1. Introduction
Recovery of cardiac function in human myocarditis is a common event and may spontaneously occur in as many as 50% of patients [
1]. Various mechanisms are known to contribute, including decline of cell death, re-synthesis of contractile proteins and then cell proliferation [
2]. Specifically, apoptosis and necrosis of cardiomyocytes have been found to decrease by 85% and 62% respectively in patients with virus-negative myocarditis responding to immuno-suppressive therapy. Myofibrillar content of myocardiocytes evaluated by ultrastructural morphometry was shown to increase by 33% in myocarditis subjects recovering and decline in those with further functional impairment. Finally, the level of myocyte replication has been reported to increase by 43% for Ki-67 and by 38% for mini-chromosome maintenance 5 (MCM5) positive cells after 6 months of successful immunosuppression [
2].
However, the mechanisms through which newly generated cardiomyocytes (NGCs) are recruit-ed and properly integrated into the myocardium are still unknown.
Miyawaki et al. showed that the signal transducer and activator of transcription 3 (STAT3) has been involved in cell cycle re-entry of adult cardiomyocytes in an experimental autoimmune myocarditis (EAM) model [
3]. In this study, 3 weeks after EAM induction by immunization with peptides derived from mouse α-myosin heavy chain (α-MHC), cardiac tissue resulted severely injured by inflammatory cell infiltration. Cardiac tissue restoration has been attributed to cell cycle re-entry of pre-existing mononucleated cardiomyocytes through the activation of STAT3. This finding was further supported by the enhancement in fibrosis detected in STAT3 cardiomyocyte-specific KO mice following 5 weeks of immunization. Importantly, a critical role of STAT3 has been also demonstrated in zebrafish and neonatal mouse cardiomyocyte proliferation [
4,
5]. Specifically, using a zebrafish model of ventricular ablation, early myocardial activation of Jak1/Stat3 downstream mediators by tissue damage stimulated the production of the protein Rln3a necessary for cardiomyocyte proliferation and heart regeneration (Fang, PNAS 2013; PMID: 23161148). It is also interesting to note that different studies have indicated pro-regenerative roles of inflammatory cytokines in other terminally differentiated tissues like zebrafish brain (Kyritsis N, Science 2012; PMID: 23138980). Accordingly, the study by Han et al. showed that IL-6 was essential for heart regeneration in neonatal mice and, consistently, cardiomyocyte-specific ablation of STAT3, the major downstream effector of IL-6 signaling, impaired cardiomyocyte proliferative response after apical resection [
4].
The IL-6/STAT3 signaling pathway is also essential for stem/progenitor cell proliferation [
6,
7] and high mobility group box 1 (HMGB1) is able to trigger activation of this pathway [
8,
9]. In particular, HMGB1, produced by stressed cardiomyocytes, induces the secretion of IL6 that activates the phosphorylation of STAT3.
Furthermore, the influence of ageing to this process is still unclear.
We report the assessment of NGC in a large population of patients with an age-range between 18 and 80 years and a biopsy-proven myocarditis of recent (≤ 4 weeks) onset. NGCs are identified by immunostaining positivity to nuclear markers of cardiomyocyte replication (Ki67 and p-STAT3) and by evidence of intracytoplasmic synthesis of contractile material (alpha-sarcomeric-actin and connexin 43). Their number per mm2 is correlated with patients ‘age. Finally, the mechanism of integration of NGC into necrotic myocardiocytes is analyzed.
2. Methods
We retrospectively enrolled consecutive patients (age range 18-80 years) from January 2010 to June 2021 with a left ventricular endomyocardial biopsy diagnostic for active myocarditis of re-cent (≤1 month) onset of symptoms including fever, chest pain and increased Troponin I.
2.1. Cardiac studies
All subjects before cardiac biopsy had an extensive non-invasive study including serum troponin I, 2D-echo showing normal valves and cardiac magnetic resonance
(CMR) suggesting myocardial edema and/or fibrosis. Echocardiographic parameters were determined according to established criteria [
10]. In particular, left ventricular ejection fraction (LVEF) was calculated in the apical 4 and 2-chamber views from three separate cardiac cycles using the modified Simpson’s method.
CMR was performed on a 1.5 Tesla scanner. Standard cardiac magnetic resonance protocol included: (i) Cine magnetic resonance images acquired during breath-holds in the short-axis, 2-chamber, and 4-chamber; (ii) black blood T2-weighted short-tau inversion recovery images on short-axis planes covering the entire left ventricle during 6 to 8 consecutive breath-holds for myocardial edema detection; (iii) late gadolinium-enhanced imaging performed 15 minutes after injection of 0.2 nmol/kg of gadoterate meglumine and signal intensity value 2 SDs above the mean signal intensity of the remote normal myocardium was considered suggestive for myocardial fibrosis. Functional and morphological data were analyzed according to the Lake Louise criteria [
11] for which CMR diagnosis of myocarditis is suggested by the presence or at least two of the following criteria, including early enhancement (hyperemia as an equivalent of vasodilation), edema on T2-weighted/STIR sequences, and late gadolinium enhancement (LGE) in a non-ischemic pattern.
2.2. Endomyocardial biopsy studies
Cardiac catheterization with left ventricular and coronary angiography was obtained in all patients. Endomyocardial biopsy (four to 8 samples for each patient) was performed in the septal-apical region of left ventricle [
12].
2.3. Histology, Immunohistochemistry and Assessment of NGC
For histological analysis the endomyocardial samples were fixed in 10% buffered formalin and paraffin embedded. Five micron-thick sections were stained with hematoxylin and eosin (H&E)
Histologic diagnosis of myocarditis included evidence of leukocyte infiltrates in association with damage of the adjacent myocytes, according to the Dallas criteria [
13] confirmed by im-munohistochemistry [
14]. For the phenotypic characterization of the inflammatory infiltrates, immunohistochemistry for CD3, CD20, CD43, CD45RO and CD68 was performed (all Dako, Carpinteria, California, USA). The presence of an inflammatory infiltrate ≥14 leucocytes/mm2 including up to 4 monocytes/mm2, with the presence of CD3-positive T-lymphocytes ≥7 cells/mm2 associated with evidence of degeneration and/or necrosis of the adjacent cardiomyocytes, was considered diagnostic for myocarditis.
NGCs were defined as small myocytes (diameter < 10 µm) with positive cytoplasm immunostaining for α-sarcomeric actin, cell membrane expressing connexin-43 and nuclear positivity to Ki67 and p-STAT-3. To analyze the possible influence of aging on NGC production, number of NGC were compared in groups of 20 patients in advancing age. In particular, number of NGC were determined in patients between 10 and 40 years (Group 1 – G1); in those between 41 and 60 years (Group 2 – G2); in those between 61 and 80 years (Group 3 – G3). Control NGC were obtained from 20 normal surgical biopsies.
2.4. Immunofluorescence
For immunofluorescence staining, human endomyocardial paraffin embedded sections (5 μm thickness) were dewaxed in xylene, hydrated, and rinsed in phosphate-buffered saline (PBS). Antigen retrieval was performed using alternatively 1 mM ethylenediaminetetraacetic acid (EDTA) buffer (pH 8) or TRIS EDTA-citrate buffer (pH 7.8) at 98 °C for 20 minutes, depending on the antigen localization (nuclear or cytoplasmatic, respectively). After cooling, sections were permeabilized for 10 min with 0.1% Triton X-100 in PBS. After a 1h block with antibody diluent with background reducing components (Dako, Agilent, Santa Clara, California, USA), sections were incubated in a humidified chamber at 4°C overnight with the following primary antibodies: mouse monoclonal anti-ki67 (ready to use, Leica Biosystems, Milan, Italy) rabbit polyclonal anti-Connexin 43 (1:750, Sigma-Aldrich, MERCK, St. Louis, Missouri, USA) or rabbit monoclonal anti-phospho STAT3 (1:200, Cell Signaling Technology, Danvers, Massachusetts, USA). After-wards, slides were washed with PBS for 3 times (5 minutes/wash) and incubated for 1 hour respectively with a goat anti-mouse IgG Alexa Fluor 488 and a goat anti-rabbit IgG Alexa Fluor 555 fluorescent secondary antibodies (1:200, Invitrogen, Thermo Fisher Scientific, Carlsbad, California, US). After that, to perform a double staining, biopsies were further incubated with an anti α-sarcomeric actin primary antibody (rabbit or mouse monoclonal respectively for double staining with ki67 or connexin43) (1:100 or 1:50, Sigma-Aldrich, MERCK, St. Louis, Missouri, USA) for 1h at 37°C, followed by a properly combined fluorescent secondary antibody incubation.
Finally, sections were washed with PBS for 3 times (5 minutes/wash) and nuclei were stained using DAPI (1:10000, 5min; Molecular Probes, USA). Slides were mounted in ProLong Diamond Antifade Mountant (Life Technologies, Thermo Fisher Scientific, Carlsbad, California, USA). For the analysis of the immunofluorescence, endocardial biopsies were imaged on LSM 501 confocal microscopy equipped with a digital camera (Zeiss, Jena, Germany).
2.5. Western blot assay
Proteins from endomyocardial biopsies were homogenized and extracted with Lysis buffer [50 mM Tris-HCl pH 7.4, 5 mM EDTA, 250 mM sodium chloride (NaCl) and 0.1% Triton® X-100] freshly added with protease and phosphatase inhibitors [0.1 mMol/L Dithiothreitol (DTT), 50 mM sodium fluoride (NaF), 0.1 mM sodium orthovanadate (Na3VO4), 1 mM phenylmethyl-sulfonyl fluoride (PMSF) and 1X protease inhibitor cocktail] (all components were purchased from Sigma-Aldrich_MERCK, St. Louis, Missouri, USA). Protein concentration was determined by Bradford assay (Bio-Rad, Hercules, California, USA) using the 96-well plater reader Glo-Max®-Multi Detection System (Promega Corporation, Madison, Wisconsin, USA).
Equal amounts of total cellular proteins (30 µg/lane) were boiled for 5 minutes at 95°C, resolved by denaturizing SDS-polyacrylamide gel electrophoresis and transferred to 0.45 µm nitrocellulose membrane (Bio-Rad, Hercules, California, USA). After blocking with 5% non-fat dry milk (Bio-Rad, Hercules, California, USA), membranes were incubated with a mouse monoclonal an-ti-HMGB1 primary antibody (1:500; MA5-17278, Invitrogen, Waltham, Massachusetts, USA) and a mouse monoclonal anti-GAPDH primary antibody (1:750; sc-137179, Santa Cruz Biotechnology Inc., Dallas, Texas, USA) overnight at 4°C, followed by appropriated horseradish peroxidase-coupled secondary antibodies and developed by a chemiluminescence-based detection system (Lite Ablot® TURBO; EuroClone, Milan, Italy). Densitometric analysis of the bands, relative to GAPDH, was determined by ImageJ Software (National Institutes of Health).
2.6. Real time PCR
In all patients at baseline a Real time PCR [
15] for the most common cardiotropic viruses (adenovirus, enterovirus, influenza A and B viruses, Epstein Barr virus, Parvovirus B19, Hepatitis C virus, Cytomegalovirus, Human Herpes Virus 6, Herpes Simplex virus type 1 and 2) was per-formed to determine whether a viral infection was the cause of myocarditis.
2.7. Statistical Analysis
For continuous variables, descriptive statistics were provided (number of available observations, mean, standard deviation), while median (interquartile range) was used for non-normal data. Categorical data were described as number (percentage). The normal distribution of all continuous variables was checked by visual methods (Q-Q plot and histogram) and by the significance test (Kolmogorov-Smirnov normality test and Shapiro-Wilk’s test). Comparisons among groups were performed using Pearson’s bivariate test and chi-square tests for categorical covariates; 1- way analysis of variance for continuous normally distributed covariates and non-parametric test of Kruskal Wallis for non-normally distributed continuous variable. For all tests, a p-value <0.05 was considered statistically significant. The relationship between the number of NGC and age was assessed using Pearson correlation coefficient (r) and p value. All statistical analyses were performed using R statistical analysis software (version 2023.09.0).
3. Results
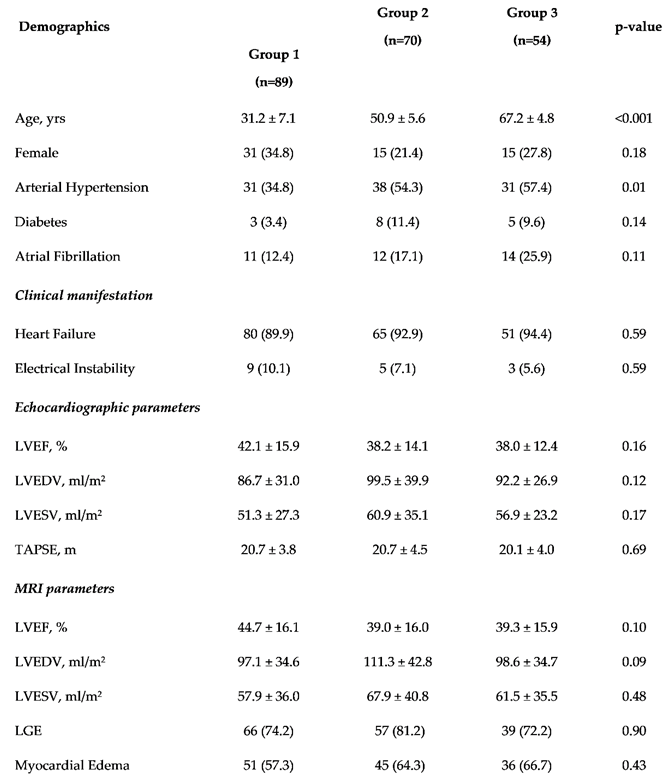
Two-hundred-thirteen patients with conclusive diagnosis of acute myocarditis were enrolled in our study [mean age: 46.8 ± 15.9; female: 61 (28.6%)]. As reported in
Table 1, the incidence of hypertension was significant difference between groups [Group 1: 31 (34.8%) vs Group 2: 38 (54.3%) vs Group 3: 31 (57.4%); p-value 0.01]; no significant difference between groups was observed in terms of clinical presentation, echocardiographic and CMR findings.
CMR documented in all, abnormal myocardial areas of T2 signaling and LGE enhancement in a non-ischemic pattern indicative of myocardial oedema and necrosis compatible with acute myocardial inflammation (Lake Louise criteria).
All patients met the histological criteria for active lymphocytic myocarditis (Dallas criteria implemented with immunohistochemical characterization of inflammatory cells) at endomyocardial biopsy.
No major complications were reported following invasive cardiac studies including cardiac biopsy.
Viral genomes were detected by PCR in 34 patients. Specifically, an adenovirus was found in 12 cases, an enterovirus in 8, Influenza virus A in 7, Epstein Barr virus in 5 and human herpes virus 6 in 2.
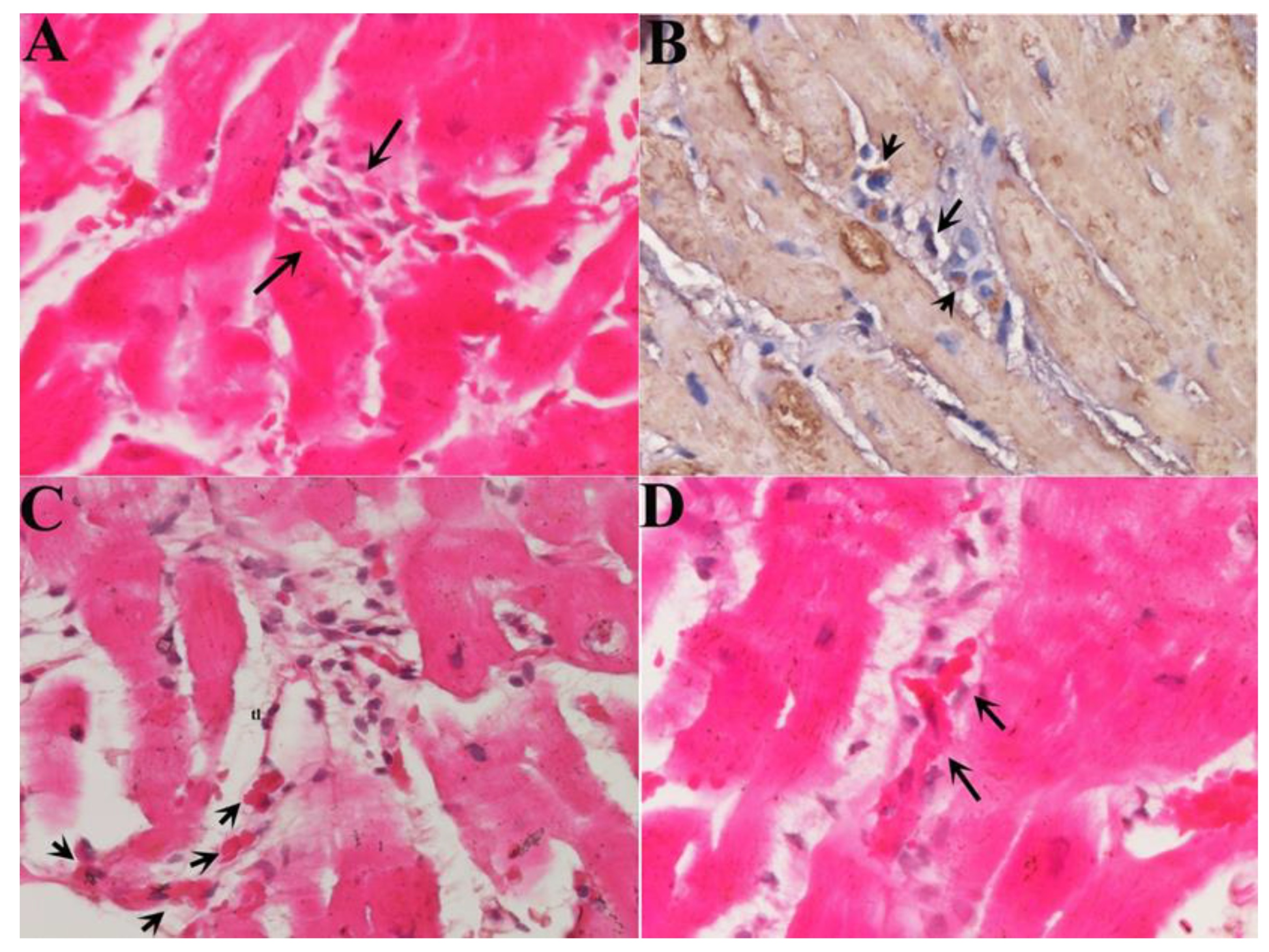
Small cells (with a diameter at nuclear level of 6.6± 3.34 µm, compared with adult myocytes of 22.5 ± 3.11 µm,
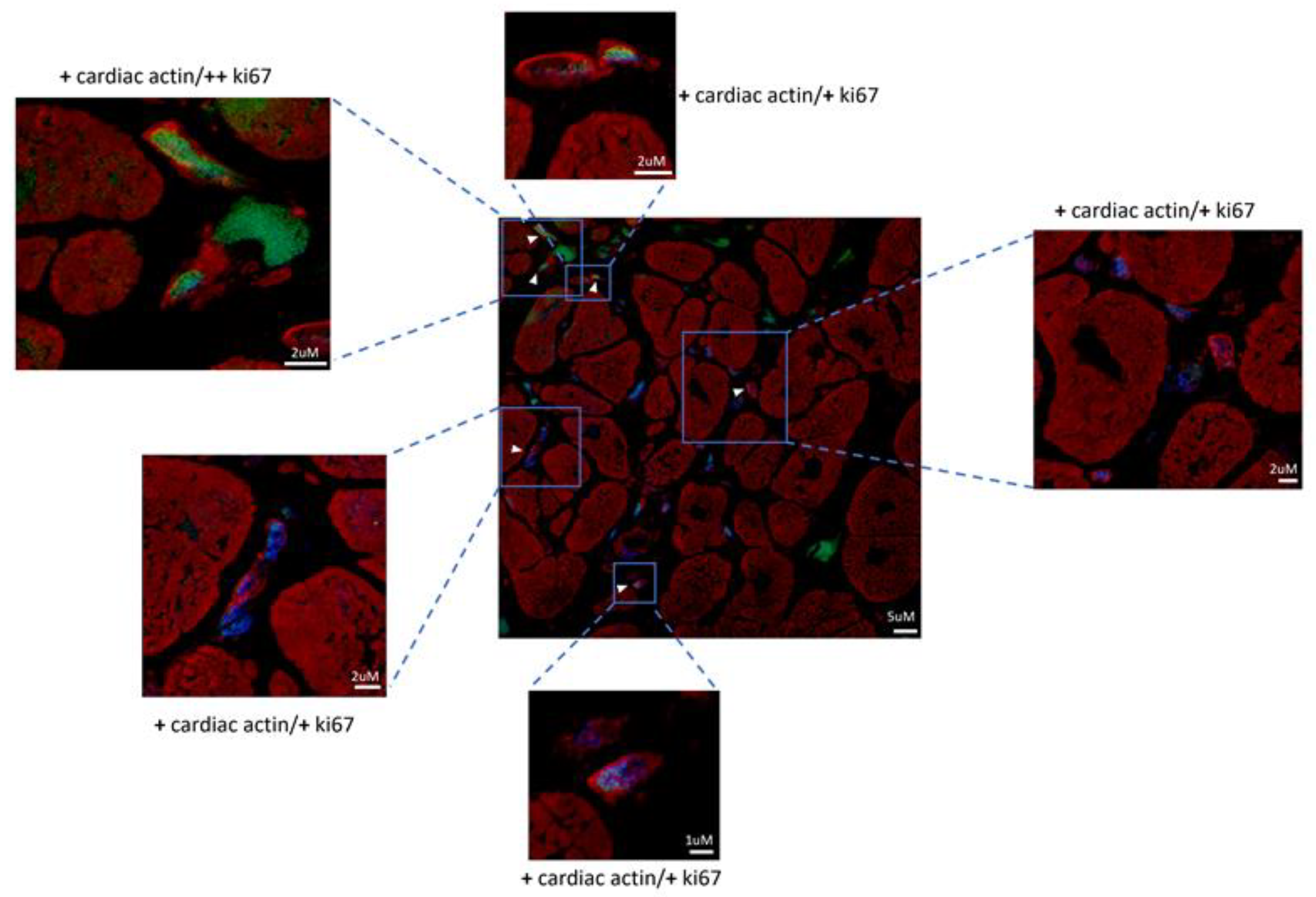
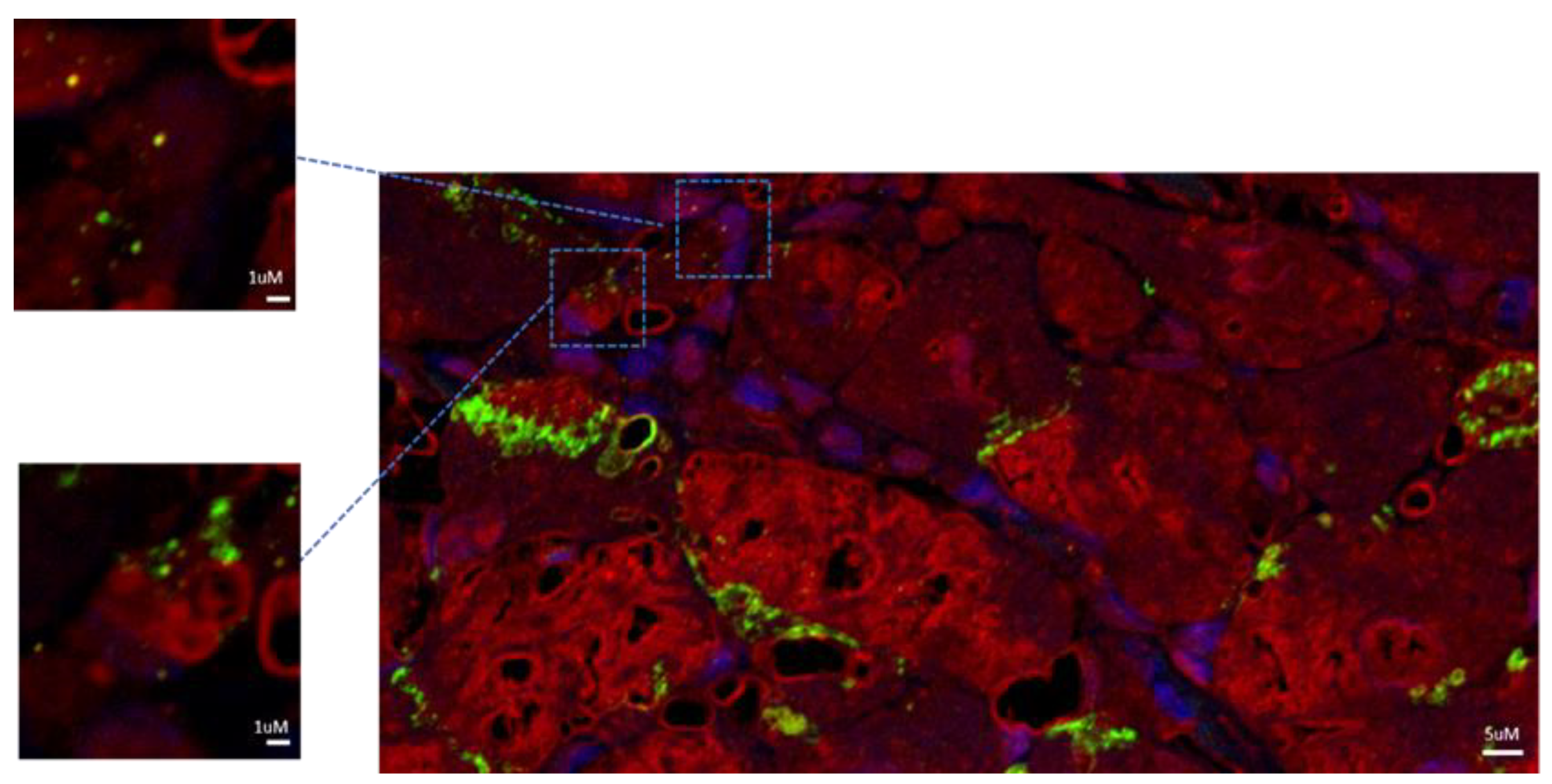
Figure 1) were observed in the setting of necrotic areas identifiable as NGC as expressing Ki-67 and pSTAT-3 in the nuclei, alpha-sarcomeric actin in the cytoplasm and con-nexin-43 at membrane level (
Figure 2 and
Figure 3). Ki-67 is a nuclear marker of cell proliferation that is no more detectable in adult, differentiated cells. Alpha-sarcomeric actin is a staining that recognizes contractile material characteristic of myocytes. Connexin-43 is a molecule that is part of the structure of the gap-junction responsible for electrical coupling between cardiomyocytes. Connexin-43 was abundantly distributed at the junctions between mature cardiomyocytes, but it was also detected as a punctuate staining at the surface of some newly formed cardiomyocytes (
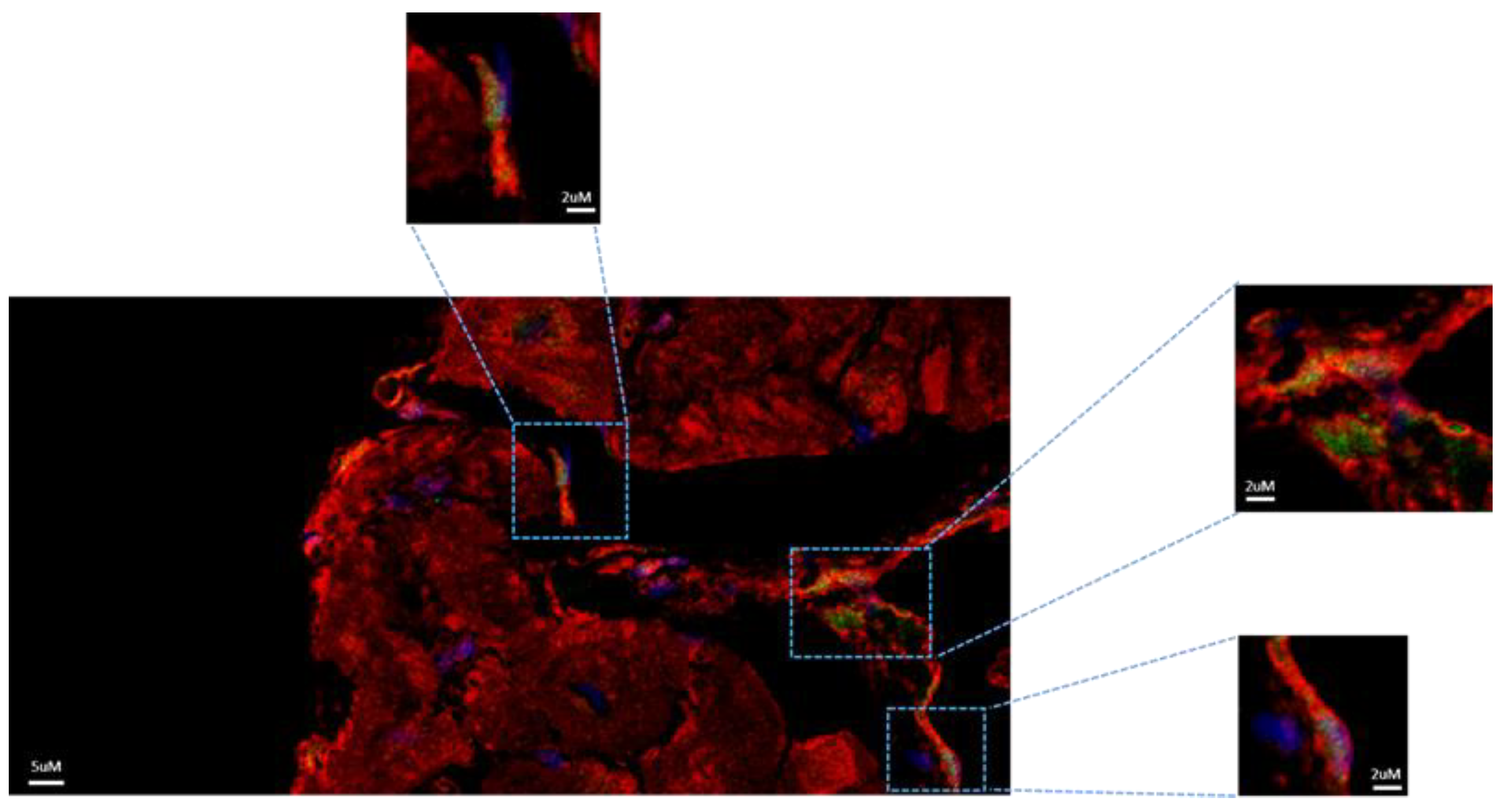
Figure 3). Immunofluorescence also revealed the translocation of STAT3 to the nucleus in several NGC as evidenced by nuclei expression of pSTAT3 (Y705) (
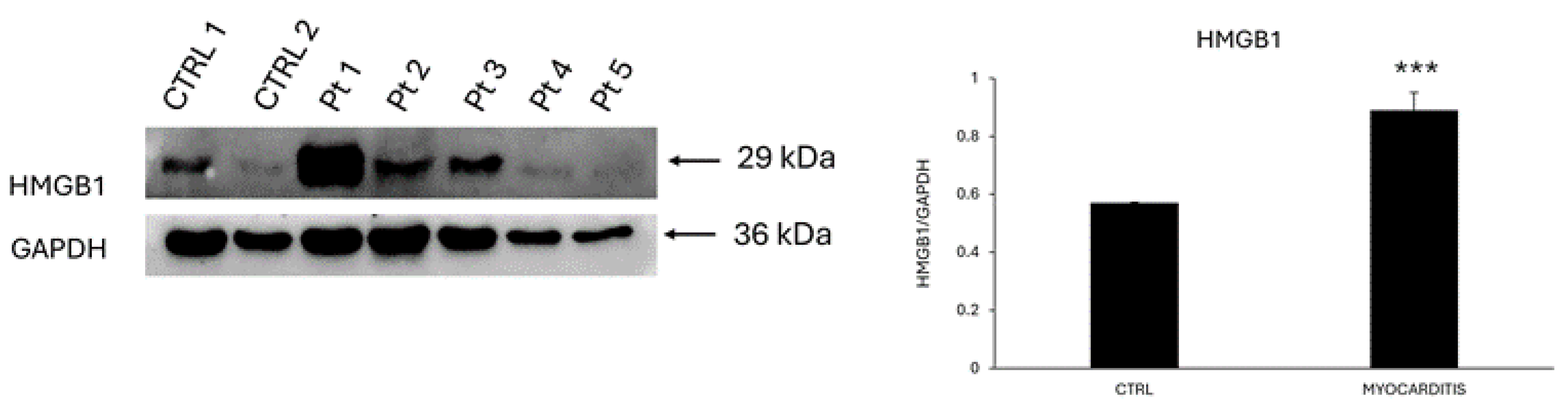
Figure 4). Translocation of pSTAT-3 has been correlated with cell proliferation. Interestingly, the protein expression level of HMGB1, an alarmin that activate the IL-6/STAT3 signaling pathway, was significantly higher (1.6 folds; *** P<0.001) in endomyocardial biopsies from patients diagnosed with myocarditis compared to controls (
Figure 5).
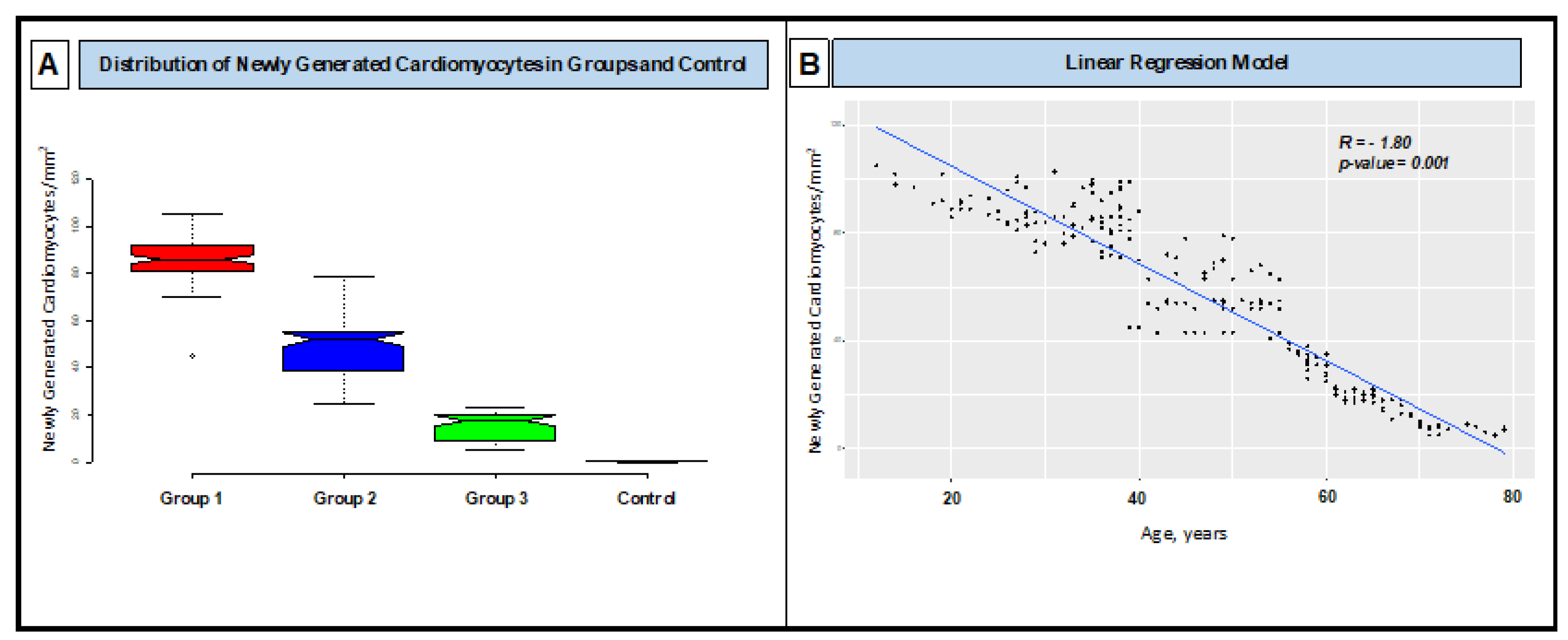
As NGC number is concerned, in G1 (patients between 10 and 40 years) were 86.3 ± 10.3/mm2; in G2 (those between 41 and 60 years) were 50.4 ±13.8/mm2; in G3 (those between 61 and 80 years) were 15.1 ± 5.7/ mm2; control NGC were 0.2± 0.2/mm2 (
Figure 6, Panel A). NGC number was not statistically different in viral and non-viral myocarditis. Value of NGC was significant and inversely associated with age (R – 1.80; p-value: 0.001) (
Figure 6, Panel B); no other significantly associations were reported.
In morphological terms, NGCs were in close contact with the scaffolds of empty cardiomyocytes deriving from myocytolytic activity of perforins bound to toxic, CD3+, T lymphocytes. They were found, in sequential histological sections, to cross the cytoplasmic membrane and grow inside the necrotic cell (
Figure 1).
4. Discussion
Improvement of cardiac dimension and function in patients with acute myocarditis is common either in response to supportive measures (as diuretics, ACE-inhibitors, carvedilol, digitalis, gliflozins etc) and/or specific treatments as anti-viral and immunosuppressive agents. In particular, the BICC trial has demonstrated the ability of Beta-interferon to improve the myocarditis promoted by entero- and adeno-virus [
16], while the TIMIC study reported the beneficial influence of immunosuppression on virus-negative inflammatory cardiomyopathy [
17,
18].
Nevertheless, complete cardiac recovery may occur spontaneously in up to 50% of cases with this affection [
1], suggesting an important contribute from natural pathways of repair. To this last regard, mechanisms involved are not yet completely clarified, although they include decline of operating noxa with decrease of cell death, cardiomyocyte repair by re-synthesis of contractile protein and then myocyte proliferation. In a previous report [
2] myocyte replication has been reported to increase by 43% for Ki67 and by 38% for MCM5 positive cells after 6 months of successful immunosuppression. However, the morphologic characterization of NGC, their modality of integration in the inflamed myocardium and their proliferative reserve with age have not been addressed so far.
Studying the histologic substrate of an extensive number of patients with acute myocarditis we were able to detect systematically NGCs in proximity of necrotic cells (
Figure 1). They were of small dimension (6.6± 3.34 µm, at nuclear level), were recognizable for the cytoplasm containing α-sarcomeric actin positive material, for cell membrane expressing connexin-43 and positive nuclear immunostaining for Ki67 and phospho STAT-3 denoting a proliferative state of cells.
It is likely that cardiomyocyte-oriented stem cells, resident in the myocardium or released by bone marrow might migrate to the site of cell necrosis following specific chemotactic stimuli, like HMGB1 [
19]. Accordingly, we detected an up-regulation in the expression of this protein in the endomyocardial biopsies from patients with myocarditis compared to controls [
20]. Our finding is supported by a preclinical study that adopted a mouse model of EAM. Specifically, Su and colleagues demonstrated that HMGB1 increased in the cardiac tissue and in the blood of EAM mice and its blockage with an anti-HMGB1 antibody reduced infiltration of T helper-17 cells, serum levels of inflammatory molecules and cardiac fibrosis. Cardiac fibro-blast/myofibroblasts represented the source of the secreted HMGB1 that contributed to collagen deposition. Interestingly, the authors showed that HMGB1 could also promote proliferation and migration of these cells. It is tempting to speculate that this proliferative effect exerted by HMGB1 toward cardiac fibroblasts/myofibroblasts might include also other cell types such as resident stem cells.
In our study, we were able to demonstrate that NGCs colonize the damaged myocardium crossing the scaffolds of necrotic myocytes (
Figure 1) resulting from perforating activity of toxic T lymphocytes. NGCs were able to grow inside empty cells (
Figure 1) realizing a perfect integration which preserves structure and function of the inflamed myocardium. To obtain homing and integration of NGCs into the inflamed myocardium, it is likely the need of at least two pre-requisites: 1) a preserved coronary circulation; 2) a partial integrity of plasma membrane of necrotic myocytes. Both these requisites are lacking in human myocardial infarction and it likely explains the lack of structural recovery in this clinical instance.
Importantly, our results suggest that myocarditis might promote regeneration at least partially by stimulating HMGB1/IL-6/STAT3-dependent NGC proliferation. This is in accordance with previous studies showing the ability of IL6/STAT signaling to induce cartilage regeneration mediated by cartilage stem/progenitor cells [
6] and the key role of this signaling in reactive cardiomyocyte proliferation induced by myocardial injury in neonatal mice [
4]. Moreover, it is well known that HMGB1 propagates the inflammatory response through the induction and secretion of inflammatory cytokines and chemokines, including IL6, and promotes the activation and proliferation of stem/progenitor cells [
21].
Furthermore, we analyzed the proliferative reserve of NGC with age. We found out a progressive decline of cardiomyocyte replication and then the ability to repair cardiac damage and dysfunction caused by myocarditis. Specifically, group of patients aged 61-80 years had an NGC number of 15.1 ± 5.7/ mm2 compared with 86.3 ± 10.3/mm2 of patients aged 18-40 years having the same degree of cardiac compromise (LVEF ≤ 30%). This suggests an increased risk of disability and death in aged patients undergoing acute myocarditis. Ultimately, the proliferative ability of NGC in the context of an acute cardiac inflammation seems independent from the cause as we did not find a difference in NGC number between viral and non-viral myocarditis (p < 0.05).
In conclusion, NGCs are constantly recognizable in acute human myocarditis. Their number de-clines with age. Their integration within necrotic myocytes consents preservation of cardiac structure and function.
Author Contributions
Author Contributions: AF designed the study, participated in and lead the categorization of the subjects, and wrote the manuscript, RV, EF, and FL categorized and organized the data and experiment. AF, and RV discussed and verified the cases, MM performed the statistics and collected the data. EF and LL obtained ultrastructural pictures All authors participated in drafting the manuscript and approved the final manuscript.
Funding
The study has been supported by FABRY SHIRE II, Ricerca corrente IRCCS L Spallanzani, and partially by Italian Health Ministry (IRCCS San Raffaele Roma–Ricerca Corrente #2020/1).
Institutional Review Board Statement
The study was conducted in accordance with the Declaration of Helsinki, and approved by the locally appointed ethics committee (opinion number 6/2019) approved the research protocol, and informed consent was obtained from all subjects.
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
The datasets used and analyzed during the current study are avail-able from the corresponding author upon reasonable request.
Conflicts of Interest
The authors declare that they have no competing interests.
Consent to publication: Informed consent to publication was obtained from relevant participants.
Abbreviations
| CMR |
Cardiac Magnetic Resonance. |
| EAM |
Experimental Autoimmune Myocarditis. |
| EDTA |
Ethylenediaminetetraacetic Acid. |
| HMGB1 |
High Mobility Group Box 1. |
| LGE |
Late Gadolinium Enhancement. |
| LVEF |
Left Ventricular Ejection Fraction. |
| MCM5 |
Mini-Chromosome Maintenance 5. |
| NGC |
Newly Generated Cardiomyocytes. |
| PBS |
Phosphate-Buffered Saline. |
| STAT3 |
Signal Transducer and Activator Transcription 3. |
References
- D’Ambrosio, A. The Fate of Acute Myocarditis between Spontaneous Improvement and Evolution to Dilated Cardiomyopathy: A Review. Heart 2001, 85, 499–504. [Google Scholar] [CrossRef]
- Frustaci, A.; Chimenti, C.; Pieroni, M.; Salvatori, L.; Morgante, E.; Sale, P.; et al. Cell Death, Proliferation and Repair in Human Myocarditis Responding to Immunosuppressive Therapy. Modern Pathology 2006, 19, 755–765. [Google Scholar] [CrossRef]
- Miyawaki, A.; Obana, M.; Mitsuhara, Y.; Orimoto, A.; Nakayasu, Y.; Yamashita, T.; et al. Adult Murine Cardiomyocytes Exhibit Regenerative Activity with Cell Cycle Reentry through STAT3 in the Healing Process of Myocarditis. Sci Rep 2017, 7, 1407. [Google Scholar] [CrossRef]
- Han, C.; Nie, Y.; Lian, H.; Liu, R.; He, F.; Huang, H.; et al. Acute Inflammation Stimulates a Regenerative Response in the Neonatal Mouse Heart. Cell Res 2015, 25, 1137–1151. [Google Scholar] [CrossRef]
- Fang, Y.; Gupta, V.; Karra, R.; Holdway, J.E.; Kikuchi, K.; Poss, K.D. Translational Profiling of Cardiomyocytes Identifies an Early Jak1/Stat3 Injury Response Required for Zebrafish Heart Regeneration. Proc. Natl. Acad. Sci. U.S.A. 2013, 110, 13416–13421. [Google Scholar] [CrossRef]
- Liu, T.; Li, X.; Wang, T.; Chen, X.; Zhang, S.; Liao, J.; et al. Kartogenin Mediates Cartilage Regeneration by Stimulating the IL-6/Stat3-Dependent Proliferation of Cartilage Stem/Progenitor Cells. Biochem Biophys Res Commun 2020, 532, 385–392. [Google Scholar] [CrossRef]
- Khaliq, M.; Ko, S.; Liu, Y.; Wang, H.; Sun, Y.; Solnica-Krezel, L.; et al. Stat3 Regulates Liver Progenitor Cell-Driven Liver Regeneration in Zebrafish. Gene Expr 2018, 18, 157–170. [Google Scholar] [CrossRef]
- Ghigo, A.; Franco, I.; Morello, F.; Hirsch, E. Myocyte Signalling in Leucocyte Recruitment to the Heart. Cardiovasc Res 2014, 102, 270–280. [Google Scholar] [CrossRef]
- Zhu, X.; Liu, L.; Wang, Y.; Cong, J.; Lin, Z.; Wang, Y.; et al. lncRNA MIAT/HMGB1 Axis Is Involved in Cisplatin Resistance via Regulating IL6-Mediated Activation of the JAK2/STAT3 Pathway in Nasopharyngeal Carcinoma. Front Oncol 2021, 11, 651693. [Google Scholar] [CrossRef]
- Lang, R.M.; Badano, L.P.; Mor-Avi, V.; Afilalo, J.; Armstrong, A.; Ernande, L.; et al. Recommendations for Cardiac Chamber Quantification by Echocardiography in Adults: An Update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. Journal of the American Society of Echocardiography 2015, 28, 1–39e14. [Google Scholar] [CrossRef]
- Friedrich, M.G.; Sechtem, U.; Schulz-Menger, J.; Holmvang, G.; Alakija, P.; Cooper, L.T.; et al. Cardiovascular Magnetic Resonance in Myocarditis: A JACC White Paper. Journal of the American College of Cardiology 2009, 53, 1475–1487. [Google Scholar] [CrossRef] [PubMed]
- Chimenti, C.; Frustaci, A. Contribution and Risks of Left Ventricular Endomyocardial Biopsy in Patients With Cardiomyopathies: A Retrospective Study Over a 28-Year Period. Circulation 2013, 128, 1531–1541. [Google Scholar] [CrossRef] [PubMed]
- Aretz, H.T.; Billingham, M.E.; Edwards, W.D.; Factor, S.M.; Fallon, J.T.; Fenoglio, J.J. ; Myocarditis. A Histopathologic Definition and Classification. Am J Cardiovasc Pathol 1987, 1, 3–14. [Google Scholar] [PubMed]
- Caforio, A.L.P.; Pankuweit, S.; Arbustini, E.; Basso, C.; Gimeno-Blanes, J. ; Felix; et al. Current State of Knowledge on Aetiology, Diagnosis, Management, and Therapy of Myocarditis: A Position Statement of the European Society of Cardiology Working Group on Myocardial and Pericardial Diseases. European Heart Journal 2013, 34, 2636–2648. [Google Scholar] [CrossRef]
- Maron, B.J.; Towbin, J.A.; Thiene, G.; Antzelevitch, C.; Corrado, D.; Arnett, D.; et al. Contemporary Definitions and Classification of the Cardiomyopathies: An American Heart Association Scientific Statement From the Council on Clinical Cardiology, Heart Failure and Transplantation Committee; Quality of Care and Outcomes Research and Functional Genomics and Translational Biology Interdisciplinary Working Groups; and Council on Epidemiology and Prevention. Circulation 2006, 113, 1807–1816. [Google Scholar] [CrossRef] [PubMed]
- Schultheiss, H.-P.; Piper, C.; Sowade, O.; Waagstein, F.; Kapp, J.-F.; Wegscheider, K.; et al. Betaferon in Chronic Viral Cardiomyopathy (BICC) Trial: Effects of Interferon-β Treatment in Patients with Chronic Viral Cardiomyopathy. Clin Res Cardiol 2016, 105, 763–773. [Google Scholar] [CrossRef] [PubMed]
- Chimenti, C.; Russo, M.A.; Frustaci, A. Immunosuppressive Therapy in Virus-Negative Inflammatory Cardiomyopathy: 20-Year Follow-up of the TIMIC Trial. European Heart Journal 2022, 43, 3463–3473. [Google Scholar] [CrossRef] [PubMed]
- Frustaci, A.; Chimenti, C. Immunosuppressive Therapy in Myocarditis. Circ J 2014, 79, 4–7. [Google Scholar] [CrossRef] [PubMed]
- Palumbo, R.; Sampaolesi, M.; De Marchis, F.; Tonlorenzi, R.; Colombetti, S.; Mondino, A.; et al. Extracellular HMGB1, a Signal of Tissue Damage, Induces Mesoangioblast Migration and Proliferation. J Cell Biol 2004, 164, 441–449. [Google Scholar] [CrossRef]
- Su, Z.; Yin, J.; Wang, T.; Sun, Y.; Ni, P.; Ma, R.; et al. Up-Regulated HMGB1 in EAM Directly Led to Collagen Deposition by a PKCβ/Erk1/2-Dependent Pathway: Cardiac Fibroblast/Myofibroblast Might Be Another Source of HMGB1. J Cell Mol Med 2014, 18, 1740–1751. [Google Scholar] [CrossRef]
- Bianchi, M.E.; Crippa, M.P.; Manfredi, A.A.; Mezzapelle, R.; Rovere Querini, P.; et al. High-Mobility Group Box 1 Protein Orchestrates Responses to Tissue Damage via Inflammation, Innate and Adaptive Immunity, and Tissue Repair. Immunol Rev 2017, 280, 74–82. [Google Scholar] [CrossRef] [PubMed]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).