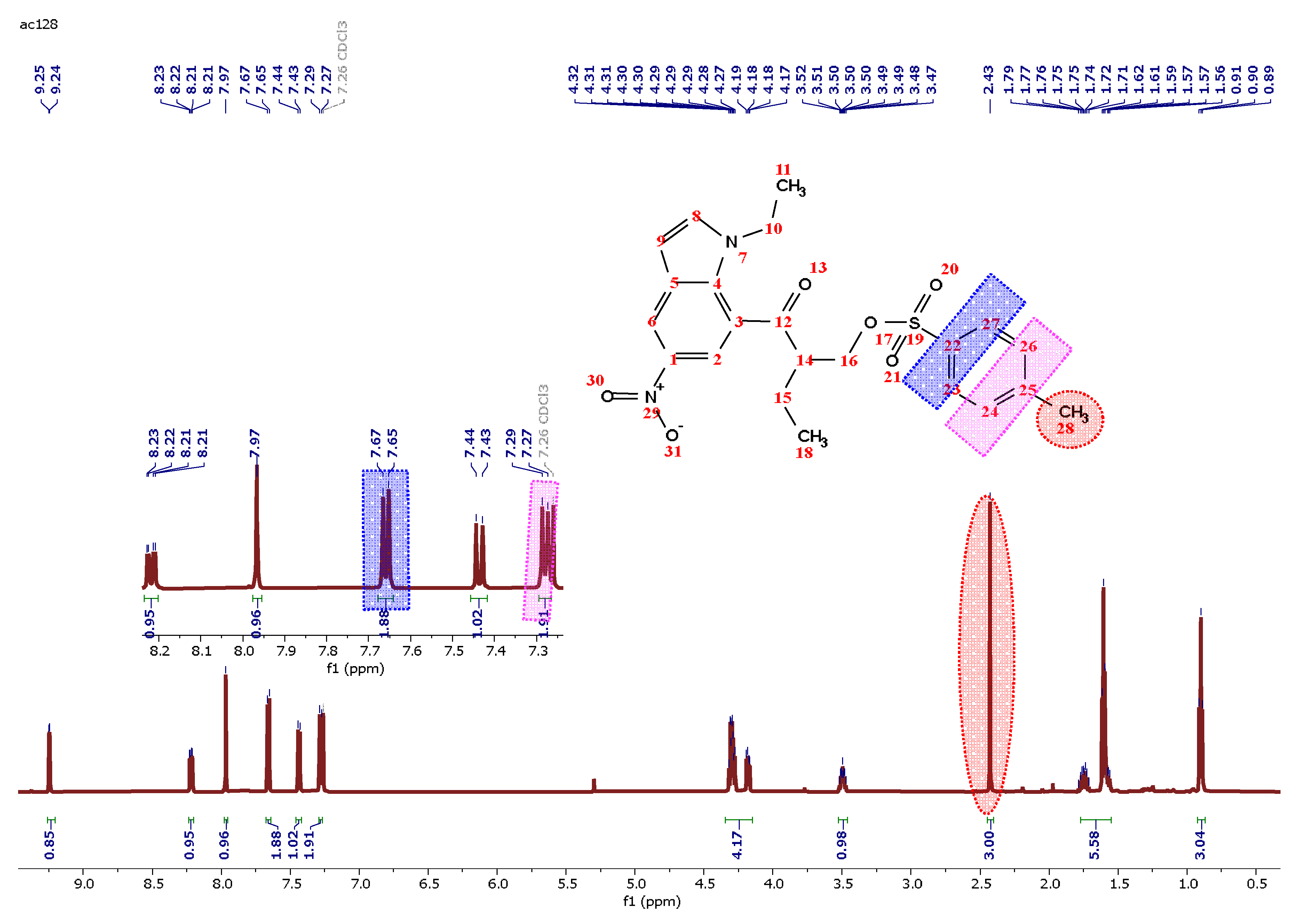
2.1. Synthesis and Characterization
2.2. Antioxidant Activity of Compound 5:
3. Materials and Methods
3.1. General Procedures
3.2. Synthesis
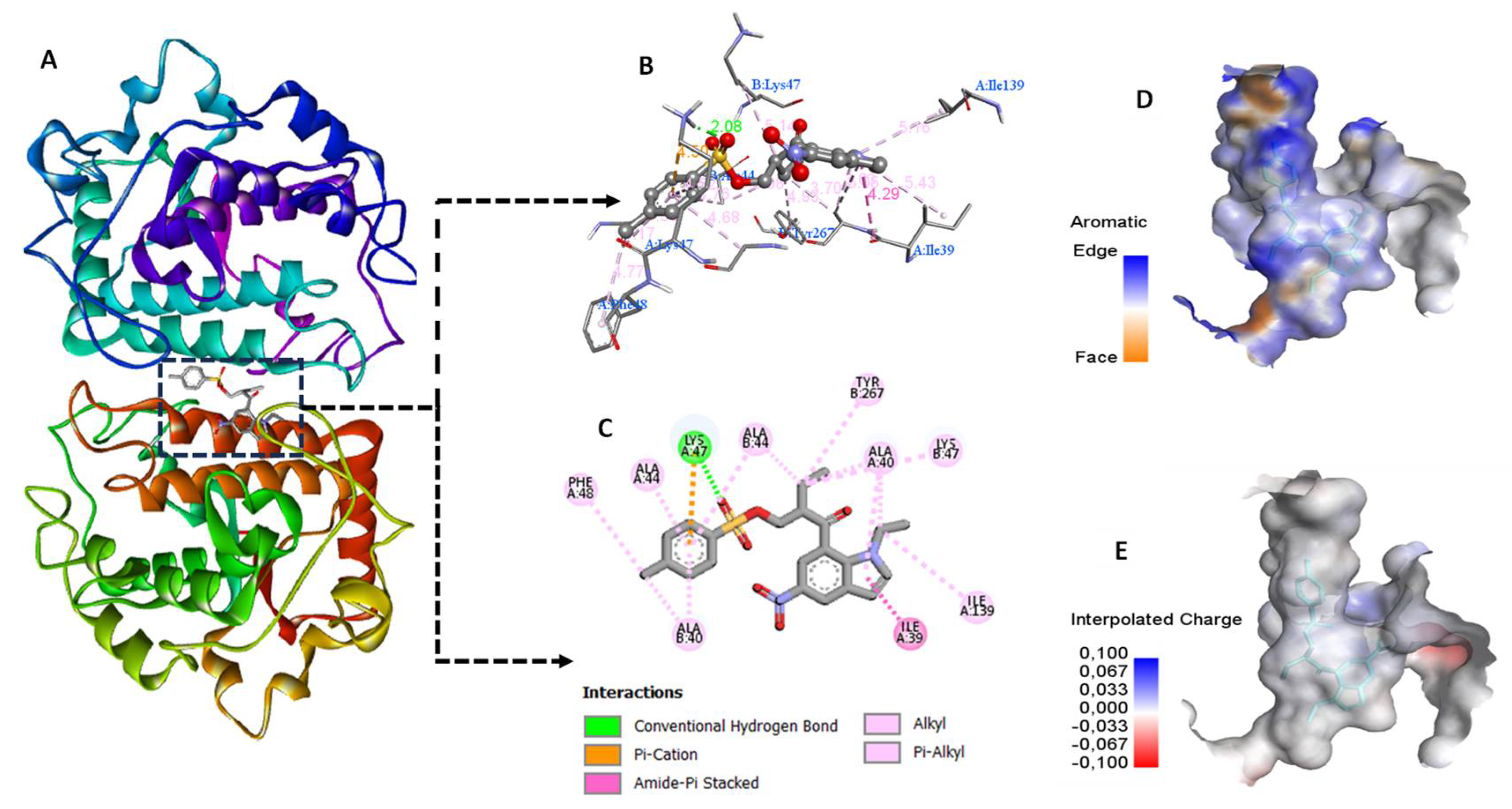
3.3. Molecular Docking
Docking study
Visualization of docking results
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Prakash, B.; Amuthavalli, A.; Edison, D.; Sivaramkumar, M.S.; Velmurugan, R. Novel Indole Derivatives as Potential Anticancer Agents: Design, Synthesis and Biological Screening. Med. Chem. Res. 2018, 27, 321–331. [Google Scholar] [CrossRef]
- Estevão, M.S.; Carvalho, L.C.; Ribeiro, D.; Couto, D.; Freitas, M.; Gomes, A.; Ferreira, L.M.; Fernandes, E.; Marques, M.M.B. Antioxidant Activity of Unexplored Indole Derivatives: Synthesis and Screening. Eur. J. Med. Chem. 2010, 45, 4869–4878. [Google Scholar] [CrossRef] [PubMed]
- Kaur, J.; Utreja, D.; Ekta; Jain, N.; Sharma, S. Recent Developments in the Synthesis and Antimicrobial Activity of Indole and Its Derivatives. Curr. Org. Synth. 2018, 16, 17–37. [CrossRef]
- Giampieri, M.; Balbi, A.; Mazzei, M.; La Colla, P.; Ibba, C.; Loddo, R. Antiviral Activity of Indole Derivatives. Antiviral Res. 2009, 83, 179–185. [Google Scholar] [CrossRef]
- de Jesus Santos, A.; Macêdo, N.A.; de Holanda Cavalcanti, S.C.; Sarmento, V.H.V.; Moreira Lira, A.A.; dos Santos, C.P.; La Corte Santos, R.; Souza Nunes, R. de Larvicidal Formulation Containing N-Tosylindole: A Viable Alternative to Chemical Control of Aedes Aegypti. Colloids Surfaces B Biointerfaces 2022, 213. [Google Scholar] [CrossRef]
- Wet-osot, S.; Pewklang, T.; Duangkamol, C.; Muangsopa, P.; Ngivprom, U.; Chansaenpak, K.; Ngernsoungnern, A.; Sritangos, P.; Chudapongse, N.; Lai, R.Y.; et al. N-Tosylindole-Coumarin with High Fluorescence Quantum Yield and Their Potential Applications. J. Mol. Struct. 2022, 1260. [Google Scholar] [CrossRef]
- Rana, G.; Kar, A.; Kundal, S.; Musib, D.; Jana, U. DDQ/Fe(NO3)3-Catalyzed Aerobic Synthesis of 3-Acyl Indoles and an In Silico Study for the Binding Affinity of N-Tosyl-3-Acyl Indoles toward RdRp against SARS-CoV-2. J. Org. Chem. 2023, 88, 838–851. [Google Scholar] [CrossRef] [PubMed]
- Bortolozzi, R.; Carta, D.; Dal Prà, M.P.; Antoniazzi, G.; Mattiuzzo, E.; Sturlese, M.; Di Paolo, V.; Calderan, L.; Moro, S.; Hamel, E.; et al. Evaluating the Effects of Fluorine on Biological Properties and Metabolic Stability of Some Antitubulin 3-Substituted 7-Phenyl-Pyrroloquinolinones. Eur. J. Med. Chem. 2019, 178, 297–314. [Google Scholar] [CrossRef] [PubMed]
- Narsinghani, T.; Sharma, M.C.; Bhargav, S. Synthesis, Docking Studies and Antioxidant Activity of Some Chalcone and Aurone Derivatives. Med. Chem. Res. 2013, 22, 4059–4068. [Google Scholar] [CrossRef]
- Ando, H.; Kondoh, H.; Ichihashi, M.; Hearing, V.J. Approaches to Identify Inhibitors of Melanin Biosynthesis via the Quality Control of Tyrosinase. J. Invest. Dermatol. 2007, 127, 751–761. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; He, S.; Bonneil, É.; Simpson, B.K. Generation of Antioxidative Peptides from Atlantic Sea Cucumber Using Alcalase versus Trypsin: In Vitro Activity, de Novo Sequencing, and in Silico Docking for in Vivo Function Prediction. Food Chem. 2020, 306, 125581. [Google Scholar] [CrossRef] [PubMed]
| Shift (ppm) | Number of C | Class |
| 11.72 | 1 | s |
| 15.24 | 1 | s |
| 21.73 | 1 | s |
| 22.73 | 1 | s |
| 42.55 | 1 | s |
| 49.35 | 1 | s |
| 71.07 | 1 | s |
| 110.05 | 1 | s |
| 118.82 | 1 | s |
| 119.25 | 1 | s |
| 120.00 | 1 | s |
| 126.00 | 1 | s |
| 127.95 | 2 | s |
| 129.96 | 2 | s |
| 132.48 | 1 | s |
| 136.85 | 1 | s |
| 139.49 | 1 | s |
| 144.10 | 1 | s |
| 145.17 | 1 | s |
| 194.63 | 1 | s |
| compound | Docking Score (Kcal/mol) | Interactions | Interacting residues |
| P5 | -10.86 | H-bond Π- alkyl Alkyl-alkyl Amide- Π stacking |
Lys 47 Ala 44, Ala40, Lys 47, Thr267 Ile 139, Phe 48 Ile 39 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





