Submitted:
09 July 2024
Posted:
11 July 2024
You are already at the latest version
Abstract
Keywords:
Introduction
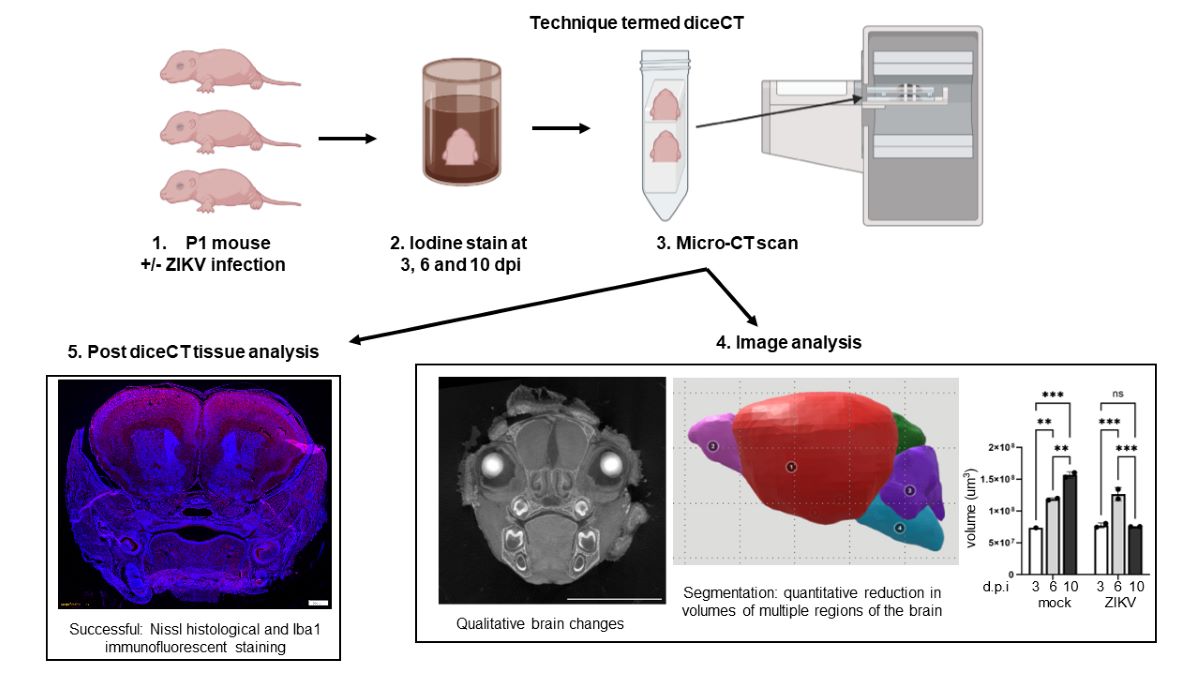
Materials and Methods
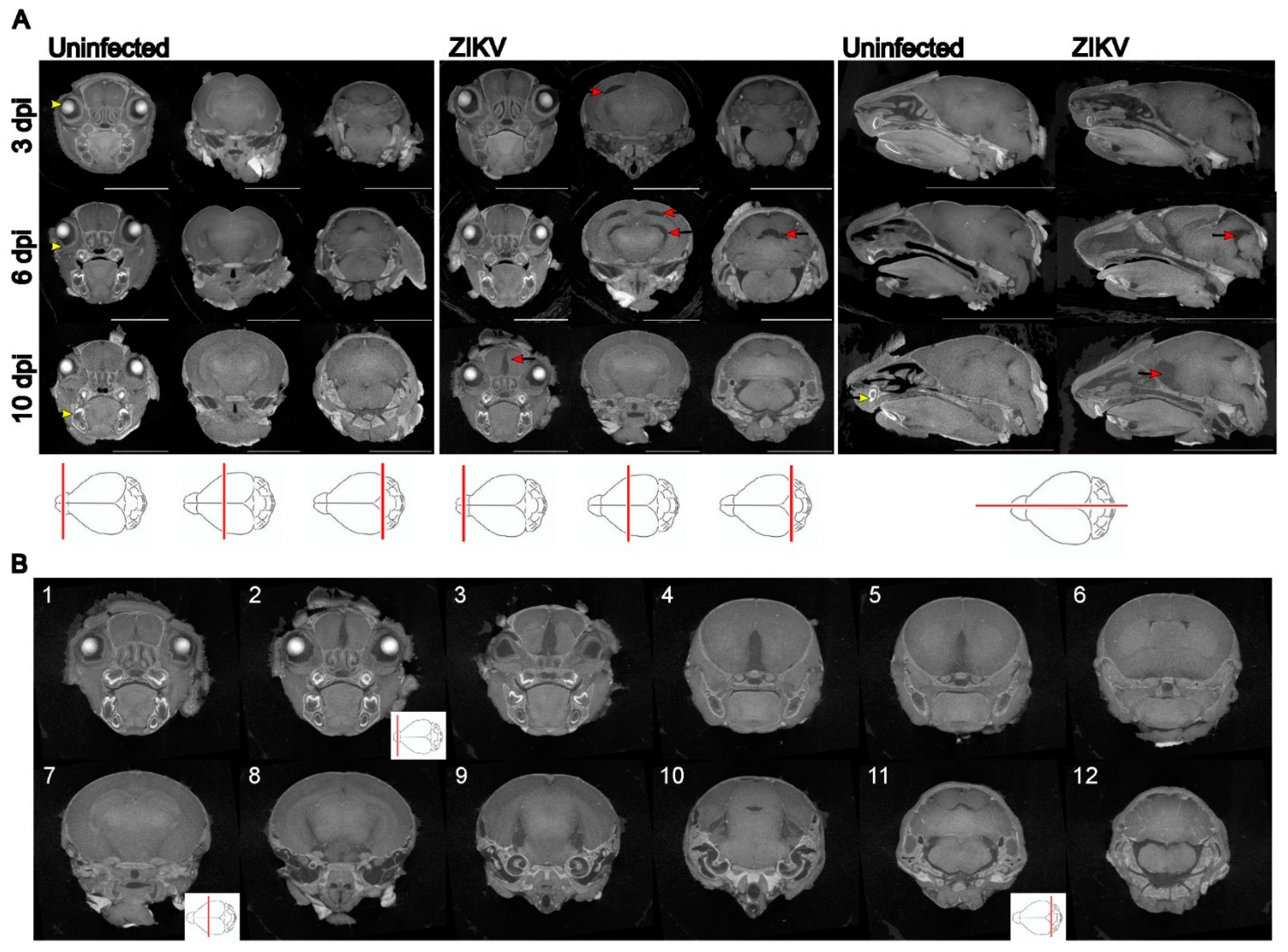
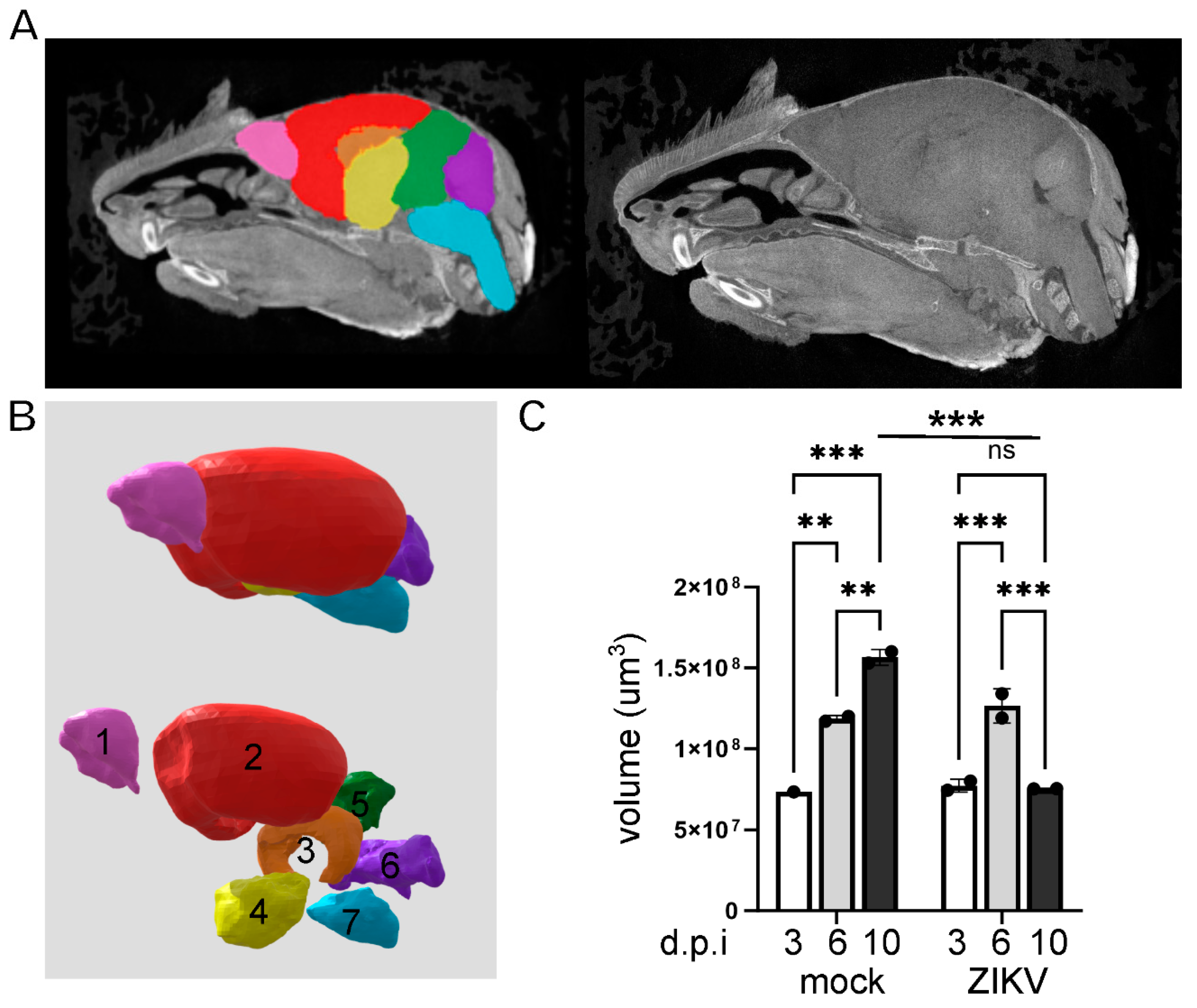
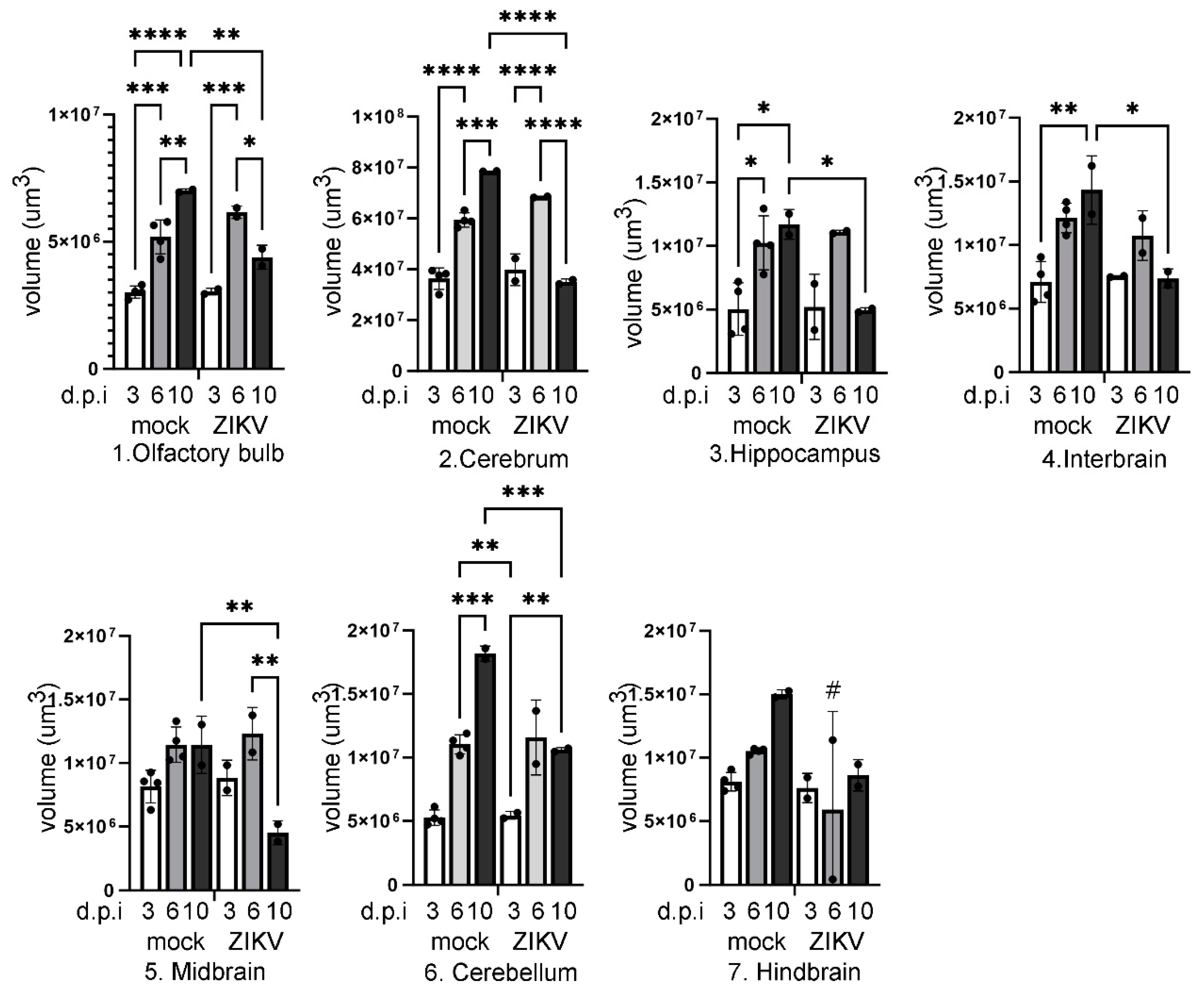
Results
Discussion
Data Availability Statement
Acknowledgements
References
- Musso, D.; Gubler, D.J. Zika Virus. Clin Microbiol Rev 2016, 29, 487–524. [Google Scholar] [CrossRef] [PubMed]
- Panchaud, A.; Stojanov, M.; Ammerdorffer, A.; Vouga, M.; Baud, D. Emerging Role of Zika Virus in Adverse Fetal and Neonatal Outcomes. Clin Microbiol Rev 2016, 29, 659–694. [Google Scholar] [CrossRef] [PubMed]
- Honein, M.A. Recognizing the Global Impact of Zika Virus Infection during Pregnancy. N Engl J Med 2018, 378, 1055–1056. [Google Scholar] [CrossRef] [PubMed]
- Pierson, T.C.; Diamond, M.S. The emergence of Zika virus and its new clinical syndromes. Nature 2018, 560, 573–581. [Google Scholar] [CrossRef] [PubMed]
- Hoen, B.; Schaub, B.; Funk, A.L.; Ardillon, V.; Boullard, M.; Cabie, A.; Callier, C.; Carles, G.; Cassadou, S.; Cesaire, R.; et al. Pregnancy Outcomes after ZIKV Infection in French Territories in the Americas. N Engl J Med 2018, 378, 985–994. [Google Scholar] [CrossRef] [PubMed]
- Freitas, D.A.; Souza-Santos, R.; Carvalho, L.M.A.; Barros, W.B.; Neves, L.M.; Brasil, P.; Wakimoto, M.D. Congenital Zika syndrome: A systematic review. PLoS ONE 2020, 15, e0242367. [Google Scholar] [CrossRef] [PubMed]
- DeSilva, M.; Munoz, F.M.; Sell, E.; Marshall, H.; Tse Kawai, A.; Kachikis, A.; Heath, P.; Klein, N.P.; Oleske, J.M.; Jehan, F.; et al. Congenital microcephaly: Case definition & guidelines for data collection, analysis, and presentation of safety data after maternal immunisation. Vaccine 2017, 35, 6472–6482. [Google Scholar] [PubMed]
- Mlakar, J.; Korva, M.; Tul, N.; Popovic, M.; Poljsak-Prijatelj, M.; Mraz, J.; Kolenc, M.; Resman Rus, K.; Vesnaver Vipotnik, T.; Fabjan Vodusek, V.; et al. Zika Virus Associated with Microcephaly. N Engl J Med 2016, 374, 951–958. [Google Scholar] [CrossRef] [PubMed]
- Marban-Castro, E.; Gonce, A.; Fumado, V.; Romero-Acevedo, L.; Bardaji, A. Zika virus infection in pregnant women and their children: A review. Eur J Obstet Gynecol Reprod Biol 2021, 265, 162–168. [Google Scholar] [CrossRef]
- Mulkey, S.B.; Arroyave-Wessel, M.; Peyton, C.; Bulas, D.I.; Fourzali, Y.; Jiang, J.; Russo, S.; McCarter, R.; Msall, M.E.; du Plessis, A.J.; et al. Neurodevelopmental Abnormalities in Children With In Utero Zika Virus Exposure Without Congenital Zika Syndrome. JAMA Pediatr 2020, 174, 269–276. [Google Scholar] [CrossRef]
- Ghosh, S.; Salan, T.; Riotti, J.; Ramachandran, A.; Gonzalez, I.A.; Bandstra, E.S.; Reyes, F.L.; Andreansky, S.S.; Govind, V.; Saigal, G. Brain MRI segmentation of Zika-Exposed normocephalic infants shows smaller amygdala volumes. PLoS ONE 2023, 18, e0289227. [Google Scholar] [CrossRef]
- Mejdoubi, M.; Monthieux, A.; Cassan, T.; Lombard, C.; Flechelles, O.; Adenet, C. Brain MRI in Infants after Maternal Zika Virus Infection during Pregnancy. N Engl J Med 2017, 377, 1399–1400. [Google Scholar] [CrossRef]
- Melo, A.S.; Aguiar, R.S.; Amorim, M.M.; Arruda, M.B.; Melo, F.O.; Ribeiro, S.T.; Batista, A.G.; Ferreira, T.; Dos Santos, M.P.; Sampaio, V.V.; et al. Congenital Zika Virus Infection: Beyond Neonatal Microcephaly. JAMA Neurol 2016, 73, 1407–1416. [Google Scholar] [CrossRef] [PubMed]
- Ribeiro, B.N.F.; Muniz, B.C.; Gasparetto, E.L.; Ventura, N.; Marchiori, E. Congenital Zika syndrome and neuroimaging findings: What do we know so far? Radiol Bras 2017, 50, 314–322. [Google Scholar] [CrossRef]
- Schuler-Faccini, L.; Del Campo, M.; Garcia-Alix, A.; Ventura, L.O.; Boquett, J.A.; van der Linden, V.; Pessoa, A.; van der Linden Junior, H.; Ventura, C.V.; Leal, M.C.; et al. Neurodevelopment in Children Exposed to Zika in utero: Clinical and Molecular Aspects. Front Genet 2022, 13, 758715. [Google Scholar] [CrossRef] [PubMed]
- de Fatima Vasco Aragao, M.; van der Linden, V.; Brainer-Lima, A.M.; Coeli, R.R.; Rocha, M.A.; Sobral da Silva, P.; Durce Costa Gomes de Carvalho, M.; van der Linden, A.; Cesario de Holanda, A.; Valenca, M.M. Clinical features and neuroimaging (CT and MRI) findings in presumed Zika virus related congenital infection and microcephaly: Retrospective case series study. BMJ 2016, 353, i1901. [Google Scholar] [CrossRef] [PubMed]
- Mulkey, S.B.; Bulas, D.I.; Vezina, G.; Fourzali, Y.; Morales, A.; Arroyave-Wessel, M.; Swisher, C.B.; Cristante, C.; Russo, S.M.; Encinales, L.; et al. Sequential Neuroimaging of the Fetus and Newborn With In Utero Zika Virus Exposure. JAMA Pediatr 2019, 173, 52–59. [Google Scholar] [CrossRef]
- Niemeyer, B.; Hollanda, R.; Muniz, B.; Marchiori, E. What We Can Find Beyond the Classic Neuroimaging Findings of Congenital Zika Virus Syndrome? Eur Neurol 2020, 83, 17–24. [Google Scholar] [CrossRef] [PubMed]
- de Paula Freitas, B.; Ventura, C.V.; Maia, M.; Belfort, R. Jr. Zika virus and the eye. Current opinion in ophthalmology 2017, 28, 595–599. [Google Scholar] [CrossRef] [PubMed]
- Oliver, G.F.; Carr, J.M.; Smith, J.R. Emerging infectious uveitis: Chikungunya, dengue, Zika and Ebola-A review. Clinical & experimental ophthalmology 2018. [Google Scholar]
- Marcelino, B.L.M.; Dos Santos, B.L.; Doerl, J.G.; Cavalcante, S.F.; Maia, S.N.; Arrais, N.M.R.; Zin, A.; Jeronimo, S.M.B.; Queiroz, C.; Hedin-Pereira, C.; et al. Zika virus infection histories in brain development. Dis Model Mech 2023, 16. [Google Scholar] [CrossRef] [PubMed]
- He, Z.; An, S.; Chen, J.; Zhang, S.; Tan, C.; Yu, J.; Ye, H.; Wu, Y.; Yuan, J.; Wu, J.; et al. Neural progenitor cell pyroptosis contributes to Zika virus-induced brain atrophy and represents a therapeutic target. Proc Natl Acad Sci U S A 2020, 117, 23869–23878. [Google Scholar] [CrossRef] [PubMed]
- Garcez, P.P.; Loiola, E.C.; Madeiro da Costa, R.; Higa, L.M.; Trindade, P.; Delvecchio, R.; Nascimento, J.M.; Brindeiro, R.; Tanuri, A.; Rehen, S.K. Zika virus impairs growth in human neurospheres and brain organoids. Science 2016, 352, 816–818. [Google Scholar] [CrossRef] [PubMed]
- Komarasamy, T.V.; Adnan, N.A.A.; James, W.; Balasubramaniam, V. Zika Virus Neuropathogenesis: The Different Brain Cells, Host Factors and Mechanisms Involved. Frontiers in immunology 2022, 13, 773191. [Google Scholar] [CrossRef]
- Li, C.; Xu, D.; Ye, Q.; Hong, S.; Jiang, Y.; Liu, X.; Zhang, N.; Shi, L.; Qin, C.F.; Xu, Z. Zika Virus Disrupts Neural Progenitor Development and Leads to Microcephaly in Mice. Cell Stem Cell 2016, 19, 120–126. [Google Scholar] [CrossRef]
- Rubio-Hernandez, E.I.; Comas-Garcia, M.; Coronado-Ipina, M.A.; Colunga-Saucedo, M.; Gonzalez Sanchez, H.M.; Castillo, C.G. Astrocytes derived from neural progenitor cells are susceptible to Zika virus infection. PLoS ONE 2023, 18, e0283429. [Google Scholar] [CrossRef]
- Veilleux, C.; Eugenin, E.A. Mechanisms of Zika astrocyte infection and neuronal toxicity. NeuroImmune Pharm Ther 2023, 2, 5–18. [Google Scholar] [CrossRef] [PubMed]
- Miner, J.J.; Cao, B.; Govero, J.; Smith, A.M.; Fernandez, E.; Cabrera, O.H.; Garber, C.; Noll, M.; Klein, R.S.; Noguchi, K.K.; et al. Zika Virus Infection during Pregnancy in Mice Causes Placental Damage and Fetal Demise. Cell 2016, 165, 1081–1091. [Google Scholar] [CrossRef]
- Cowell, E.; Kris, L.P.; Bracho-Granado, G.; Jaber, H.; Smith, J.R.; Carr, J.M. Zika virus infection of retinal cells and the developing mouse eye induces host responses that contrasts to the brain and dengue virus infection. J Neurovirol 2023, 29, 187–202. [Google Scholar] [CrossRef]
- Li, Y.; Shi, S.; Xia, F.; Shan, C.; Ha, Y.; Zou, J.; Adam, A.; Zhang, M.; Wang, T.; Liu, H.; et al. Zika virus induces neuronal and vascular degeneration in developing mouse retina. Acta Neuropathol Commun 2021, 9, 97. [Google Scholar] [CrossRef]
- van den Pol, A.N.; Mao, G.; Yang, Y.; Ornaghi, S.; Davis, J.N. Zika Virus Targeting in the Developing Brain. J Neurosci 2017, 37, 2161–2175. [Google Scholar] [CrossRef]
- Nem de Oliveira Souza, I.; Frost, P.S.; Franca, J.V.; Nascimento-Viana, J.B.; Neris, R.L.S.; Freitas, L.; Pinheiro, D.; Nogueira, C.O.; Neves, G.; Chimelli, L.; et al. Acute and chronic neurological consequences of early-life Zika virus infection in mice. Science translational medicine 2018, 10, eaar2749. [Google Scholar] [CrossRef] [PubMed]
- Snyder-Keller, A.; Bolivar, V.J.; Zink, S.; Kramer, L.D. Brain Iron Accumulation and the Formation of Calcifications After Developmental Zika Virus Infection. J Neuropathol Exp Neurol 2020, 79, 767–776. [Google Scholar] [CrossRef]
- Snyder-Keller, A.; Kramer, L.D.; Zink, S.; Bolivar, V.J. Mouse Strain and Sex-Dependent Differences in Long-term Behavioral Abnormalities and Neuropathologies after Developmental Zika Infection. J Neurosci 2019, 39, 5393–5403. [Google Scholar] [CrossRef]
- Ireland, D.D.C.; Manangeeswaran, M.; Lewkowicz, A.P.; Engel, K.; Clark, S.M.; Laniyan, A.; Sykes, J.; Lee, H.N.; McWilliams, I.L.; Kelley-Baker, L.; et al. Long-term persistence of infectious Zika virus: Inflammation and behavioral sequela in mice. PLoS Pathog 2020, 16, e1008689. [Google Scholar] [CrossRef]
- Clancy, B.; Darlington, R.B.; Finlay, B.L. Translating developmental time across mammalian species. Neuroscience 2001, 105, 7–17. [Google Scholar] [CrossRef] [PubMed]
- Semple, B.D.; Blomgren, K.; Gimlin, K.; Ferriero, D.M.; Noble-Haeusslein, L.J. Brain development in rodents and humans: Identifying benchmarks of maturation and vulnerability to injury across species. Prog Neurobiol 2013, 106–107, 1–16. [Google Scholar] [CrossRef]
- Metscher, B.D. MicroCT for comparative morphology: Simple staining methods allow high-contrast 3D imaging of diverse non-mineralized animal tissues. BMC Physiol 2009, 9, 11. [Google Scholar] [CrossRef] [PubMed]
- Gignac, P.M.; Kley, N.J. Iodine-enhanced micro-CT imaging: Methodological refinements for the study of the soft-tissue anatomy of post-embryonic vertebrates. J Exp Zool B Mol Dev Evol 2014, 322, 166–176. [Google Scholar] [CrossRef]
- Camilieri-Asch, V.; Shaw, J.A.; Mehnert, A.; Yopak, K.E.; Partridge, J.C.; Collin, S.P. diceCT: A Valuable Technique to Study the Nervous System of Fish. eNeuro 2020, 7. [Google Scholar] [CrossRef]
- Nasrullah, Q.; Renfree, M.B.; Evans, A.R. Three-dimensional mammalian tooth development using diceCT. Arch Oral Biol 2018, 85, 183–191. [Google Scholar] [CrossRef] [PubMed]
- Callahan, S.; Crowe-Riddell, J.M.; Nagesan, R.S.; Gray, J.A.; Davis Rabosky, A.R. A guide for optimal iodine staining and high-throughput diceCT scanning in snakes. Ecol Evol 2021, 11, 11587–11603. [Google Scholar] [CrossRef] [PubMed]
- Gray, J.A.; Gignac, P.M.; Stanley, E.L. The first full body diffusible iodine-based contrast-enhanced computed tomography dataset and teaching materials for a member of the Testudines. Anat Rec (Hoboken) 2024, 307, 535–548. [Google Scholar] [CrossRef] [PubMed]
- Smith, T.D.; Corbin, H.M.; King, S.E.E.; Bhatnagar, K.P.; DeLeon, V.B. A comparison of diceCT and histology for determination of nasal epithelial type. PeerJ 2021, 9, e12261. [Google Scholar] [CrossRef] [PubMed]
- Yoakum, C.; Terhune, C. The inferior alveolar nerve and its relationship to the mandibular canal. Anat Rec (Hoboken) 2024, 307, 97–117. [Google Scholar] [CrossRef] [PubMed]
- Feldman, K.M.; O’Keefe, Y.A.; Gignac, P.M.; O’Brien, H.D. Highest resolution microCT scan of the human brainstem reveals putative anatomical basis for infrequency of medial medullary syndrome. Neuroimage Clin 2022, 36, 103272. [Google Scholar] [CrossRef] [PubMed]
- Gignac, P.M.; O’Brien, H.D.; Sanchez, J.; Vazquez-Sanroman, D. Multiscale imaging of the rat brain using an integrated diceCT and histology workflow. Brain Struct Funct 2021, 226, 2153–2168. [Google Scholar] [CrossRef]
- Klaunberg, B.A.; Lizak, M.J. Considerations for setting up a small-animal imaging facility. Lab Animal 2004, 33, 28–34. [Google Scholar] [CrossRef] [PubMed]
- Szulc, K.U.; Lerch, J.P.; Nieman, B.J.; Bartelle, B.B.; Friedel, M.; Suero-Abreu, G.A.; Watson, C.; Joyner, A.L.; Turnbull, D.H. 4D MEMRI atlas of neonatal FVB/N mouse brain development. Neuroimage 2015, 118, 49–62. [Google Scholar] [CrossRef]
- Cabezas, S.; Bracho, G.; Aloia, A.L.; Adamson, P.J.; Bonder, C.S.; Smith, J.R.; Gordon, D.L.; Carr, J.M. Dengue Virus Induces Increased Activity of the Complement Alternative Pathway in Infected Cells. J Virol 2018, 92. [Google Scholar] [CrossRef]
- Dawood, Y.; Hagoort, J.; Siadari, B.A.; Ruijter, J.M.; Gunst, Q.D.; Lobe, N.H.J.; Strijkers, G.J.; de Bakker, B.S.; van den Hoff, M.J.B. Reducing soft-tissue shrinkage artefacts caused by staining with Lugol’s solution. Sci Rep 2021, 11, 19781. [Google Scholar] [CrossRef] [PubMed]
- Thompson, C.L.; Ng, L.; Menon, V.; Martinez, S.; Lee, C.K.; Glattfelder, K.; Sunkin, S.M.; Henry, A.; Lau, C.; Dang, C.; et al. A high-resolution spatiotemporal atlas of gene expression of the developing mouse brain. Neuron 2014, 83, 309–323. [Google Scholar] [CrossRef] [PubMed]
- Fedorov, A.; Beichel, R.; Kalpathy-Cramer, J.; Finet, J.; Fillion-Robin, J.C.; Pujol, S.; Bauer, C.; Jennings, D.; Fennessy, F.; Sonka, M.; et al. 3D Slicer as an image computing platform for the Quantitative Imaging Network. Magn Reson Imaging 2012, 30, 1323–1341. [Google Scholar] [CrossRef] [PubMed]
- Rolfe, S.; Pieper, S.; Porto, A.; Diamond, K.; Winchester, J.; Shan, S.; Kirveslahti, H.; Boyer, D.; Summers, A.; Maga, A.M. SlicerMorph: An open and extensible platform to retrieve, visualize and analyse 3D morphology. Methods Ecol Evol 2021, 12, 1816–1825. [Google Scholar] [CrossRef]
- Cignoni, P.; Corsini, M.; Ranzuglia, G. MeshLab: An Open-Source 3D Mesh Processing System. Ercim News 2008, (73), 45–46. [Google Scholar]
- Kazhdan, M.; Hoppe, H. Screened Poisson Surface Reconstruction. Acm T Graphic 2013, 32, 1–13. [Google Scholar] [CrossRef]
- Team, R.C. R: A language and environment for statistical computing. URL https://www.R-project.org/.
- Gignac, P.M.; Kley, N.J.; Clarke, J.A.; Colbert, M.W.; Morhardt, A.C.; Cerio, D.; Cost, I.N.; Cox, P.G.; Daza, J.D.; Early, C.M.; et al. Diffusible iodine-based contrast-enhanced computed tomography (diceCT): An emerging tool for rapid, high-resolution, 3-D imaging of metazoan soft tissues. J Anat 2016, 228, 889–909. [Google Scholar] [CrossRef] [PubMed]
- Wurm, J.; Konttinen, H.; Andressen, C.; Malm, T.; Spittau, B. Microglia Development and Maturation and Its Implications for Induction of Microglia-Like Cells from Human iPSCs. Int J Mol Sci 2021, 22, 3088. [Google Scholar] [CrossRef] [PubMed]
- Chen, V.S.; Morrison, J.P.; Southwell, M.F.; Foley, J.F.; Bolon, B.; Elmore, S.A. Histology Atlas of the Developing Prenatal and Postnatal Mouse Central Nervous System, with Emphasis on Prenatal Days E7.5 to E18.5. 5 to E18.5. Toxicol Pathol 2017, 45, 705–744. [Google Scholar] [CrossRef]
- Sequerra, E.B.; Rocha, A.J.; de Medeiros, G.O.C.; Neto, M.M.; Maia, C.R.S.; Arrais, N.M.R.; Bezerra, M.; Jeronimo, S.M.B.; Barros, A.K.; Sousa, P.S.; et al. Association between brain morphology and electrophysiological features in Congenital Zika Virus Syndrome: A cross-sectional, observational study. EClinicalMedicine 2020, 26, 100508. [Google Scholar] [CrossRef]
- Harding, A.T.; Ocwieja, K.; Jeong, M.; Zhang, Y.; Leger, V.; Jhala, N.; Stankovic, K.M.; Gehrke, L. Human otic progenitor cell models of congenital hearing loss reveal potential pathophysiologic mechanisms of Zika virus and cytomegalovirus infections. mBio 2024, 15, e0019924. [Google Scholar] [CrossRef] [PubMed]
- Ginhoux, F.; Lim, S.; Hoeffel, G.; Low, D.; Huber, T. Origin and differentiation of microglia. Front Cell Neurosci 2013, 7, 45. [Google Scholar] [CrossRef] [PubMed]
- Hattori, Y. The multifaceted roles of embryonic microglia in the developing brain. Front Cell Neurosci 2023, 17, 988952. [Google Scholar] [CrossRef] [PubMed]
- Mehl, L.C.; Manjally, A.V.; Bouadi, O.; Gibson, E.M.; Tay, T.L. Microglia in brain development and regeneration. Development 2022, 149, dev200425. [Google Scholar] [CrossRef] [PubMed]
- Wen, W.; Cheng, J.; Tang, Y. Brain perivascular macrophages: Current understanding and future prospects. Brain 2024, 147, 39–55. [Google Scholar] [CrossRef] [PubMed]
- Linsley, J.W.; Reisine, T.; Finkbeiner, S. Three dimensional and four dimensional live imaging to study mechanisms of progressive neurodegeneration. J Biol Chem 2024, 300, 107433. [Google Scholar] [CrossRef]
- Rolfe, S.M.; Whikehart, S.M.; Maga, A.M. Deep learning enabled multi-organ segmentation of mouse embryos. Biol Open 2023, 12, bio059698. [Google Scholar] [CrossRef]
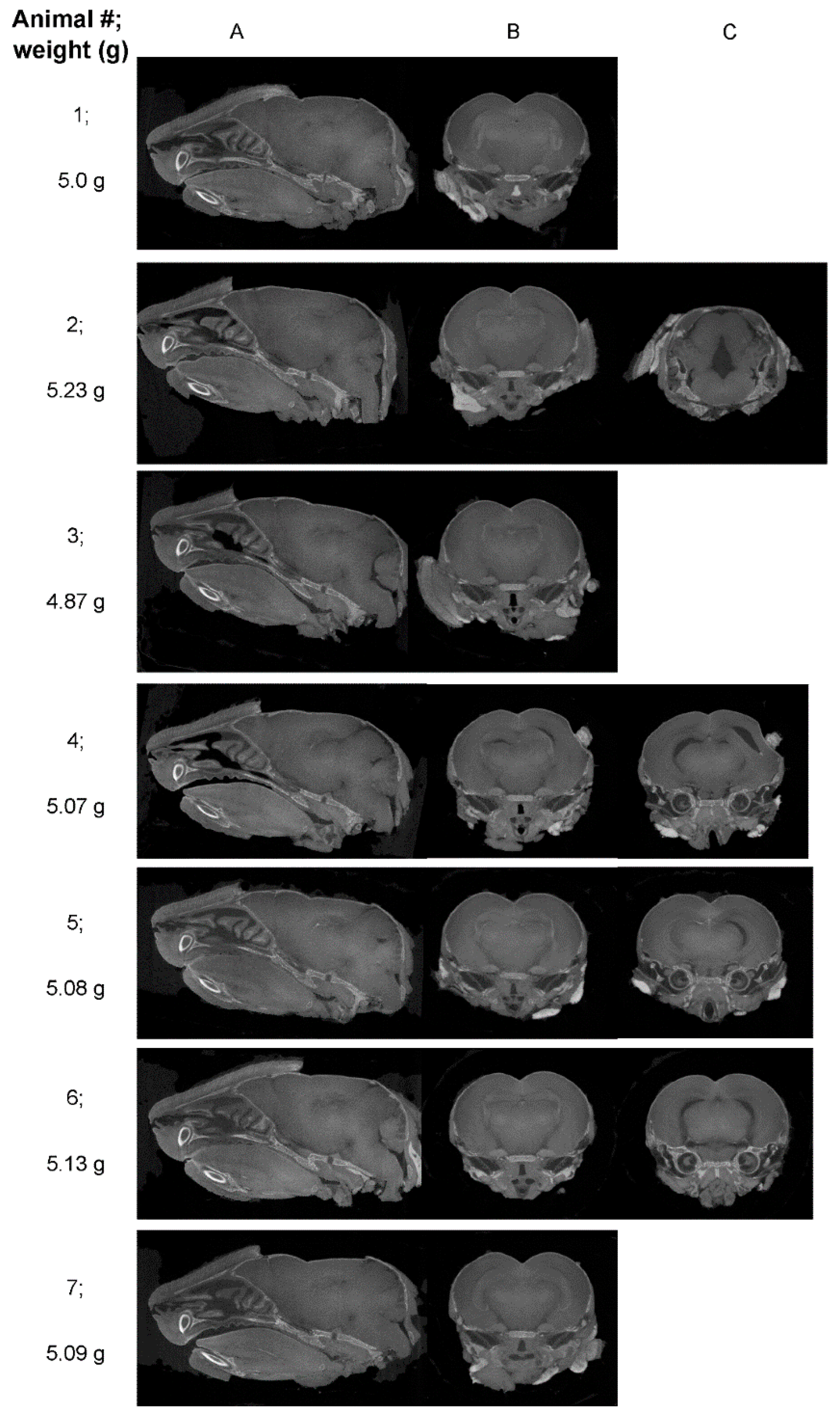
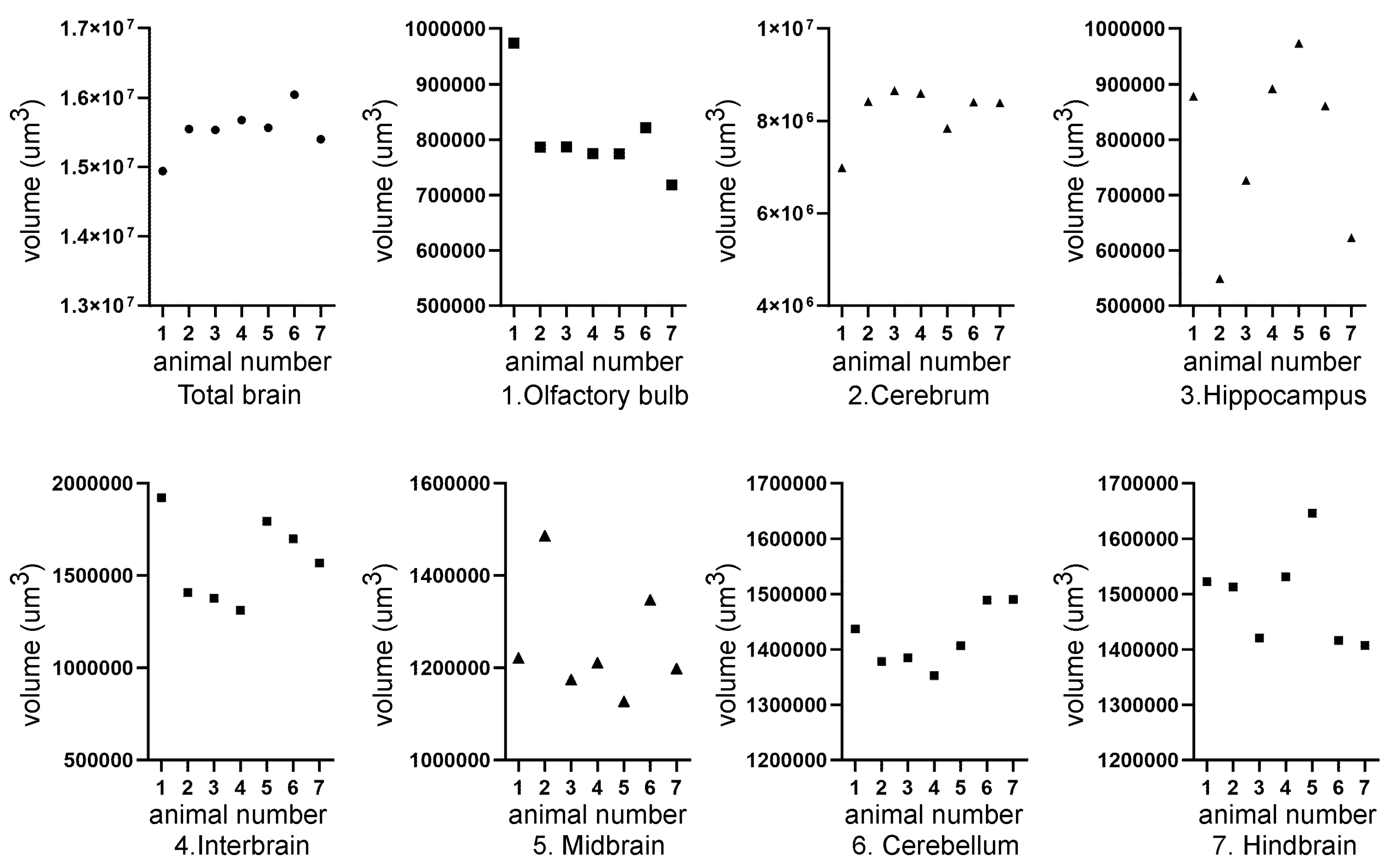
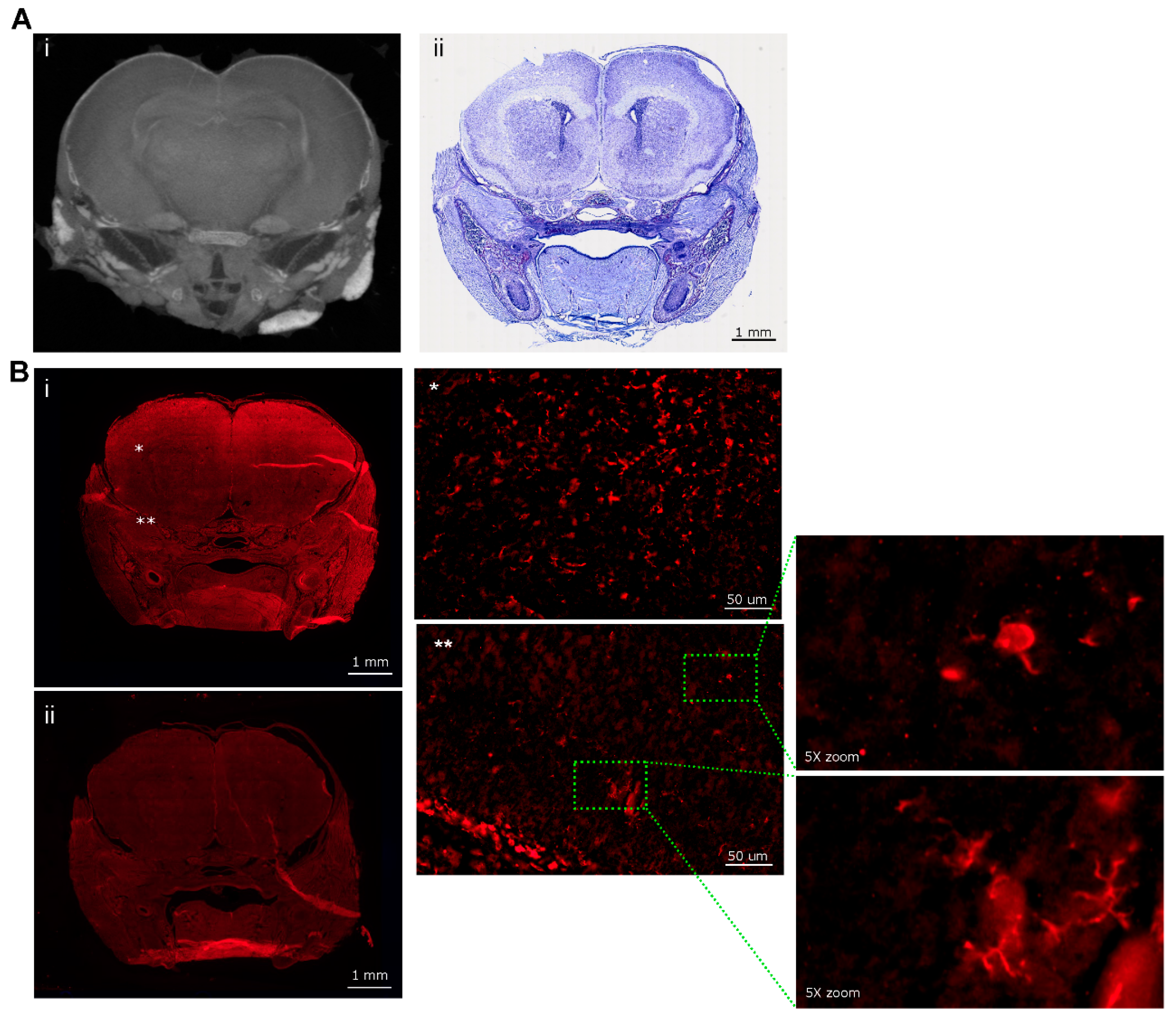
| Mouse ID | Weight (g) | Head length, L, width, W, depth, D (cm) | ZIKV RT-PCR | Movement |
|---|---|---|---|---|
| 1 | 6.40 | L=1.9; W=0.8; D=0.6 | positive | Mild hind limb dysfunction |
| 2 | 7.50 | L=2.3; W=1; D=1 | negative | normal |
| 3 | 6.64 | L=2.1; W=1.2; D=0.9 | positive | Mild hind limb dysfunction |
| 4 | 5.93 | L=2.1; W=0.9; D=0.7 | positive | Mild hind limb dysfunction |
| 5 | 7.37 | L=1.8; W=0.7; D=0.8 | negative | normal |
| 6 | 7.34 | L=2.1; W=0.9; D=0.7 | negative | normal |
| Mean ± SEM | Body Weight (g) | Head Length (cm) | Head Width (cm) | Head Depth (cm) |
| Mock (2, 5, 6) |
7.40 ± 0.05 | 2.17±0.07 | 1.03±0.09 | 0.87±0.09 |
| ZIKV (1, 3, 4) |
*6.32 ± 0.21 | 1.93±0.09 | 0.8±0.06 | 0.7±0.06 |
| p=0.007, t=5.042, df=4 | p=0.102, t=2.211, df=4 | p=0.091, t=2.214, df=4 | p=0.189, t=1.581, df=4 |
| Mouse ID | Reviewer 1 | Reviewer 2 | Reviewer 3 | Reviewer 4 |
|---|---|---|---|---|
| 1 | No pathological findings noted UI |
No pathological findings noted UI |
No pathological findings noted UI |
No pathological findings noted UI |
| 2 | Major gap at rear of brain ZIKV |
Asymmetrical gaps at the level of the hippocampus/thalamus ZIKV |
Asymmetrical, apparent volume loss in the hippocampus ZIKV |
Asymmetrical, increased space between hippocampal region and thalamus and towards the hind brain ZIKV |
| 3 | No pathological findings noted probably UI |
Some gaps, but no asymmetrical volume loss UI |
No pathological findings noted UI |
Slight gaps between hippocampal region and thalamus probably UI |
| 4 | Multiple asymmetrical gaps, penetrating across midbrain ZIKV |
Clear asymmetrical gaps between structures, with majority of abnormalities around thalamus and hippocampus, continuing through the posterior brain ZIKV |
Very clear asymmetrical abnormalities, particularly at level of posterior hippocampus, and continuing more posteriorly ZIKV |
Gaps between hippocampus and cortex (right) and thalamus (left); Gap (right) appears to go through majority of the brain ZIKV |
| 5 | Maybe minor anomalies; UD |
Asymmetrical gaps between hippocampus and thalamus ZIKV |
Abnormality in region anterior to nuc accumbens and at level of striatum (less defined); Clear asymmetry at the level of the poster hippocampus; ZIKV |
Gap in between hippocampal region and thalamus probably ZIKV |
| 6 | Symmetrical gaps, penetrating across images through midbrain ZIKV |
Clear gaps at hippocampus, continuing throughout posterior brain ZIKV |
Very clear abnormalities, particularly at level of posterior hippocampus and extending posteriorly ZIKV |
Gap in between hippocampal region and thalamus on left and right side, which runs through majority of brain ZIKV |
| 7 | Maybe minor anomalies UD |
Minor abnormalities UI |
Some minor abnormality, which may be an imaging artifact or normal anatomical variation UD |
Slight gap between hippocampal region and thalamus; probably UI |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





