Submitted:
10 July 2024
Posted:
11 July 2024
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Materials and Methods
2.1. Cell Lines
2.2. Drugs
2.3. Cell Culture Treatment and Assays
2.4. Fluorescence-Microscopic Analyses of DNA Damage in S Phase Cells
2.5. Western Blot of DNA Damage Proteins
2.6. Mouse Model, Treatment, and Imaging
2.7. Tissue Preparation and Staining
2.8. Statistics
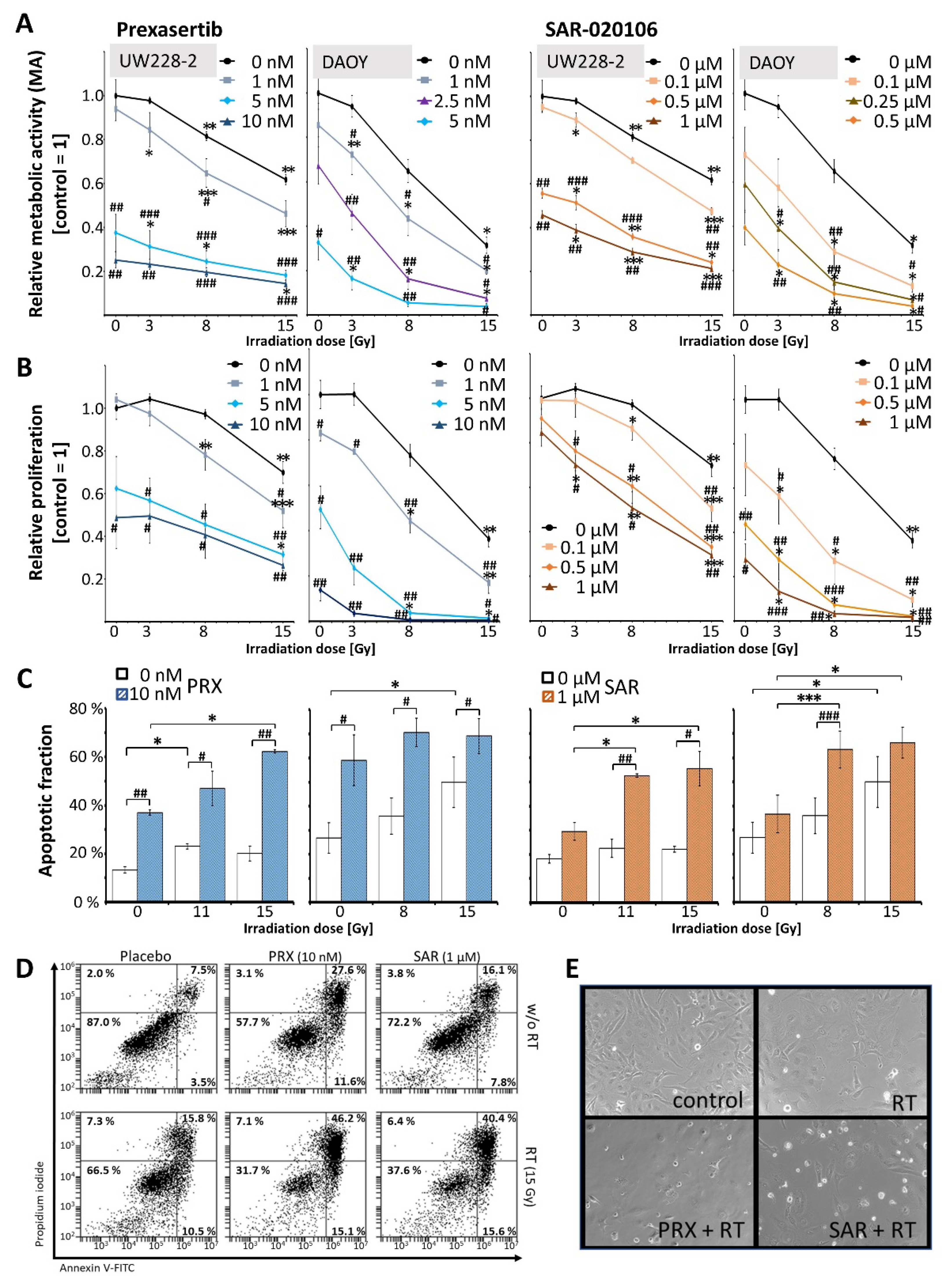
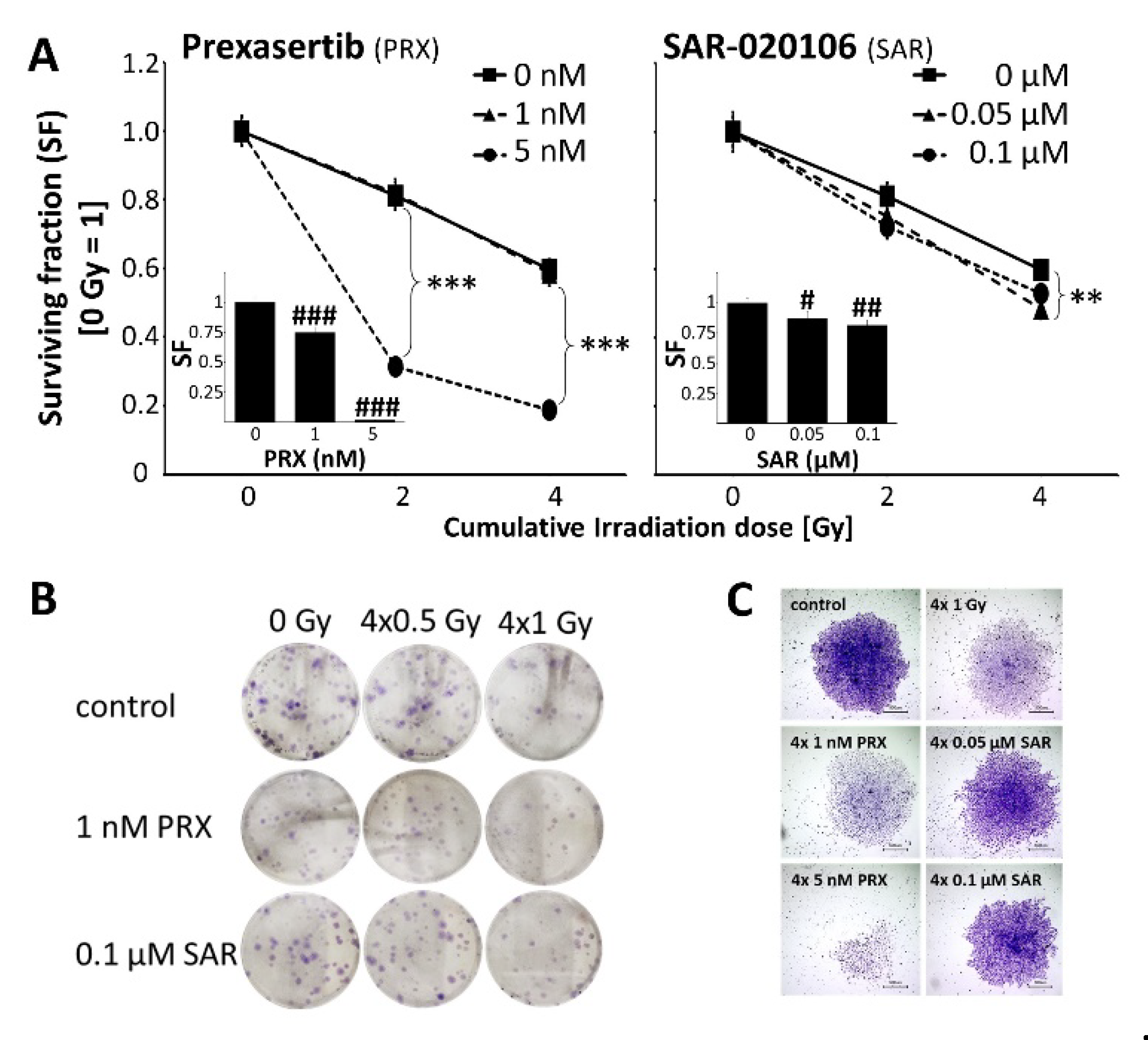
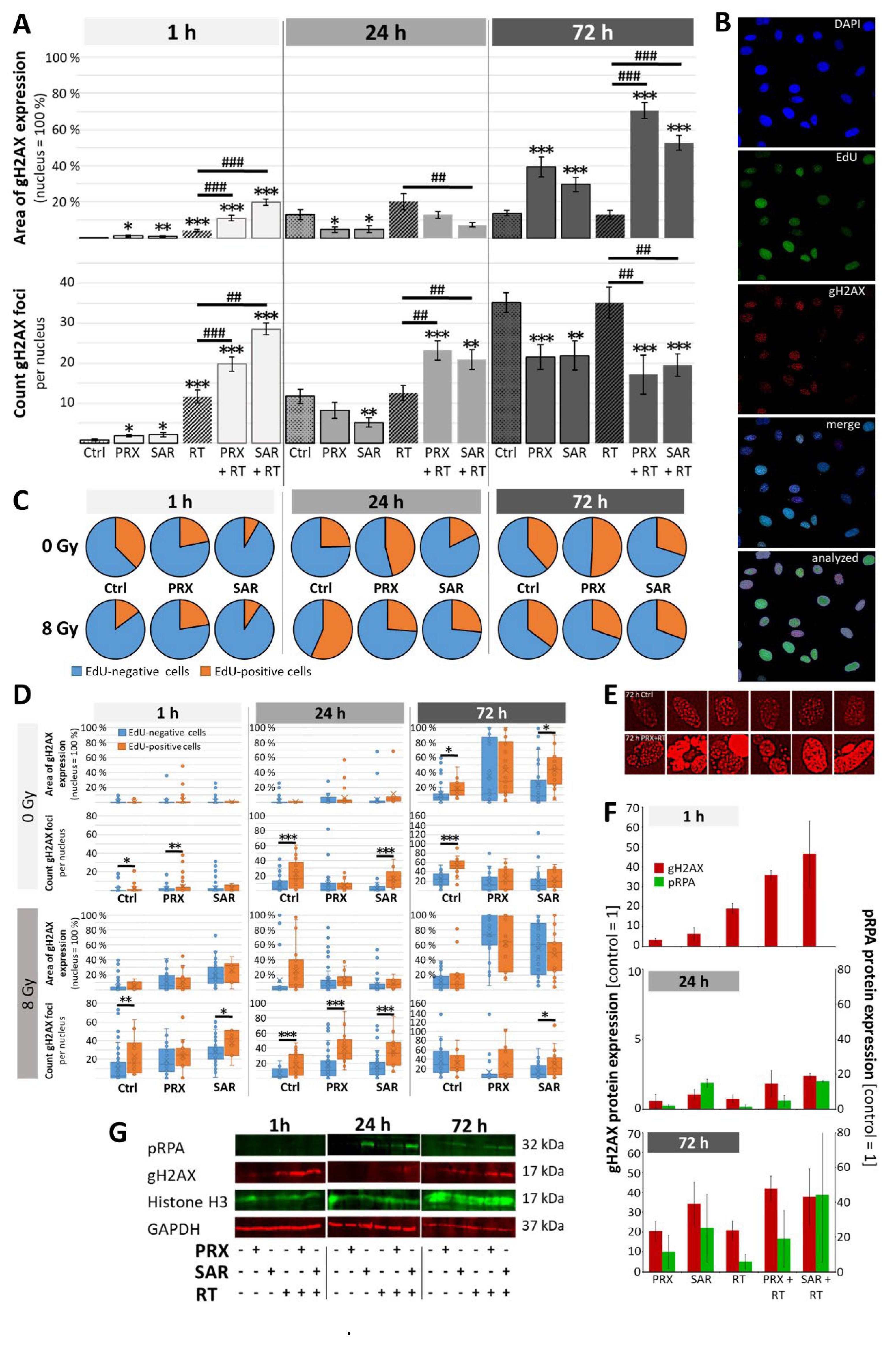
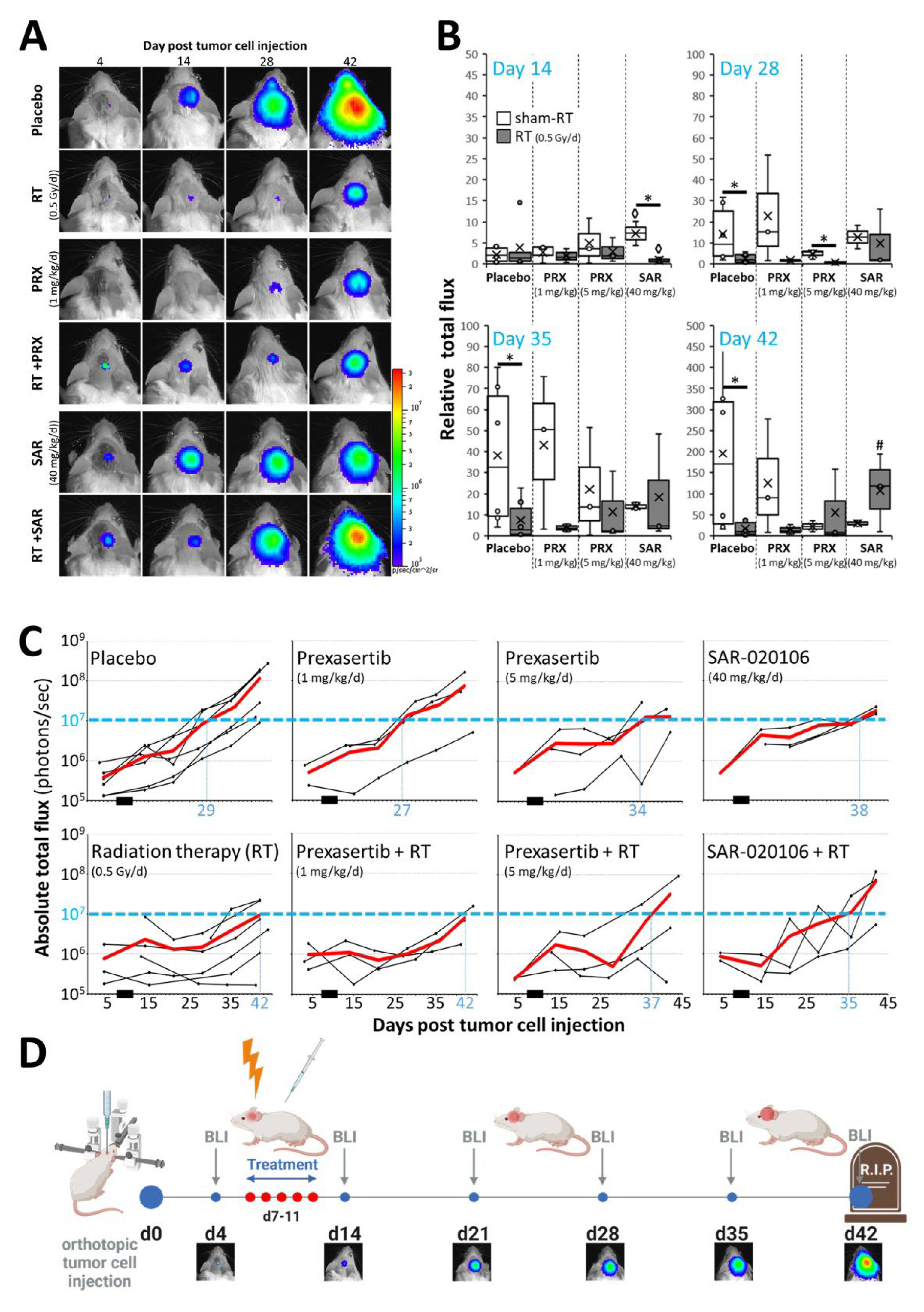
3. Results and Discussion
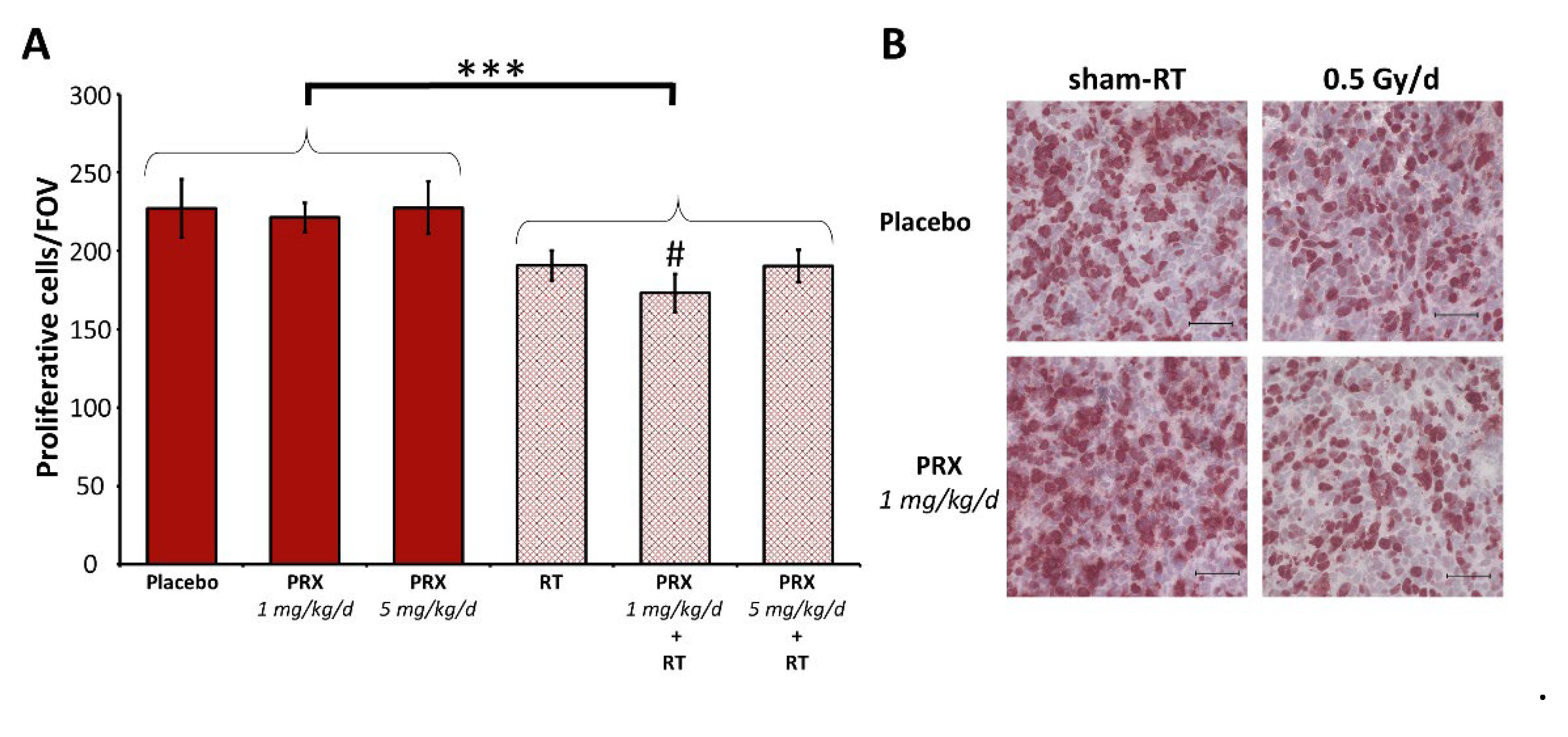
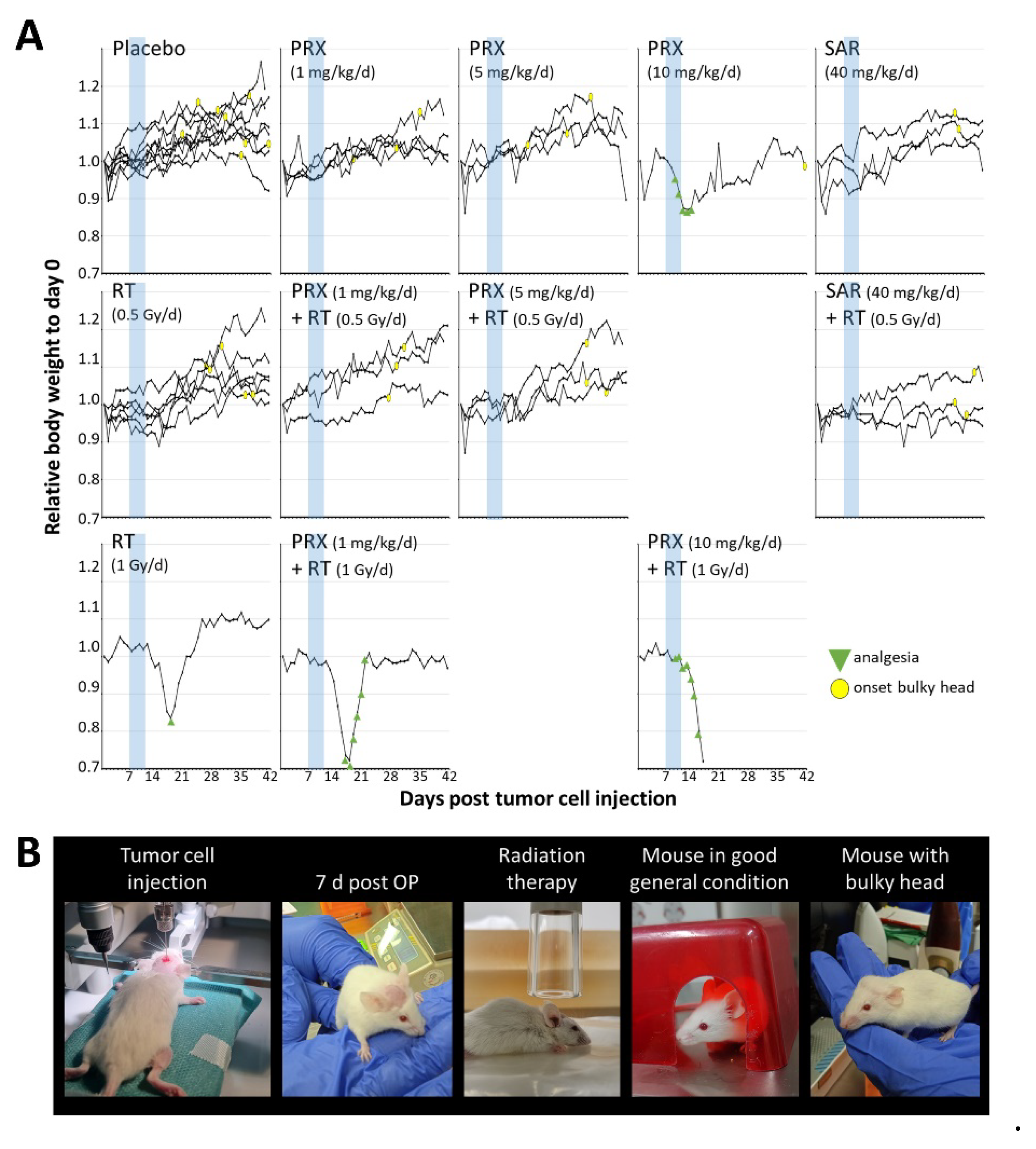
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ostrom QT, Price M, Ryan K, Edelson J, Neff C, Cioffi G et al. CBTRUS Statistical Report: Pediatric Brain Tumor Foundation Childhood and Adolescent Primary Brain and Other Central Nervous System Tumors Diagnosed in the United States in 2014-2018. Neuro Oncol 2022;24(Suppl 3):iii1-iii38. [CrossRef]
- Louis DN, Perry A, Wesseling P, Brat DJ, Cree IA, Figarella-Branger D et al. The 2021 WHO Classification of Tumors of the Central Nervous System: a summary. Neuro Oncol 2021;23(8):1231–51. [CrossRef]
- Zhukova N, Ramaswamy V, Remke M, Pfaff E, Shih DJH, Martin DC et al. Subgroup-specific prognostic implications of TP53 mutation in medulloblastoma. J Clin Oncol 2013;31(23):2927–35. [CrossRef]
- Waszak SM, Northcott PA, Buchhalter I, Robinson GW, Sutter C, Groebner S et al. Spectrum and prevalence of genetic predisposition in medulloblastoma: a retrospective genetic study and prospective validation in a clinical trial cohort. Lancet Oncol 2018;19(6):785–98. [CrossRef]
- Doz F, Milde T. AN INTERNATIONAL PROSPECTIVE TRIAL ON MEDULLOBLASTOMA (MB) IN CHILDREN OLDER THAN 3 TO 5 YEARS WITH WNT BIOLOGICAL PROFILE (PNET 5 MB - LR and PNET 5 MB - WNT-HR), AVERAGE-RISK BIOLOGICAL PROFILE (PNET 5 MB -SR), OR TP53 MUTATION, AND REGISTRY FOR MB OCCURRING IN THE CONTEXT OF GENETIC PREDISPOSITION: NCT02066220, SIOP PNET 5 MB. [June 14, 2023]; Available from: https://clinicaltrials.gov/ct2/show/NCT02066220.
- Chevignard M, Câmara-Costa H, Doz F, Dellatolas G. Core deficits and quality of survival after childhood medulloblastoma: a review. Neurooncol Pract 2017;4(2):82–97. [CrossRef]
- Castellino SM, Ullrich NJ, Whelen MJ, Lange BJ. Developing interventions for cancer-related cognitive dysfunction in childhood cancer survivors. J Natl Cancer Inst 2014;106(8). [CrossRef]
- Bucher N, Britten CD. G2 checkpoint abrogation and checkpoint kinase-1 targeting in the treatment of cancer. Br J Cancer 2008;98(3):523–8. [CrossRef]
- Tse AN, Carvajal R, Schwartz GK. Targeting checkpoint kinase 1 in cancer therapeutics. Clin Cancer Res 2007;13(7):1955–60. [CrossRef]
- King C, Diaz HB, McNeely S, Barnard D, Dempsey J, Blosser W et al. LY2606368 Causes Replication Catastrophe and Antitumor Effects through CHK1-Dependent Mechanisms. Mol Cancer Ther 2015;14(9):2004–13. [CrossRef]
- Zeng L, Beggs RR, Cooper TS, Weaver AN, Yang ES. Combining Chk1/2 Inhibition with Cetuximab and Radiation Enhances In Vitro and In Vivo Cytotoxicity in Head and Neck Squamous Cell Carcinoma. Mol Cancer Ther 2017;16(4):591–600. [CrossRef]
- Lowery CD, VanWye AB, Dowless M, Blosser W, Falcon BL, Stewart J et al. The Checkpoint Kinase 1 Inhibitor Prexasertib Induces Regression of Preclinical Models of Human Neuroblastoma. Clin Cancer Res 2017;23(15):4354–63. [CrossRef]
- Zeng L, Nikolaev A, Xing C, Della Manna DL, Yang ES. CHK1/2 Inhibitor Prexasertib Suppresses NOTCH Signaling and Enhances Cytotoxicity of Cisplatin and Radiation in Head and Neck Squamous Cell Carcinoma. Mol Cancer Ther 2020;19(6):1279–88. [CrossRef]
- Endersby R, Whitehouse J, Pribnow A, Kuchibhotla M, Hii H, Carline B et al. Small-molecule screen reveals synergy of cell cycle checkpoint kinase inhibitors with DNA-damaging chemotherapies in medulloblastoma. Sci Transl Med 2021;13(577). [CrossRef]
- Robinson GW. Evaluation of LY2606368 Therapy in Combination With Cyclophosphamide or Gemcitabine for Children and Adolescents With Refractory or Recurrent Group 3/Group 4 or SHH Medulloblastoma Brain Tumors: NCT04023669.
- Walton MI, Eve PD, Hayes A, Valenti M, Haven Brandon A de, Box G et al. The preclinical pharmacology and therapeutic activity of the novel CHK1 inhibitor SAR-020106. Mol Cancer Ther 2010;9(1):89–100. [CrossRef]
- Reader JC, Matthews TP, Klair S, Cheung K-MJ, Scanlon J, Proisy N et al. Structure-guided evolution of potent and selective CHK1 inhibitors through scaffold morphing. J Med Chem 2011;54(24):8328–42. [CrossRef]
- Borst GR, McLaughlin M, Kyula JN, Neijenhuis S, Khan A, Good J et al. Targeted radiosensitization by the Chk1 inhibitor SAR-020106. Int J Radiat Oncol Biol Phys 2013;85(4):1110–8. [CrossRef]
- Touchefeu Y, Khan AA, Borst G, Zaidi SH, McLaughlin M, Roulstone V et al. Optimising measles virus-guided radiovirotherapy with external beam radiotherapy and specific checkpoint kinase 1 inhibition. Radiother Oncol 2013;108(1):24–31. [CrossRef]
- Patties I, Kallendrusch S, Böhme L, Kendzia E, Oppermann H, Gaunitz F et al. The Chk1 inhibitor SAR-020106 sensitizes human glioblastoma cells to irradiation, to temozolomide, and to decitabine treatment. J Exp Clin Cancer Res 2019;38(1):420. [CrossRef]
- Patties I, Kortmann R-D, Glasow A. Inhibitory effects of epigenetic modulators and differentiation inducers on human medulloblastoma cell lines. J Exp Clin Cancer Res 2013;32(1):27. [CrossRef]
- Patties I, Kortmann R-D, Menzel F, Glasow A. Enhanced inhibition of clonogenic survival of human medulloblastoma cells by multimodal treatment with ionizing irradiation, epigenetic modifiers, and differentiation-inducing drugs. J Exp Clin Cancer Res 2016;35(1):94. [CrossRef]
- Oppermann H, Purcz K, Birkemeyer C, Baran-Schmidt R, Meixensberger J, Gaunitz F. Carnosine’s inhibitory effect on glioblastoma cell growth is independent of its cleavage. Amino Acids 2019;51(5):761–72. [CrossRef]
- Gringmuth M, Walther J, Greiser S, Toussaint M, Schwalm B, Kool M et al. Enhanced Survival of High-Risk Medulloblastoma-Bearing Mice after Multimodal Treatment with Radiotherapy, Decitabine, and Abacavir. Int J Mol Sci 2022;23(7). [CrossRef]
- Ramaswamy V, Remke M, Bouffet E, Faria CC, Perreault S, Cho Y-J et al. Recurrence patterns across medulloblastoma subgroups: an integrated clinical and molecular analysis. Lancet Oncol 2013;14(12):1200–7. [CrossRef]
- Lowery CD, Dowless M, Renschler M, Blosser W, VanWye AB, Stephens JR et al. Broad Spectrum Activity of the Checkpoint Kinase 1 Inhibitor Prexasertib as a Single Agent or Chemopotentiator Across a Range of Preclinical Pediatric Tumor Models. Clin Cancer Res 2019;25(7):2278–89. [CrossRef]
- Chaudhary R, Slebos RJC, Song F, McCleary-Sharpe KP, Masannat J, Tan AC et al. Effects of checkpoint kinase 1 inhibition by prexasertib on the tumor immune microenvironment of head and neck squamous cell carcinoma. Mol Carcinog 2021;60(2):138–50. [CrossRef]
- Manic G, Signore M, Sistigu A, Russo G, Corradi F, Siteni S et al. CHK1-targeted therapy to deplete DNA replication-stressed, p53-deficient, hyperdiploid colorectal cancer stem cells. Gut 2018;67(5):903–17. [CrossRef]
- Parmar K, Kochupurakkal BS, Lazaro J-B, Wang ZC, Palakurthi S, Kirschmeier PT et al. The CHK1 Inhibitor Prexasertib Exhibits Monotherapy Activity in High-Grade Serous Ovarian Cancer Models and Sensitizes to PARP Inhibition. Clin Cancer Res 2019;25(20):6127–40. [CrossRef]
- Nair J, Huang T-T, Murai J, Haynes B, Steeg PS, Pommier Y et al. Resistance to the CHK1 inhibitor prexasertib involves functionally distinct CHK1 activities in BRCA wild-type ovarian cancer. Oncogene 2020;39(33):5520–35. [CrossRef]
- Nie S, Wan Y, Wang H, Liu J, Yang J, Sun R et al. CXCL2-mediated ATR/CHK1 signaling pathway and platinum resistance in epithelial ovarian cancer. J Ovarian Res 2021;14(1):115. [CrossRef]
- Moureau S, Luessing J, Harte EC, Voisin M, Lowndes NF. A role for the p53 tumour suppressor in regulating the balance between homologous recombination and non-homologous end joining. Open Biol 2016;6(9). [CrossRef]
- Ma CX, Janetka JW, Piwnica-Worms H. Death by releasing the breaks: CHK1 inhibitors as cancer therapeutics. Trends Mol Med 2011;17(2):88–96. [CrossRef]
- Syljuåsen RG, Sørensen CS, Hansen LT, Fugger K, Lundin C, Johansson F et al. Inhibition of human Chk1 causes increased initiation of DNA replication, phosphorylation of ATR targets, and DNA breakage. Mol Cell Biol 2005;25(9):3553–62. [CrossRef]
- Sidi S, Sanda T, Kennedy RD, Hagen AT, Jette CA, Hoffmans R et al. Chk1 suppresses a caspase-2 apoptotic response to DNA damage that bypasses p53, Bcl-2, and caspase-3. Cell 2008;133(5):864–77. [CrossRef]
- Manzl C, Fava LL, Krumschnabel G, Peintner L, Tanzer MC, Soratroi C et al. Death of p53-defective cells triggered by forced mitotic entry in the presence of DNA damage is not uniquely dependent on Caspase-2 or the PIDDosome. Cell Death Dis 2013;4(12):e942. [CrossRef]
- Wang W, Macaulay RJB. Cell-cycle gene expression in lovastatin-induced medulloblastoma apoptosis. Can J Neurol Sci 2003;30(4):349–57. [CrossRef]
- Ditano JP, Eastman A. Comparative Activity and Off-Target Effects in Cells of the CHK1 Inhibitors MK-8776, SRA737, and LY2606368. ACS Pharmacol Transl Sci 2021;4(2):730–43. [CrossRef]
- Zhao H, Watkins JL, Piwnica-Worms H. Disruption of the checkpoint kinase 1/cell division cycle 25A pathway abrogates ionizing radiation-induced S and G2 checkpoints. Proc Natl Acad Sci U S A 2002;99(23):14795–800. [CrossRef]
- Campagne O, Davis A, Maharaj AR, Zhong B, Stripay J, Farmer D et al. CNS penetration and pharmacodynamics of the CHK1 inhibitor prexasertib in a mouse Group 3 medulloblastoma model. Eur J Pharm Sci 2020;142:105106. [CrossRef]
- Yang ES, Deutsch E, Mehmet A, Fayette J, Tao Y, Nabell L et al. A Phase 1b trial of prexasertib in combination with chemoradiation in patients with locally advanced head and neck squamous cell carcinoma. Radiother Oncol 2021;157:203–9. [CrossRef]
- Moore KN, Hong DS, Patel MR, Pant S, Ulahannan SV, Jones S et al. A Phase 1b Trial of Prexasertib in Combination with Standard-of-Care Agents in Advanced or Metastatic Cancer. Target Oncol 2021;16(5):569–89. [CrossRef]
- Lampert EJ, Cimino-Mathews A, Lee JS, Nair J, Lee M-J, Yuno A et al. Clinical outcomes of prexasertib monotherapy in recurrent BRCA wild-type high-grade serous ovarian cancer involve innate and adaptive immune responses. J Immunother Cancer 2020;8(2). [CrossRef]
- Iwasa S, Yamamoto N, Shitara K, Tamura K, Matsubara N, Tajimi M et al. Dose-finding study of the checkpoint kinase 1 inhibitor, prexasertib, in Japanese patients with advanced solid tumors. Cancer Sci 2018;109(10):3216–23. [CrossRef]
- Hong DS, Moore K, Patel M, Grant SC, Burris HA, William WN et al. Evaluation of Prexasertib, a Checkpoint Kinase 1 Inhibitor, in a Phase Ib Study of Patients with Squamous Cell Carcinoma. Clin Cancer Res 2018;24(14):3263–72. [CrossRef]
- Hong D, Infante J, Janku F, Jones S, Nguyen LM, Burris H et al. Phase I Study of LY2606368, a Checkpoint Kinase 1 Inhibitor, in Patients With Advanced Cancer. J Clin Oncol 2016;34(15):1764–71. [CrossRef]
- Lee J-M, Nair J, Zimmer A, Lipkowitz S, Annunziata CM, Merino MJ et al. Prexasertib, a cell cycle checkpoint kinase 1 and 2 inhibitor, in BRCA wild-type recurrent high-grade serous ovarian cancer: a first-in-class proof-of-concept phase 2 study. Lancet Oncol 2018;19(2):207–15. [CrossRef]
- Gatti-Mays ME, Karzai FH, Soltani SN, Zimmer A, Green JE, Lee M-J et al. A Phase II Single Arm Pilot Study of the CHK1 Inhibitor Prexasertib (LY2606368) in BRCA Wild-Type, Advanced Triple-Negative Breast Cancer. Oncologist 2020;25(12):1013-e1824. [CrossRef]
- Konstantinopoulos PA, Lee J-M, Gao B, Miller R, Lee J-Y, Colombo N et al. A Phase 2 study of prexasertib (LY2606368) in platinum resistant or refractory recurrent ovarian cancer. Gynecol Oncol 2022. [CrossRef]
- Cash T, Fox E, Liu X, Minard CG, Reid JM, Scheck AC et al. A phase 1 study of prexasertib (LY2606368), a CHK1/2 inhibitor, in pediatric patients with recurrent or refractory solid tumors, including CNS tumors: A report from the Children’s Oncology Group Pediatric Early Phase Clinical Trials Network (ADVL1515). Pediatr Blood Cancer 2021;68(9):e29065. [CrossRef]
- Di Giulio S, Colicchia V, Pastorino F, Pedretti F, Fabretti F, Di Nicolis Robilant V et al. A combination of PARP and CHK1 inhibitors efficiently antagonizes MYCN-driven tumors. Oncogene 2021;40(43):6143–52. References must be numbered in order of appearance in the text (including citations in tables and legends) and listed individually at the end of the manuscript. We recommend preparing the references with a bibliography software package, such as EndNote, ReferenceManager or Zotero to avoid typing mistakes and duplicated references. Include the digital object identifier (DOI) for all references where available. [CrossRef]
| Nuclei | EdU | gH2AX | |
| Fluorochrome | DAPI | Alexa Fluor 488 | Alexa Fluor 568 |
| Area description | Target area 200-500 µm² |
Extraction area 1 EdU-positive/negative nuclei |
Extraction area 2 0.1-2 µm² |
| Selected color | Blue | Green | Red |
| Extraction settings | |||
| Threshold | 10 Smoothed edges |
30 Smoothed edges |
40 |
| Adjust/Correct area | Separate areas Value = 100 |
Fill cracks Value = 10 |
Separate areas Value = 100 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





