1. Introduction
Familial hypercholesterolemia (FH) stands as a genetic disorder caused by mutations in cholesterol metabolism genes, primarily low-density lipoprotein receptor gene (LDLR), and to a lesser extent, apolipoprotein B gene (APOB) and proprotein convertase subtilisin-kexin 9 gene (PCSK9)1,2. Its prevalence is estimated at one in every 250 individuals, impacting over 25 million people globally3. This disease constitutes the most prevalent genetic predisposition to premature atherosclerotic cardiovascular disease (ASCVD), with affected patients exhibiting a 3 to 13 times higher risk of ASCVD compared to the general population. Their life expectancy may be shortened by 20-30 years due to persistent exposure to elevated blood cholesterol levels since birth4. Treatment with lipid-lowering therapies initiated at an early age can ameliorate cardiovascular morbidity and mortality up to tenfold1. However, presently, nine out of ten individuals born worldwide with FH remain undiagnosed5. A recent systematic review by Beath Jhan et al.6 outlined various strategies for FH diagnosis, concluding that the most cost-effective approach is universal screening in childhood alongside diagnosing first-line relatives through reverse cascade. In an economic evaluation using a decision tree analysis, the results showed that a national program for FH based on molecular testing is a cost-effective diagnostic and management strategy that supports government expenditure aimed at preventing coronary artery disease in FH patients7. Despite such recommendations, FH diagnosis has not increased substantially to date.
Newborn metabolic and endocrine disease screening program constitutes a successful public health initiative attaining a coverage exceeding 99%. FH is categorized as a level 1 genomic alteration by the Centers for Disease Control and Prevention Office of Public Health Genomics8.
Several authors have investigated biomarkers that evaluate the probability of presenting FH. Held et al.9-10 have contributed studies regarding the validity of determining total cholesterol, low-density lipoprotein cholesterol (LDLc), and apolipoprotein B in dried blood spots (DBS). Corso et al.11 devised and validated a method for determining cholesterol in DBS.
In our Clinical Analysis and Vascular Risk Laboratory at the Infanta Elena Hospital in Huelva we have enhanced a technique with specific modifications based on literature models 9-11. The aim of our study is to ascertain the precision and stability of cholesterol levels using our methodological approach, correlate cholesterol levels in DBS with the reference method in serum, and conduct an internal validation of the regression formula; in order to carry out a pilot project to implement national FH screening in Spain.
2. Methods
2.1. Study Design
This study adopts a prospective cross-sectional design, comparing a series of samples using two distinct techniques for measuring cholesterol levels. The study adheres to the STARD 2015 guidelines12 for diagnostic accuracy studies. The protocol of this investigation (Protocol code: CHOLESTEROL BDS-2023-01, titled “Accuracy of Blood Cholesterol Determination on Whatman 903 Paper (DBS Cholesterol Validation)”) was conducted in accordance with the Declaration of Helsinki and received approval from the Ethics Committee of the province of Huelva on July 21, 2023.
Participants were selected through consecutive non-probabilistic sampling. Individuals were eligible for the study if they were over 18 years of age, with a requisition for serum cholesterol determination from their attending physician, and consenting to participate when visiting the Clinical Analysis laboratory of the Infanta Elena Hospital. Those who did not provide informed consent and individuals with insufficient or altered samples for both methods were excluded. Sample collection occurred during December 2023.
The measured variables included total cholesterol in DBS and serum.
2.2. Serum Total Cholesterol Method
Serum cholesterol gold standard method was enzymatic colorimetric determination with total cholesterol reagents manufactured by Roche Diagnostics SA in a COBAS PRO (Roche Diagnostics SA) instrument. Both the reagent and equipment possess appropriate authorizations and Conformité Européene marking for cholesterol determination. Rigorous quality control measures are in place, including daily internal controls to verify operational integrity and result validity. Monthly external controls were conducted, comparing equipment performance with other national laboratories to ensure result accuracy.
2.3. Cholesterol Method in DBS
Cholesterol concentration in DBS was determined through a manual enzymatic colorimetric method employing Roche reagent (CHOL2 for Cobas equipment by Roche Diagnostics). For each determination, two disks of three-millimeter were extracted from each patient’s DBS card. The determination was conducted in duplicate, needing a total of four discs per patient. These discs were placed in a single well of a 96-well plate. To extract the blood, 125 µL of methanol (methanol-anhydrous, 99.8%, EMSURE® ACS, ISO, Reag. Ph Eur) were added to each well. The plate was then covered with protective plastic and incubated at 37°C for 30 minutes with gentle shaking, followed by 15 minutes without agitation. Subsequently, 100 µL of reagent and 50 µL of the extracted solution from the previous plate were added to another 96-well plate. The reaction was incubated at 37°C for ten minutes, after which absorbance was measured at 492 nm using a microplate reader (AMR-100T, Allsheng®).
In each plate, a calibration curve was established using five controls with known cholesterol levels ranging from 130 to 300 mg/dL. An internal control of known value was analyzed at the beginning and at the end of each plate. Absorbance levels for each patient were extrapolated to the calibration curve equation, thereby calculating cholesterol concentration in DBS. The median of all cholesterol values determined in each plate was computed and assessed in a Levey-Jennings chart, depicting the mean of all medians and standard deviation. Results were deemed acceptable if the median fell within the two standard deviations depicted in the chart.
The laboratorian technician conducting DBS cholesterol determination was not privy to serum cholesterol values for each patient. Likewise, the technician performing serum cholesterol determination remained unaware of DBS cholesterol values.
2.4. Method Validation
For the analysis of diagnostic variability, three samples were selected for quality control (QC). Their serum cholesterol levels were 149, 220, and 283 mg/dL, respectively. These samples underwent an average of 25 replications on the same day (within-day) and 27 replications over different consecutive days (between-day).
2.5. Methods Comparison: SERUM/DBS
The precision assessment involved the determination of cholesterol in DBS as the index test. After obtaining informed consent, 394 patients were selected. Serum cholesterol values were collected and measured on the same day of blood collection using the COBAS PRO autoanalyzer at the Clinical Analysis Laboratory of the Infanta Elena Hospital. One tube of ethylenediaminetetraacetic acid (EDTA) was collected from each subject to prepare the DBS.
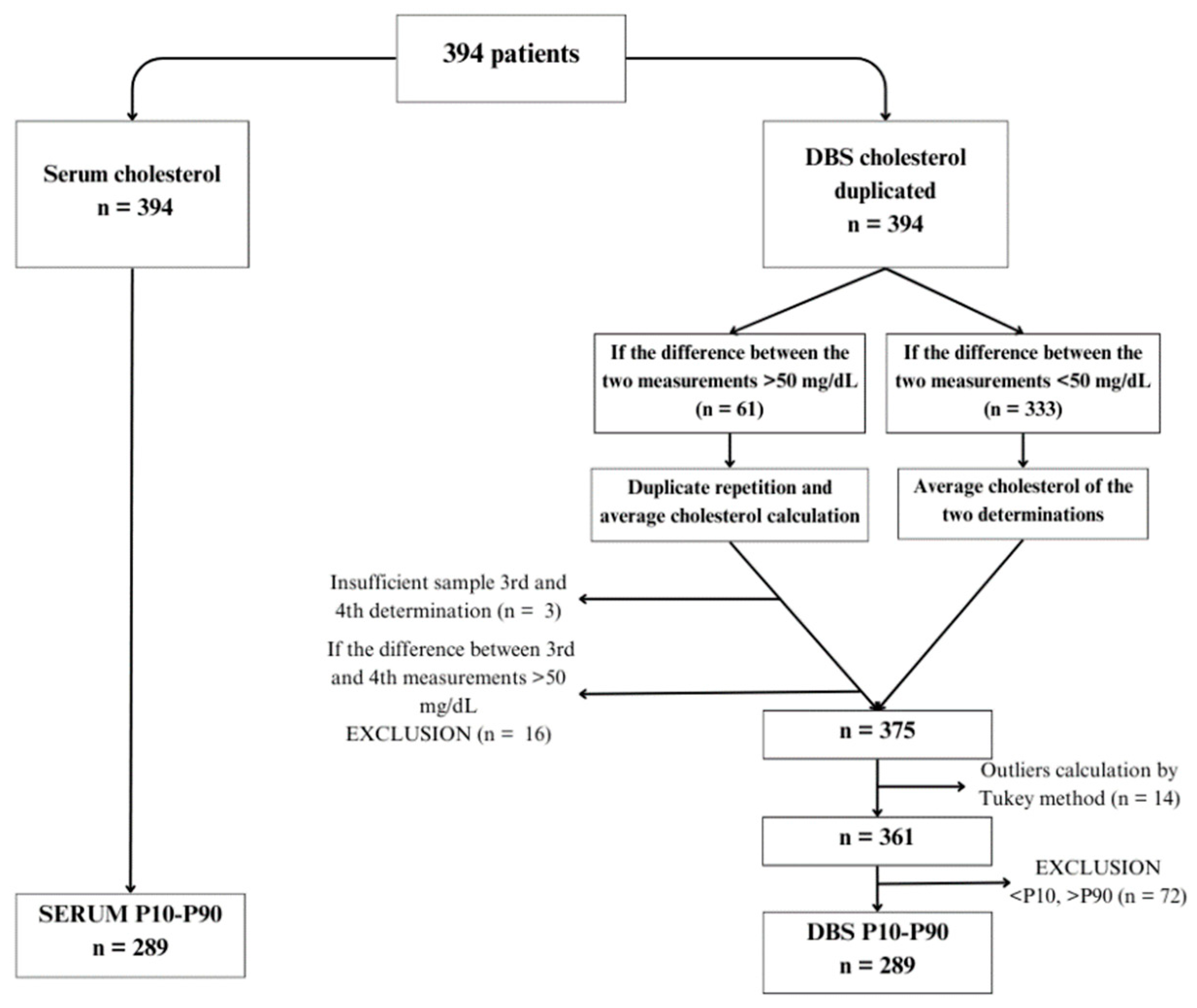
Figure 1 shows the flow chart of serum and DBS samples. Cholesterol values in DBS were determined in duplicate following the previously described methodology. Subsequently, a method comparison was conducted using Passing-Bablok regression, resulting in a regression line enabling the prediction of calculated cholesterol values in DBS.
2.6. Internal Validation
For the internal validation of our regression formula, cholesterol was determined in DBS from 155 samples. The predicted cholesterol was then calculated based on the predictive formula. A Passing-Bablok regression compared the cholesterol results obtained using the predictor formula with those obtained using the serum reference method. Additionally, an external validation utilizing the formula proposed by Corso et al.11 was conducted using the same samples.
2.7. Statistical Analysis
Mean, standard deviation, and coefficient of variation were employed as statistical tools for method validation. To compare methods, a Bland-Altman plot was constructed to elucidate differences between cholesterol determination in DBS and serum. Data were analyzed using a Mann-Whitney U test. A Passing-Bablok regression was conducted between cholesterol values measured in serum and DBS, resulting in a regression line. To validate this line, a box and whisker plot and another Passing-Bablok regression were used. Statistical differences in the validation cholesterol data were assessed using a Mann-Whitney U test. Cholesterol outlier values were identified using the Tukey method. Statistical analysis was performed using SPSS version 23 for data descriptions and the R4 4.0 statistical software for method comparison.
3. Results
Table 1 presents imprecision data, expressed as the coefficient of variation (CV) of DBS samples analyzed using the enzymatic method. Within-day imprecision was calculated based on three quality control samples with known values (149, 220, and 283 mg/dL), analyzed 23, 25, and 26 times, respectively. The CVs were 10.14%, 8.9%, and 8.11% for each respective level. For the between-day imprecision study, the same control samples were analyzed over 27 working days, resulting in CVs of 14.09% for QC 149 mg/dL, 10.57% for QC 220 mg/dL, and 13.29% for QC 283 mg/dL.
To compare methods, 394 patients were selected (
Figure 1). Serum cholesterol levels were determined for all patients, along with DBS analysis. DBS samples were analyzed in duplicate. In cases where the difference between the two DBS measurements was <50 mg/dL (n=333), the average cholesterol value was calculated. For cases where the difference exceeded 50 mg/dL (n=61), a third and fourth determination were performed. Three subjects had insufficient samples for repeated analysis, and 16 exhibited differences exceeding 50 mg/dL between determinations, leading to their exclusion from the study. Consequently, the final sample size comprised 375 subjects with serum cholesterol determination and mean DBS values, which reduced to 361 patients after identifying outliers using the Tukey test.
Furthermore, to conduct method comparison analysis, we decided to discard values exceeding the 90th percentile or falling below the 10th percentile of the difference between serum cholesterol and DBS to capture the central variability of data and provide a balanced representation of the studied population. The final sample comprised 289 subjects. The Kolmogorov-Smirnov test assessed the Gaussian distribution of serum cholesterol and DBS data. Cholesterol values in DBS exhibited a normal distribution, while serum values did not. Thus, non-parametric tests (Mann-Whitney U test) were applied to analyze differences between both methods.
Table 2 describes cholesterol results obtained using the serum reference method and the manual DBS method, indicating a median serum cholesterol of 162 ± 41 mg/dL and a median DBS cholesterol of 173.96 ± 44.10 mg/dL (p=0.001).
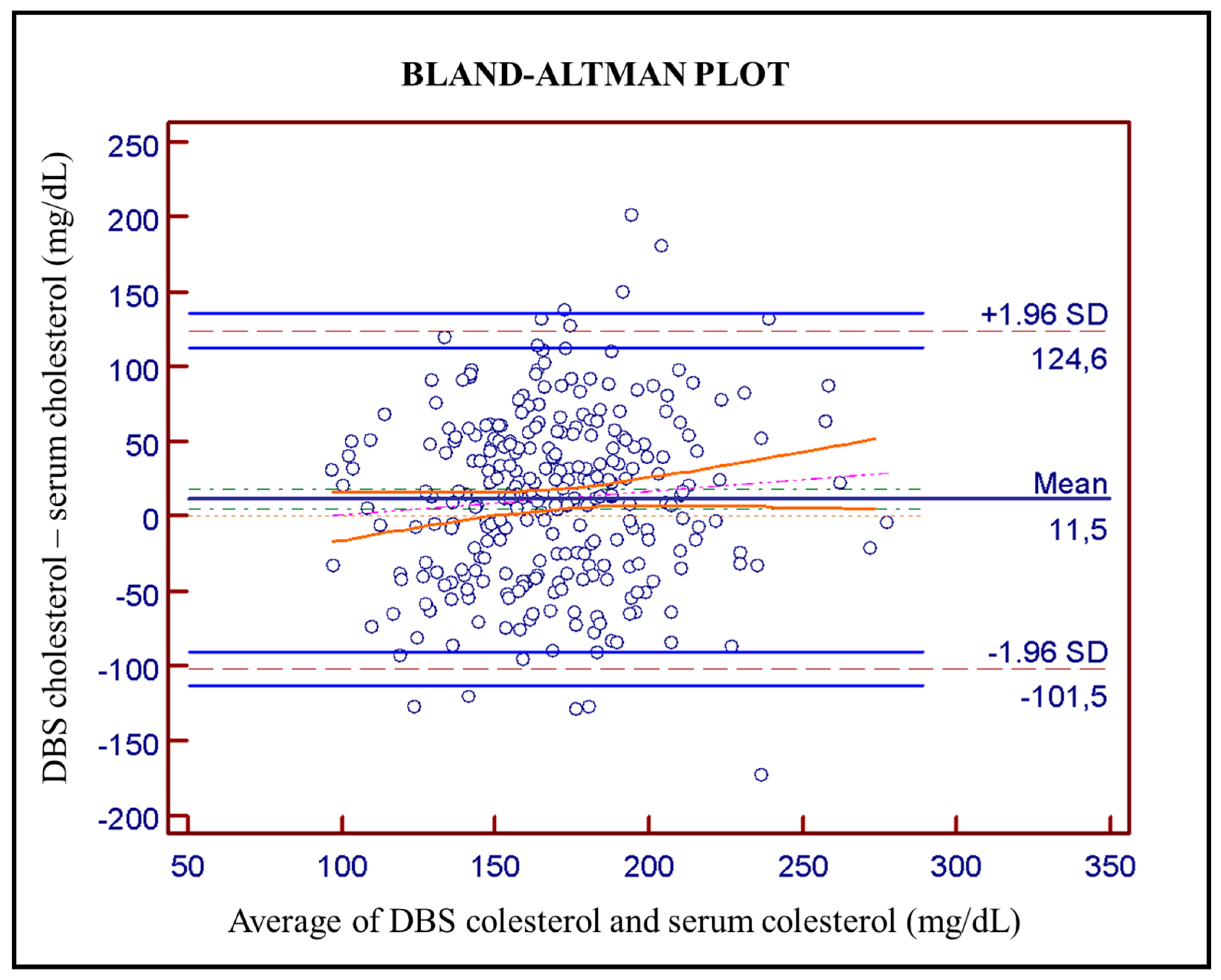
Bland-Altman plot (
Figure 2) was used to evaluate agreement between DBS and serum cholesterol determination, revealing an average bias of 11.5 mg/dL, with a range of 101.5 to 124.6 mg/dL and a 95% confidence interval.
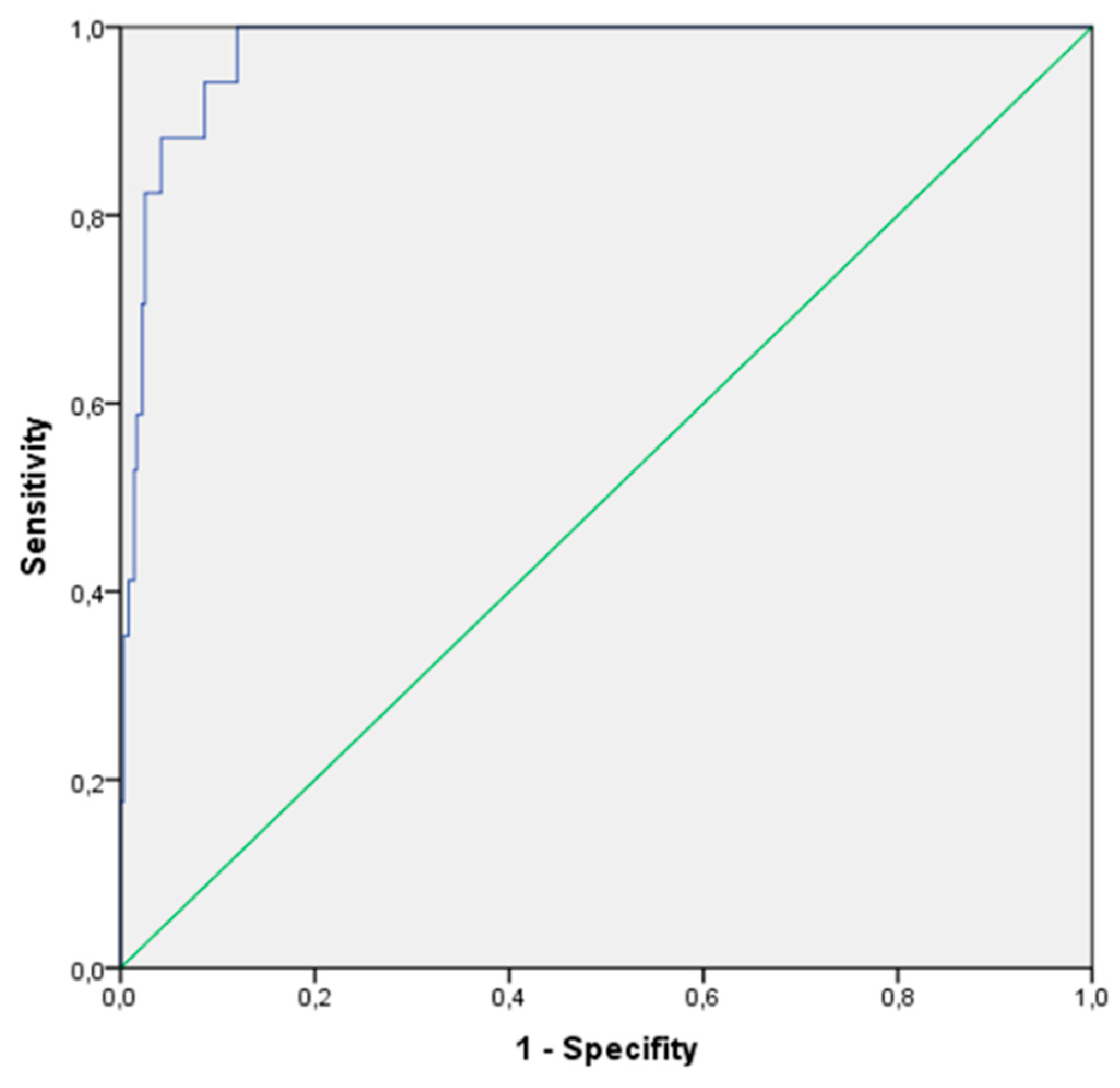
Figure 3 illustrates the area under the curve (AUC) of the obtained data, which was 0.976, suggesting excellent discriminative capacity of the diagnostic test model. The standard error was 0.009, indicating precise determination. The value obtained was significant at a 95% confidence interval (0.958-0.994). At the DBS cholesterol cut-off point of 223.71 mg/dL, the sensitivity of the test was 100%, identifying all positive cases, with a specificity of 88%.
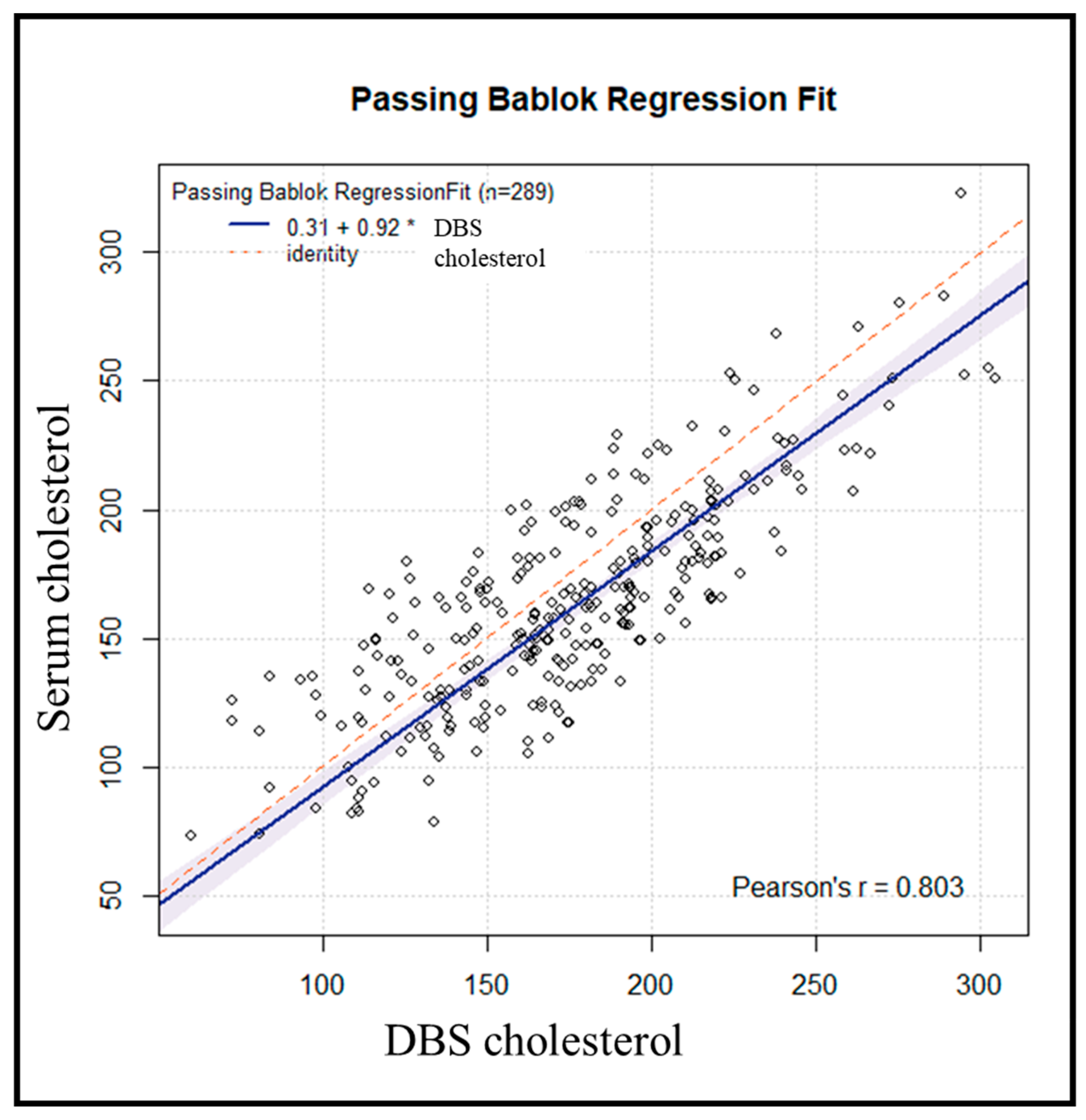
We used the Passing-Bablok regression (
Figure 4) to assess the agreement, accuracy, and precision of cholesterol measurement using DBS versus serum as the standard method. The Pearson correlation coefficient of 0.80 suggests a strong and consistent association between DBS and serum measurements. The accuracy value was 0.9604, suggesting high accuracy of the DBS method. The resulting regression line had a slope of 0.9174, with the Passing-Bablok regression plot yielding a regression equation (y = 0.31 + 0.92x, r = 0.80).
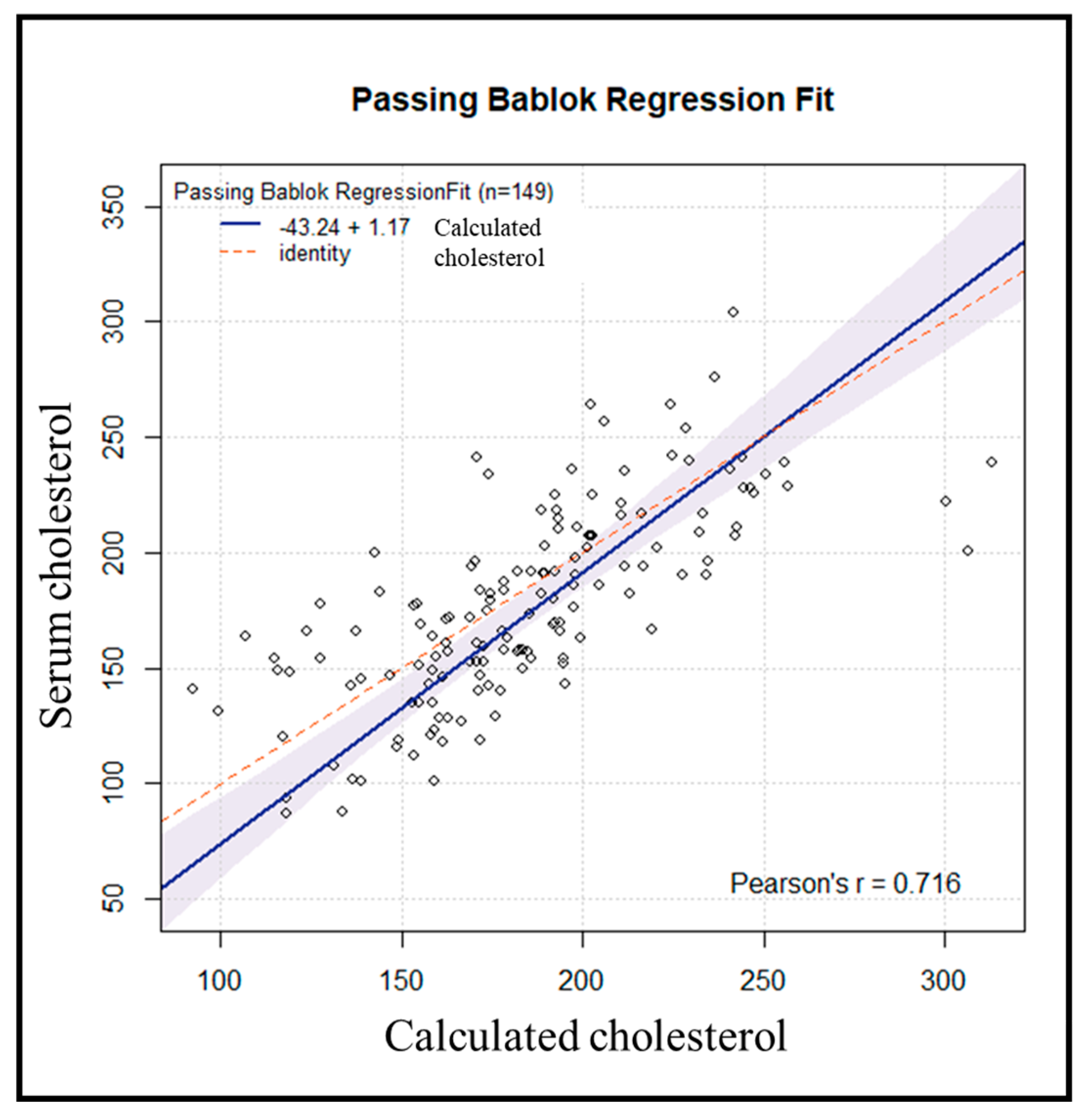
Subsequently, an internal validation of the method was conducted on 155 samples processed in the same manner. Six samples were excluded due to variations > 50 mg/dL between repetitions. Using the previously obtained regression line, the cholesterol value for each subject was estimated from the resulting DBS cholesterol value. Comparing the results of serum cholesterol versus calculated cholesterol yielded a Passing-Bablok regression line (y = -43.24 + 1.17x, r = 0.72) (
Figure 5).
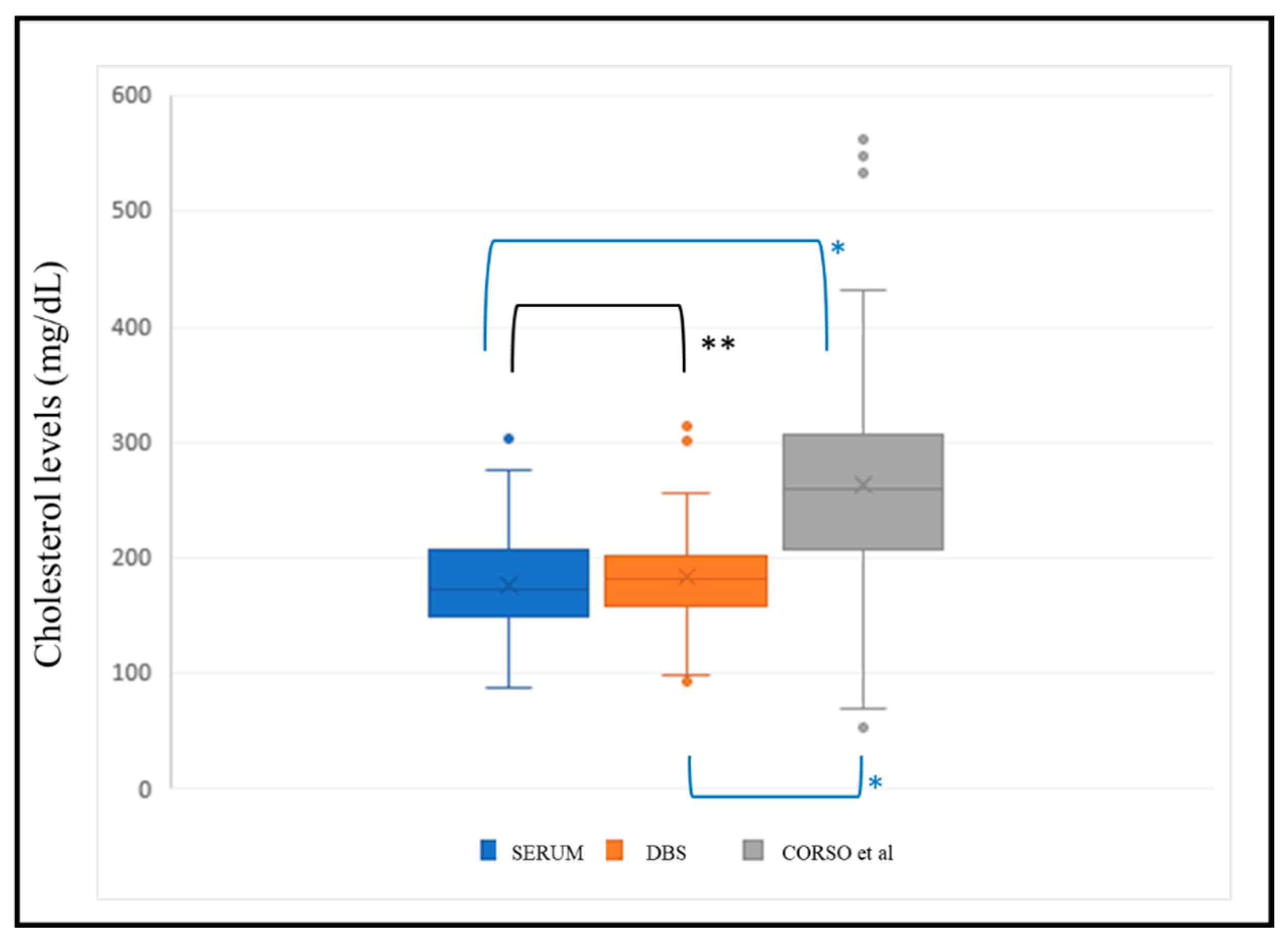
We applied the regression line (y = 0.5217x + 64.86) with x = estimated serum cholesterol, y = DBS cholesterol from Corso et al.
10 to the same validation population (n=149) for external validation of the methodology. The Kolmogorov-Smirnov test indicated that cholesterol data estimated by both our formula and that of Corso et al.
11 did not follow a normal distribution. Consequently, non-parametric tests were conducted to determine significant differences between these data and the corresponding serum cholesterol value. The results revealed significant differences between the serum cholesterol value and that estimated by Corso et al.
10 (p<0.001), while there were no significant differences between the value estimated by our formula and the serum value (p=0.053) (
Figure 6).
4. Discussion
Familial hypercholesterolemia (FH) remains underdiagnosed despite being recognized as a public health priority by the World Health Organization (WHO) in 198813. Approximately 450,000 children are born with FH annually worldwide, yet only 2.1% of adults with FH are diagnosed before the age of 18 using current diagnostic approaches14.
The increase in cholesterol levels since birth presents an opportunity for early FH diagnosis. Early identification and cholesterol reduction can prevent premature ASCVD. Implementing universal screening for FH in childhood is a logical approach to close the gap between prevalence and detection. This approach is in line with the 2020 WHO-UNICEF-Lancet Commission Strategy15, which emphasized the importance of early preventive interventions in childhood over corrective actions in adulthood.
In this study, we developed and validated a method for extracting and analyzing total cholesterol from DBS. DBS analysis reduces the invasiveness of blood sampling and requires only a small sample volume, facilitating easy transportation to laboratories.
The precision results obtained were within acceptable limits (CV 10%) for total cholesterol determination. Cholesterol concentrations remained stable over 27 consecutive days of measurement.
Sample preparation quality significantly influences both analysis and cholesterol extraction. The use of calibrated and certified filter paper cards is crucial for the analysis of blood biomarkers, in addition, the amount of blood that is deposited must be equal to or greater than 70 μL with homogeneous distribution to minimize variability. Each determination was accompanied by a calibration curve and internal controls analyzed under consistent conditions on the same day. Sample determination was performed in duplicate. Comparisons of daily medians on a Levey-Jenning chart was performed, excluding those that exceeded two standard deviations. The final result was accepted if it fulfilled all the quality conditions.
Comparisons between serum cholesterol and DBS determinations of concurrently obtained samples from research participants using the applied algorithm showed positive agreement. DBS cholesterol values were consistently higher, attributed to the presence of cholesterol in red blood cells. This finding is consistent with reports by Corso11 and Held9-10. In our study, the observed difference was smaller (11 mg/dL) compared to 30 mg/dL in previous studies10, possibly due to the 16% residual cholesterol present in the paper post-extraction, as described by Corso et al.11
4.1. Study Limitations
Manual determination increases imprecision compared to automated analyzers, but currently, no autoanalyzers offer such technology. Standardizing sample collection with paper is challenging, requiring prior extraction and analyte dilution.
4.2. Advantages
Universal neonatal screening uses DBS. The validation of cholesterol determination in this sample can be used as a screening test for the diagnosis of FH. The determination of cholesterol in DBS has been carried out with instrumentation already available in neonatal screening laboratories and the cost of the determination is equivalent to the cost of the reagent, facilitating adoption in all screening laboratories for this disease.
4.3. Future
Further population studies with neonatal DBS samples are needed to establish cholesterol level cutoffs for confirmatory genetic testing. Evaluating cost-effectiveness and healthcare circuits will aid in expanding this procedure.
5. Conclusions
Cholesterol determination using DBS is a cost-effective, accessible, reproducible screening method for selecting individuals at risk of FH. Confirmatory genetic testing remains essential for diagnosis.
Author Contributions
Conceptualization, Bonet Estruch, E.; Gutiérrez-Cortizo, E.N.; Mata, P. and Romero-Jiménez, M.J.; methodology, Bonet Estruch, E.; López-Lara, M.J and Castaño López, M.A.; software, Castaño López, M.A.; validation, Bonet Estruch, E.; López-Lara, M.J. and Romero-Jiménez, M.J; formal analysis, López-Lara, M.J; investigation, Bonet Estruch, E. and López-Lara, M.J.; resources, Mata, P.; data curation, Bonet Estruch, E.; López-Lara, M.J and Castaño López, M.A.; writing—original draft preparation, Bonet Estruch, E., Gutiérrez-Cortizo, E.N. and Romero-Jiménez, M.J.; writing—review and editing, all authors.; visualization, all authors.; supervision, Romero-Jiménez, M.J; project administration, Romero-Jiménez, M.J.; funding acquisition, Mata P. and Romero-Jiménez, M.J. All authors have read and agreed to the published version of the manuscript.
Funding
The Lipid and Vascular Risk Unit of the Infanta Elena Hospital has received funding support from the project with protocol code : CRINEO HF HUELVA-2022-01 presented in public call FPS 2020 -I+I projects in primary care, county hospitals and CHARES with identification code: AP-0407-2023-C4-F2.
Institutional Review Board Statement
The study was conducted in accordance with the Declaration of Helsinki, and received approval from the Ethics Committee of the province of Huelva on July 21, 2023. (Protocol code: CHOLESTEROL BDS-2023-01, titled “Accuracy of Blood Cholesterol Determination on Whatman 903 Paper (DBS Cholesterol Validation)”)
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study
Data Availability Statement
The original contributions presented in the study are included in the article, further inquiries can be directed to the corresponding author.
Conflicts of Interest
The authors declare no conflicts of interest.
Abbreviations
| Familial Hypercholesterolemia |
FH |
| Atherosclerotic Cardiovascular Disease |
ASCVD |
| Dried Blood Spot |
DBS |
| Quality Control |
QC |
| Ethylenediaminetetraacetic acid |
EDTA |
| Coefficient of Variation |
CV |
| Standard Deviation |
SD |
| Interquartile Range |
IQR |
| Area Under the Curve |
AUC |
| World Health Organization |
WHO |
References
- Watts, G.F.; Gidding, S.S.; Hegele, R.A.; Raal, F.J.; Sturm, A.C.; Jones, L.K.; Sarkies, M.N.; Al-Rasadi, K.; Blom, D.J.; Daccord, M.; et al. International Atherosclerosis Society guidance for implementing best practice in the care of familial hypercholesterolaemia. Nat. Rev. Cardiol. 2023, 20, 845–869. [Google Scholar] [CrossRef] [PubMed]
- Wald, D.S.; Bestwick, J.P. Reaching detection targets in familial hypercholesterolaemia: Comparison of identification strategies. Atherosclerosis 2020, 293, 57–61. [Google Scholar] [CrossRef]
- Hu P, Dharmayat KI, Stevens CAT, Sharabiani MTA, Jones RS, Watts GF, et al. Prevalence of Familial Hypercholesterolemia among the General Population and Patients with Atherosclerotic Cardiovascular Disease: A Systematic Review and Meta-Analysis. Circulation. 2020;1742–59.
- Elis, A.; Leventer-Roberts, M.; Bachrach, A.; Lieberman, N.; Durst, R.; Knobler, H.; Balicer, R. The characteristics of patients with possible familial hypercholesterolemia—screening a large payer/provider healthcare delivery system. Qjm: Int. J. Med. 2019, 113, 411–417. [Google Scholar] [CrossRef]
- Representatives of the Global Familial Hypercholesterolemia Community; Wilemon, K. A.; Patel, J.; Aguilar-Salinas, C.; Ahmed, C.D.; Alkhnifsawi, M.; Almahmeed, W.; Alonso, R.; Al-Rasadi, K.; Badimon, L.; et al. Reducing the Clinical and Public Health Burden of Familial Hypercholesterolemia. JAMA Cardiol. 2020, 5, 217–229. [Google Scholar] [CrossRef]
- B. Jahn et al. Familial hypercholesterolemia: A systematic review of modeling studies on screening interventions Atherosclerosis 355 (2022) 15–29.
- Lázaro, P.; de Isla, L.P.; Watts, G.F.; Alonso, R.; Norman, R.; Muñiz, O.; Fuentes, F.; Mata, N.; López-Miranda, J.; González-Juanatey, J.R.; et al. Cost-effectiveness of a cascade screening program for the early detection of familial hypercholesterolemia. J. Clin. Lipidol. 2017, 11, 260–271. [Google Scholar] [CrossRef] [PubMed]
- Desai, N.R.; Farbaniec, M.; Karalis, D.G. Nonadherence to lipid-lowering therapy and strategies to improve adherence in patients with atherosclerotic cardiovascular disease. Clin. Cardiol. 2022, 46, 13–21. [Google Scholar] [CrossRef]
- Held, P.K.; Campbell, K.; Wiberley-Bradford, A.E.; Lasarev, M.; Horner, V.; Peterson, A. Analytical Validation of Familial Hypercholesterolemia Biomarkers in Dried Blood Spots. Int. J. Neonatal Screen. 2022, 8, 14. [Google Scholar] [CrossRef]
- Held, P.K.; Lasarev, M.; Zhang, X.; Wiberley-Bradford, A.E.; Campbell, K.; Horner, V.; Shao, X.; Benoy, M.; Dodge, A.M.; Peterson, A.L. Familial Hypercholesterolemia Biomarker Distribution in Dried Blood Spots. J. Pediatr. 2023, 259, 113469. [Google Scholar] [CrossRef]
- Corso, G.; Papagni, F.; Gelzo, M.; Gallo, M.; Barone, R.; Graf, M.; Scarpato, N.; Russo, A.D. Development and Validation of an Enzymatic Method for Total Cholesterol Analysis Using Whole Blood Spot. J. Clin. Lab. Anal. 2015, 30, 517–523. [Google Scholar] [CrossRef] [PubMed]
- Bossuyt PM, Reitsma JB, Bruns DE, Gatsonis CA, Glasziou PP, Irwig L, et al; STARD Group. STARD 2015: an updated list of essential items for reporting diagnostic accuracy studies. BMJ. 2015 Oct 28;351:h5527.
- Wilemon KA, Patel J, Aguilar-Salinas C, et al. Reducing the clinical and public health burden of familial hypercholesterolemia: a global call to action. JAMA Cardiol 2020; 5: 217–29.
- Mary P McGowan,Marina Cuche. Familial hypercholesterolaemia in children and adolescents from 48 countries: a cross-sectional study. Lancet 2024; 403: 55–66.
- Clark H, Coll-Seck AM, Banerjee A, et al. A future for the world’s children? A WHO–UNICEF–Lancet Commission. Lancet 2020; 395: 605–58.
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).