Submitted:
08 July 2024
Posted:
09 July 2024
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Results
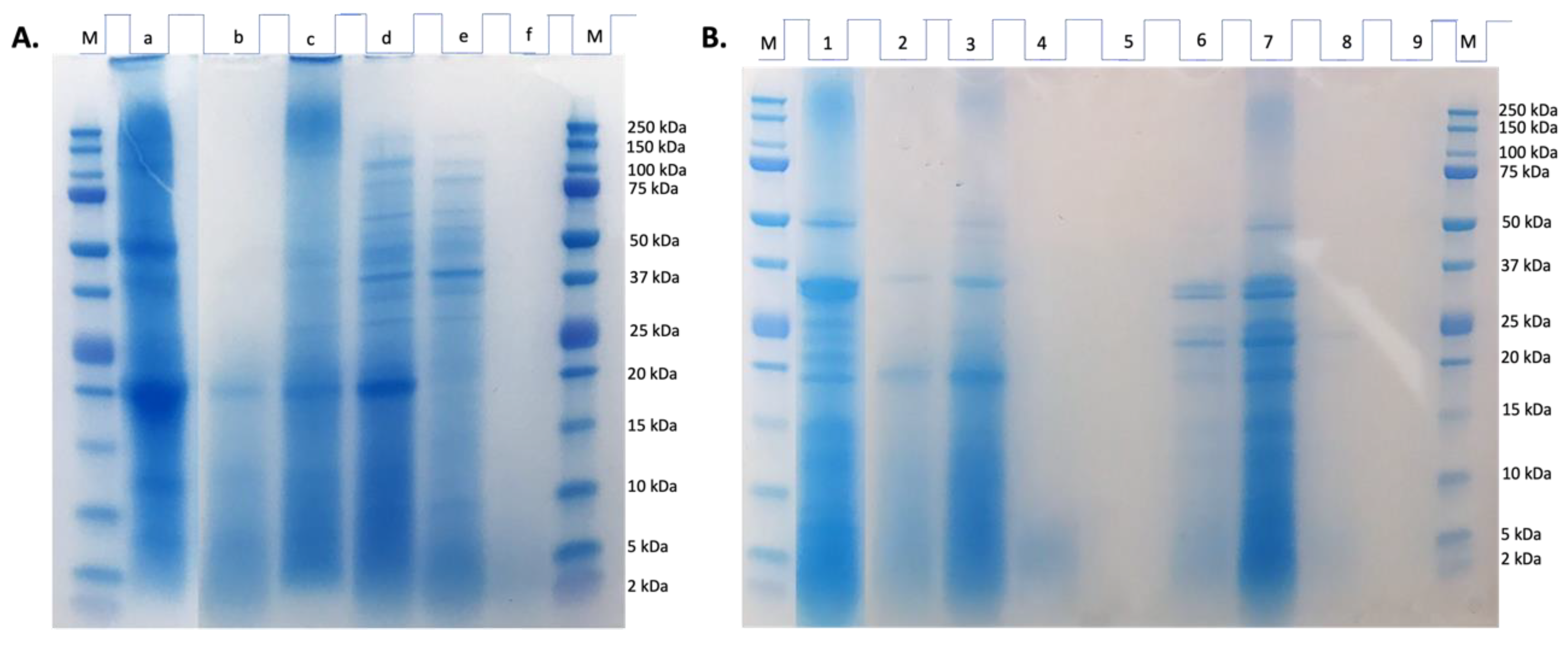
2.1. Enzymatic Hydrolysis of Chachafruto Seed Protein Concentrate (CPC)
2.2. Characterization of Chachafruto Protein Concentrate Hydrolyzate (HES)
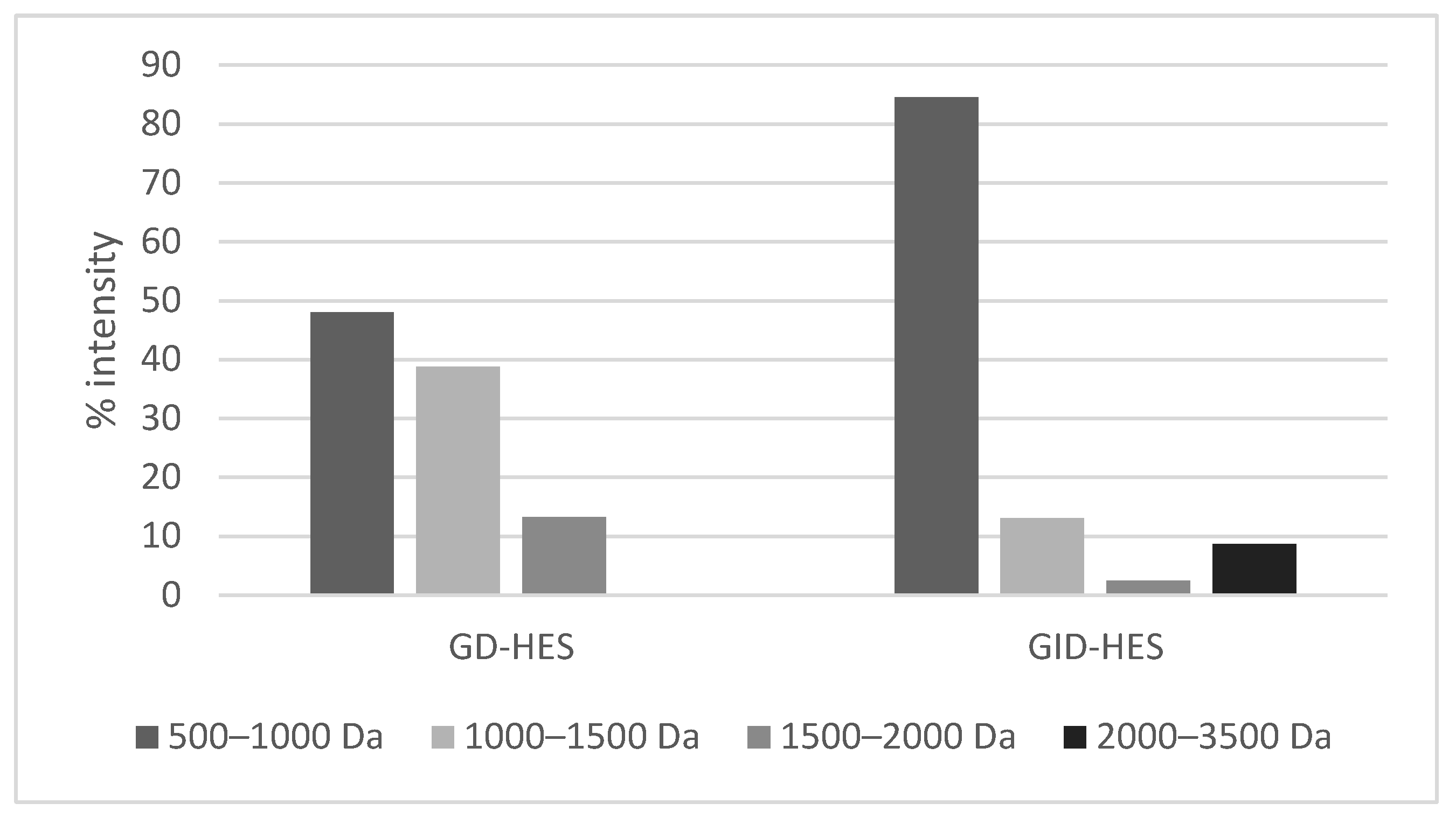
2.3. Behavior of Chachafruto Protein Concentrate Hydrolysate (HES) under Simulated Gastrointestinal Digestion
2.4. Effect of the Simulated Gastrointestinal Digestion on the Antioxidant Activity of Chachafruto Protein Hydrolyzate (HES)
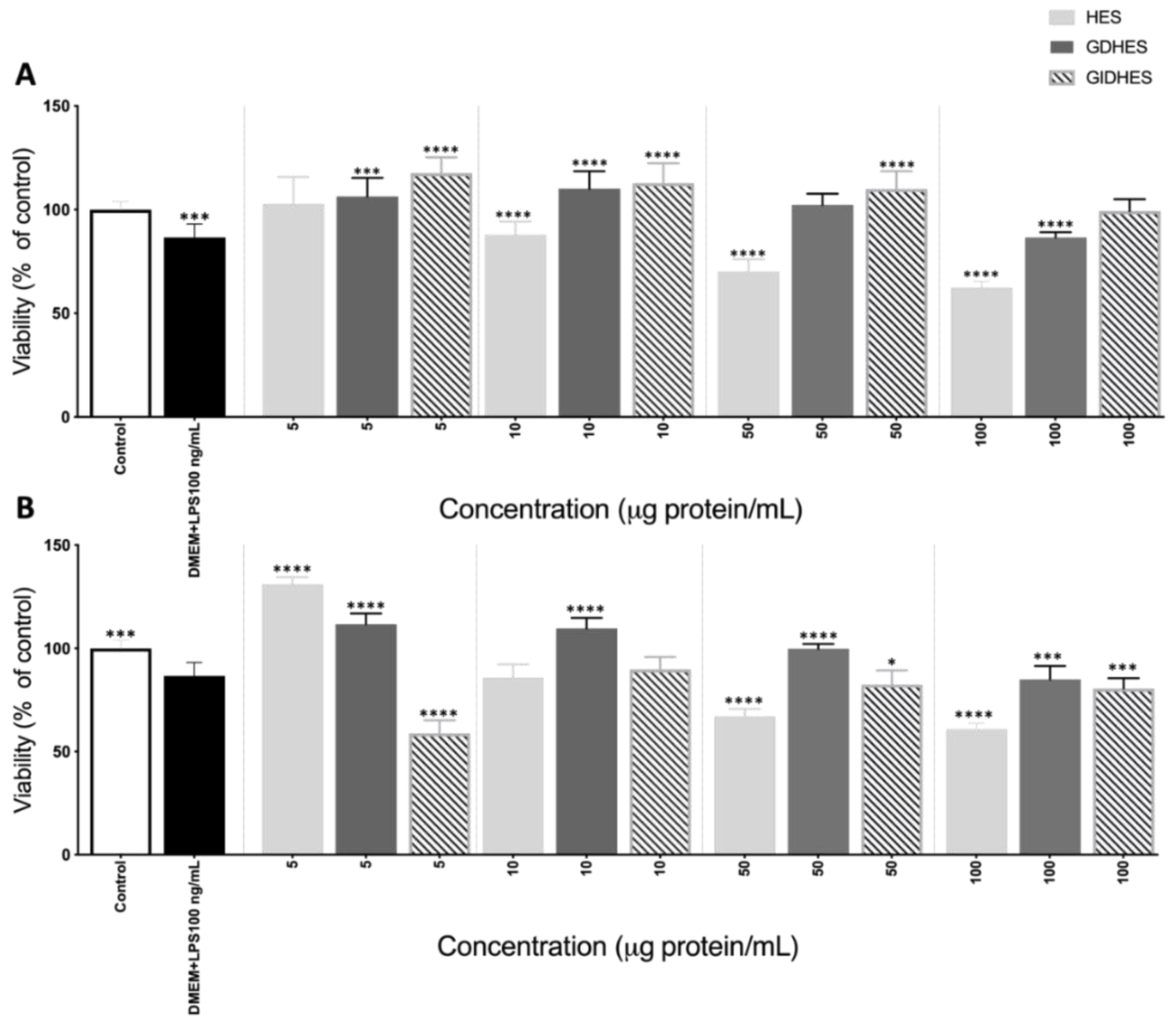
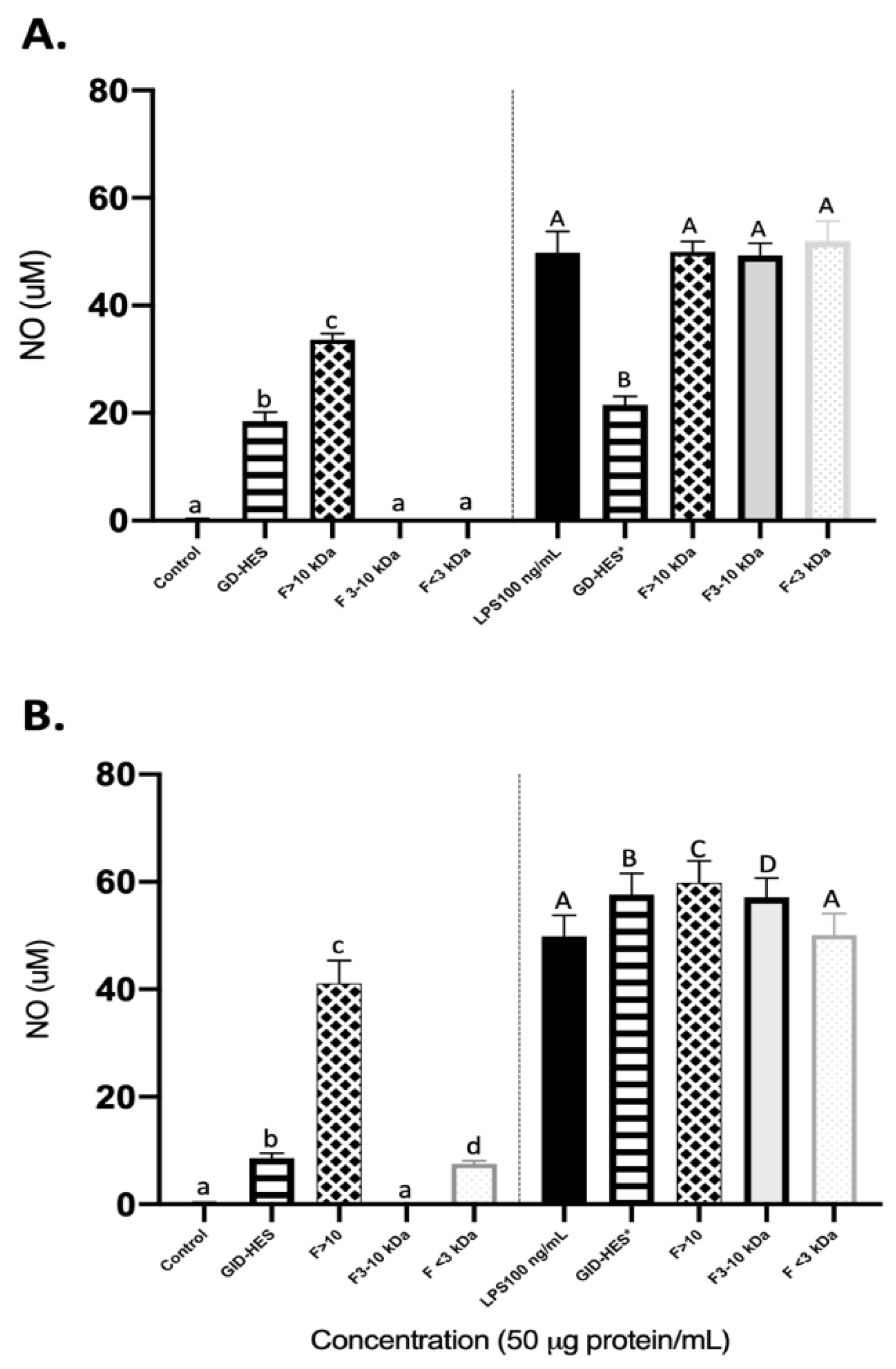
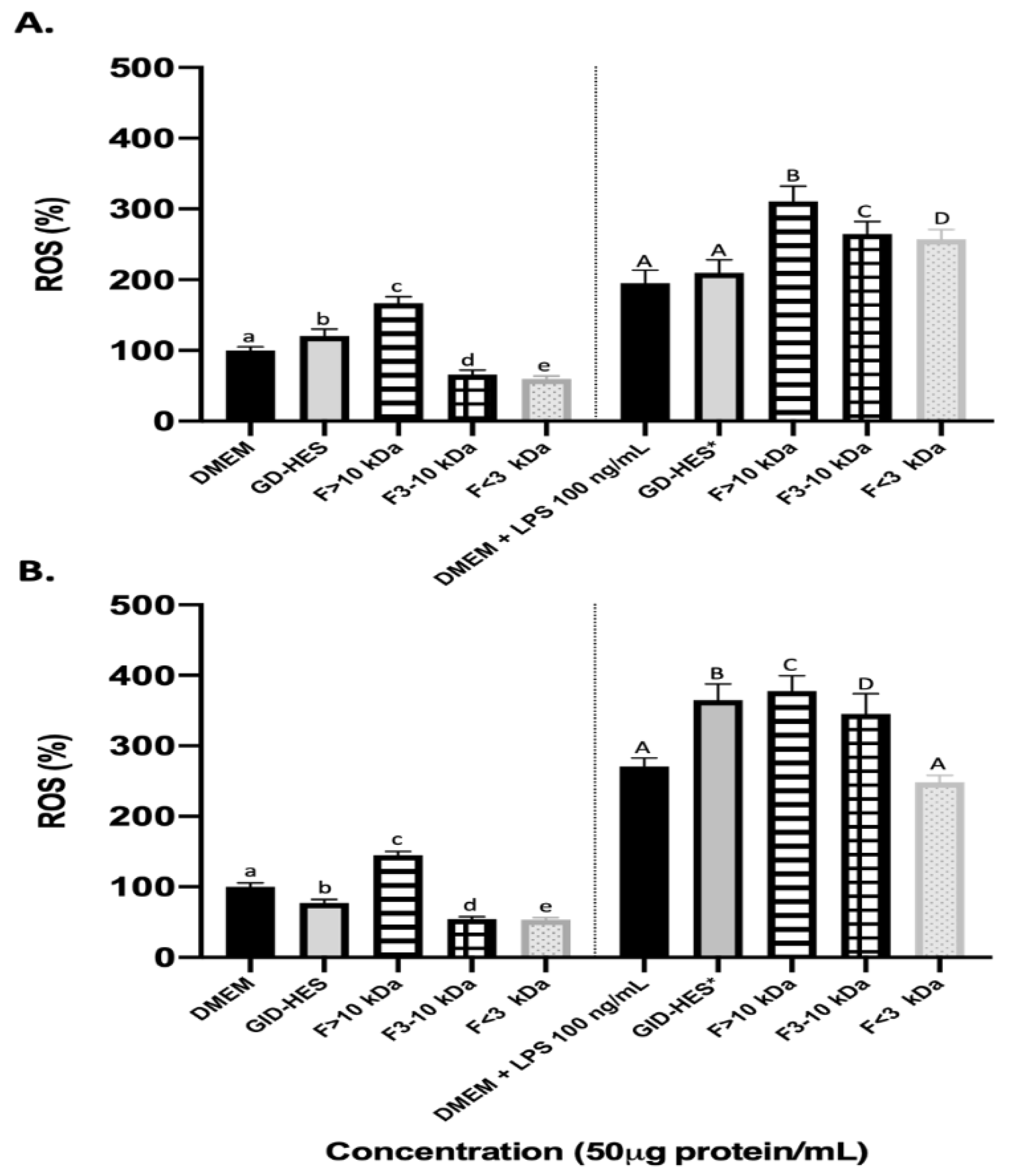
2.5. Impact of the Simulated Gastrointestinal Digestion on the Modulatory Effects of HES in an Immune Cell Model
3. Materials and Methods
3.1. Preparation of Chachafruto Seed Protein Concentrate (CPC)
3.2. Enzymatic Hydrolysis of Chachafruto Seed Protein Concentrate (CPC)
3.3. Characterization of the Chachafruto Protein Hydrolyzate (HES)
3.3.1. Degree of Hydrolysis (DH)
3.3.2. Amino Acid Content
3.3.3. Electrophoretic (SDS-PAGE) of Chachafruto Seed Protein Hydrolyzate (HES)
3.4. Simulated Gastrointestinal Digestion
3.5. Distribution of Molecular Weight
3.6. Antioxidant Activity by Biochemical Assays
3.7. Protective Effects in Macrophage RAW264.7 Cells
3.7.1. Cell Culture
3.7.2. Effects on Cell Viability
3.7.3. Effects on Reactive Oxygen Species (ROS) Generation
3.7.4. Effects on Nitric Oxide (NO) Levels
3.8. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Banwo, K.; Olojede, A.O.; Adesulu-Dahunsi, A.T.; Verma, D.K.; Thakur, M.; Tripathy, S.; Singh, S.; Patel, A.R.; Gupta, A.K.; Aguilar, C.N. Functional importance of bioactive compounds of foods with potential health benefits: a review on recent trends. Food Biosci. 2021, 43, 101320. [Google Scholar] [CrossRef]
- Guiné, R.P.F.; Florença, S.G.; Barroca, M.J.; Anjos, O. The link between the consumer and the innovations in food product development. Foods 2020, 9, 1317. [Google Scholar] [CrossRef] [PubMed]
- Mora, L.; Toldrá, F. Advanced enzymatic hydrolysis of food proteins for the production of bioactive peptides. Curr. Opin. Food Sci. 2022, 100973. [Google Scholar] [CrossRef]
- Augusto-Jimenez, Y.E.; González-Montoya, M.; Naranjo-Feliciano, D.; Uribe-Ramírez, D.; Cristiani-Urbina, E.; Díaz-Águila, C.; Yee-Madeira, H.; Mora-Escobedo, R. Antioxidant activity of bioactive peptide fractions from germinated soybeans conjugated to Fe3O4 nanoparticles by the Ugi multicomponent reaction. Molecules 2021, 26, 5726. [Google Scholar] [CrossRef] [PubMed]
- Bhandari, D.; Rafiq, S.; Gat, Y.; Gat, P.; Waghmare, R.; Kumar, V. A review on bioactive peptides: physiological functions, bioavailability and safety. Int. J. Pept. Res. Ther. 2020, 26, 139–150. [Google Scholar] [CrossRef]
- do Nascimento, E.S.; Anaya, K.; de Oliveira, J.M.C.; de Lacerda, J.T.J.G.; Miller, M.E.; Dias, M.; Mendes, M.A.; Pallone, J. de A.L.; Arns, C.W.; Juliano, M.A. Identification of bioactive peptides released from in vitro gastrointestinal digestion of yam proteins (Dioscorea cayennensis). Food Res. Int. 2021, 143, 110–286. [Google Scholar]
- Gao, Y.; Zhang, X.; Ren, G.; Wu, C.; Qin, P.; Yao, Y. Peptides from extruded lupin (Lupinus albus L.) regulate inflammatory activity via the P38 MAPK signal transduction pathway in RAW 264.7 cells. J. Agric. Food Chem. 2020, 68, 11702–11709. [Google Scholar] [CrossRef] [PubMed]
- Patil, S.M.; Sujay, S.; Tejaswini, M.; Sushma, P.P.; Prithvi, S.; Ramu, R. Bioactive peptides: its production and potential role on health. Innov. Food Sci. Emerg. Technol. 2020, 7, 167–182. [Google Scholar]
- Ahmed, T.; Sun, X.; Udenigwe, C.C. Role of structural properties of bioactive peptides in their stability during simulated gastrointestinal digestion: a systematic review. Trends Food Sci. Technol. 2022, 120, 265–273. [Google Scholar] [CrossRef]
- Acero, L.E. Guía para el cultivo y aprovechamiento del Chachafruto o Balú: Erythrina edulis triana ex micheli; Convenio Andres Bello, 2000.
- Serrano Cervantes, L.K. Multifuncionalidad de hidrolizados proteicos a partir de semillas de Erythrina edulis de diferentes regiones del Perú. 2022.
- Velásquez Holguín, L.F. Evaluación del potencial antioxidante e inhibitorio de la enzima convertidora de angiotensina (ECA) de hidrolizados proteicos de semillas de Chachafruto (Erythrina edulis Micheli. ). 2019. [Google Scholar]
- Guerra-Almonacid, C.M.; Torruco-Uco, J.G.; Murillo-Arango, W.; Méndez-Arteaga, J.J.; Rodríguez-Miranda, J. Effect of ultrasound pretreatment on the antioxidant capacity and antihypertensive activity of bioactive peptides obtained from the protein hydrolysates of Erythrina edulis. Emir. J. Food Agric. 2019, 288–296. [Google Scholar] [CrossRef]
- Intiquilla, A.; Jiménez-Aliaga, K.; Guzmán, F.; Alvarez, C.A.; Zavaleta, A.I.; Izaguirre, V.; Hernández-Ledesma, B. Novel antioxidant peptides obtained by alcalase hydrolysis of Erythrina edulis (Pajuro) protein. J. Sci. Food Agric. 2019, 99, 2420–2427. [Google Scholar] [CrossRef] [PubMed]
- Palma-Albino, C.; Intiquilla, A.; Jiménez-Aliaga, K.; Rodríguez-Arana, N.; Solano, E.; Flores, E.; Zavaleta, A.I.; Izaguirre, V.; Hernández-Ledesma, B. Albumin from Erythrina edulis (Pajuro) as a promising source of multifunctional peptides. Antioxidants 2021, 10, 1722. [Google Scholar] [CrossRef] [PubMed]
- Rutherfurd, S.M. Methodology for determining degree of hydrolysis of proteins in hydrolysates: a review. J. AOAC Int. 2010, 93, 1515–1522. [Google Scholar] [CrossRef] [PubMed]
- Nielsen, P.M.; Petersen, D.; Dambmann, C. Improved method for determining food protein degree of hydrolysis. J. Food Sci. 2001, 66, 642–646. [Google Scholar] [CrossRef]
- Spellman, D.; McEvoy, E.; O’cuinn, G.; FitzGerald, R.J. Proteinase and exopeptidase hydrolysis of whey protein: comparison of the TNBS, OPA and pH stat methods for quantification of degree of hydrolysis. Int. Dairy J. 2003, 13, 447–453. [Google Scholar] [CrossRef]
- Vioque, J.; Millán, F. Los hidrolizados proteicos en alimentación: suplementos alimenticios de gran calidad funcional y nutricional. AgroCSIC 2005, 2–8. [Google Scholar]
- Rodríguez, M.; Fillería, S.F.G.; Tironi, V.A. Simulated gastrointestinal digestion of Amaranth flour and protein isolate: comparison of methodologies and release of antioxidant peptides. Food Res. Int. 2020, 138, 109735. [Google Scholar] [CrossRef] [PubMed]
- Barbana, C.; Boye, J.I. In vitro protein digestibility and physico-chemical properties of flours and protein concentrates from two varieties of lentil (Lens culinaris). Food Funct. 2013, 4, 310–321. [Google Scholar] [CrossRef]
- Correa, J.L.; Zapata Montoya, J.E.; Hernández-Ledesma, B. Release of bioactive peptides from Erythrina edulis (Chachafruto) proteins under simulated gastrointestinal digestion. 2022.
- Trumbo, P.; Schlicker, S.; Yates, A.A.; Poos, M. Dietary reference intakes for energy, carbohydrate, fiber, fat, fatty acids, cholesterol, protein and amino acids. (Commentary). J. Amer. Diet. Assoc. 2002, 102, 1621–1631. [Google Scholar] [CrossRef]
- Li, Y.; Yu, Y.; Song, Y.; Wei, C. Influence of different planting combinations on the amino acid concentration in pericarp of Zanthoxylum planispinum ‘Dintanensis’ and soil. Forests 2023, 14, 843. [Google Scholar] [CrossRef]
- Pownall, T.L.; Udenigwe, C.C.; Aluko, R.E. Amino acid composition and antioxidant properties of pea seed (Pisum sativum L.) enzymatic protein hydrolysate fractions. J. Agric. Food Chem. 2010, 58, 4712–4718. [Google Scholar] [CrossRef] [PubMed]
- Suzen, S.; Cihaner, S.S.; Coban, T. Synthesis and comparison of antioxidant properties of indole-based melatonin analogue indole amino acid derivatives. Chem. Biol. Drug Des. 2012, 79, 76–83. [Google Scholar] [CrossRef] [PubMed]
- Martínez, E.P.; Costa, J.F.; Pérez, T.L.G.; Soret, N.L.G. Proteasas gástricas. Identificación y aislamiento, método directo en gel de agarosa. Rev. Cubana Med. 2020, 17, 207–217. [Google Scholar]
- Shen, C.-R.; Liu, C.-L.; Lee, H.-P.; Chen, J.-K. The identification and characterization of chitotriosidase activity in pancreatin from porcine pancreas. Molecules 2013, 18, 2978–2987. [Google Scholar] [CrossRef] [PubMed]
- Vásquez, P.; Zapata, J.E.; Chamorro, V.C.; Fillería, S.F.G.; Tironi, V.A. Antioxidant and angiotensin I-converting enzyme (ACE) inhibitory peptides of rainbow trout (Oncorhynchus mykiss) viscera hydrolysates subjected to simulated gastrointestinal digestion and intestinal absorption. LWT 2022, 154, 112834. [Google Scholar] [CrossRef]
- Zhang, Q.; Tong, X.; Qi, B.; Wang, Z.; Li, Y.; Sui, X.; Jiang, L. Changes in antioxidant activity of alcalase-hydrolyzed soybean hydrolysate under simulated gastrointestinal digestion and transepithelial transport. J. Funct. Foods 2018, 42, 298–305. [Google Scholar] [CrossRef]
- Mudgil, P.; Kamal, H.; Kilari, B.P.; Salim, M.A.S.M.; Gan, C.-Y.; Maqsood, S. Simulated gastrointestinal digestion of camel and bovine casein hydrolysates: identification and characterization of novel anti-diabetic bioactive peptides. Food Chem. 2021, 353, 129374. [Google Scholar] [CrossRef] [PubMed]
- Vilcacundo, R.; Martínez-Villaluenga, C.; Miralles, B.; Hernández-Ledesma, B. Release of multifunctional peptides from Kiwicha (Amaranthus caudatus) protein under in vitro gastrointestinal digestion. J. Sci. Food Agric. 2019, 99, 1225–1232. [Google Scholar] [CrossRef]
- Garcia-Mora, P.; Frias, J.; Peñas, E.; Zieliński, H.; Giménez-Bastida, J.A.; Wiczkowski, W.; Zielińska, D.; Martínez-Villaluenga, C. Simultaneous release of peptides and phenolics with antioxidant, ACE-inhibitory and anti-inflammatory activities from Pinto bean (Phaseolus vulgaris L. Var. Pinto) proteins by subtilisins. J. Funct. Foods 2015, 18, 319–332. [Google Scholar] [CrossRef]
- Hjellnes, V.; Rustad, T.; Jensen, I.-J.; Eiken, E.; Pettersen, S.M.; Falch, E. Ultrafiltration of saithe (Pollachius virens) protein hydrolysates and its effect on antioxidative activity. Catalysts 2021, 11, 1053. [Google Scholar] [CrossRef]
- Li, W.; Ye, S.; Zhang, Z.; Tang, J.; Jin, H.; Huang, F.; Yang, Z.; Tang, Y.; Chen, Y.; Ding, G. Purification and characterization of a novel pentadecapeptide from protein hydrolysates of Cyclina sinensis and its immunomodulatory effects on RAW264. 7 cells. Mar. Drugs 2019, 17, 30. [Google Scholar] [CrossRef] [PubMed]
- Jeong, S.H.; Kim, J.; Min, H. In vitro anti-inflammatory activity of the Artemisia montana leaf ethanol extract in macrophage RAW 264.7 cells. Food Agric. Immun. 2018, 29, 688–698. [Google Scholar] [CrossRef]
- Yu, Q.; Nie, S.; Li, W.; Zheng, W.; Yin, P.; Gong, D.; Xie, M. Macrophage immunomodulatory activity of a purified polysaccharide isolated from Ganoderma atrum. Phytother. Res. 2013, 27, 186–191. [Google Scholar] [CrossRef] [PubMed]
- de Oliveira Rodrigues, R.; Yaochite, J.N.U.; Braga, M.A.; Sasahara, G.L.; da Cruz Fonseca, S.G.; de Vasconcelos Araújo, T.D.; Santiago, M.P.; de Sousa, L.M.; de Carvalho, J.L.; Aires, F.B.S. Antioxidant and anti-inflammatory activities of Bauhinia ungulata L. (Fabaceae) on LPS-stimulated RAW 264.7 cells. Pharm. J. 2019, 11. [Google Scholar]
- Han, Y.-K.; Kim, Y.-S.; Natarajan, S.B.; Kim, W.-S.; Hwang, J.-W.; Jeon, N.-J.; Jeong, J.-H.; Moon, S.-H.; Jeon, B.-T.; Park, P.-J. Antioxidant and anti-inflammatory effects of Chaenomeles sinensis leaf extracts on LPS-stimulated RAW 264.7 cells. Molecules 2016, 21, 422. [Google Scholar] [CrossRef] [PubMed]
- Checa, J.; Aran, J.M. Reactive oxygen species: drivers of physiological and pathological processes. J. Inflamm. Res. 2020, 13, 1057–1073. [Google Scholar] [CrossRef] [PubMed]
- Du, C.; Hui, Z.; Li, N.; Liu, K.; Wang, Y.; Wang, P.; Sun, S. Investigation of the antioxidative potential of Meretrix meretrix L. derived peptides from simulated gastrointestinal digestion: in vitro and in silico insights. LWT 2024, 198, 115939. [Google Scholar] [CrossRef]
- Delgado, M.C.O.; Nardo, A.; Pavlovic, M.; Rogniaux, H.; Añón, M.C.; Tironi, V.A. Identification and characterization of antioxidant peptides obtained by gastrointestinal digestion of Amaranth proteins. Food Chem. 2016, 197, 1160–1167. [Google Scholar] [CrossRef]
- Zhang, M.; Huang, T.-S.; Mu, T.-H. Production and in vitro gastrointestinal digestion of antioxidant peptides from enzymatic hydrolysates of sweet potato protein affected by pretreatment. Plant Foods Hum. Nutr. 2019, 74, 225–231. [Google Scholar] [CrossRef]
- Shahidi, F. Natural antioxidants: chemistry, health effects, and applications; The American Oil Chemists Society, 1997; ISBN 978-0-935315-77-6.
- Cheng, X.; Gao, D.-X.; Song, J.-J.; Ren, F.-Z.; Mao, X.-Y. Casein glycomacropeptide hydrolysate exerts cytoprotection against H2O2-induced oxidative stress in RAW 264.7 macrophages via ROS-dependent heme oxygenase-1 expression. RSC Adv. 2015, 5, 4511–4523. [Google Scholar] [CrossRef]
- Allegra, M.; D’Acquisto, F.; Tesoriere, L.; Attanzio, A.; Livrea, M.A. Pro-oxidant activity of indicaxanthin from Opuntia ficus indica modulates arachidonate metabolism and prostaglandin synthesis through lipid peroxide production in LPS-stimulated RAW 264.7 macrophages. Redox Biol. 2014, 2, 892–900. [Google Scholar] [CrossRef] [PubMed]
- Adler-Nissen, J. Determination of the degree of hydrolysis of food protein hydrolysates by trinitrobenzenesulfonic acid. J. Agric. Food Chem. 1979, 27, 1256–1262. [Google Scholar] [CrossRef] [PubMed]
- Minekus, M.; Alminger, M.; Alvito, P.; Ballance, S.; Bohn, T.; Bourlieu, C.; Carrière, F.; Boutrou, R.; Corredig, M.; Dupont, D. A standardised static in vitro digestion method suitable for food–an international consensus. Food Funct. 2014, 5, 1113–1124. [Google Scholar] [CrossRef] [PubMed]
- Lozano-Ojalvo, D.; Molina, E.; López-Fandiño, R. Hydrolysates of egg white proteins modulate T- and B-cell responses in mitogen-stimulated murine cells. Food Funct. 2016, 7, 1048–1056. [Google Scholar] [CrossRef] [PubMed]
- Re, R.; Pellegrini, N.; Proteggente, A.; Pannala, A.; Yang, M.; Rice-Evans, C. Antioxidant activity applying an improved ABTS radical cation decolorization assay. Free Rad. Biol. Med. 1999, 26, 1231–1237. [Google Scholar] [CrossRef] [PubMed]
- Hernández-Ledesma, B.; Dávalos, A.; Bartolomé, B.; Amigo, L. Preparation of antioxidant enzymatic hydrolysates from α-lactalbumin and β-lactoglobulin. Identification of active peptides by HPLC-MS/MS. J. Agric. Food Chem. 2005, 53, 588–593. [Google Scholar] [CrossRef] [PubMed]
- LeBel, C.P.; Ischiropoulos, H.; Bondy, S.C. Evaluation of the probe 2’,7’-dichlorofluorescin as an indicator of reactive oxygen species formation and oxidative stress. Chem. Res. Toxicol. 1992, 5, 227–231. [Google Scholar] [CrossRef]
- Martinez-Villaluenga, C.; Dia, V.P.; Berhow, M.; Bringe, N.A.; Gonzalez de Mejia, E. Protein hydrolysates from β-conglycinin enriched soybean genotypes inhibit lipid accumulation and inflammation in vitro. Mol. Nutr. Food Res. 2009, 53, 1007–1018. [Google Scholar] [CrossRef]
| Amino acid | Content (g/100 g protein) | Content (g/100 g product) | FAO recommendations |
|---|---|---|---|
| Essential | |||
| Lys | 2.99 ± 0.08 | 2.18 ± 0.06 | 5.20 |
| Trp | n.d. | n.d. | 0.70 |
| Phe | 3.56 ± 0.09 | 2.59 ± 0.06 | 4.60a |
| Tyr | 3.77 ± 0.15 | 2.75 ± 0.11 | |
| Met | 0.82 ± 0.03 | 0.60 ± 0.02 | 2.60b |
| Cys | 0.64 ± 0.10 | 0.47 ± 0.08 | |
| Thr | 2.21 ± 0.14 | 1.61 ± 0.10 | 2.70 |
| Leu | 6.10 ± 0.20 | 4.45 ± 0.15 | 6.30 |
| Ile | 2.15 ± 0.09 | 1.57 ± 0.07 | 3.10 |
| Val | 2.96 ± 0.10 | 2.16 ± 0.07 | 4.20 |
| Non-essential | |||
| Asxa | 7.49 ± 0.29 | 5.46 ± 0.21 | |
| Glxb | 9.48 ± 0.35 | 6.91 ± 0.25 | |
| Ser | 3.85 ± 0.17 | 2.81 ± 0.12 | |
| His | 1.35 ± 0.03 | 0.98 ± 0.03 | |
| Arg | 3.06 ± 0.08 | 2.23 ± 0.06 | |
| Ala | 2.77 ± 0.07 | 2.02 ± 0.05 | |
| Pro | 3.80 ± 0.06 | 2.77 ± 0.04 | |
| Gly | 2.98 ± 0.11 | 2.17 ± 0.08 | |
| EAA | 25.20 | 18.38 | |
| NEAA | 34.78 | 25.35 | |
| TAA | 59.98 | 43.73 | |
| EAA*100/TAA(%) | 42.0 | ||
| EAA*100/NEAA (%) | 72.5 | ||
| HAA | 26.57 | 19.37 | |
| AAA | 7.32 | 5.34 | |
| HAA*100/TAA (%) | 44.3 | ||
| AAA*100/TAA (%) | 12.2 | ||
| SAMPLE | TEAC (μmol TE/mg protein) | ORAC (μmol TE/mg protein) | ||||||
|---|---|---|---|---|---|---|---|---|
| Whole sample | F > 10 kDa | F 3-10 kDa | F < 3 kDa | Whole sample | F > 10 kDa | F 3-10 kDa | F < 3 kDa | |
| HES | 0.64 ± 0.04a | 0.42 ± 0.01b,e | 0.43 ± 0.02c,e | 0.74 ± 0.03d | 1.95 ± 0.11a | 1.31 ± 0.02b | 1.48 ± 0.01c | 3.08 ± 0.10d |
| GD-HES | 0.73 ± 0.01a | 0.39 ± 0.001b,d | 0.37 ± 0.004c,d | 0.72 ± 0.004a | 2.72 ± 0.22a | 1.02 ± 0.09b | 1.22 ± 0.07c | 2.27 ± 0.02d |
| GID-HES | 0.68 ± 0.01a | 0.33 ± 0.004b,d | 0.34 ± 0.01c,d | 0.56 ± 0.01a | 2.96 ± 0.07a | 1.23 ± 0.08b | 1.17 ± 0.01c | 2.46 ± 0.11d |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





