Submitted:
03 July 2024
Posted:
06 July 2024
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Materials and Methods
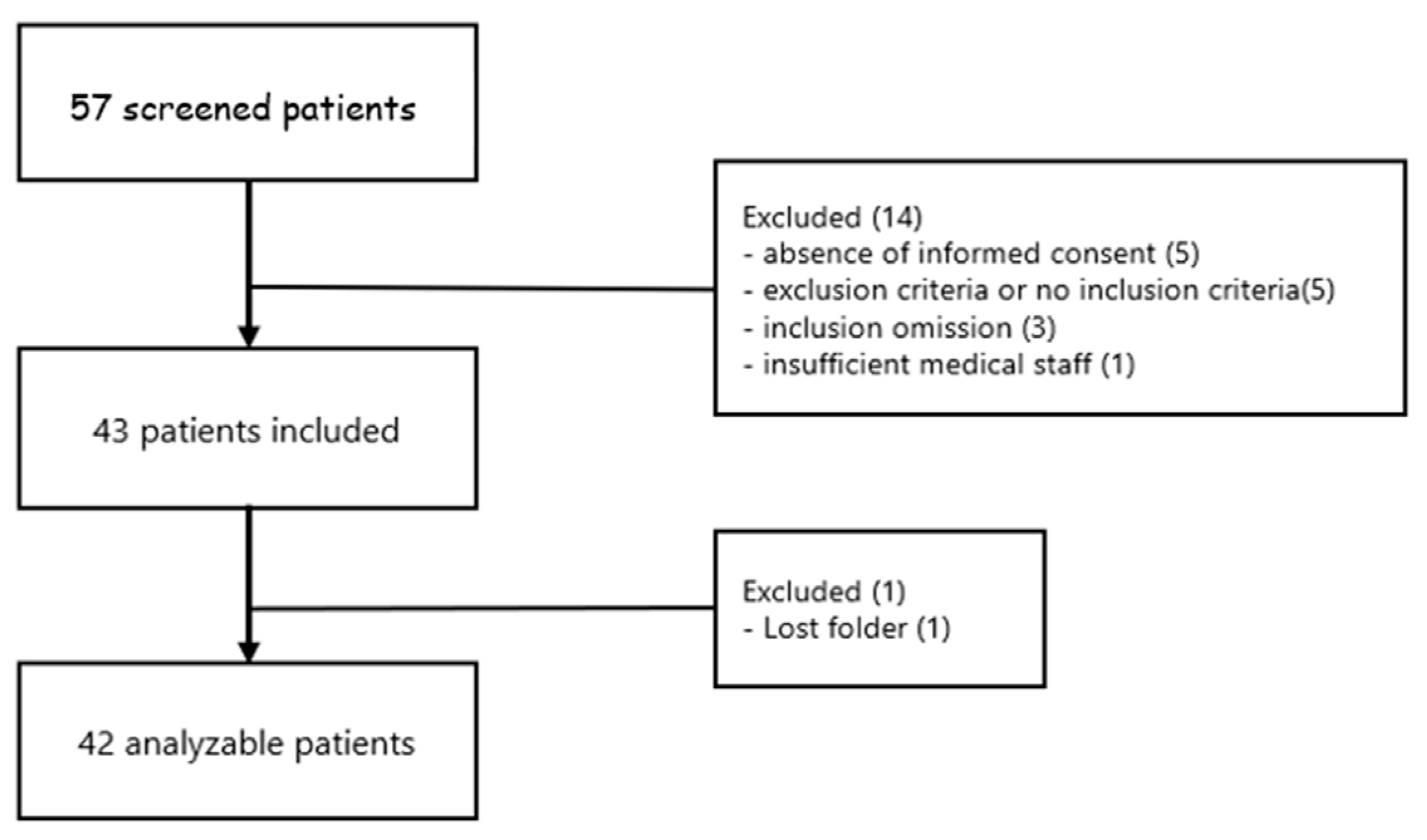
2.1. Study Design
2.2. Population
2.3. Heart Failure Treatment and Protocol
2.4. Lung Ultrasound Protocol
2.5. Follow-Up / Data Collection
2.6. Study Outcomes
2.7. Statistical Analysis
2.8. Ethical Aspects
3. Results
3.1. General Characteristics
3.2. Reproducibility and Feasibility
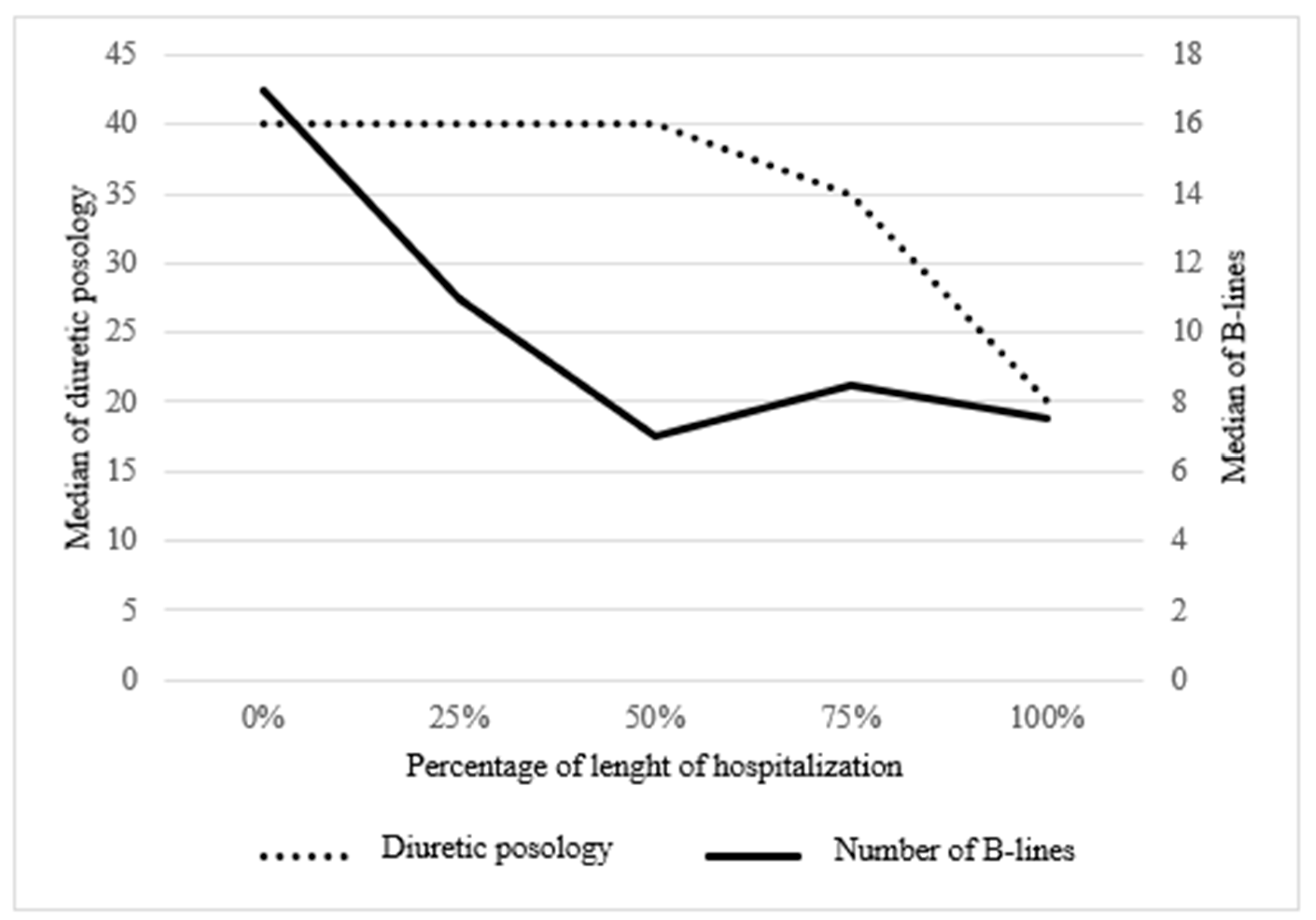
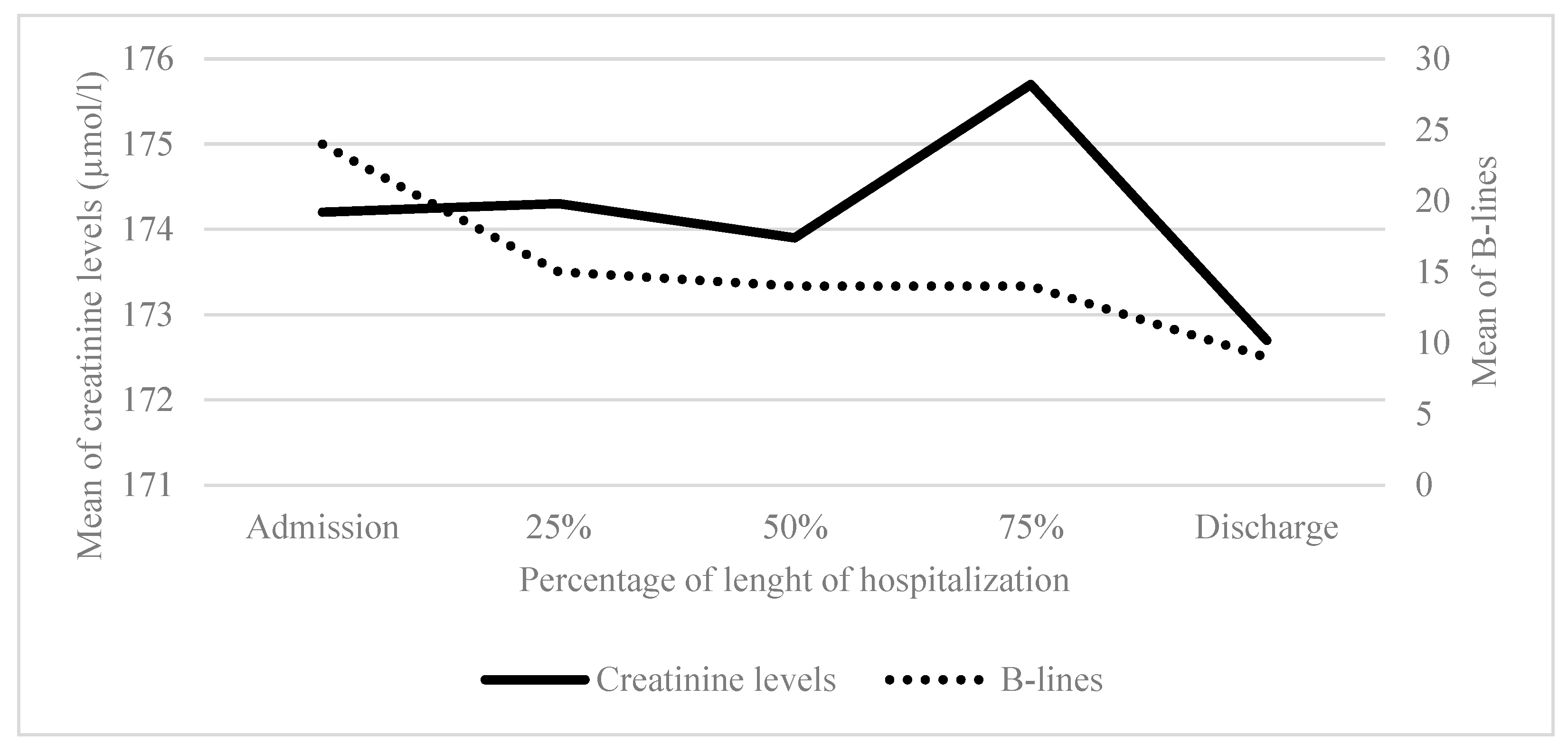
3.3. LUS B-Lines, Posology Diuretics, Clinical and Biological Parameters
4. Discussion
4.1. Strenghs
4.2. Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
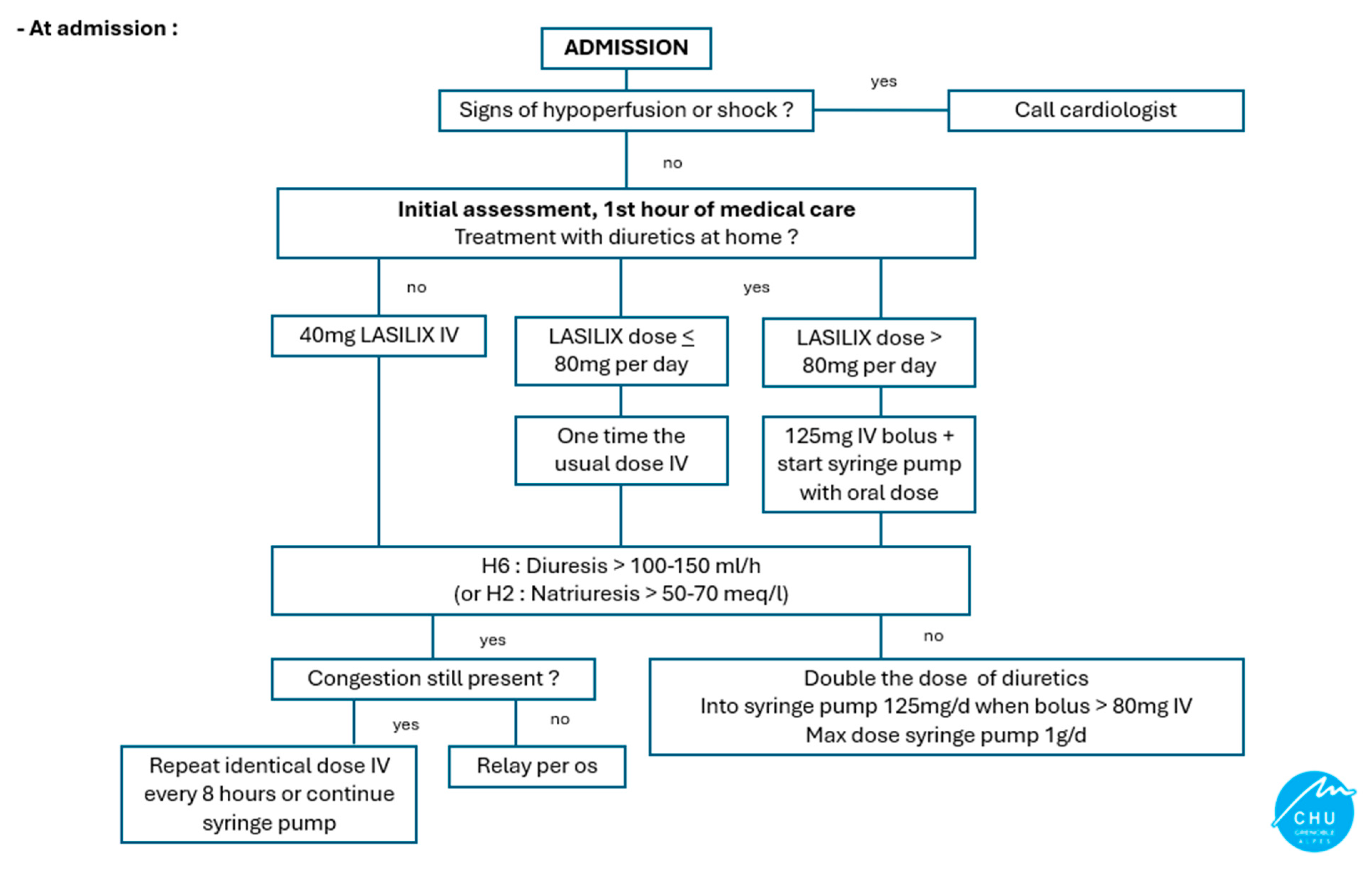
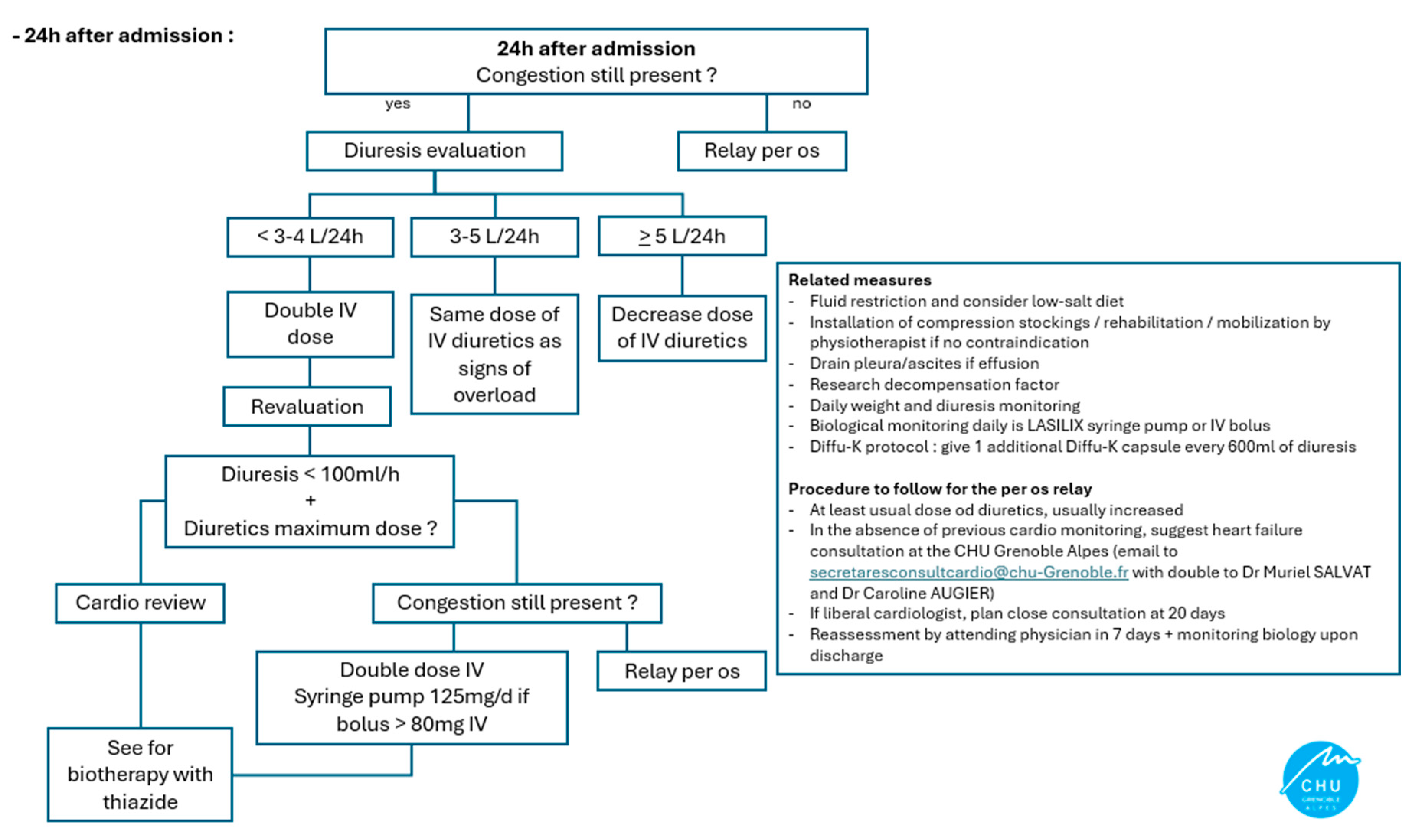
Appendix A. Diuretic Adaptation Protocol
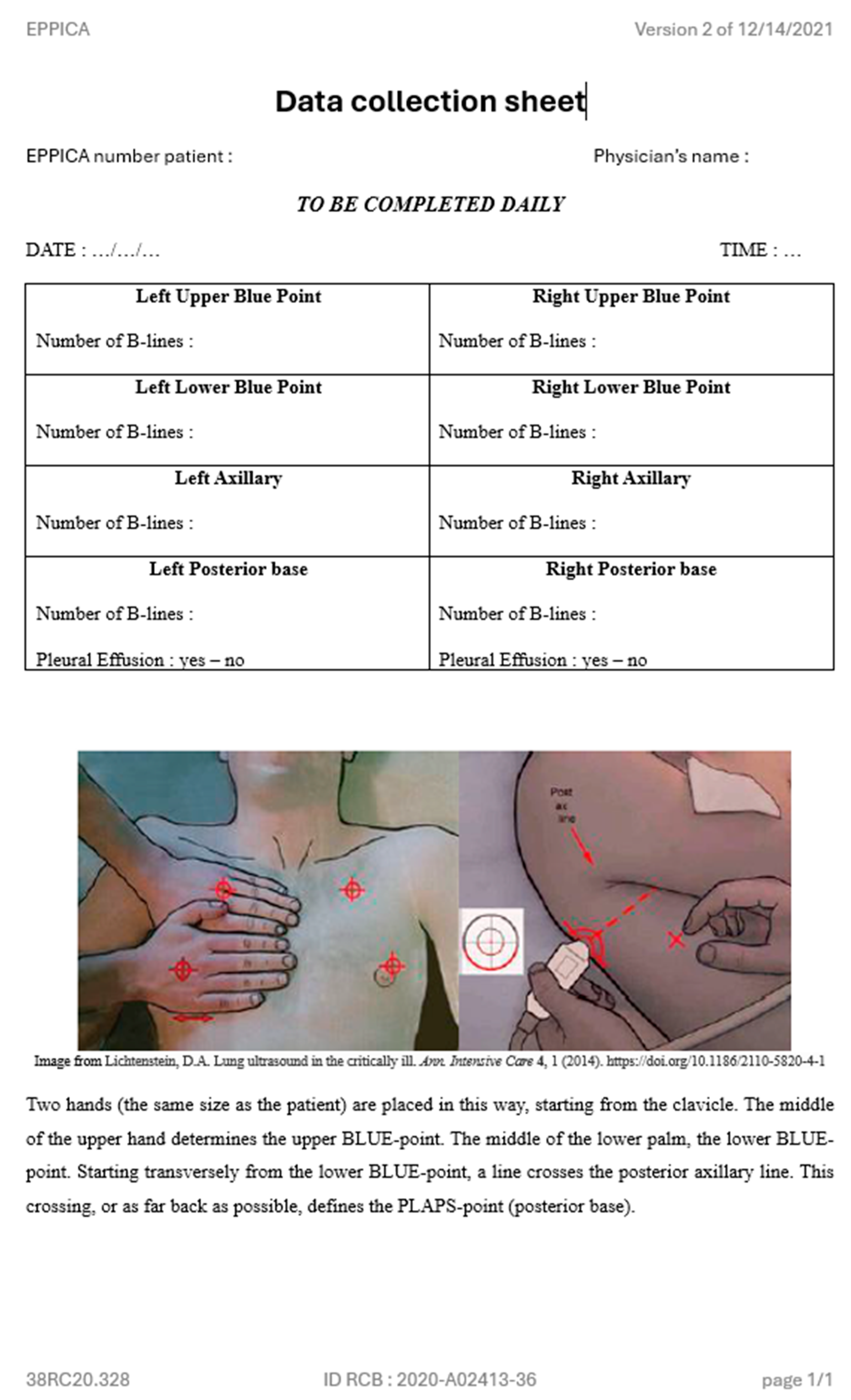
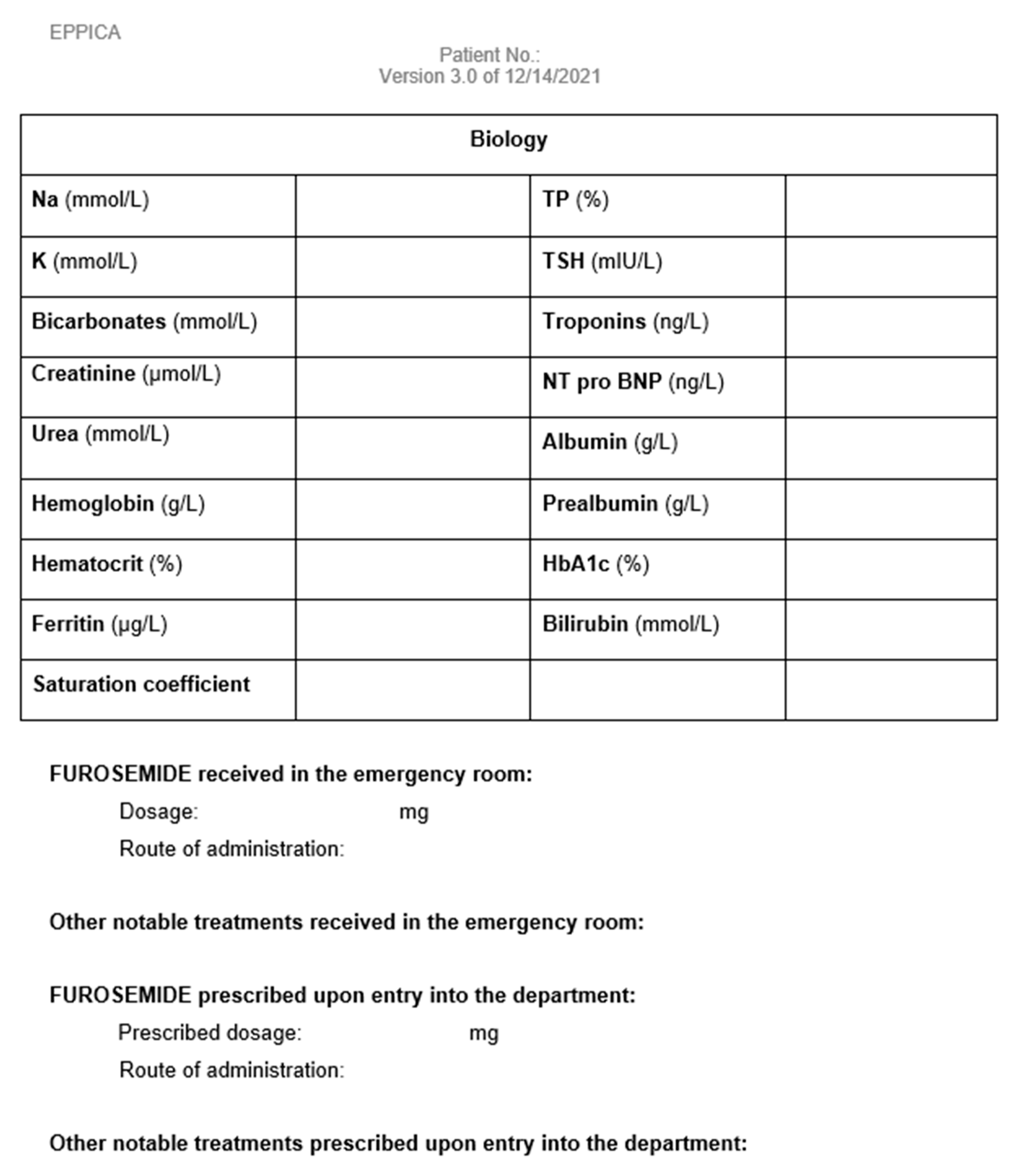
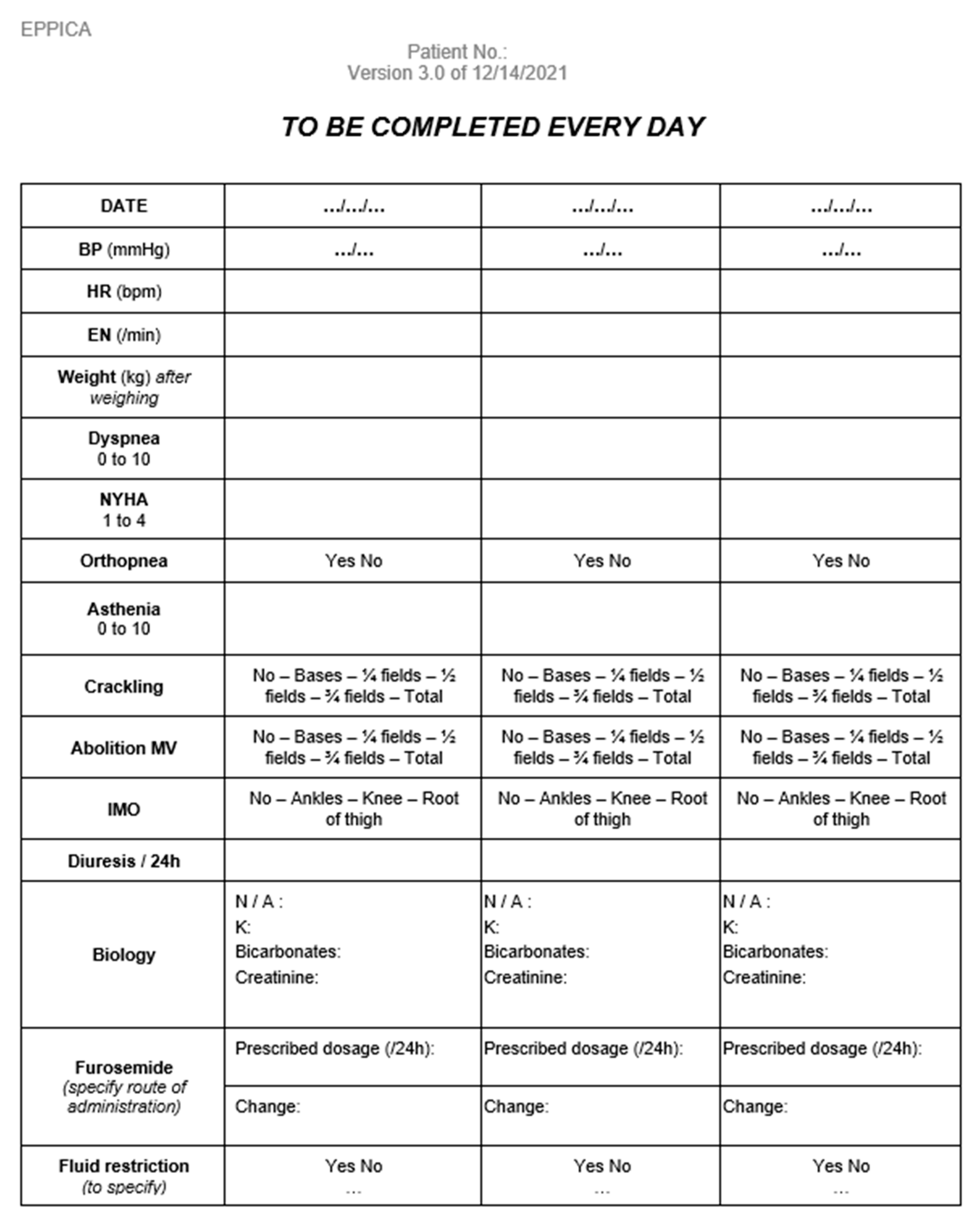
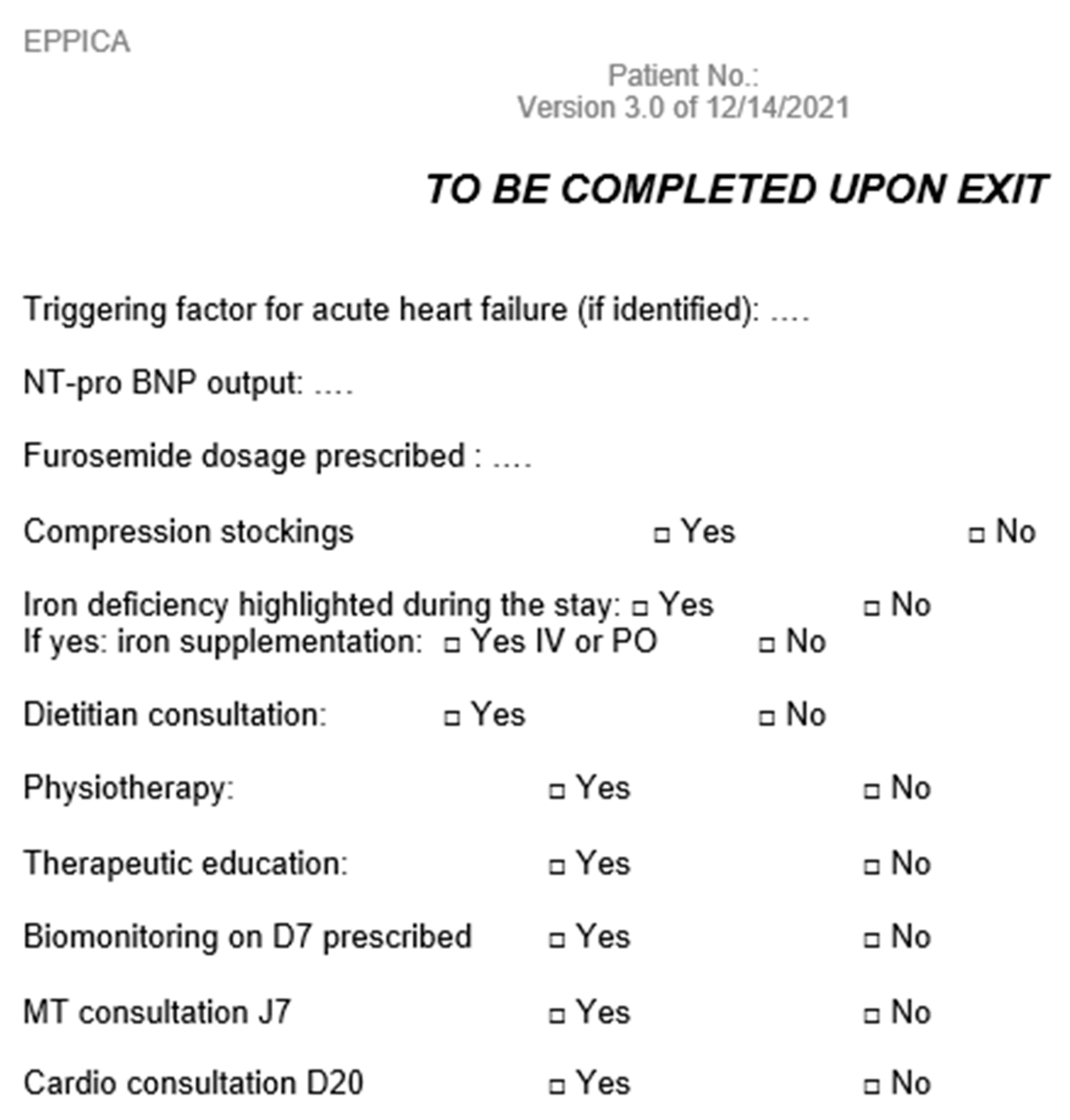
Appendix B. Paper Form to Report LUS Results
Appendix C. Follow-Up Table
| Description of the data collected |
Pre inclusion J0 (entry into service) |
Inclusion T0 |
Daily during hospitalization |
Follow-up visit 30 days (+/- 3 days) after the last ultrasound |
| Subject Information | ✔ | |||
| Checking eligibility criteria | ✔ | ✔ | ||
| Collection of clinical data | ✔ | ✔ | ||
| Collection of biological data | ✔ | ✔* | ||
| Ultrasound | ✔ | ✔ | ||
| Collection of rehospitalizations or vital status | ✔ | |||
| Medical record review | ✔ |
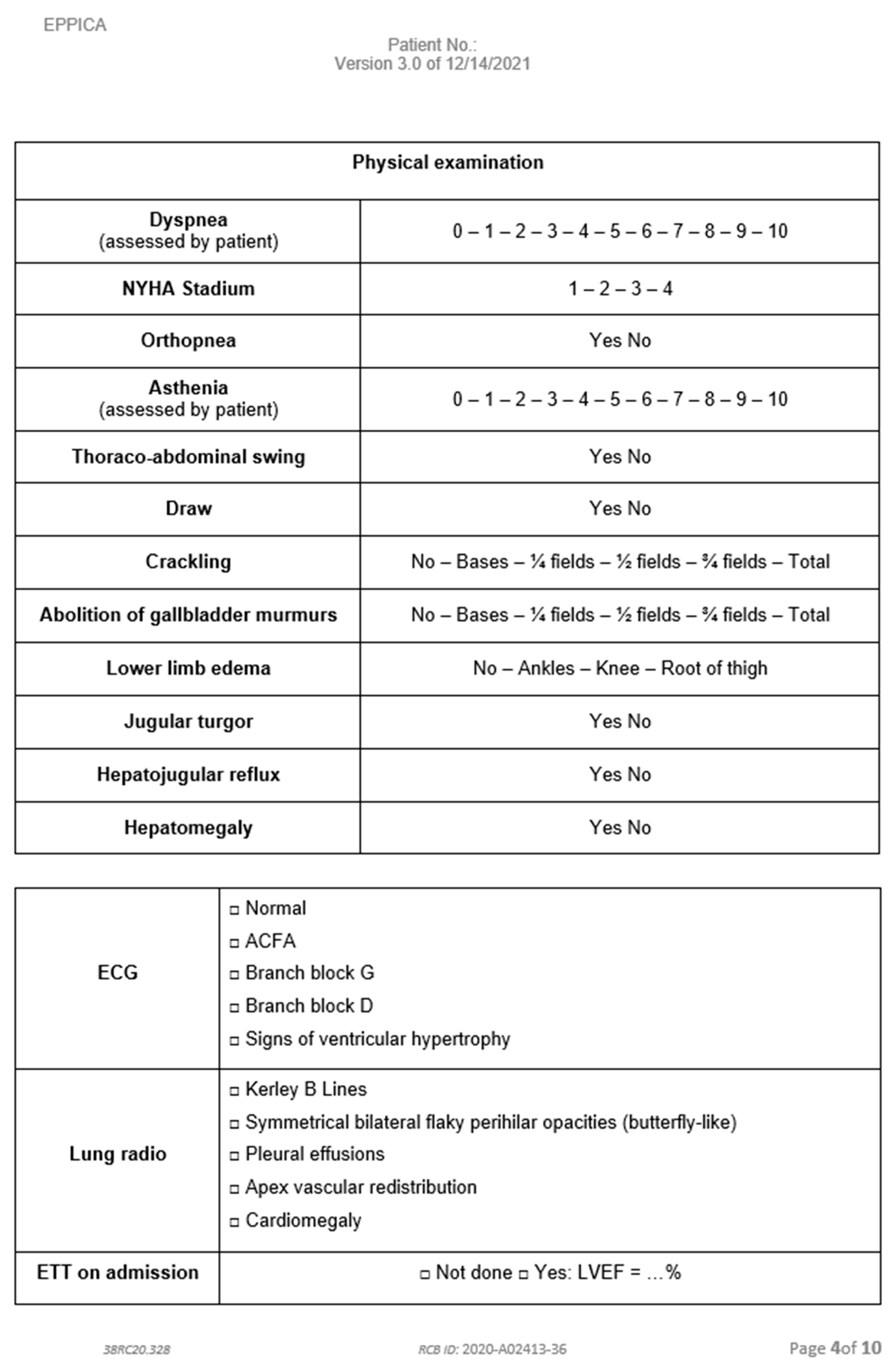
Appendix D. Data Collection Notebook



Appendix E. Notice of the Committee for the Protection of Persons – November the 3rd 2020
Appendix G. Notice of the Committee for the Protection of persons – Review of protocol on March the 1rd 2022
References
- L’état de santé de la population en France - Rapport 2017 | Direction de la recherche, des études, de l’évaluation et des statistiques [Internet]. [cité 18 mars 2022]. Disponible sur: https://drees.solidarites-sante.gouv.fr/publications-documents-de-reference/rapports/letat-de-sante-de-la-population-en-france-rapport-2017.
- Coiro S, Rossignol P, Ambrosio G, Carluccio E, Alunni G, Murrone A, et al. Prognostic value of residual pulmonary congestion at discharge assessed by lung ultrasound imaging in heart failure. Eur J Heart Fail. nov 2015;17(11):1172-81. [CrossRef]
- Cogliati C, Casazza G, Ceriani E, Torzillo D, Furlotti S, Bossi I, et al. Lung ultrasound and short-term prognosis in heart failure patients. Int J Cardiol. 1 sept 2016;218:104-8. [CrossRef]
- Gargani L, Pang PS, Frassi F, Miglioranza MH, Dini FL, Landi P, et al. Persistent pulmonary congestion before discharge predicts rehospitalization in heart failure: a lung ultrasound study. Cardiovasc Ultrasound. 4 sept 2015;13:40. [CrossRef]
- Torino C, Gargani L, Sicari R, Letachowicz K, Ekart R, Fliser D, et al. The Agreement between Auscultation and Lung Ultrasound in Hemodialysis Patients: The LUST Study. Clin J Am Soc Nephrol. 7 nov 2016;11(11):2005-11. [CrossRef]
- Riocreux CAM. Thèse de médecine : Optimisation des diurétiques dans la décompensation de l’insuffisance cardiaque chronique : rationnel e tdescription de l’étude ProDUCT-HF. 2019;134.
- Platz E, Merz AA, Jhund PS, Vazir A, Campbell R, McMurray JJ. Dynamic changes and prognostic value of pulmonary congestion by lung ultrasound in acute and chronic heart failure: a systematic review. Eur J Heart Fail. sept 2017;19(9):1154-63. [CrossRef]
- Girerd N, Seronde MF, Coiro S, Chouihed T, Bilbault P, Braun F, et al. Integrative Assessment of Congestion in Heart Failure Throughout the Patient Journey. JACC Heart Fail. avr 2018;6(4):273-85. [CrossRef]
- Martindale JL, Wakai A, Collins SP, Levy PD, Diercks D, Hiestand BC, et al. Diagnosing Acute Heart Failure in the Emergency Department: A Systematic Review and Meta-analysis. Acad Emerg Med. mars 2016;23(3):223-42. [CrossRef]
- Lichtenstein DA, Mezière GA. Relevance of lung ultrasound in the diagnosis of acute respiratory failure: the BLUE protocol. Chest. juill 2008;134(1):117-25. [CrossRef]
- Öhman J, Harjola VP, Karjalainen P, Lassus J. Focused echocardiography and lung ultrasound protocol for guiding treatment in acute heart failure. ESC Heart Fail. févr 2018;5(1):120-8. [CrossRef]
- 2021 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure | European Heart Journal | Oxford Academic [Internet]. [cité 1 juin 2022]. Disponible sur: https://academic.oup.com/eurheartj/article/42/36/3599/6358045?login=true.
- Frasure SE, Matilsky DK, Siadecki SD, Platz E, Saul T, Lewiss RE. Impact of patient positioning on lung ultrasound findings in acute heart failure. Eur Heart J Acute Cardiovasc Care. août 2015;4(4):326-32. [CrossRef]
- Buessler A, Chouihed T, Duarte K, Bassand A, Huot-Marchand M, Gottwalles Y, et al. Accuracy of Several Lung Ultrasound Methods for the Diagnosis of Acute Heart Failure in the ED: A Multicenter Prospective Study. Chest. janv 2020;157(1):99-110.
- Platz E, Jhund PS, Girerd N, Pivetta E, McMurray JJV, Peacock WF, et al. Expert consensus document: Reporting checklist for quantification of pulmonary congestion by lung ultrasound in heart failure. Eur J Heart Fail. juill 2019;21(7):844-51. [CrossRef]
- Pang PS, Russell FM, Ehrman R, Ferre R, Gargani L, Levy PD, et al. Lung Ultrasound-Guided Emergency Department Management of Acute Heart Failure (BLUSHED-AHF): A Randomized Controlled Pilot Trial. JACC Heart Fail. sept 2021;9(9):638-48.
- Panuccio V, Tripepi R, Parlongo G, Mafrica A, Caridi G, Catalano F, et al. Lung ultrasound to detect and monitor pulmonary congestion in patients with acute kidney injury in nephrology wards: a pilot study. J Nephrol. avr 2020;33(2):335-41. [CrossRef]
- Rivas-Lasarte M, Álvarez-García J, Fernández-Martínez J, Maestro A, López-López L, Solé-González E, et al. Lung ultrasound-guided treatment in ambulatory patients with heart failure: a randomized controlled clinical trial (LUS-HF study). Eur J Heart Fail. déc 2019;21(12):1605-13. [CrossRef]
- Rivas-Lasarte M, Maestro A, Fernández-Martínez J, López-López L, Solé-González E, Vives-Borrás M, et al. Prevalence and prognostic impact of subclinical pulmonary congestion at discharge in patients with acute heart failure. ESC Heart Fail. oct 2020;7(5):2621-8. [CrossRef]
- Ramos-Hernández C, Botana-Rial M, Núñez-Fernández M, Lojo-Rodríguez I, Mouronte-Roibas C, Salgado-Barreira Á, et al. Validity of Lung Ultrasound: Is an Image Worth More Than a Thousand Sounds? J Clin Med. 25 mai 2021;10(11):2292.
- Ruggenenti P, Remuzzi G. Worsening kidney function in decompensated heart failure: treat the heart, don’t mind the kidney. Eur Heart J. oct 2011;32(20):2476-8. [CrossRef]
- Blair JEA, Pang PS, Schrier RW, Metra M, Traver B, Cook T, et al. Changes in renal function during hospitalization and soon after discharge in patients admitted for worsening heart failure in the placebo group of the EVEREST trial. Eur Heart J. oct 2011;32(20):2563-72. [CrossRef]
- Virani SS, Alonso A, Aparicio HJ, Benjamin EJ, Bittencourt MS, Callaway CW, et al. Heart Disease and Stroke Statistics—2021 Update. Circulation. 23 févr 2021;143(8):e254-743. [CrossRef]
- Platz E, Campbell RT, Claggett B, Lewis EF, Groarke JD, Docherty KF, et al. Lung Ultrasound in Acute Heart Failure: Prevalence of Pulmonary Congestion and Short- and Long-Term Outcomes. JACC Heart Fail. oct 2019;7(10):849-58.
- Miglioranza MH, Gargani L, Sant’Anna RT, Rover MM, Martins VM, Mantovani A, et al. Lung ultrasound for the evaluation of pulmonary congestion in outpatients: a comparison with clinical assessment, natriuretic peptides, and echocardiography. JACC Cardiovasc Imaging. nov 2013;6(11):1141-51.
- Komajda M, Hanon O, Hochadel M, Lopez-Sendon JL, Follath F, Ponikowski P, et al. Contemporary management of octogenarians hospitalized for heart failure in Europe: Euro Heart Failure Survey II. Eur Heart J. févr 2009;30(4):478-86. [CrossRef]
- Formiga F, Moreno-Gonzalez R, Chivite D, Franco J, Montero A, Corbella X. High comorbidity, measured by the Charlson Comorbidity Index, associates with higher 1-year mortality risks in elderly patients experiencing a first acute heart failure hospitalization. Aging Clin Exp Res. août 2018;30(8):927-33. [CrossRef]
- Bannay A, Chaignot C, Blotière PO, Basson M, Weill A, Ricordeau P, et al. The Best Use of the Charlson Comorbidity Index With Electronic Health Care Database to Predict Mortality. Med Care. févr 2016;54(2):188-94. [CrossRef]
- Freund Y, Cachanado M, Delannoy Q, Laribi S, Yordanov Y, Gorlicki J, et al. Effect of an Emergency Department Care Bundle on 30-Day Hospital Discharge and Survival Among Elderly Patients With Acute Heart Failure: The ELISABETH Randomized Clinical Trial. JAMA. 17 nov 2020;324(19):1948-56.
- Teixeira A, Parenica J, Park JJ, Ishihara S, AlHabib KF, Laribi S, et al. Clinical presentation and outcome by age categories in acute heart failure: results from an international observational cohort. Eur J Heart Fail. nov 2015;17(11):1114-23. [CrossRef]
- Russell FM, Ferre R, Ehrman RR, Noble V, Gargani L, Collins SP, et al. What are the minimum requirements to establish proficiency in lung ultrasound training for quantifying B-lines? ESC Heart Fail. oct 2020;7(5):2941-7.
- Mazzola M, Pugliese NR, Zavagli M, De Biase N, Bandini G, Barbarisi G, et al. Diagnostic and Prognostic Value of Lung Ultrasound B-Lines in Acute Heart Failure With Concomitant Pneumonia. Front Cardiovasc Med. 2021;8:693912. [CrossRef]
- Yang F, Wang Q, Zhi G, Zhang L, Huang D, Shen D, et al. The application of lung ultrasound in acute decompensated heart failure in heart failure with preserved and reduced ejection fraction. Echocardiography. oct 2017;34(10):1462-9. [CrossRef]
- Palazzuoli A, Ruocco G, Beltrami M, Nuti R, Cleland JG. Combined use of lung ultrasound, B-type natriuretic peptide, and echocardiography for outcome prediction in patients with acute HFrEF and HFpEF. Clin Res Cardiol. juill 2018;107(7):586-96. [CrossRef]
| No | Mean (SD) or No. (%) | |
| Baseline characteristics | ||
| Age, years (extremes) | 42 | 85.8 (SD 8.2) (71.7 - 98.8) |
| Male gender | 42 | 26 (61.9%) |
| BMI (kg/m2) | 39 | 26.5 (SD 6.2) |
| Charlson Comorbidity Index | 42 | 8.5 (SD 2.3) |
| Chronic kidney disease | 42 | 22 (52%) |
| Hypertension | 42 | 32 (76%) |
| Diabetes | 42 | 18 (43%) |
| Previous cardiac history | ||
| Know heart disease | 42 | 31 (73.8%) |
| LVEF | 25 | 49.8 (SD 13.5) |
| Daily per os furosemide posology (mg) | 42 | 132.5 (SD 231.5) |
| Triggering factor of AHF | ||
| Unknown | 42 | 8 (19,0%) |
| Anaemia | 42 | 9 (21,4%) |
| Pneumopathy | 42 | 8 (21,4%) |
| Atrial fibrillation | 42 | 5 (11,9%) |
| Other bacterial infections (pyelonephritis, erysipelas) | 42 | 4 (9,5%) |
| Recent decrease in diuretic treatment | 42 | 2 (4,8%) |
| Non-compliance in healthcare | 42 | 2 (4,8%) |
| Pulmonary embolism | 42 | 1 (2,4%) |
| Altitude hypoxemia | 42 | 1 (2,4%) |
| Covid-19 | 42 | 1 (2,4%) |
| Biological and radiography characteristics at admission | ||
| Haemoglobin (g/dL) | 42 | 11.2 ( SD 1.9) |
| Ferritin (ng/ml) | 30 | 167.1 (SD 199.2) |
| Transferrin saturation (%) | 30 | 12.4 (SD 9.1) |
| Creatinine (µmol/L) | 42 | 134.9 (SD 55.0) |
| Urea (mmol/L) | 42 | 14.7 (SD 9.5) |
| Natremia (mmol/L) | 42 | 139.8 (SD 3.8) |
| Kalemia (mmol/L) | 41 | 4.2 (SD 0.7) |
| NT-pro BNP (ng/L) | 41 | 9041 (SD 8770.6) |
| Albumin (g/L) | 35 | 32.1 (SD 5.6) |
| Signs of pulmonary oedema on chest radiography | 36 | 30 (83.3%) |
| During hospitalization | ||
| Length of hospitalization (days) | 42 | 8.0 (5.1) |
| Furosemide posology IV received in ED (mg) | 42 | 122.4 (SD 214.5) |
| Furosemide posology IV/24h on admission (mg) | 42 | 159.2 (SD 256.5) |
| Intra-venous iron supplementation | 42 | 24 (57.1%) |
| Dietetic consultation | 42 | 15 (35,7%) |
| Physiotherapy | 42 | 25 (59,5%) |
| Therapeutic patient education | 42 | 5 (11,9%) |
| At discharge | ||
| Daily per os furosemide posology (mg) | 42 | 153.9 (SD 268.5) |
| Biological check-up at 7 days | 42 | 22 (52,4%) |
| Consultation with attending physician at 7 days | 42 | 18 (42,9%) |
| Consultation with cardiologist at 20 days | 42 | 20 (47,6%) |
| Admission (0%) | 25% | 50% | 75% | Discharge (100%) | ||
|---|---|---|---|---|---|---|
|
Weight loss Mean (SD) [count] |
- | -0,8 (-0,4) [24] | -1,7 (-0,8) [22] | -1,6 (-1,2) [21] | -2,7 (-1,6) [20] | |
|
Dyspnoea (self-evaluation) Mean (SD) [count] |
5.1 (3.2) [34] | 3.3 (2.9) [25] | 2.1 (2.5) [26] | 1.8 (2.3) [25] | 1.7 (2.2) [28] | |
|
NHYA Mean (SD) [count] |
3.1 (1.0) [35] | 2.3 (1.0) [27] | 2.2 (1.0) [27] | 2.1 (0.9) [23] | 2.2 (0.9) [27] | |
|
Orthopnoea % of total [count] |
Yes | 36.6% [15] | 14.7% [5] | 9.7% [3] | 7.4% [2] | 2.6% [1] |
| No | 63.4% [26] | 85.3% [29] | 90.3% [28] | 92.6% [25] | 97.4% [37] | |
|
Asthenia (self-evaluation) Mean (SD) [count] |
5.6 (3.0) [31] | 4.4 (2.7) [23] | 3.9 (3.0) [20] | 3.3 (3.2) [18] | 3.2 (2.6) [21] | |
|
Crackles % of total [count] |
No | 24.4% [10] | 32.3% [10] | 35.5% [11] | 30.0% [9] | 44.7% [17] |
| Base | 26.8% [11] | 38.7% [12] | 48.4% [15] | 53.3% [16] | 31.6% [12] | |
| 1/4 lung | 12.2% [5] | 6.5% [2] | 6.5% [2] | 10.0% [3] | 21.1% [8] | |
| 1/2 lung | 29.3% [12] | 22.6% [7] | 9.7% [3] | 6.7% [2] | 2.6% [1] | |
| 3/4 lung | 7.3% [3] | 0.0% [0] | 0.0% [0] | 0.0% [0] | 0.0% [0] | |
|
VM abolition % of total [count] |
No | 61.0% [25] | 75.0% [24] | 63.3% [19] | 63.3% [19] | 84.2% [32] |
| Base | 22.0% [9] | 9.4% [3] | 23.3% [7] | 26.7% [8] | 10.5% [4] | |
| 1/4 lung | 4.9% [2] | 6.2% [2] | 3.3% [1] | 3.3% [1] | 5.3% [2] | |
| 1/2 lung | 7.3% [3] | 6.2% [2] | 6.7% [2] | 6.7% [2] | 0.0% [0] | |
| 3/4 lung | 2.4% [1] | 3.1% [1] | 3.3% [1] | 0.0% [0] | 0.0% [0] | |
| All lung | 2.4% [1] | 0.0% [0] | 0.0% [0] | 0.0% [0] | 0.0% [0] | |
|
Lower limbs oedema % of total [count] |
No | 33.3% [14] | 50.0% [16] | 14 (46.7%) | 48.3% [14] | 56.8% [21] |
| Ankle | 28.6% [12] | 21.9% [7] | 12 (40.0%) | 44.8% [13] | 45.1% [13] | |
| Knee | 28.6% [12] | 25.0% [8] | 3 (10.0%) | 6.9% [2] | 5.4% [2] | |
| Thigh | 9.5% [4] | 3.1% [1] | 1 (3.3%) | 0.0% [0] | 2.7% [1] | |
|
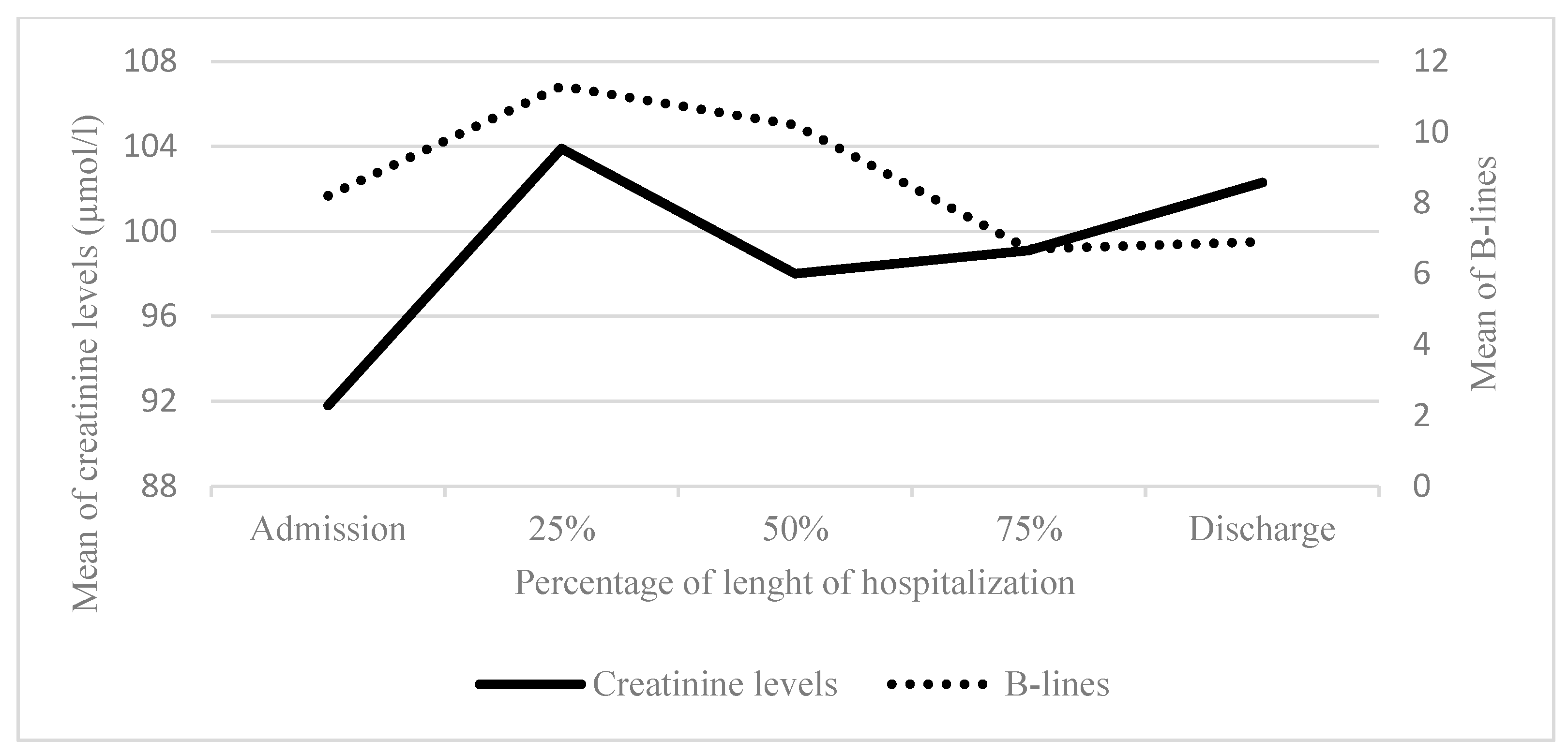
Creatinine (µmol/l) Mean (SD) [count] |
133.7 (55.1) [42] | 139.1 (51.8) [30] | 139.8 (54.0) [26] | 144.1 (58.5) [21] | 142.8 (57.5) [30] | |
|
Furosemide posology (IV, mg) Mean (SD) [count] |
162.1 (SD 259.0) [42] | 147.4 (224.7) [35] | 124.0 (220.2) [36] | 80.5 (137.4) [34] | 77.0 (134.2) [42] | |
|
Pleural effusion % of total [count] |
Yes | 61.5% [8] | 60.0% [15] | 48.0% [12] | 46.2% [12] | 35.7% [10] |
| No | 38.5% [5] | 40.0% [10] | 52.0% [13] | 53.8% [14] | 64.3% [18] | |
|
B-lines left Mean (SD) [count] |
9.1 (7.5) [13] | 6.8 (5.1) [25] | 5.6 (5.1) [25] | 4.8 (4.1) [26] | 4.5 (3.5) [28] | |
|
B-lines right Mean (SD) [count] |
9.8 (7.1) [13] | 8.2 (6.9) [25] | 6.3 (4.8) [25] | 6.0 (4.2) [26] | 3.9 (4.0) [28] | |
|
Total B-lines Mean (SD) [count] |
18.1 (13.0) [13] | 13.4 (10.2) [25] | 11.9 (9.4) [25] | 10.8 (7.8) [26] | 8.4 (6.8) [28] | |
| Hospitalization time | Intensity of clinic sign | No. | B-lines mean (SD) [count] | p value1 |
|---|---|---|---|---|
| Admission (0%) | Absent | 13 | 14.7 (9.1) [3] | 0.295 |
| Moderate a | 12.8 (14.4) [5] | |||
| Severe b | 25.4 (12.3) [5] | |||
| 25% | Absent | 31 | 10.3 (11.4) [10] | 0.824 |
| Moderate a | 8.7 (9.9) [14] | |||
| Severe b | 11.7 (11.1) [7] | |||
| 50% | Absent | 31 | 9.8 (11.8) [11] | 0.539 |
| Moderate a | 9.1 (8.3) [17] | |||
| Severe b | 3.0 (3.6) [3] | |||
| 75% | Absent | 30 | 12.8 (9.4) [9] | 0.223 |
| Moderate a | 7.4 (7.9) [19] | |||
| Severe b | 4.5 (6.4) [2] | |||
| At discharge (100%) | Absent | 39 | 6.6 (7.1) [17] | 0.055 |
| Moderate a | 3.9 (6.4) [21] | |||
| Severe b | 20.0 (NA) [1] |
| At discharge | No crackles | Bases crackles | ¼ lung crackles | ½ lung crackles | ¾ lung crackles | All lung crackles | p-value1 |
|---|---|---|---|---|---|---|---|
|
B lines Mean (SD) [count] |
6.6 (7.1) [17] | 4.1 (7.2) [13] | 3.6 (5.4) [8] | 20.0 (NA) [1] | 0.0 (NA) [0] | 0.0 (NA) [0] | 0.124 |
| Hospitalization time | CDK history | No. | Creatinine in µmol/l mean (SD) [count] | B-lines mean (SD) [count] | p value1 |
|---|---|---|---|---|---|
| Admission (0%) | Yes | 22 | 174.2 (43.1) [22] | 24.2 (11.6) [8] | 0.023 |
| No | 20 | 91.8 (26.9) [20] | 8.2 (8.8) [5] | ||
| 25% | Yes | 22 | 174.3 (43.8) [15] | 14.6 (9.4) [16] | 0.450 |
| No | 20 | 103.9 (31.4) [15] | 11.3 (11.7) [9] | ||
| 50% | Yes | 22 | 170.5 (49.8) [15] | 13.7 (10.2)[12] | 0.373 |
| No | 20 | 98.0 (21.7) [11] | 10.2 (8.7) [13] | ||
| 75% | Yes | 22 | 171.8 (56.6) [13] | 14.4 (8.5) [14] | 0.008 |
| No | 20 | 99.1 (23.3) [8] | 6.7 (3.9) [12] | ||
| At discharge (100%) | Yes | 22 | 169.8 (57) [18] | 9.2 (7.5) [18] | 0.406 |
| No | 20 | 102.3 (26.9) [12] | 6.9 (5.2) [10] |
| At 30-days after discharge | B-lines mean (SD) [count] at discharge | p-value1 | |
|---|---|---|---|
| Vital status | Death (all causes) | 15.2 (10.1) [5] | <0.001 |
| Alive | 3.9 (5.4) [37] | ||
| Hospital status for AHF | Readmission | 4.9 (5.4) [8] | 0.469 |
| No readmission | 4.1 (5.7) [30] | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





