Submitted:
11 July 2024
Posted:
11 July 2024
You are already at the latest version
Abstract
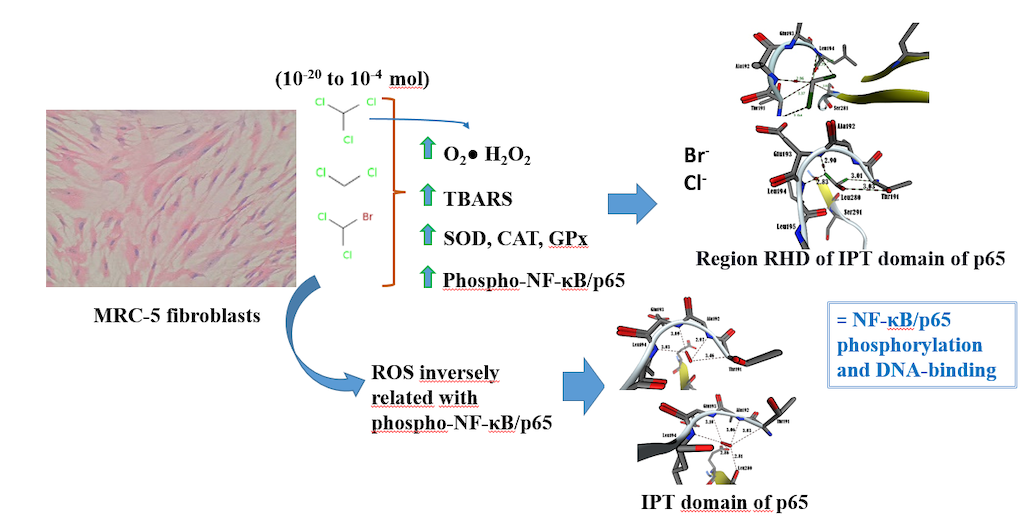
Keywords:
Introduction
Materials & Methods
Culture of Human Lung MRC-5 Fibroblasts and Treatments
Evaluation of Biomarkers
Quantification of ROS (O2• and H2O2) and Level of Lipid Peroxidation as Thiobarbituric Acid Reactive Substances (TBARS)
Activity of the Antioxidant Defenses (SOD, CAT and GPx)
Assay of Phosphorylated NF-κB/p65 Levels
Molecular Docking Analysis
Statistical Analysis
Results
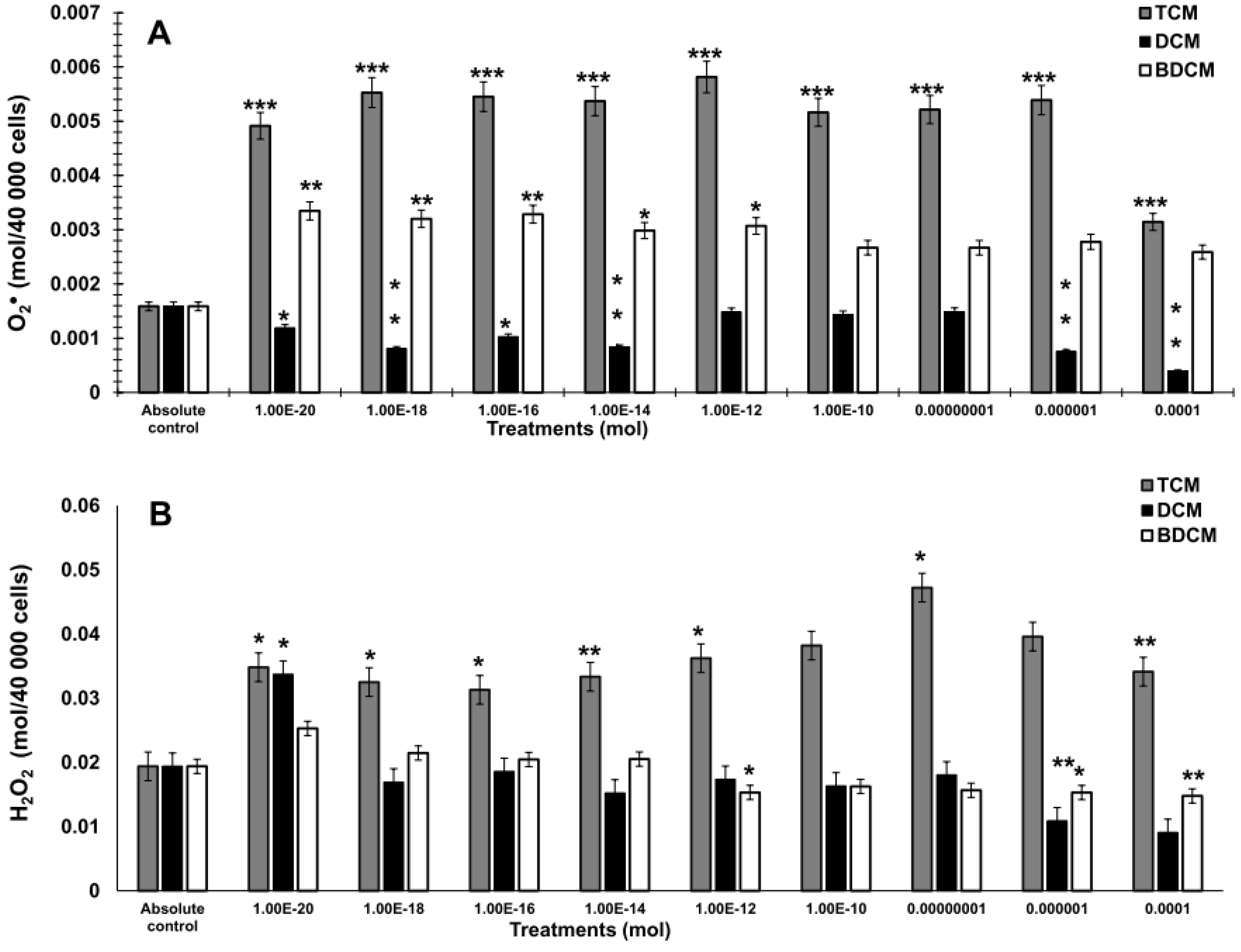
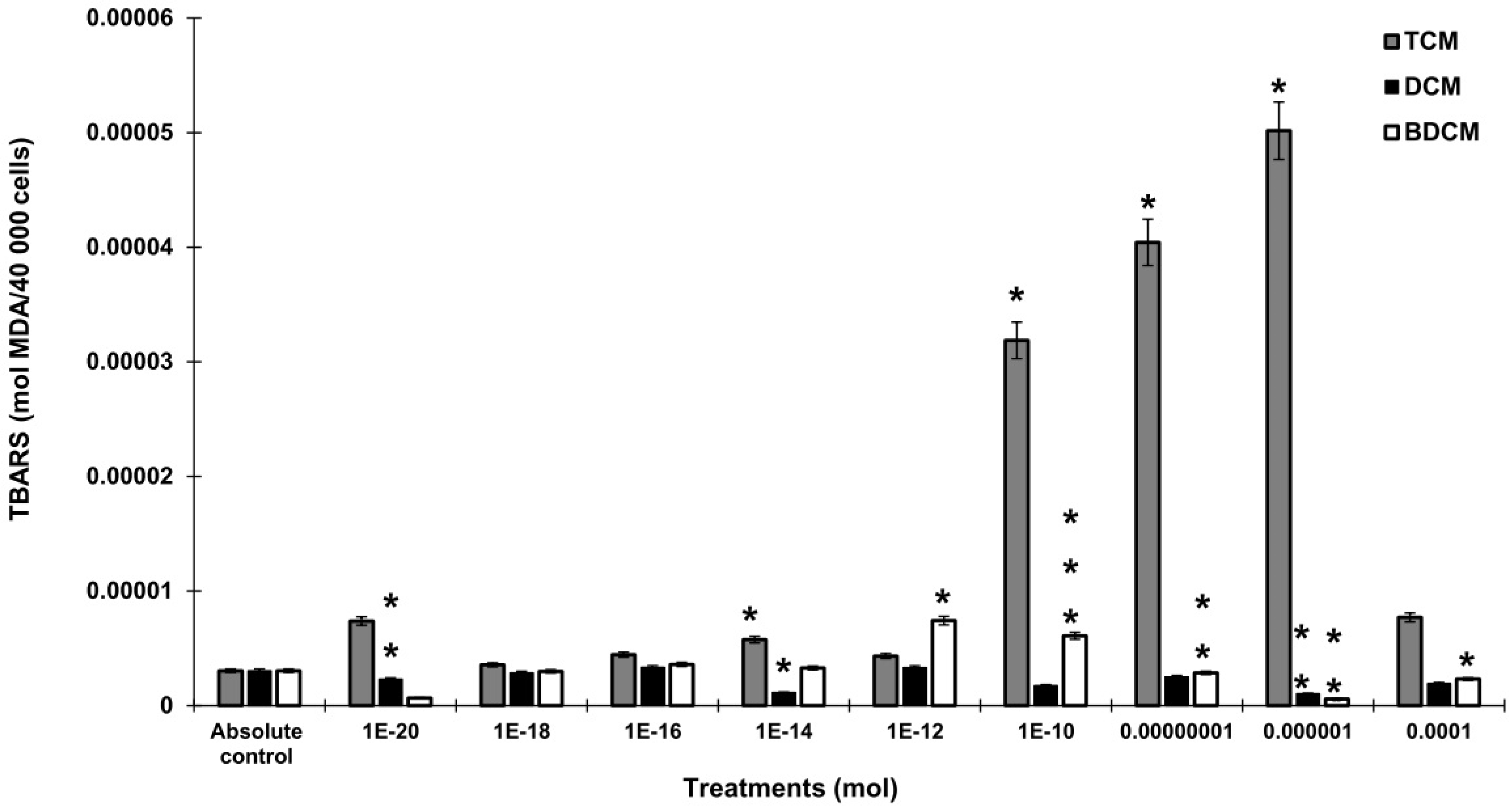
The Generation of Hydrogen Peroxide (H2O2), Superoxide Anion (O2•), and Lipid Peroxidation by Exposure to Halomethanes (CH2Cl2, CHCl3, BrCHCl2) in Human Lung Fibroblasts (MRC-5)
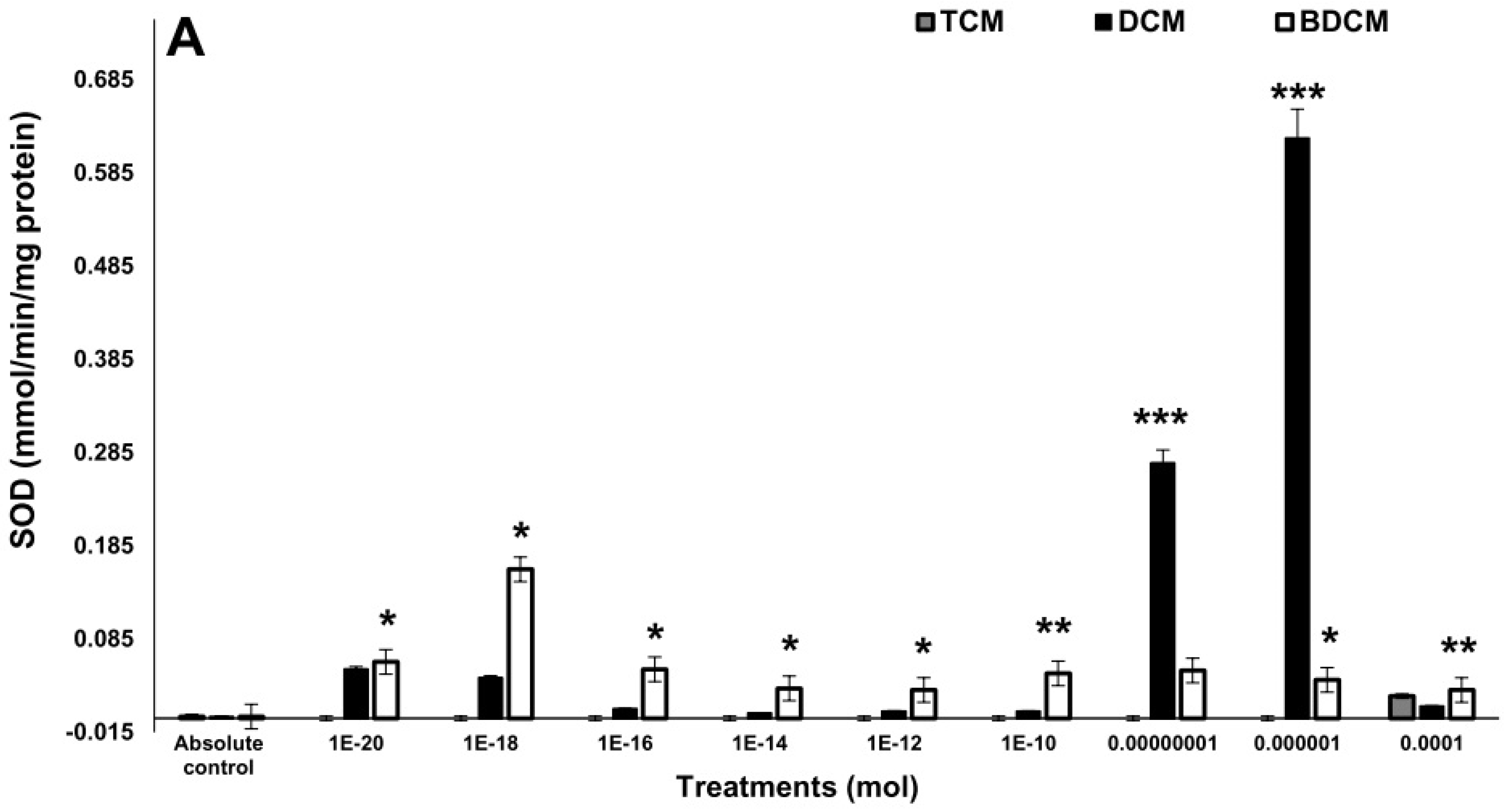
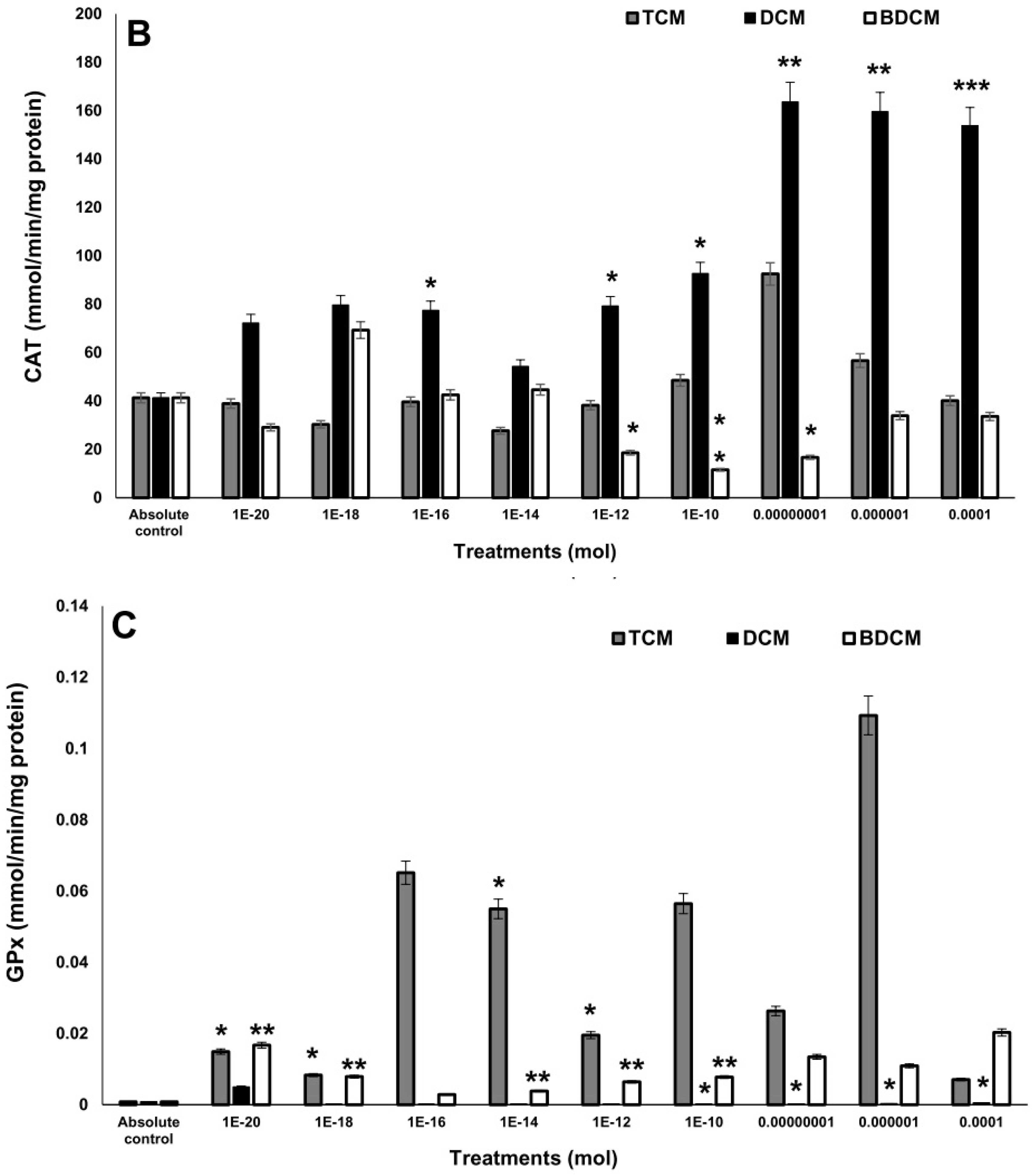
The Activity of the Antioxidant Enzymes
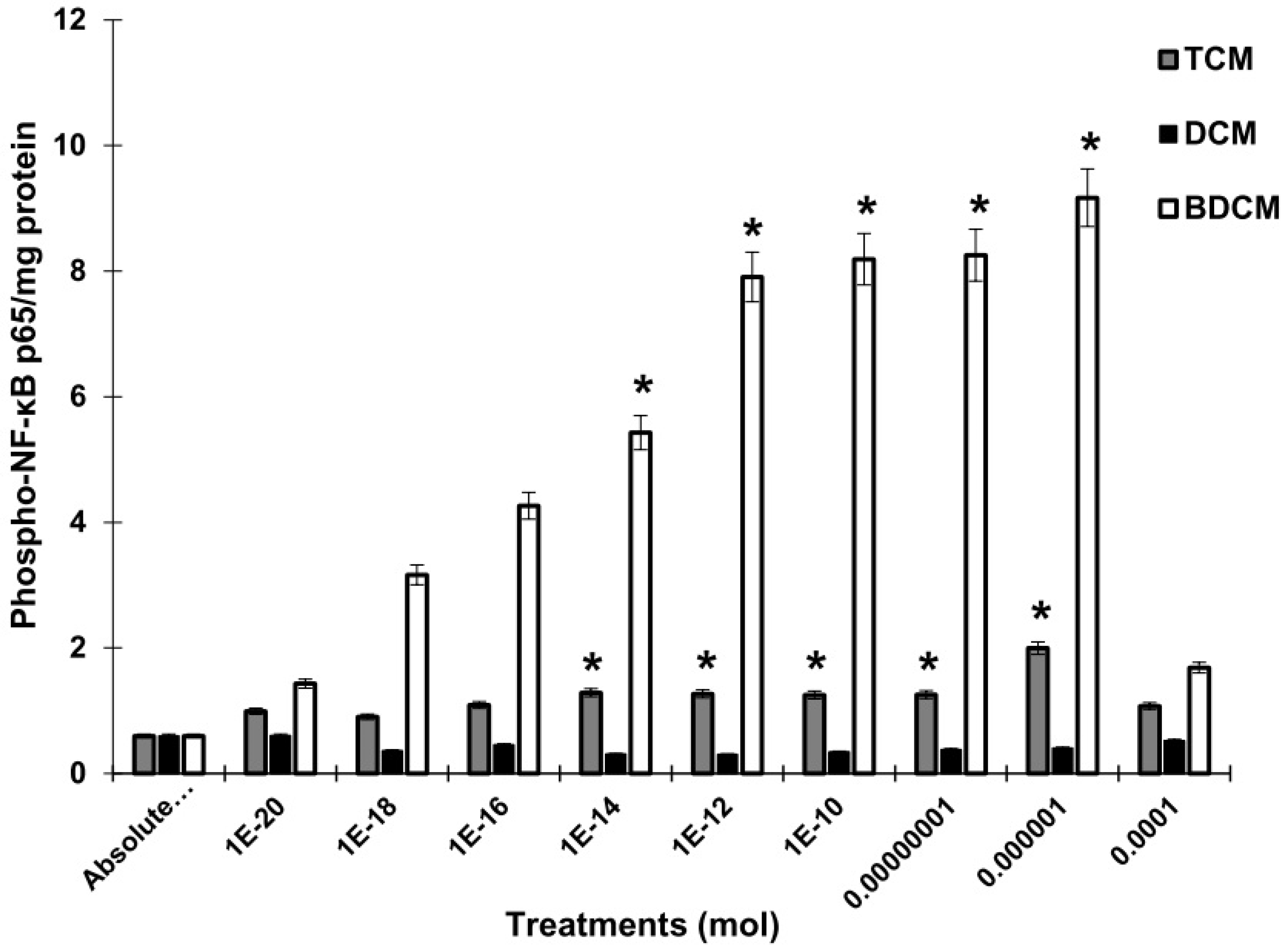
Evaluation of Phosphorylated NF-κB/p65 Levels
Relation between Biomarkers
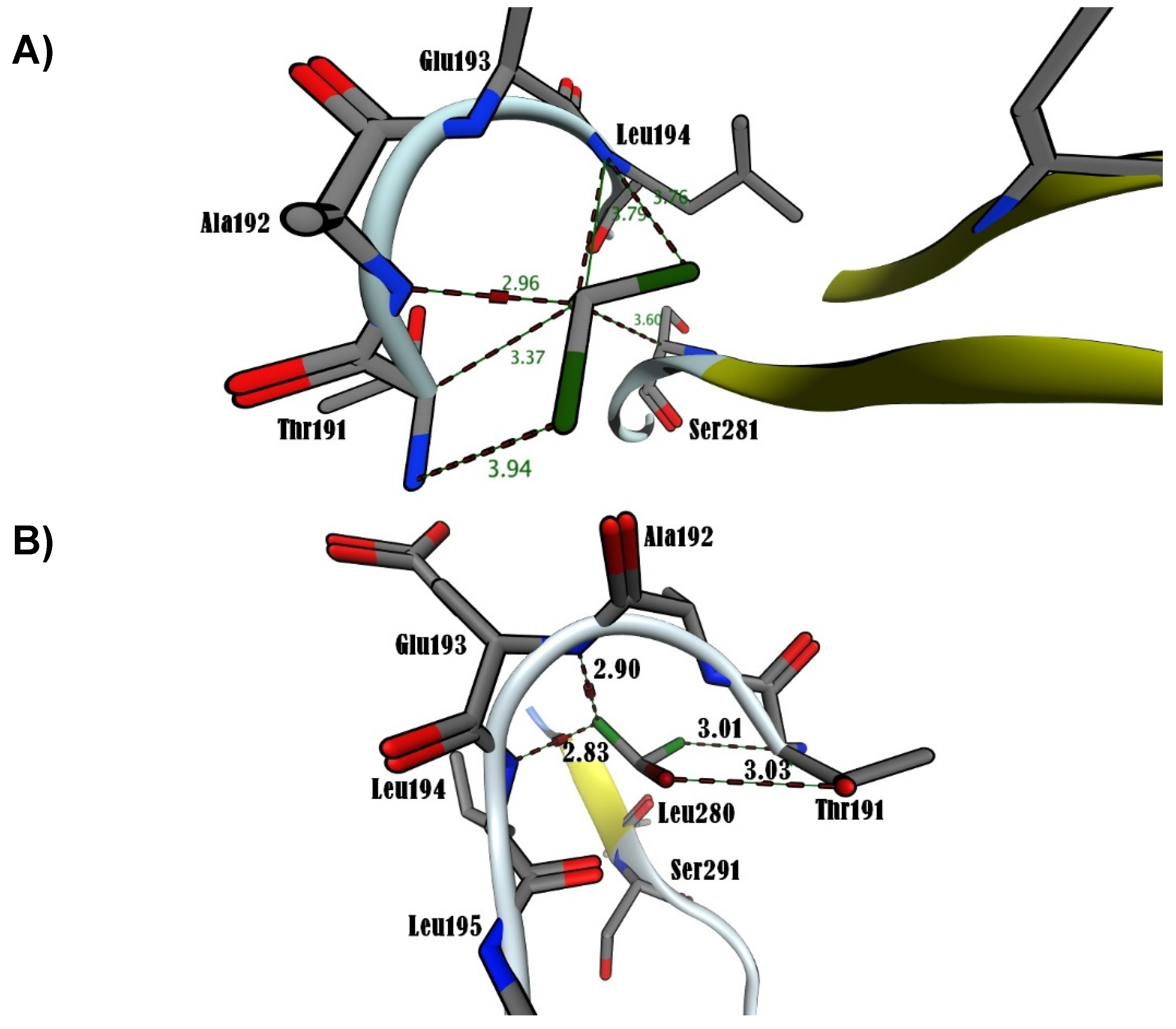
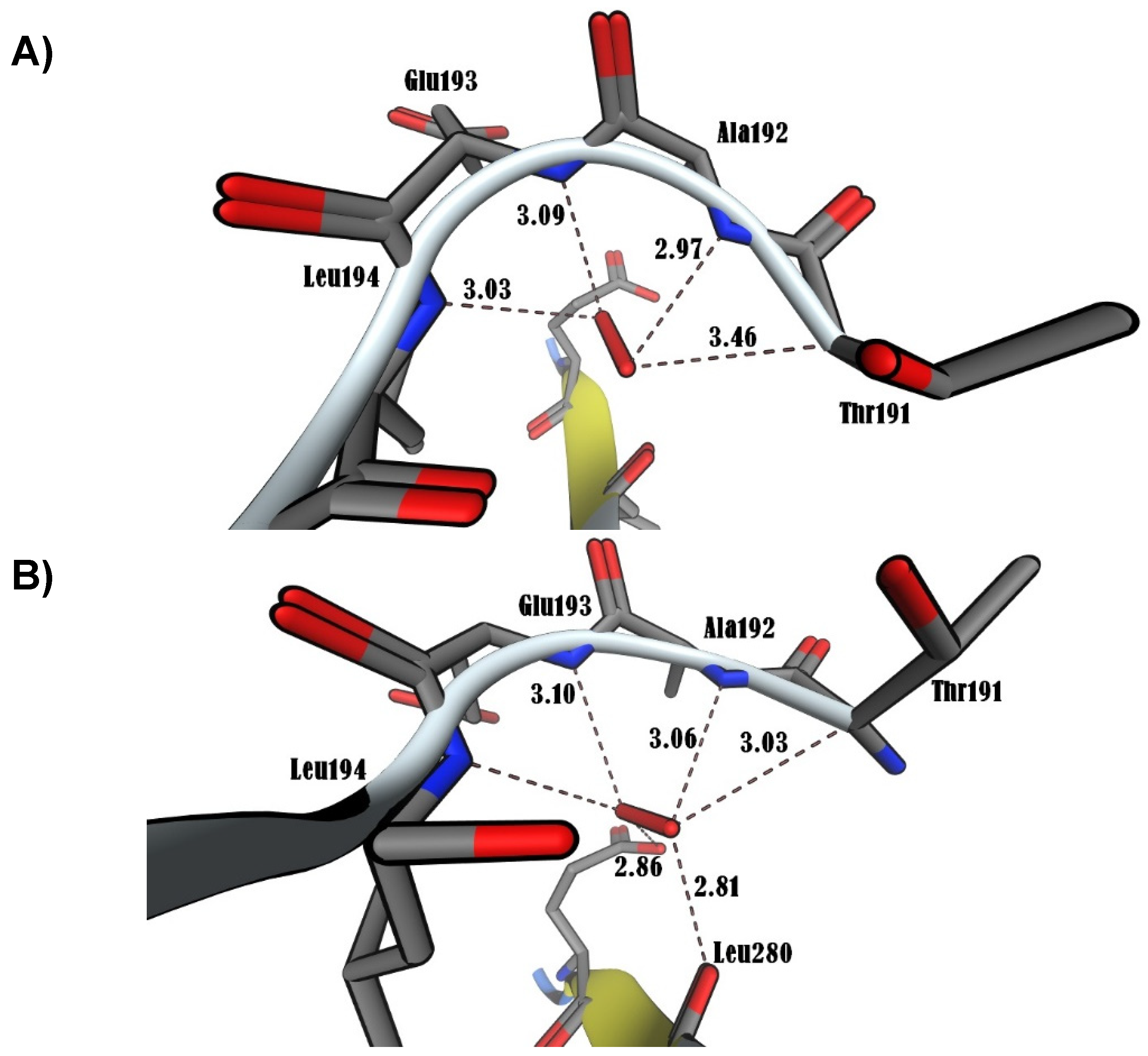
Molecular Docking Analysis
Discussion
Conclusions
Author Contributions
Funding and Acknowledgements
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Snezhkina, A.V., Kudryavtseva, A.V., Kardymon, O.L., Savvateeva, M.V., Melnikova, N.V., Krasnov, G.S., Dmitriev, A.A. (2019). ROS Generation and Antioxidant Defense Systems in Normal and Malignant Cells. Oxid. Med. Cell. Longev. (2019) 6175804 . [CrossRef]
- Schieber, M., Chandel, N.S. ROS function in redox signaling and oxidative stress. Curr. Biol. 24(2014), R453-62 . [CrossRef]
- Fulda, S., Gorman, A. M., Hori, O., Samali, A. Cellular Stress Responses: Cell Survival and Cell Death. International J. Cell Biol. 2010, 214074 . [CrossRef]
- Reuter, S., Gupta, S.C., Chaturvedi, M.M., Aggarwal, B.B. Oxidative stress, inflammation, and cancer: How are they linked? Free Radic. Biol. Med. 49 (2010), 1603–1616 . [CrossRef]
- Wang, L., Li, H., Yang, S. Role of oxygen free radicals in the proliferation of myofibroblasts induced by AngII. Acta Pharm. Sin. B, 3 (2013), 32-37 . [CrossRef]
- Kinnula, V. L., Fattman, C. L., Tan, R. J., Oury, T. D. Oxidative Stress in Pulmonary Fibrosis. Am. J. Respir. Crit. Care Med. 172 (2005), 417–422 . [CrossRef]
- Fukushima, K., Satoh, T., Kida, H., Kumanogoh, A. Revisiting Cell Death Responses in Fibrotic Lung Disease: Crosstalk between Structured and Non-Structured Cells. Diagnostic, 10 (2020), 504 . [CrossRef]
- Nájera-Martínez, M., García-Latorre, E.A., Reyes-Maldonado, E., Domínguez-López, M.L., Vega-López, A. Halomethane-induced cytotoxicity and cell proliferation in human lung MRC-5 fibroblasts and NL20-TA epithelial cells. Inh. Toxicol. 24 (2012), 762–773 . [CrossRef]
- Htwe, S. S., Harrington, H., Knox, A., Rose, F., Aylott, J., Haycock, J.W., Ghaemmaghami, A.M. Investigating NF-κB signaling in lung fibroblasts in 2D and 3D culture systems. “Respir. Res. 16 (2015), 144 . [CrossRef]
- Thompson, J. E., Phillips, R. J., Erdjument-Bromage, H., Tempst, P., Ghosh, S. I. kappa B-beta regulates the persistent response in a biphasic activation of NF-kappa B. Cell. 80 (1995), 573–582 . [CrossRef]
- Hayden, M.S., Ghosh, S. Shared principles in NF-kappaB signaling. Cell. 132(2008), 344-362 . [CrossRef]
- Ghosh, S., Dass, F.P.J. Non-canonical pathway network modelling and ubiquitination site prediction through homology modelling of NF-κB. Gene 581 (2016), 48-56 . [CrossRef]
- Liu, T., Zhang, L., Joo, D., Sun, S.C. NF-κB signaling in inflammation. Signal Transduct. Target. Ther. 2 (2017a), 17023 . [CrossRef]
- Huxford, T., Mishler, D., Phelps, C. B., Huang, D. B., Sengchanthalangsy, L. L., Reeves, R., Hughes, C. A., Elizabeth A. Komives, E. A., Ghosh, G. Solvent Exposed Non-contacting Amino Acids Play a Critical Role in NF-kB/IkBa Complex Formation. J. Mol. Biol. 324 (2002), 587–597 . [CrossRef]
- Chen, Z. J., Parent, L., Maniatis, T. Site-specific phosphorylation of IκB by a novel ubiquitination-dependent protein kinase activity. Cell. 84 (1996), 853–862 . [CrossRef]
- Whiteside, S.T., Epinat, J. C., Rice, N. R., Israel, A. I kappa B epsilon, a novel member of the I kappa B family, controls RelA and cRel NF-kappa B activity. EMBO J. 16 (1997), 1413–1426. [CrossRef]
- Christian, F., Smith, E., Carmody, R. The Regulation of NF-κB Subunits by Phosphorylation. Cells. 5 (2016), 12. [CrossRef]
- Sun, X. F. & Zhang, H. NFKB and NFKBI polymorphisms in relation to susceptibility of tumour and other diseases. Histol. Histopathol. 22 (2007), 1387-98. [CrossRef]
- Uraga-Tovar, D. I., Domíguez-López, M. L., Madera-Sandoval, R. L., Nájera-Martínez, M., García-Latorre, E., Vega-López, A. (2014). Generation of oxyradicals O2• and H2O2), mitochondrial activity and induction of apoptosis of PBMC of Cyprinus carpio carpio treated in vivo with halomethanes and with recombinant HSP60 kDa and with LPS of Klebsiella pneumoniae. Immunopharmacol. Immunotoxicol. 36 (2014), 329–340. [CrossRef]
- Dzul-Caamal, R., Salazar-Coria, L., Olivares-Rubio, H.F., Rocha-Gómez, M.A., Girón-Pérez, M.I., Vega-López, A. Oxidative stress response in the skin mucus layer of Goodea gracilis (Hubbs and Turner, 1939) exposed to crude oil: A non-invasive approach. Comp. Biochem. Physiol., Part A Mol. Integr. Physiol. 200 (2016), 9-20. [CrossRef]
- Buege, J. A., Aust, S. D. Microsomal lipid peroxidation. Methods Enzymol. 52 (1978), 302-310. [CrossRef]
- Misra, H. P., Fridovich, I. The role of superoxide dismutase anion in the autooxidation of epinephrine and a simple assay for superoxide dismutase. J. Biol. Chem. 247 (1972), 3170-3175. [CrossRef]
- Radi, R., Turrens, J. F., Chang, L.Y., Bush, K. M., Crapo, J. D., Freeman, B.A. Detection of catalase in rat heart mitochondria. J. Biol. Chem. 266 (1991), 22028-22034. PMID: 1657986.
- Lei, X. G., Evenson, J. K., Thompson, K. M., Sunde, R. A. Glutathione peroxidase and phospholipid hydroperoxide glutathione peroxidase are differentially regulated in rats by dietary selenium. J. Nutr. 125 (1995), 1438–1446. [CrossRef]
- Mackerell, A.D. Jr., Feig, M., Brooks, C.L. 3rd. Extending the treatment of backbone energetics in protein force fields: limitations of gas-phase quantum mechanics in reproducing protein conformational distributions in molecular dynamics simulations. J. Comput. Chem. 25(2004), 1400-15. [CrossRef]
- Phillips, J.C., Hardy, D.J., Maia, J.D.C., Stone, J.E., Ribeiro, J.V., Bernardi, R.C., Buch, R., et al. Scalable molecular dynamics on CPU and GPU architectures with NAMD. J. Chem. Phys. 153 (2020), 044130. [CrossRef]
- MacKerell, A.D., Bashford, D., Bellott, M., Dunbrack, R.L., Evanseck, J.D., Field, M.J., et al. All-atom empirical potential for molecular modeling and dynamics studies of proteins. J. Phys. Chem. B. 102 (1998), 3586-616. [CrossRef]
- Jorgensen, W.L., Chandrasekhar, J., Madura, J.D. Comparison of simple potential functions for simulating liquid water. J. Chem. Phys. 79 (1983), 926-935. [CrossRef]
- Grubmüller, H., Heller, H., Windemuth, A., Schulten, K. Generalized Verlet Algorithm for Efficient Molecular Dynamics Simulations with Long-range Interactions. Mol. Simul. 6 (1991), 121-142. [CrossRef]
- Schlick, T., Skeel, R.D., Brunger, A.T., Kalé, L.V., Board, J.A., Hermans, J., Schulten, K. Algorithmic challenges in computational molecular biophysics. J. Comput. Phys. 151 (1999), 9–48. [CrossRef]
- Brunger, A.T. X-PLOR Version 3.1, A System for X-ray Crystallography and NMR. Yale University, New Haven, CT, USA, 405 p. ISBN: 9780300054026. 1992.
- Hsu, C. C., Lien, J. C., Chang, C.W., Chang, C. H., Kuo, S. C., Huang, T. F. Yuwen02f1 suppresses LPS-induced endotoxemia and adjuvant-induced arthritis primarily through blockade of ROS formation, NFkB and MAPK activation. Biochem. Pharmacol. 85(2013), 385-95. [CrossRef]
- Tkaczyk, J., Vízek, M. Oxidative Stress in the Lung Tissue – Sources of Reactive Oxygen Species and Antioxidant Defence. Prague Med. Rep. 108(2007), 105–114. PMID: 18225638.
- Rimal, B., Greenberg, A. K., Rom, W. N. Basic pathogenetic mechanisms in silicosis: current understanding. Curr. Opin. Pulm. Med. 11(2005), 169-73. [CrossRef]
- Mittal, M., Siddiqui, M.R., Tran, K., Reddy, S.P., Malik, A.B. Reactive oxygen species in inflammation and tissue injury. Antioxid. Redox Signal. 20(2014), 1126-67. [CrossRef]
- Becklake, M. R. Occupational exposures: evidence for a causal association with chronic obstructive pulmonary disease. Am. Rev. Respir. Dis. 140 (1989), S85-91. [CrossRef]
- Omland, O., Würtz, E.T., Aasen, T.B., Blanc, P., Brisman, J., Miller, M.R., Pederson, O.F., Schlünssen, V., Sigsgaard, T., Ulrik, C.S., Viskum, S. Occupational chronic obstructive pulmonary disease: a systematic literature review. Scand. J. Work Environ. Health. 40(2011), 19-35. [CrossRef]
- Melnick, R. L., Kohn, M. C., Dunnick, J.K. Regenerative hyperplasia is not required for liver tumor induction in female B6C3F1 mice exposed to trihalomethanes. Toxicol. Appl. Pharmacol. 148(1998), 137–147. [CrossRef]
- Vega-López, A., Carrillo-Morales, C. I., Olivares-Rubio, H.F. Evidence of bioactivation of halomethanes and its relation to oxidative stress response in Chirostoma riojai, an endangered fish from a polluted lake in Mexico. Arch. Environ. Cont. Toxicol. 62(2012), 479–493. [CrossRef]
- Dzul-Caamal, R., Olivares-Rubio, H.F., López-Tapia, P., Vega-López, A. Pro-oxidant and antioxidant response elicited by CH2Cl2, CHCl3 and BrCHCl2 in Goodea gracilis using non-invasive methods. Comp. Bioch. Physiol. A Mol. Integr. Physiol. 165(2013), 515–527. [CrossRef]
- Dekant, W., Vamvakas, S. Glutathione-dependent bioactivation of xenobiotics. Xenobiotica. 23(1993), 873-87. [CrossRef]
- Zhang, H., Zhang, J., Zhu, Y. In vitro investigations for the QSAR mechanism of lymphocytes apoptosis induced by substituted aromatic toxicants. Fish Shellfish Immunol. 25(2008), 710–717. [CrossRef]
- Crapo, J. D., Tierney, D. F. Superoxide dismutase and pulmonary oxygen toxicity. Am. J. Physiol. 226(1974), 1401–1407. [CrossRef]
- Birben, E., Sahiner, E. M., Sackesen, C., Erzurum, S., Kalayci, O. Oxidative Stress and Antioxidant Defense. World Allergy Org. J. 5 (2012), 9–19. [CrossRef]
- Hermes-Lima, M. Oxygen in biology and biochemistry: role free radicals. Functional Metabolism: Regulation and Adaptation. Edited by Kenneth B. Storey. John Wiley & Sons, Inc. 319-368. 2004. [CrossRef]
- Finkel, T., Holbrook, N. J. Oxidants, oxidative stress and the biology of ageing. Nature. 408(2000), 239–247. [CrossRef]
- Klaunig, J. E., Kamendulis, L. M. The role of oxidative stress in carcinogenesis. Ann. Rev. Pharmacol. Toxicol. 44(2004), 239-67. [CrossRef]
- Gao, F., Kinnula, L. V., Marjukka Myllärniemi, M., Oury, D.T. Extracellular Superoxide Dismutase in Pulmonary Fibrosis. Antioxid. Redox Signal. 10(2008), 343–354. [CrossRef]
- Brand, M.D. Mitochondrial generation of superoxide and hydrogen peroxide as the source of mitochondrial redox signaling. Free Rad. Biol. Med. 100(2016), 14-31. [CrossRef]
- McCord, J. M., Fridovich, I. Superoxide dismutase. An enzymic function for erythrocuprein (hemocuprein). J. Biol. Chem. 244(1969), 6049–6055.
- Sauer, H., Rahimi, G., Hescheler, J., Wartenberg, M. Role of reactive oxygen species and phosphatidylinositol 3-kinase in cardiomyocyte differentiation of embryonic stem cells. FEBS Lett. 476(2000), 218–223. [CrossRef]
- Foreman, J., Demidchik, V., Bothwell, J. H., Mylona, P., Miedema, H., Torres, M. A., Linstead, P., Costa, S., Brownlee, C., Jones, J. D. Reactive oxygen species produced by NADPH oxidase regulate plant cell growth. Nature, 422(2003), 442–446. [CrossRef]
- Geiszt, M., Leto, T.L. The Nox family of NAD(P)H oxidases: host defense and beyond. J. Biol. Chem. 279(2004), 51715–51718. [CrossRef]
- Cai, H. Hydrogen peroxide regulation of endothelial function: origins, mechanisms, and consequences. Cardiovasc. Res. 68(2005), 26–36. [CrossRef]
- Li, J., Stouffs, M., Serrander, L., Banfi, B., Bettiol, E., Charnay, Y., Steger, K., Krause, K. H., Jaconi, M. E. The NADPH oxidase NOX4 drives cardiac differentiation: role in regulating cardiac transcription factors and MAP kinase activation. Mol. Biol. Cell. 17(2006), 3978– 3988. [CrossRef]
- Cantin, A. M., North, S. L., Hubbard, R. C., Crystal, R.G. Normal alveolar epithelial lining fluid contains high levels of glutathione. J. Appl. Physiol. 63(1987), 152–157. [CrossRef]
- Ayala, A., Muñoz, M. F., Argüelles, S. Lipid Peroxidation: Production, Metabolism, and Signaling Mechanisms of Malondialdehyde and 4-Hydroxy-2-Nonenal. Oxidative Medicine and Cellular Longevity. Oxid. Med. Cell. Longev. (2014)360438. [CrossRef]
- Kornbrust, D. J., Mavis, R. D. Relative susceptibility of microsomes from lung, heart, liver, kidney, brain and testes to lipid peroxidation: correlation with vitamin E content. Lipids. 15(1980), 315-22. [CrossRef]
- Cardoso, W. V., Lu, J. Regulation of early lung morphogenesis: questions, facts and controversies. Development. 133(2006), 1611–1624. [CrossRef]
- Cardoso, W. V., Whitsett, J. A. Resident cellular components of the lung: developmental aspects. Proc. Am. Thorac. Soc. 5(2008), 767–771. [CrossRef]
- Morrisey, E. E., Hogan, B. L. Preparing for the first breath: genetic and cellular mechanisms in lung development. Dev. Cell. 18(2010), 8–23. [CrossRef]
- O’shea, J. M., Perkins, N. D. Regulation of the RelA (p65) transactivation domain. Biochem. Soc. Trans. 36(2008), 603–608. [CrossRef]
- Cramer, P., Müller, C.W. A firm hand on NFkappaB: structures of the IkappaBalpha-NFkappaB complex. Structure, 7(1999), R1-6. [CrossRef]
- Li, X., Stark, G.R. NFkappaB-dependent signaling pathways. Exp. Hematol. 30(2002), 285-96. [CrossRef]
- Zhou, Z., Wang, M., Zhao, J., Wang, L., Gao, Y., Zhang, H., Liu, R., Song, L. increased transcriptional response and translocation of a Rel/NF-κB homologue in scallop Chlamys farreri during the immune stimulation. Fish Shellfish Immunol. 34(2013), 1209-15. [CrossRef]
- Du, R.H., Tan, J., Yan, N., Wang, L., Qiao, C., Ding, J.H., Lu, M., Hu, G. Kir6.2 knockout aggravates lipopolysaccharide-induced mouse liver injury via enhancing NLRP3 inflammasome activation. J. Gastroenterol. 49(2014), 727-36. [CrossRef]
- He, J.Y., Li, P.H., Huang, X., Sun, Y.H., He, X.P., Huang, W., Yu, Z.H. & Sun, H.Y.. Molecular cloning, expression and functional analysis of NF-kB1 p105 from sea cucumber Holothuria leucospilota. Dev. Comp. Immunol. 114(2021), 103801. [CrossRef]
- Huang, D-B., Huxford, T., Chen, Y-Q., Ghosh, G. The role of DNA in the mechanism of NFkB dimer formation: crystal structures of the dimerization domains of the p50 and p65 subunits. Structure. 5(1997), 1427–1436. [CrossRef]
- Vermeulen, L., De Wilde, G., Notebaert, S., Vanden Berghe, W., Haegeman, G. Regulation of the transcriptional activity of the nuclear factor-κB p65 subunit. Bioch. Pharmacol. 64(2002), 963–970. [CrossRef]
- Jacobs, M. D., Harrison, S. C. Structure of an IkBa/NF-kB Complex. Cell. 95(1998), 749–758. [CrossRef]
- Wu, C., Ghosh, S. Differential phosphorylation of the signal-responsive domain of I kappa B alpha and I kappa B beta by I kappa B kinases. J. Biol. Chem. 278(2003), 31980-7. [CrossRef]
- Kanarek, N., Ben-Neriah, Y. Regulation of NF-κB by ubiquitination and degradation of the IκBs. Immunol. Rev. 246(2012), 77-94. [CrossRef]
- Anrather, J., Racchumi, G. Iadecola, C. cis-Acting Element-specific Transcriptional Activity of Differentially Phosphorylated Nuclear Factor-κB. J. Biol. Chem. 280(2004), 244–252. [CrossRef]
- Cramer, P., Larson, C.J., Verdine, G.L., Müller, C.W. Structure of the human NF-kappaB p52 homodimer-DNA complex at 2.1 A resolution. EMBO J. 16(1997), 7078-90. [CrossRef]
- Milanovic, M., Kracht, M., Schmitz, M. L. The cytokine-induced conformational switch of nuclear factor κB p65 is mediated by p65 phosphorylation. Bioch. J. 457(2014), 401–413. [CrossRef]
- Schwartz, J.L., Antoniades, D.Z., Zhao, S. Molecular and biochemical reprogramming of oncogenesis through the activity of prooxidants and antioxidants. Ann. N. Y. Acad. Sci. 28(1993), 686:262-78, discussion 278-9. [CrossRef]
- Dominici, S., Visvikis, A., Pieri, L., Paolicchi, A., Valentini, M.A., Comporti, M. & Pompella, A. Redox modulation of NF-kappaB nuclear translocation and DNA binding in metastatic melanoma. The role of endogenous and gamma-glutamyl transferase-dependent oxidative stress. Tumori. 89(2003), 426-33. PMID: 14606649.
- Yao, H., Yang, S.R., Kode, A., Rajendrasozhan, S., Caito, S., Adenuga, D., Henry, R., Edirisinghe, I., Rahman, I. Redox regulation of lung inflammation: role of NADPH oxidase and NF-kappaB signalling. Biochem. Soc. Trans. 35Pt 5(2007), 1151-5. [CrossRef]
- Zhong, Z.M., Bai, L., Chen, J.T. Advanced oxidation protein products inhibit proliferation and differentiation of rat osteoblast-like cells via NF-kappaB pathway. Cell. Physiol. Biochem. 24(2009), 105-14. [CrossRef]
- Ali, F., Sultana, S. Repeated short-term stress synergizes the ROS signalling through up regulation of NFkB and iNOS expression induced due to combined exposure of trichloroethylene and UVB rays. Mol. Cell. Biochem. 360(2012), 133-45. [CrossRef]
- Li, Y., Liu, Y.J., Lv, G., Zhang, D.L., Zhang, L., Li, D. Propofol protects against hydrogen peroxide-induced apoptosis in cardiac H9c2 cells is associated with the NF-κB activation and PUMA expression. Eur. Rev. Med. Pharmacol. Sci. 18(2014), 1517-24. PMID: 24899612.
- Fenga, C., Gangemi, S., Giambò, F., Tsitsimpikou, C., Golokhvast, K., Tsatsakis, A., Costa, C. Low-dose occupational exposure to benzene and signal transduction pathways involved in the regulation of cellular response to oxidative stress. Life Sci. 147(2016), 67-70. [CrossRef]
- Hu, W., Shi, L., Li, M.Y., Zhou, P.H., Qiu, B., Yin, K., Zhang, H.H., Gao, Y., Kang, R., Qin, S.L., Ning, J.Z., Wang, W., Zhang, L.J. Adrenomedullin protects Leydig cells against lipopolysaccharide-induced oxidative stress and inflammatory reaction via MAPK/NF-κB signalling pathways. Sci. Rep. 7(2017), 16479. [CrossRef]
- Liu, L., Zuo, Z., Lu, S., Liu, A., Liu, X. Naringin attenuates diabetic retinopathy by inhibiting inflammation, oxidative stress and NF-κB activation in vivo and in vitro. Iran. J. Basic Med. Sc. 20(2017b):813-821. [CrossRef]
- He J, Zhou D., Yan B. Eriocitrin alleviates oxidative stress and inflammatory response in cerebral ischemia reperfusion rats by regulating phosphorylation levels of Nrf2/NQO-1/HO-1/NF-κB p65 proteins. Annals of Translational Medicine, 8(2020), 757. [CrossRef]
- Li, Q., Sun, Y., Liu, B., Li, J., Hao, X., Ge, W., Zhang, X., Bao, S., Gong, J., Jiang, Z., Qiu, C., Zhao, L., Zhao, Y., Chen, Y., Yang, X., Ding, Y., Wu, Z. ACT001 modulates the NF-κB/MnSOD/ROS axis by targeting IKKβ to inhibit glioblastoma cell growth. J. Mol. Med. (Berl), 98(2020), 263-277. [CrossRef]
- Kamata, H., Manabe, T., Oka, S., Kamata, K., Hirata, H. Hydrogen peroxide activates IkappaB kinases through phosphorylation of serine residues in the activation loops. FEBS Lett. 519(2002), 231–237. [CrossRef]
- Oliveira-Marques, V., Marinho, H.S., Cyrne, L., Antunes, F. Role of hydrogen peroxide in NF-B activation: From inducer to modulator. Antioxid. Redox Signal. 11(2009), 2223–2243. [CrossRef]
| R, p | R, p | R, p | R, p | R, p | R, p | |
| CH2Cl2 | ||||||
| H2O2 | TBARS | SOD | CAT | GPx | NF-κB | |
| O2• | 0.590, <0.001 | 0.728, <0.001 | 0.596, <0.001 | -0.533, <0.01 | ||
| H2O2 | 0.736, <0.001 | 0.541, <0.01 | ||||
| TBARS | 0.408, <0.05 | -0.471, <0.01 | ||||
| SOD | 0.584, <0.001 | |||||
| CHCl3 | ||||||
| H2O2 | TBARS | SOD | CAT | GPx | NF-κB | |
| O2• | 0.884, <0.001 | 0.504, <0.01 | -0.611, <0.001 | |||
| H2O2 | 0.465, <0.05 | 0.592, <0.001 | -0.672, <0.001 | |||
| TBARS | 0.771, <0.001 | 0.514, <0.01 | ||||
| BrCHCl2 | ||||||
| H2O2 | TBARS | SOD | CAT | GPx | NF-κB | |
| O2• | 0.915, <0.001 | 0.418, <0.05 | 0.539, <0.01 | 0.682, <0.001 | 0.407, <0.05 | |
| H2O2 | 0.404, <0.05 | 0.396, <0.05 | 0.653, <0.001 | 0.451, <0.05 | ||
| SOD | 0.536, <0.01 | |||||
| CAT | -0.406, <0.05 | |||||
| Compound | SCF (au) | ΔH (kcal/mol) | ΔE(eV) | ΔG (kcal/mol) |
| CHCl3 | -46.4 | -23.6 | 11.6 | -3.5 |
| BrCHCl2 | -23.9 | -17.9 | 12.5 | -3.3 |
| O2• | -24.4 | -35.3 | 0.5 | -3.6 |
| H2O2 | -45 | -15.5 | 0.3 | -3.6 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





